Multi-Targeting Intranasal Nanoformulation as a Therapeutic for Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Materials
2.2. MIT Nanoformulation Preparation
2.3. Intranasal Treatment
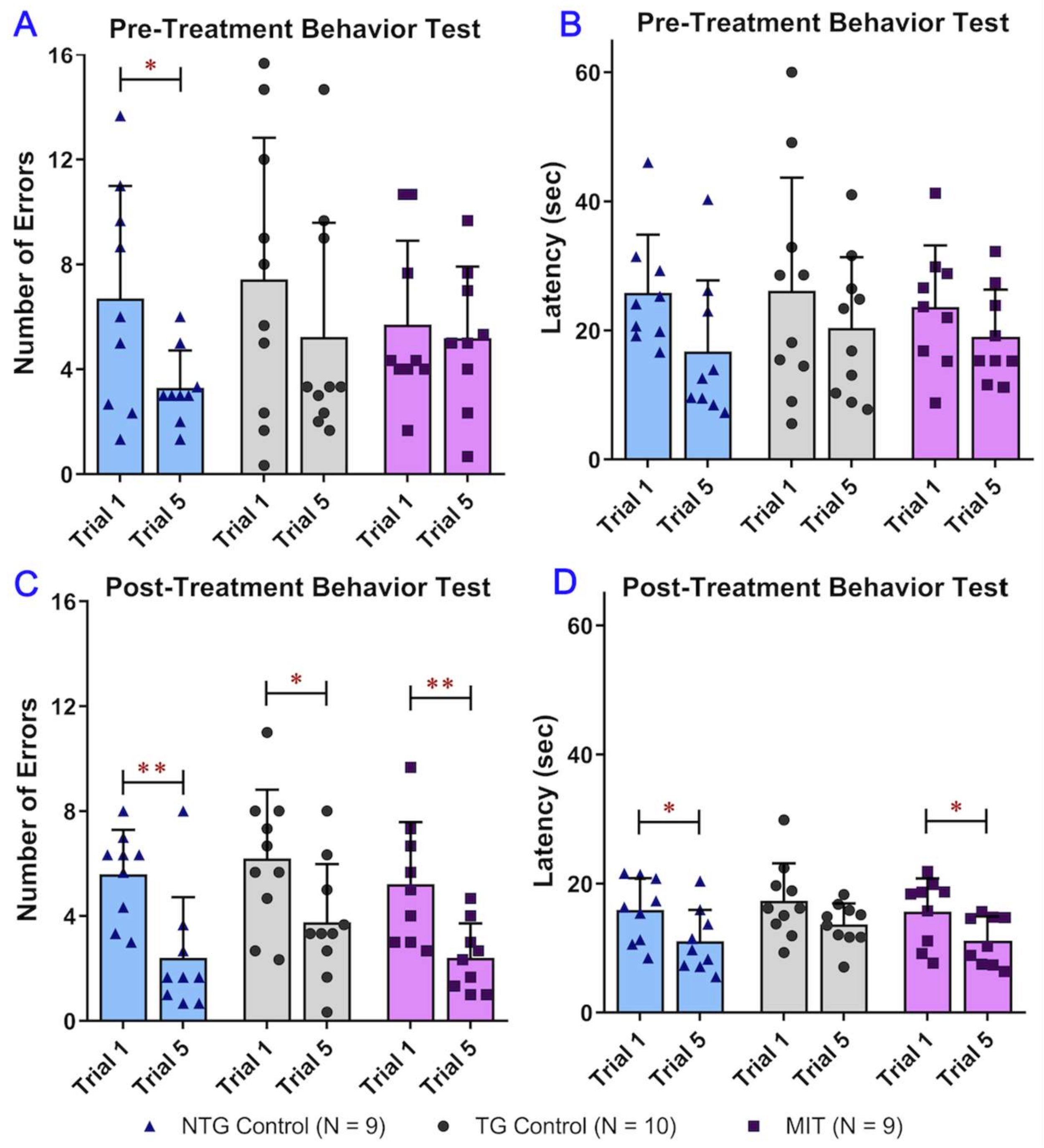
2.4. Radial Arm Water Maze Behavior Study
2.5. Tissue Preparation and Sample Collection
2.6. Brain Tissue Protein Extraction
2.7. Aβ1-40 and Aβ1-42 Peptide Measurement
2.8. Immunoblotting Detection for Protein Expression
2.9. Immunohistochemistry
2.10. Data Analysis
3. Results
3.1. MIT Nanoformulation Continuous Treatment Improves APP/PS1 Aged MICE Cognitive Performance
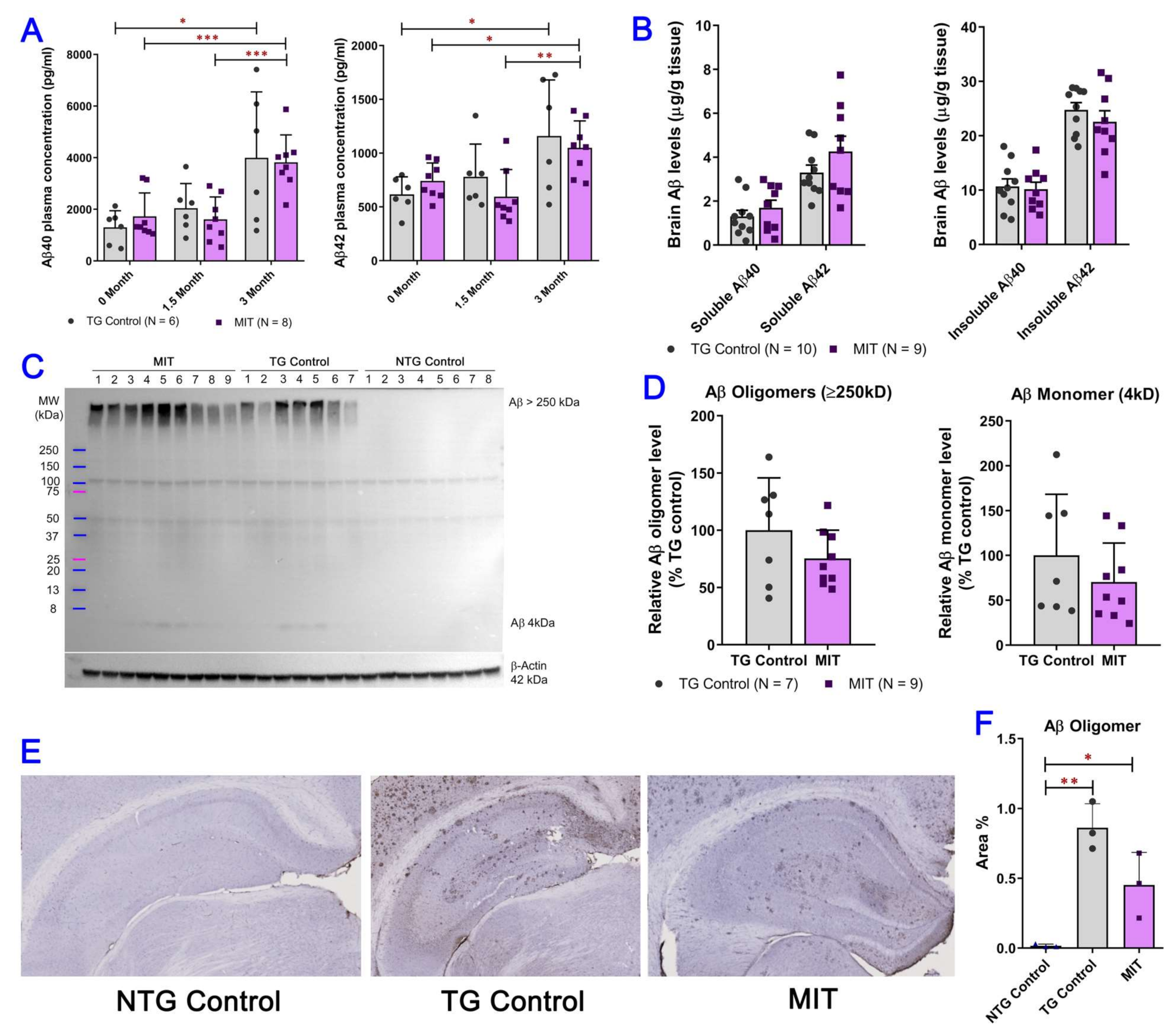
3.2. Intranasal MIT Treatment Induces a Decrease in Aβ Levels in the Brain
3.3. Long-Term Intranasal MIT Nanoformulation Treatment Reduced Tau Phosphorylation and Increased GSK3β Phosphorylation at Ser9
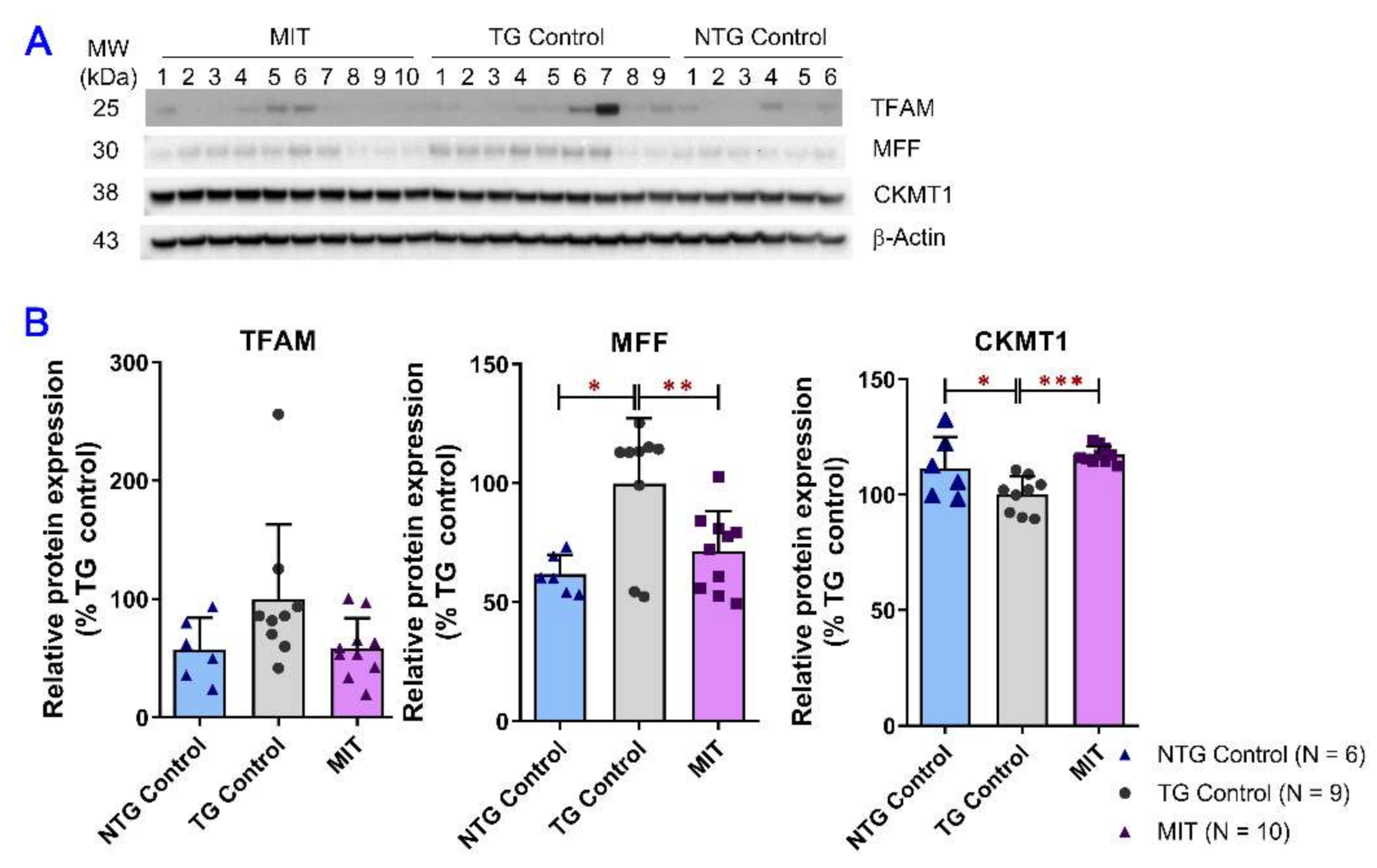
3.4. Modulation of Mitochondrial Proteins by MIT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anitua, E.; Pascual, C.; Antequera, D.; Bolos, M.; Padilla, S.; Orive, G.; Carro, E. Plasma rich in growth factors (PRGF-Endoret) reduces neuropathologic hallmarks and improves cognitive functions in an Alzheimer’s disease mouse model. Neurobiol. Aging 2014, 35, 1582–1595. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Liu, F.; Iqbal, K. Multifactorial Hypothesis and Multi-Targets for Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64 (Suppl. 1), S107–S117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, V.; Anand, P.; Kumar, V.; Ranjan Dwivedi, A.; Kumar, V. Advancements in the development of multi-target directed ligands for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2022, 61, 116742. [Google Scholar] [CrossRef]
- Macdonald, R.; Barnes, K.; Hastings, C.; Mortiboys, H. Mitochondrial abnormalities in Parkinson’s disease and Alzheimer’s disease: Can mitochondria be targeted therapeutically? Biochem. Soc. Trans. 2018, 46, 891–909. [Google Scholar] [CrossRef]
- Neth, B.J.; Craft, S. Insulin Resistance and Alzheimer’s Disease: Bioenergetic Linkages. Front. Aging Neurosci. 2017, 9, 345. [Google Scholar] [CrossRef]
- Nudelman, K.N.H.; McDonald, B.C.; Lahiri, D.K.; Saykin, A.J. Biological Hallmarks of Cancer in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 7173–7187. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Dai, C.L.; Liu, F.; Iqbal, K. Multi-Targets: An Unconventional Drug Development Strategy for Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 837649. [Google Scholar] [CrossRef]
- Schmitt, B.; Bernhardt, T.; Moeller, H.J.; Heuser, I.; Frölich, L. Combination therapy in Alzheimer’s disease: A review of current evidence. CNS Drugs 2004, 18, 827–844. [Google Scholar] [CrossRef]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Cabrera, J.; D’Arpa, D.; Sainz, R.M.; Mayo, J.C.; Ramos, S. The oxidant/antioxidant network: Role of melatonin. Biol. Signals Recept. 1999, 8, 56–63. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, M.O. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus. J. Pineal Res. 2015, 59, 47–59. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef]
- Benedict, C.; Frey, W.H., 2nd; Schiöth, H.B.; Schultes, B.; Born, J.; Hallschmid, M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp. Gerontol. 2011, 46, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef]
- Reger, M.A.; Watson, G.S.; Green, P.S.; Wilkinson, C.W.; Baker, L.D.; Cholerton, B.; Fishel, M.A.; Plymate, S.R.; Breitner, J.C.; DeGroodt, W.; et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 2008, 70, 440–448. [Google Scholar] [CrossRef]
- Jauch-Chara, K.; Friedrich, A.; Rezmer, M.; Melchert, U.H.; Scholand-Engler, H.S.; Hallschmid, M.; Oltmanns, K.M. Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes 2012, 61, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, M.H.; Barclay, T.R.; Pyle, M.; Owens, B.L.; Cagan, A.B.; Anderson, C.P.; Frey, W.H., 2nd; Hanson, L.R. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs 2014, 28, 1185–1189. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. 3), S5. [Google Scholar] [CrossRef]
- Sava, V.; Fihurka, O.; Khvorova, A.; Sanchez-Ramos, J. Kinetics of HTT lowering in brain of YAC 128 mice following single and repetitive intranasal dosing of siRNA packaged in chitosan-based nanoparticle. J. Drug Deliv. Sci. Technol. 2021, 63, 102517. [Google Scholar] [CrossRef]
- Currais, A.; Quehenberger, O.; Armando, M.A.; Daugherty, D.; Maher, P.; Schubert, D. Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids. NPJ Aging Mech Dis 2016, 2, 16012. [Google Scholar] [CrossRef]
- Abate, G.; Uberti, D.; Tambaro, S. Potential and Limits of Cannabinoids in Alzheimer’s Disease Therapy. Biology 2021, 10, 542. [Google Scholar] [CrossRef]
- Sarne, Y.; Toledano, R.; Rachmany, L.; Sasson, E.; Doron, R. Reversal of age-related cognitive impairments in mice by an extremely low dose of tetrahydrocannabinol. Neurobiol. Aging 2018, 61, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, Y.; Yan, J.; Brown, B.; Lin, X.; Zhang, X.; Shen, N.; Li, M.; Cai, J.; Gordon, M.; et al. Low-Dose Delta-9-Tetrahydrocannabinol as Beneficial Treatment for Aged APP/PS1 Mice. Int. J. Mol. Sci. 2022, 23, 2757. [Google Scholar] [CrossRef] [PubMed]
- Fihurka, O.; Hong, Y.; Yan, J.; Brown, B.; Lin, X.; Shen, N.; Wang, Y.; Zhao, H.; Gordon, M.N.; Morgan, D.; et al. The Memory Benefit to Aged APP/PS1 Mice from Long-Term Intranasal Treatment of Low-Dose THC. Int. J. Mol. Sci. 2022, 23, 4253. [Google Scholar] [CrossRef] [PubMed]
- Fihurka, O.; Sava, V.; Sanchez-Ramos, J. Dual-function hybrid nanoparticles with gene silencing and anti-inflammatory effects. Nanomedicine 2022, 17, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Sava, V.; Fihurka, O.; Khvorova, A.; Sanchez-Ramos, J. Data on enrichment of chitosan nanoparticles for intranasal delivery of oligonucleotides to the brain. Data Brief 2020, 28, 105093. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, B.; Rao, J. Nutraceutical potential of industrial hemp (Cannabis sativa L.) extracts: Physicochemical stability and bioaccessibility of cannabidiol (CBD) nanoemulsions. Food Funct. 2022, 13, 4502–4512. [Google Scholar] [CrossRef]
- Iqubal, A.; Iqubal, M.K.; Fazal, S.A.; Pottoo, F.H.; Haque, S.E. Nutraceuticals and their Derived Nano-Formulations for the Prevention and Treatment of Alzheimer’s Disease. Curr. Mol. Pharmacol. 2022, 15, 23–50. [Google Scholar] [CrossRef]
- Kiran, P.; Debnath, S.K.; Neekhra, S.; Pawar, V.; Khan, A.; Dias, F.; Pallod, S.; Srivastava, R. Designing nanoformulation for the nose-to-brain delivery in Parkinson’s disease: Advancements and barrier. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1768. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Gerges, N.Z.; Aleisa, A.M.; Alkadhi, K.A. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus 2009, 19, 66–78. [Google Scholar] [CrossRef]
- Izco, M.; Pesini, P.; Pérez-Grijalba, V.; Fandos, N.; Sarasa, M. Optimized protocol for amyloid-β extraction from the brain. J. Alzheimers Dis. 2013, 34, 835–839. [Google Scholar] [CrossRef]
- Golde, T.E.; Eckman, C.B.; Younkin, S.G. Biochemical detection of Abeta isoforms: Implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochim. Biophys. Acta 2000, 1502, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Garzon-Rodriguez, W.; Sepulveda-Becerra, M.; Milton, S.; Glabe, C.G. Soluble amyloid Abeta-(1-40) exists as a stable dimer at low concentrations. J. Biol. Chem. 1997, 272, 21037–21044. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, M.; Takashima, A.; Noguchi, K.; Murayama, M.; Sato, M.; Kondo, S.; Saitoh, Y.; Ishiguro, K.; Hoshino, T.; Imahori, K. Regulation of mitochondrial pyruvate dehydrogenase activity by tau protein kinase I/glycogen synthase kinase 3beta in brain. Proc. Natl. Acad. Sci. USA 1996, 93, 2719–2723. [Google Scholar] [CrossRef]
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef]
- Stambolic, V.; Woodgett, J.R. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem. J. 1994, 303 Pt 3, 701–704. [Google Scholar] [CrossRef]
- Jope, R.S.; Johnson, G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004, 29, 95–102. [Google Scholar] [CrossRef]
- Johri, A.; Beal, M.F. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Datler, C.; Pazarentzos, E.; Mahul-Mellier, A.L.; Chaisaklert, W.; Hwang, M.S.; Osborne, F.; Grimm, S. CKMT1 regulates the mitochondrial permeability transition pore in a process that provides evidence for alternative forms of the complex. J. Cell Sci. 2014, 127 Pt 8, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Chu, C.T.; Kaufman, B.A. The mitochondrial transcription factor TFAM in neurodegeneration: Emerging evidence and mechanisms. FEBS Lett. 2018, 592, 793–811. [Google Scholar] [CrossRef]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Ihenacho, U.K.; Meacham, K.A.; Harwig, M.C.; Widlansky, M.E.; Hill, R.B. Mitochondrial Fission Protein 1: Emerging Roles in Organellar Form and Function in Health and Disease. Front. Endocrinol. 2021, 12, 660095. [Google Scholar] [CrossRef]
- Nedelcovych, M.T.; Gadiano, A.J.; Wu, Y.; Manning, A.A.; Thomas, A.G.; Khuder, S.S.; Yoo, S.W.; Xu, J.; McArthur, J.C.; Haughey, N.J.; et al. Pharmacokinetics of Intranasal versus Subcutaneous Insulin in the Mouse. ACS Chem. Neurosci. 2018, 9, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Hamidovic, A.; Khafaja, M.; Brandon, V.; Anderson, J.; Ray, G.; Allan, A.M.; Burge, M.R. Reduction of smoking urges with intranasal insulin: A randomized, crossover, placebo-controlled clinical trial. Mol. Psychiatry 2017, 22, 1413–1421. [Google Scholar] [CrossRef]
- Pepper, A.R.; Gall, C.; Mazzuca, D.M.; Melling, C.W.; White, D.J. Diabetic rats and mice are resistant to porcine and human insulin: Flawed experimental models for testing islet xenografts. Xenotransplantation 2009, 16, 502–510. [Google Scholar] [CrossRef]
- Alamed, J.; Wilcock, D.M.; Diamond, D.M.; Gordon, M.N.; Morgan, D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat. Protoc. 2006, 1, 1671–1679. [Google Scholar] [CrossRef]
- Hascup, K.N.; Findley, C.A.; Sime, L.N.; Hascup, E.R. Hippocampal alterations in glutamatergic signaling during amyloid progression in AβPP/PS1 mice. Sci. Rep. 2020, 10, 14503. [Google Scholar] [CrossRef]
- Mak, K.; Yang, F.; Vinters, H.V.; Frautschy, S.A.; Cole, G.M. Polyclonals to beta-amyloid(1-42) identify most plaque and vascular deposits in Alzheimer cortex, but not striatum. Brain Res. 1994, 667, 138–142. [Google Scholar] [CrossRef]
- Gu, L.; Guo, Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Copes, N.; O’Neal-Moffitt, G.; Jin, J.; Buzzeo, R.; Mamcarz, M.; Tan, J.; Cao, C.; Olcese, J.M.; Arendash, G.W.; et al. Melatonin treatment restores mitochondrial function in Alzheimer’s mice: A mitochondrial protective role of melatonin membrane receptor signaling. J. Pineal Res. 2011, 51, 75–86. [Google Scholar] [CrossRef]
- Watson, G.S.; Peskind, E.R.; Asthana, S.; Purganan, K.; Wait, C.; Chapman, D.; Schwartz, M.W.; Plymate, S.; Craft, S. Insulin increases CSF Abeta42 levels in normal older adults. Neurology 2003, 60, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Yin, X.; Yu, D.; Cao, M.; Gong, C.X.; Iqbal, K.; Ding, F.; Gu, X.; Liu, F. Truncation and activation of GSK-3β by calpain I: A molecular mechanism links to tau hyperphosphorylation in Alzheimer’s disease. Sci. Rep. 2015, 5, 8187. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zeng, J.; Li, Z.; Chen, Q.; Hang, W.; Xia, L.; Wu, Y.; Chen, J.; Shi, A. Melatonin Mitigates Kainic Acid-Induced Neuronal Tau Hyperphosphorylation and Memory Deficits through Alleviating ER Stress. Front. Mol. Neurosci. 2018, 11, 5. [Google Scholar] [CrossRef]
- Luengo, E.; Buendia, I.; Fernandez-Mendivil, C.; Trigo-Alonso, P.; Negredo, P.; Michalska, P.; Hernandez-Garcia, B.; Sanchez-Ramos, C.; Bernal, J.A.; Ikezu, T.; et al. Pharmacological doses of melatonin impede cognitive decline in tau-related Alzheimer models, once tauopathy is initiated, by restoring the autophagic flux. J. Pineal Res. 2019, 67, e12578. [Google Scholar] [CrossRef]
- Chen, D.; Mei, Y.; Kim, N.; Lan, G.; Gan, C.L.; Fan, F.; Zhang, T.; Xia, Y.; Wang, L.; Lin, C.; et al. Melatonin directly binds and inhibits death-associated protein kinase 1 function in Alzheimer’s disease. J. Pineal Res. 2020, 69, e12665. [Google Scholar] [CrossRef]
- Das, R.; Balmik, A.A.; Chinnathambi, S. Melatonin Reduces GSK3beta-Mediated Tau Phosphorylation, Enhances Nrf2 Nuclear Translocation and Anti-Inflammation. ASN Neuro 2020, 12, 1759091420981204. [Google Scholar] [CrossRef]
- Planel, E.; Tatebayashi, Y.; Miyasaka, T.; Liu, L.; Wang, L.; Herman, M.; Yu, W.H.; Luchsinger, J.A.; Wadzinski, B.; Duff, K.E.; et al. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J. Neurosci. 2007, 27, 13635–13648. [Google Scholar] [CrossRef]
- Liu, C.; Song, X.; Nisbet, R.; Gotz, J. Co-immunoprecipitation with Tau Isoform-specific Antibodies Reveals Distinct Protein Interactions and Highlights a Putative Role for 2N Tau in Disease. J. Biol. Chem. 2016, 291, 8173–8188. [Google Scholar] [CrossRef]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, B.; Lee, H.G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Wang, C.; Cleland, M.M.; Setoguchi, K.; Yokota, S.; Youle, R.J.; Mihara, K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010, 191, 1141–1158. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Shirihai, O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 20. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fihurka, O.; Wang, Y.; Hong, Y.; Lin, X.; Shen, N.; Yang, H.; Brown, B.; Mommer, M.; Zieneldien, T.; Li, Y.; et al. Multi-Targeting Intranasal Nanoformulation as a Therapeutic for Alzheimer’s Disease. Biomolecules 2023, 13, 232. https://doi.org/10.3390/biom13020232
Fihurka O, Wang Y, Hong Y, Lin X, Shen N, Yang H, Brown B, Mommer M, Zieneldien T, Li Y, et al. Multi-Targeting Intranasal Nanoformulation as a Therapeutic for Alzheimer’s Disease. Biomolecules. 2023; 13(2):232. https://doi.org/10.3390/biom13020232
Chicago/Turabian StyleFihurka, Oksana, Yanhong Wang, Yuzhu Hong, Xiaoyang Lin, Ning Shen, Haiqiang Yang, Breanna Brown, Marcus Mommer, Tarek Zieneldien, Yitong Li, and et al. 2023. "Multi-Targeting Intranasal Nanoformulation as a Therapeutic for Alzheimer’s Disease" Biomolecules 13, no. 2: 232. https://doi.org/10.3390/biom13020232
APA StyleFihurka, O., Wang, Y., Hong, Y., Lin, X., Shen, N., Yang, H., Brown, B., Mommer, M., Zieneldien, T., Li, Y., Kim, J., Li, M., Cai, J., Zhou, Q., & Cao, C. (2023). Multi-Targeting Intranasal Nanoformulation as a Therapeutic for Alzheimer’s Disease. Biomolecules, 13(2), 232. https://doi.org/10.3390/biom13020232








