Effects of High Glucose on Human Endothelial Cells Exposed to Simulated Microgravity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culturing
2.2. Three-Dimensional Clinostat
2.3. Sample Collection and Protein Extraction
2.4. Western Blot Analysis
2.5. Immunofluorescence Staining and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labelling (TUNEL) Assay
2.6. RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
2.7. STRING Analysis
2.8. Statistical Analyses
3. Results
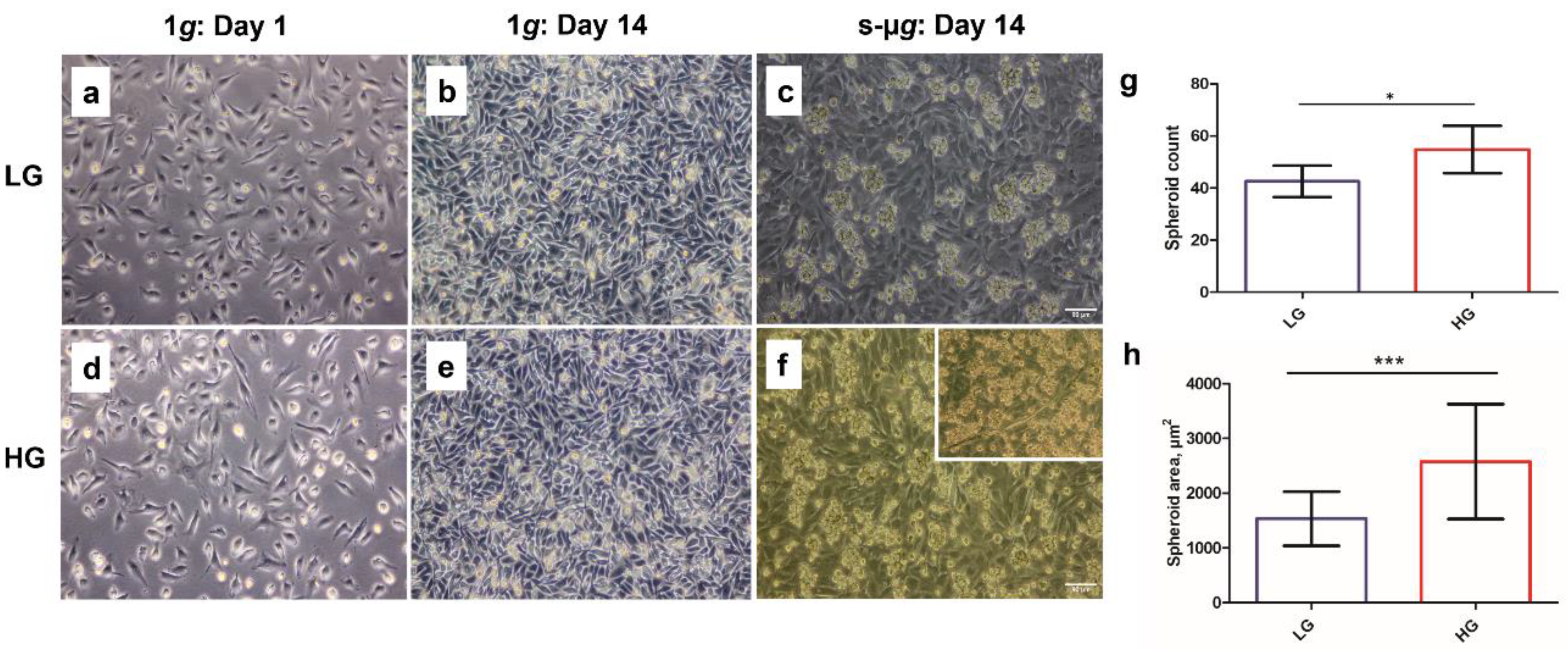
3.1. Hyperglycaemia in Microgravity Induces the Formation of Bigger and a Greater Number of Multicellular Structures
3.2. Extracellular Matrix Proteins Collagen IV and Fibronectin Are Downregulated in Microgravity
3.3. Microgravity Has a Stronger Effect on Glucose Metabolism Than Hyperglycaemia
3.3.1. Glucose Transporters
3.3.2. Triosephosphate Isomerase 1
3.4. Transglutaminase-2 Expression Is Modulated by Microgravity and Hyperglycaemia
3.5. NADPH Oxidase 4 and Interleukin-8 Are Upregulated on the Clinostat
3.5.1. NADPH Oxidase 4
3.5.2. Interleukin-8
3.6. Endothelial Cells Undergo Apoptosis on the Clinostat
3.6.1. Osteopontin
3.6.2. NF-κB p65
3.6.3. Caspase-3
3.6.4. TUNEL Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Antibody | Host Animal | Dilution for ICC | Dilution for WB | Supplier, Cat. No. |
| NOX4 | rabbit | 1:250 | 1:4000 | Abcam, Cambridge, UK, ab133303 |
| GLUT-1 | rabbit | 1:200 | - | Sigma-Aldrich, 07-1401 |
| GLUT3 | rabbit | 1:250 | - | Abcam, ab41525 |
| TIM | rabbit | 1:250 | - | Santa Cruz Biotechnology, Dallas, TX, USA, sc-30145 |
| TG2 | mouse | 1:250 | 1:1000 | Zedira, Darmstadt, Germany, A033 |
| COL4A6 | rabbit | 1:250 | - | ThermoFischer, PA5-50939 |
| Anti-Fibronectin | rabbit | 1:250 | 1:800 | Abcam, ab2413 |
| CASP3 Activated CASP3 | rabbit rabbit | 1:250 - | - 1:1000 | Cell Signaling Technology, Danvers, MA, USA, 9662S Sigma-Aldrich, C8487 |
| OPN | mouse | 1:250 | - | Sigma-Aldrich, SAB4200018 |
| CXCL8 (IL-8) | mouse | 1:250 | - | ThermoFischer, AHC0762 |
| NF-κB p65 | rabbit | 1:200 | - | Cell Signaling Technology, 4764S |
| Anti-Rabbit (Alexa Fluor® 488) | goat | 1:500 | - | Abcam, ab150077 |
| Anti-Rabbit (Alexa Fluor® 594) | goat | 1:500 | - | Abcam, ab150080 |
| Anti-Mouse (Alexa Fluor® 488) | goat | 1:500 | - | Invitrogen™, A11029 |
| Anti-mouse IgG, HRP-linked | horse | - | 1:4000 | Cell Signaling Technology, 7076S |
| Anti-rabbit IgG (H+L) Cross-Adsorbed, HRP | goat | - | 1:4000 | Invitrogen™, G21234 |
| Gene Name | Primer Name | Sequence * |
| 18S-rRNA | 18S-F | GGAGCCTGCGGCTTAATTT |
| 18S-R | CAACTAAGAACGGCCATGCA | |
| CASP3 | CASP3-F | AACTGCTCCTTTTGCTGTGATCT |
| CASP3-R | GCAGCAAACCTCAGGGAAAC | |
| CASP8 | CASP8-F | TGCAAAAGCACGGGAGAAAG |
| CASP8-R | CTCTTCAAAGGTCGTGGTCAAAG | |
| CASP9 | CASP9-F | CTCCAACATCGACTGTGAGAAGTT |
| CASP9-R | GCGCCAGCTCCAGCAA | |
| COL1A1 | COL1A1-F | CGATGGATTCCCGTTCGAGT |
| COL1A1-R | GAGGCCTCGGTGGACATTAG | |
| COL4A6 | COL4-F | GGTACCTGTAACTACTATGCCAACTCCTA |
| COL4-R | CGGCTAATTCGTGTCCTCAAG | |
| CXCL8 | CXCL8-F | TGGCAGCCTTCCTGATTTCT |
| CXCL8-R | GGGTGGAAAGGTTTGGAGTATG | |
| ENO1 | ENO1-F | TGGGAAAGATGCCACCAATGT |
| ENO1-R | GCAGCTCCAGGCCTTCTTTA | |
| FAK1 | FAK1-F | TGTGGGTAAACCAGATCCTGC |
| FAK1-R | CTGAAGCTTGACACCCTCGT | |
| FN1 | FN1-F | AGATCTACCTGTACACCTTGAATGACA |
| FN1-R | CATGATACCAGCAAGGAATTGG | |
| GAPDH | GAPDH-F | CCACATCGCTCAGACACCAT |
| GAPDH-R | GCAACAATATCCACTTTACCAGAGTTAA | |
| GLUT1 | GLUT1-F | TTCACTGTCGTGTCGCTGTT |
| GLUT1-R | TGAGTATGGCACAACCCGC | |
| GLUT3 | GLUT3-F | ACATTTTGAAGGTTTTGTTGGCTG |
| GLUT3-R | TCAGAGCTGGGGTGACCTTC | |
| JNK1 | JNK1-F | TCTCCTTTAGGTGCAGCAGTG |
| JNK1-R | CAGAGGCCAAAGTCGGATCT | |
| NOX4 | NOX4-F | ACCCTCACAATGTGTCCAAC |
| NOX4-R | CTCGAAATCGTTCTGTCCAGTC | |
| PARP1 | PARP1-F | CGAGTCGAGTACGCCAAGAG |
| PARP1-R | CATCAAACATGGGCGACTGC | |
| RELA | RELA-F | ACTGCCGGGATGGCTTCT |
| RELA-R | CGCTTCTTCACACACTGGATTC | |
| SPP1 | SPP1-F | CCGAGGTGATAGCTTGGCTT |
| SPP1-R | TGTGGCATCAGGATACTGTTCA | |
| TGM2 | TGM2-F | AAGAGGAGCGGCAGGAGTATG |
| TGM2-R | GCCCAAAATTCCAAGGTATGTTC | |
| TPI1 | TPI1-F | CAAGGTCGTCCTGGCCTATG |
| TPI1-R | TGTACTTCCTGGGCCTGTTG |

References
- Grimm, D. Microgravity and space medicine. Int. J. Mol. Sci. 2021, 22, 6697. [Google Scholar] [CrossRef] [PubMed]
- Löbrich, M.; Jeggo, P.A. Hazards of human spaceflight. Science 2019, 364, 127–128. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Shi, J.; Wang, J.; Hao, J.; Pang, X.; Huang, X.; Chen, X.; Li, Y.; Jin, R.; et al. Intestinal microbiota contributes to altered glucose metabolism in simulated microgravity mouse model. FASEB J. 2019, 33, 10140–10151. [Google Scholar] [CrossRef] [Green Version]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA twins study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef]
- Tobin, B.W.; Uchakin, P.N.; Leeper-Woodford, S.K. Insulin secretion and sensitivity in space flight: Diabetogenic effects. Nutrition 2002, 18, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Hughson, R.L.; Robertson, A.D.; Arbeille, P.; Shoemaker, J.K.; Rush, J.W.E.; Fraser, K.S.; Greaves, D.K. Increased postflight carotid artery stiffness and inflight insulin resistance resulting from 6-mo spaceflight in male and female astronauts. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H628–H638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Chen, J.; Wu, F.; Li, J.; Liang, P.; Zhang, H.; Wang, H.; Li, Y.; Wan, Y.; Qin, L.; et al. Effects of 60-day head-down bed rest on osteocalcin, glycolipid metabolism and their association with or without resistance training. Clin. Endocrinol. 2014, 81, 671–678. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, S.; Amirova, L.; Murphy, R.; Wallace, R.; Twomey, L.; Gauquelin-Koch, G.; Raverot, V.; Larcher, F.; Custaud, M.A.; Navasiolava, N. Multi-System Deconditioning in 3-Day Dry Immersion without Daily Raise. Front. Physiol. 2017, 8, 799. [Google Scholar] [CrossRef] [Green Version]
- Strollo, F.; Gentile, S.; Pipicelli, A.M.V.; Mambro, A.; Monici, M.; Magni, P. Space Flight-Promoted Insulin Resistance as a Possible Disruptor of Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 868999. [Google Scholar] [CrossRef]
- Mapanga, R.F.; Essop, M.F. Damaging effects of hyperglycemia on cardiovascular function: Spotlight on glucose metabolic pathways. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H153–H173. [Google Scholar] [CrossRef]
- Allen, D.A.; Yaqoob, M.M.; Harwood, S.M. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J. Nutr. Biochem. 2005, 16, 705–713. [Google Scholar] [CrossRef]
- Bhedi, C.D.; Nasirova, S.; Toksoz, D.; Warburton, R.R.; Morine, K.J.; Kapur, N.K.; Galper, J.B.; Preston, I.R.; Hill, N.S.; Fanburg, B.L.; et al. Glycolysis regulated transglutaminase 2 activation in cardiopulmonary fibrogenic remodeling. FASEB J. 2020, 34, 930–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, M.P.; Lim, Y.-C.; Hwang, J.; Na, S.; Kim, Y.-M.; Ha, K.-S. C-Peptide Prevents Hyperglycemia-Induced Endothelial Apoptosis Through Inhibition of Reactive Oxygen Species–Mediated Transglutaminase 2 Activation. Diabetes 2013, 62, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Du, X.; Yao, X.; Qiu, Y.; Jiang, W.; Shen, J.; Li, L.; Liu, X. Mechanism of cell death of endothelial cells regulated by mechanical forces. J. Biomech. 2022, 131, 110917. [Google Scholar] [CrossRef]
- Edgell, C.J.S.; Haizlip, J.E.; Bagnell, C.R.; Packenham, J.P.; Harrison, P.; Wilbourn, B.; Madden, V.J. Endothelium specific Weibel-Palade bodies in a continuous human cell line, EA.hy926. In Vitro Cell. Dev. Biol. 1990, 26, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Gass, S.; Nebuloni, S.; Echegoyen, D.; Riwaldt, S.; Baake, C.; Bauer, J.; Corydon, T.J.; Egli, M.; Infanger, M.; et al. Three-dimensional growth of human endothelial cells in an automated cell culture experiment container during the SpaceX CRS-8 ISS space mission—The SPHEROIDS project. Biomaterials 2017, 124, 126–156. [Google Scholar] [CrossRef]
- Baran, R.; Marchal, S.; Campos, S.G.; Rehnberg, E.; Tabury, K.; Baselet, B.; Wehland, M.; Grimm, D.; Baatout, S. The Cardiovascular System in Space: Focus on In Vivo and In Vitro Studies. Biomedicines 2022, 10, 59. [Google Scholar] [CrossRef]
- Grimm, D.; Schulz, H.; Krüger, M.; Cortés-Sánchez, J.L.; Egli, M.; Kraus, A.; Sahana, J.; Corydon, T.J.; Hemmersbach, R.; Wise, P.M.; et al. The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. Int. J. Mol. Sci. 2022, 23, 3073. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Sahana, J.; Krüger, M.; Wehland, M.; Bauer, T.J.; Liemersdorf, C.; Hemmersbach, R.; et al. Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef] [Green Version]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Krüger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schütte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef]
- Snel, B.; Lehmann, G.; Bork, P.; Huynen, M.A. STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000, 28, 3442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotrupi, S.; Ranzani, D.; Maier, J.A.M. Impact of modeled microgravity on microvascular endothelial cells. Biochim. Biophys. Acta-Mol. Cell Res. 2005, 1746, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, J.A.M.; Cialdai, F.; Monici, M.; Morbidelli, L. The Impact of Microgravity and Hypergravity on Endothelial Cells. Biomed Res. Int. 2015, 2015, 434803. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D.; Wehland, M.; Pietsch, J.; Aleshcheva, G.; Wise, P.; Van Loon, J.; Ulbrich, C.; Magnusson, N.E.; Infanger, M.; Bauer, J. Growing tissues in real and simulated microgravity: New methods for tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 555–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Loon, J.J.W.A. Centrifuges for Microgravity Simulation. The Reduced Gravity Paradigm. Front. Astron. Sp. Sci. 2016, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; De Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-Based Facilities for Simulation of Microgravity: Organism-Specific Recommendations for Their Use, and Recommended Terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Oluwafemi, F.A.; Neduncheran, A. Analog and simulated microgravity platforms for life sciences research: Their individual capacities, benefits and limitations. Adv. Sp. Res. 2022, 69, 2921–2929. [Google Scholar] [CrossRef]
- Andreeva, E.; Matveeva, D.; Zhidkova, O.; Zhivodernikov, I.; Kotov, O.; Buravkova, L. Real and Simulated Microgravity: Focus on Mammalian Extracellular Matrix. Life 2022, 12, 1343. [Google Scholar] [CrossRef]
- Dittrich, A.; Grimm, D.; Sahana, J.; Bauer, J.; Kröger, M.; Infanger, M.; Magnusson, N.E. Key Proteins Involved in Spheroid Formation and Angiogenesis in Endothelial Cells After Long-Term Exposure to Simulated Microgravity. Cell. Physiol. Biochem. 2018, 45, 429–445. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Sun, S.; Zhang, C.; Lü, D.; Chen, Q.; Long, M. Microgravity-Induced Alterations of Inflammation-Related Mechanotransduction in Endothelial Cells on Board SJ-10 Satellite. Front. Physiol. 2018, 9, 1025. [Google Scholar] [CrossRef]
- Luo, E.; Wang, D.; Yan, G.; Qiao, Y.; Zhu, B.; Liu, B.; Hou, J.; Tang, C. The NF-κB/miR-425-5p/MCT4 axis: A novel insight into diabetes-induced endothelial dysfunction. Mol. Cell. Endocrinol. 2020, 500, 110641. [Google Scholar] [CrossRef]
- Daniel, B.; Livne, A.; Cohen, G.; Kahremany, S.; Sasson, S. Endothelial Cell–Derived Triosephosphate Isomerase Attenuates Insulin Secretion From Pancreatic Beta Cells of Male Rats. Endocrinology 2021, 162, bqaa234. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, P.M.; Pedrini, E.; Hughes, D.; McDonnell, S.; Pathak, V.; Peixoto, E.; Guduric-Fuchs, J.; Stitt, A.W.; Medina, R.J. Long term high glucose exposure induces premature senescence in retinal endothelial cells. Front. Physiol. 2022, 13, 929118. [Google Scholar] [CrossRef]
- Fernandes, R.; Suzuki, K.; Kumagai, A.K. Inner blood-retinal barrier GLUT1 in long-term diabetic rats: An immunogold electron microscopic study. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3150–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, B.; Rösen, P. Diabetes mellitus induces long lasting changes in the glucose transporter of rat heart endothelial cells. Horm. Metab. Res. 1999, 31, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Y.; Sin-Malia, Y.; Cohen, G.; Alpert, E.; Gruzman, A.; Eckel, J.; Staels, B.; Guichardant, M.; Sasson, S. The Natural Protective Mechanism Against Hyperglycemia in Vascular Endothelial CellsRoles of the Lipid Peroxidation Product 4-Hydroxydodecadienal and Peroxisome Proliferator–Activated Receptor δ. Diabetes 2010, 59, 808–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leão, L.L.; Tangen, G.; Barca, M.L.; Engedal, K.; Santos, S.H.S.; Machado, F.S.M.; de Paula, A.M.B.; Monteiro-Junior, R.S. Does hyperglycemia downregulate glucose transporters in the brain? Med. Hypotheses 2020, 139, 109614. [Google Scholar] [CrossRef]
- Akimov, S.S.; Krylov, D.; Fleischmana, L.F.; Belkin, A.M. Tissue Transglutaminase Is an Integrin-Binding Adhesion Coreceptor for Fibronectin. J. Cell Biol. 2000, 148, 825. [Google Scholar] [CrossRef] [Green Version]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.W.; Mehta, K. Transglutaminase Regulation of Cell Function. Physiol. Rev. 2014, 94, 383. [Google Scholar] [CrossRef] [Green Version]
- Nadalutti, C.; Viiri, K.M.; Kaukinen, K.; Mäki, M.; Lindfors, K. Extracellular transglutaminase 2 has a role in cell adhesion, whereas intracellular transglutaminase 2 is involved in regulation of endothelial cell proliferation and apoptosis. Cell Prolif. 2011, 44, 49–58. [Google Scholar] [CrossRef]
- Bisserier, M.; Shanmughapriya, S.; Rai, A.K.; Gonzalez, C.; Brojakowska, A.; Garikipati, V.N.S.; Madesh, M.; Mills, P.J.; Walsh, K.; Arakelyan, A.; et al. Cell-free mitochondrial DNA as a potential biomarker for astronauts’ health. J. Am. Heart Assoc. 2021, 10, 22055. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Ran, H.H.; Zhang, Y.; Zhao, Y.; Fan, Y.Y.; Peng, L.; Zhang, R.; Cao, F. NADPH Oxidase Accounts for Changes in Cerebrovascular Redox Status in Hindlimb Unweighting Rats. Biomed. Environ. Sci. 2015, 28, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ran, H.H.; Peng, L.; Xu, F.; Sun, J.F.; Zhang, L.N.; Fan, Y.Y.; Peng, L.; Cui, G. Mitochondrial regulation of NADPH oxidase in hindlimb unweighting rat cerebral arteries. PLoS ONE 2014, 9, e95916. [Google Scholar] [CrossRef]
- Takahashi, K.; Okumura, H.; Guo, R.; Naruse, K. Effect of Oxidative Stress on Cardiovascular System in Response to Gravity. Int. J. Mol. Sci. 2017, 18, 1426. [Google Scholar] [CrossRef] [Green Version]
- Dietrichs, D.; Grimm, D.; Sahana, J.; Melnik, D.; Corydon, T.J.; Wehland, M.; Krüger, M.; Vermeesen, R.; Baselet, B.; Baatout, S.; et al. Three-Dimensional Growth of Prostate Cancer Cells Exposed to Simulated Microgravity. Front. Cell Dev. Biol. 2022, 10, 234. [Google Scholar] [CrossRef]
- Kopp, S.; Krüger, M.; Bauer, J.; Wehland, M.; Corydon, T.J.; Sahana, J.; Nassef, M.Z.; Melnik, D.; Bauer, T.J.; Schulz, H.; et al. Microgravity Affects Thyroid Cancer Cells during the TEXUS-53 Mission Stronger than Hypergravity. Int. J. Mol. Sci. 2018, 19, 4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favaro, F.; Luciano-Mateo, F.; Moreno-Caceres, J.; Hernández-Madrigal, M.; Both, D.; Montironi, C.; Püschel, F.; Nadal, E.; Eldering, E.; Muñoz-Pinedo, C. TRAIL receptors promote constitutive and inducible IL-8 secretion in non-small cell lung carcinoma. Cell Death Dis. 2022, 13, 1046. [Google Scholar] [CrossRef]
- Kang, C.Y.; Zou, L.; Yuan, M.; Wang, Y.; Li, T.Z.; Zhang, Y.; Wang, J.F.; Li, Y.; Deng, X.W.; Liu, C.T. Impact of simulated microgravity on microvascular endothelial cell apoptosis. Eur. J. Appl. Physiol. 2011, 111, 2131–2138. [Google Scholar] [CrossRef]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Baatout, S.; Witzing, A.; Grosse, J.; Bauer, J.; Cogoli, A.; Faramarzi, S.; Derradji, H.; et al. Induction of three-dimensional assembly and increase in apoptosis of human endothelial cells by simulated microgravity: Impact of vascular endothelial growth factor. Apoptosis 2006, 11, 749–764. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Rudimov, E.G.; Andreeva, E.R.; Grigoriev, A.I. The ICAM-1 expression level determines the susceptibility of human endothelial cells to simulated microgravity. J. Cell. Biochem. 2018, 119, 2875–2885. [Google Scholar] [CrossRef]
- Mamazhakypov, A.; Sartmyrzaeva, M.; Sarybaev, A.S.; Schermuly, R.; Sydykov, A. Clinical and Molecular Implications of Osteopontin in Heart Failure. Curr. Issues Mol. Biol. 2022, 44, 3573–3597. [Google Scholar] [CrossRef] [PubMed]
- Vorwald, C.E.; Murphy, K.C.; Leach, J.K. Restoring Vasculogenic Potential of Endothelial Cells from Diabetic Patients Through Spheroid Formation. Cell. Mol. Bioeng. 2018, 11, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.M.; Liu, S.H.; Liau, C.S.; Huang, P.J.; Lin-Shiau, S.Y. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation 2000, 101, 2618–2624. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Sala, R.; Cagliero, E.; Lorenzi, M. Overexpression of fibronectin induced by diabetes or high glucose: Phenomenon with a memory. Proc. Natl. Acad. Sci. USA 1990, 87, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Uruski, P.; Mikuła-Pietrasik, J.; Drzewiecki, M.; Budkiewicz, S.; Gładki, M.; Kurmanalina, G.; Tykarski, A.; Książek, K. Diverse functional responses to high glucose by primary and permanent hybrid endothelial cells in vitro. J. Mol. Cell. Cardiol. 2021, 156, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.A.; Shangraw, R.E.; Prince, M.J.; Peters, E.J.; Wolfe, R.R. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. 1988, 37, 802–806. [Google Scholar] [CrossRef]
- Hirose, M.; Kaneki, M.; Sugita, H.; Yasuhara, S.; Martyn, J.A.J. Immobilization depresses insulin signaling in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yang, Z.; Li, W.; Xue, Y.; Xu, H.; Li, J.; Shang, P. Glucocorticoid: A potential role in microgravity-induced bone loss. Acta Astronaut. 2017, 140, 206–212. [Google Scholar] [CrossRef]
- Vinken, M. Hepatology in space: Effects of spaceflight and simulated microgravity on the liver. Liver Int. 2022, 42, 2599–2606. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jokšienė, J.; Sahana, J.; Wehland, M.; Schulz, H.; Cortés-Sánchez, J.L.; Prat-Duran, J.; Grimm, D.; Simonsen, U. Effects of High Glucose on Human Endothelial Cells Exposed to Simulated Microgravity. Biomolecules 2023, 13, 189. https://doi.org/10.3390/biom13020189
Jokšienė J, Sahana J, Wehland M, Schulz H, Cortés-Sánchez JL, Prat-Duran J, Grimm D, Simonsen U. Effects of High Glucose on Human Endothelial Cells Exposed to Simulated Microgravity. Biomolecules. 2023; 13(2):189. https://doi.org/10.3390/biom13020189
Chicago/Turabian StyleJokšienė, Justina, Jayashree Sahana, Markus Wehland, Herbert Schulz, José Luis Cortés-Sánchez, Judit Prat-Duran, Daniela Grimm, and Ulf Simonsen. 2023. "Effects of High Glucose on Human Endothelial Cells Exposed to Simulated Microgravity" Biomolecules 13, no. 2: 189. https://doi.org/10.3390/biom13020189
APA StyleJokšienė, J., Sahana, J., Wehland, M., Schulz, H., Cortés-Sánchez, J. L., Prat-Duran, J., Grimm, D., & Simonsen, U. (2023). Effects of High Glucose on Human Endothelial Cells Exposed to Simulated Microgravity. Biomolecules, 13(2), 189. https://doi.org/10.3390/biom13020189








