The Role of Platelets in Hypoglycemia-Induced Cardiovascular Disease: A Review of the Literature
Abstract
1. Introduction
2. Platelet Biology
2.1. Thrombopoiesis
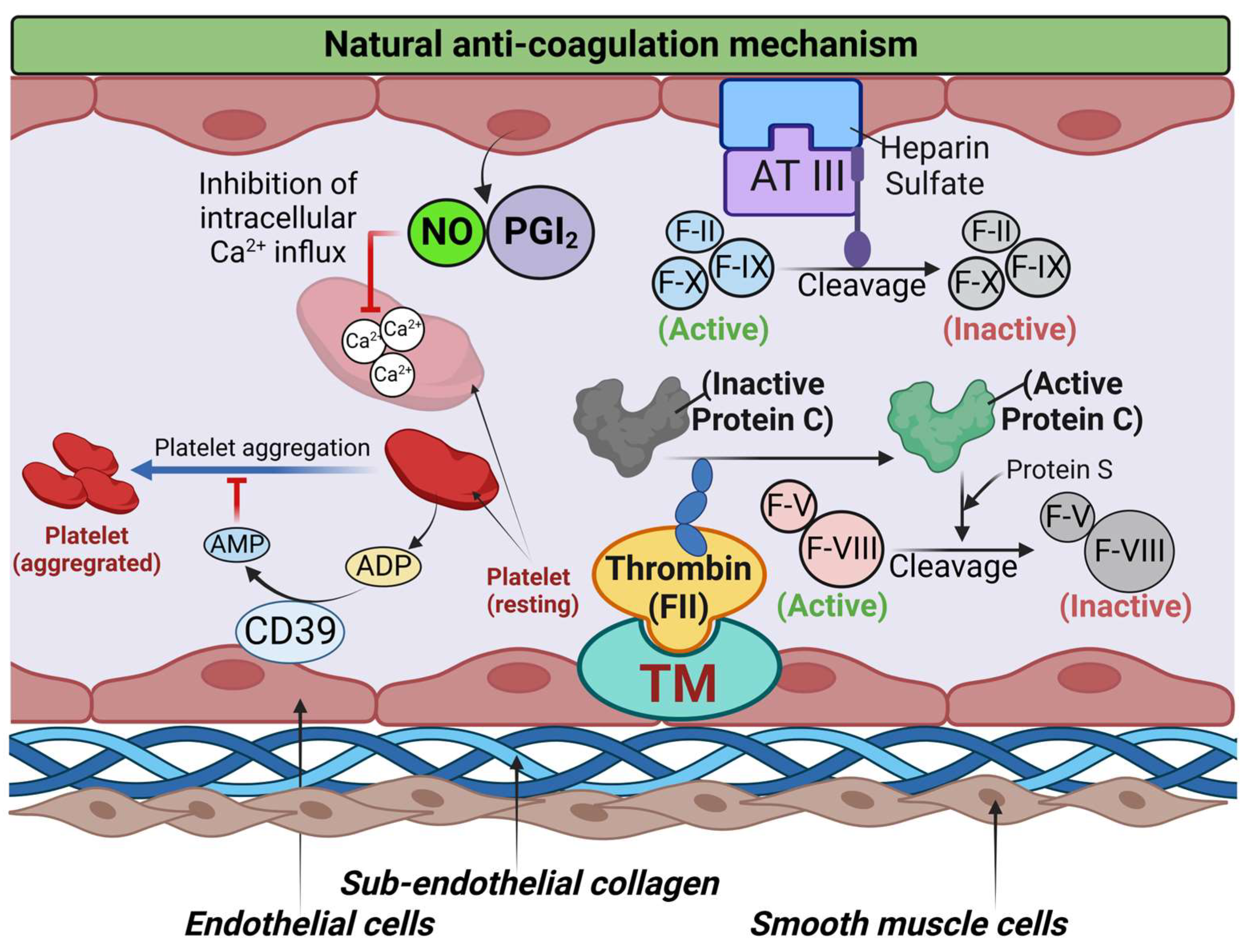
2.2. Platelets in Hemostasis and Thrombosis
2.3. Platelet Priming
3. Role of Platelets in Cardiovascular Diseases
4. Hypoglycemia and Platelet Dysfunction
5. The Association between Hypoglycemia and Cardiovascular Diseases
6. Therapies for the Prevention of Cardiovascular Disease
6.1. Avoiding the Use of Sulfonylureas
6.2. New Insulin Preparations
6.3. Altering the Method of Insulin Delivery
6.4. Individualizing HbA1c Targets
7. Future Directions and Perspectives
8. Clinical Implications
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathew, P.; Thoppil, D. Hypoglycemia. In StatPearls; StatPearls Publishing Copyright © 2023; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- American Diabetes Association. Glycemic Targets: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S66–S76. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.M.; Andersen, H.U.; Thorsteinsson, B.; Pedersen-Bjergaard, U. Hypoglycemic Exposure and Risk of Asymptomatic Hypoglycemia in Type 1 Diabetes Assessed by Continuous Glucose Monitoring. J. Clin. Endocrinol. Metab. 2018, 103, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.R.; Peyrot, M.; Oates, S.K.; Taylor, A.D. Hypoglycemia in patient with type 2 diabetes treated with insulin: It can happen. BMJ Open Diabetes Res. Care 2020, 8, e001194. [Google Scholar] [CrossRef] [PubMed]
- Melo, K.F.S.; Bahia, L.R.; Pasinato, B.; Porfirio, G.J.M.; Martimbianco, A.L.; Riera, R.; Calliari, L.E.P.; Minicucci, W.J.; Turatti, L.A.A.; Pedrosa, H.C.; et al. Short-acting insulin analogues versus regular human insulin on postprandial glucose and hypoglycemia in type 1 diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Oyer, D.S. The science of hypoglycemia in patients with diabetes. Curr. Diabetes Rev. 2013, 9, 195–208. [Google Scholar] [CrossRef]
- Nirantharakumar, K.; Marshall, T.; Hodson, J.; Narendran, P.; Deeks, J.; Coleman, J.J.; Ferner, R.E. Hypoglycemia in non-diabetic in-patients: Clinical or criminal? PLoS ONE 2012, 7, e40384. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Yamamoto-Honda, R.; Kajio, H.; Kishimoto, M.; Noto, H.; Hachiya, R.; Kimura, A.; Kakei, M.; Noda, M. Prediction of 90-day mortality in patients without diabetes by severe hypoglycemia: Blood glucose level as a novel marker of severity of underlying disease. Acta Diabetol. 2015, 52, 307–314. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H., Jr.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef]
- Sanon, V.P.; Sanon, S.; Kanakia, R.; Yu, H.; Araj, F.; Oliveros, R.; Chilton, R. Hypoglycemia from a cardiologist’s perspective. Clin. Cardiol. 2014, 37, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.S.M.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. The severity and duration of Hypoglycemia affect platelet-derived protein responses in Caucasians. Cardiovasc. Diabetol. 2022, 21, 202. [Google Scholar] [CrossRef]
- Kahal, H.; Halama, A.; Aburima, A.; Bhagwat, A.M.; Butler, A.E.; Graumann, J.; Suhre, K.; Sathyapalan, T.; Atkin, S.L. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci. Rep. 2020, 10, 4750. [Google Scholar] [CrossRef] [PubMed]
- Lebas, H.; Yahiaoui, K.; Martos, R.; Boulaftali, Y. Platelets Are at the Nexus of Vascular Diseases. Front. Cardiovasc. Med. 2019, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, A.; Bertaggia Calderara, D.; Zermatten, M.G.; Alberio, L. High-Dose Epinephrine Enhances Platelet Aggregation at the Expense of Procoagulant Activity. Thromb. Haemost. 2021, 121, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- von Känel, R.; Heimgartner, N.; Stutz, M.; Zuccarella-Hackl, C.; Hänsel, A.; Ehlert, U.; Wirtz, P.H. Prothrombotic response to norepinephrine infusion, mimicking norepinephrine stress-reactivity effects, is partly mediated by α-adrenergic mechanisms. Psychoneuroendocrinology 2019, 105, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, T.; Jain, K.; Gu, S.X.; Qiu, M.; Gu, V.W.; Melchinger, H.; Rinder, H.; Martin, K.A.; Gardiner, E.E.; Lee, A.I.; et al. A guide to molecular and functional investigations of platelets to bridge basic and clinical sciences. Nat. Cardiovasc. Res. 2022, 1, 223–237. [Google Scholar] [CrossRef]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Quach, M.E.; Chen, W.; Li, R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018, 131, 1512–1521. [Google Scholar] [CrossRef]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017, 38, 785–791. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef]
- Dahlbäck, B.; Villoutreix, B.O. The anticoagulant protein C pathway. FEBS Lett. 2005, 579, 3310–3316. [Google Scholar] [CrossRef] [PubMed]
- Brass, L.F.; Tomaiuolo, M.; Welsh, J.; Poventud-Fuentes, I.; Zhu, L.; Diamond, S.L.; Stalker, T.J. 20—Hemostatic Thrombus Formation in Flowing Blood. In Platelets, 4th ed.; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 371–391. [Google Scholar]
- Offermanns, S. Activation of platelet function through G protein-coupled receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Targher, G. Arterial thrombus formation in cardiovascular disease. Nat. Rev. Cardiol. 2011, 8, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Baaten, C.; Ten Cate, H.; van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet populations and priming in hematological diseases. Blood Rev. 2017, 31, 389–399. [Google Scholar] [CrossRef]
- Raslan, Z.; Naseem, K.M. The control of blood platelets by cAMP signalling. Biochem. Soc. Trans. 2014, 42, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, L.; Bergmeier, W. Negative regulators of platelet activation and adhesion. J. Thromb. Haemost. 2018, 16, 220–230. [Google Scholar] [CrossRef]
- Frier, B.M. Hypoglycaemia in diabetes mellitus: Epidemiology and clinical implications. Nat. Rev. Endocrinol. 2014, 10, 711–722. [Google Scholar] [CrossRef]
- Papazafiropoulou, A.; Papanas, N.; Pappas, S.; Maltezos, E.; Mikhailidis, D.P. Effects of oral hypoglycemic agents on platelet function. J. Diabetes Complicat. 2015, 29, 846–851. [Google Scholar] [CrossRef]
- Dandona, P.; Chaudhuri, A.; Dhindsa, S. Proinflammatory and prothrombotic effects of hypoglycemia. Diabetes Care 2010, 33, 1686–1687. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ito, T.; Nagasato, T.; Shinnakasu, A.; Kurano, M.; Arimura, A.; Arimura, H.; Hashiguchi, H.; Deguchi, T.; Maruyama, I.; et al. Effects of glycemic control and hypoglycemia on Thrombus formation assessed using automated microchip flow chamber system: An exploratory observational study. Thromb. J. 2019, 17, 17. [Google Scholar] [CrossRef]
- Chow, E.; Iqbal, A.; Walkinshaw, E.; Phoenix, F.; Macdonald, I.A.; Storey, R.F.; Ajjan, R.; Heller, S.R. Prolonged Prothrombotic Effects of Antecedent Hypoglycemia in Individuals with Type 2 Diabetes. Diabetes Care 2018, 41, 2625–2633. [Google Scholar] [CrossRef]
- Trovati, M.; Anfossi, G.; Cavalot, F.; Vitali, S.; Massucco, P.; Mularoni, E.; Schinco, P.; Tamponi, G.; Emanuelli, G. Studies on mechanisms involved in hypoglycemia-induced platelet activation. Diabetes 1986, 35, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Kahal, H.; Aburima, A.; Spurgeon, B.; Wraith, K.S.; Rigby, A.S.; Sathyapalan, T.; Kilpatrick, E.S.; Naseem, K.M.; Atkin, S.L. Platelet function following induced hypoglycaemia in type 2 diabetes. Diabetes Metab. 2018, 44, 431–436. [Google Scholar] [CrossRef]
- Dong, C.; Bu, X.; Liu, J.; Wei, L.; Ma, A.; Wang, T. Cardiovascular disease burden attributable to dietary risk factors from 1990 to 2019: A systematic analysis of the Global Burden of Disease study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Lievens, D.; von Hundelshausen, P. Platelets in atherosclerosis. Thromb. Haemost. 2011, 106, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Gawaz, M. Platelets in Atherosclerosis; Springer International Publishing © 2023; Springer International Publishing AG: Basel, Switzerland; pp. 993–1013.
- Dalsgaard-Nielsen, J.; Madsbad, S.; Hilsted, J. Changes in platelet function, blood coagulation and fibrinolysis during insulin-induced hypoglycaemia in juvenile diabetics and normal subjects. Thromb. Haemost. 1982, 47, 254–258. [Google Scholar] [CrossRef]
- Wright, R.J.; Newby, D.E.; Stirling, D.; Ludlam, C.A.; Macdonald, I.A.; Frier, B.M. Effects of acute insulin-induced hypoglycemia on indices of inflammation: Putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 2010, 33, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Gogitidze Joy, N.; Hedrington, M.S.; Briscoe, V.J.; Tate, D.B.; Ertl, A.C.; Davis, S.N. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010, 33, 1529–1535. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Al-Qaissi, A.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. Platelet Protein-Related Abnormalities in Response to Acute Hypoglycemia in Type 2 Diabetes. Front. Endocrinol. 2021, 12, 651009. [Google Scholar] [CrossRef]
- Hutton, R.A.; Mikhailidis, D.; Dormandy, K.M.; Ginsburg, J. Platelet aggregation studies during transient hypoglycaemia: A potential method for evaluating platelet function. J. Clin. Pathol. 1979, 32, 434–438. [Google Scholar] [CrossRef]
- Aberer, F.; Pferschy, P.N.; Tripolt, N.J.; Sourij, C.; Obermayer, A.M.; Prüller, F.; Novak, E.; Reitbauer, P.; Kojzar, H.; Prietl, B.; et al. Hypoglycaemia leads to a delayed increase in platelet and coagulation activation markers in people with type 2 diabetes treated with metformin only: Results from a stepwise hypoglycaemic clamp study. Diabetes Obes. Metab. 2020, 22, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, S.E.; Gjesdal, K.; Eide, I.; Aakesson, I.; Amundsen, R.; Foss, O.P.; Leren, P. Increased beta-thromboglobulin in essential hypertension: Interactions between arterial plasma adrenaline, platelet function and blood lipids. Acta Med. Scand. 1983, 213, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Lande, K.; Gjesdal, K.; Fønstelien, E.; Kjeldsen, S.E.; Eide, I. Effects of adrenaline infusion on platelet number, volume and release reaction. Thromb. Haemost. 1985, 54, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Joy, N.G.; Mikeladze, M.; Younk, L.M.; Tate, D.B.; Davis, S.N. Effects of equivalent sympathetic activation during hypoglycemia on endothelial function and pro-atherothrombotic balance in healthy individuals and obese standard treated type 2 diabetes. Metabolism 2016, 65, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aljada, A.; Dandona, P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef]
- Hoak, J.C.; Warner, E.D.; Connor, W.E. Platelets, fatty acids and thrombosis. Circ. Res. 1967, 20, 11–17. [Google Scholar] [CrossRef]
- Burstein, Y.; Berns, L.; Heldenberg, D.; Kahn, Y.; Werbin, B.Z.; Tamir, I. Increase in platelet aggregation following a rise in plasma free fatty acids. Am. J. Hematol. 1978, 4, 17–22. [Google Scholar] [CrossRef]
- Nordøy, A.; Svensson, B. The simultaneous effect of albumin bound fatty acids on platelets and endothelial cells. Thromb. Res. 1979, 15, 215–226. [Google Scholar] [CrossRef]
- Nordøy, A. Albumin-bound fatty acids, platelets and endothelial cells in thrombogenesis. Haemostasis 1979, 8, 193–202. [Google Scholar] [CrossRef]
- Barbano, B.; Gigante, A.; Amoroso, A.; Cianci, R. Thrombosis in nephrotic syndrome. Semin. Thromb. Hemost. 2013, 39, 469–476. [Google Scholar] [CrossRef]
- Dhindsa, S.; Ghanim, H.; Dandona, P. Nonesterified Fatty Acids, Albumin, and Platelet Aggregation. Diabetes 2015, 64, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Blache, D.; Bourdon, E.; Salloignon, P.; Lucchi, G.; Ducoroy, P.; Petit, J.M.; Verges, B.; Lagrost, L. Glycated albumin with loss of fatty acid binding capacity contributes to enhanced arachidonate oxygenation and platelet hyperactivity: Relevance in patients with type 2 diabetes. Diabetes 2015, 64, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Martini, F.; Riondino, S.; La Farina, F.; Magnapera, A.; Ciatti, F.; Guadagni, F. Soluble P-selectin as a marker of in vivo platelet activation. Clin. Chim. Acta 2009, 399, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Eyileten, C.; Wicik, Z.; Keshwani, D.; Aziz, F.; Aberer, F.; Pferschy, P.N.; Tripolt, N.J.; Sourij, C.; Prietl, B.; Prüller, F.; et al. Alteration of circulating platelet-related and diabetes-related microRNAs in individuals with type 2 diabetes mellitus: A stepwise hypoglycaemic clamp study. Cardiovasc. Diabetol. 2022, 21, 79. [Google Scholar] [CrossRef]
- Tanaka, K.; Okada, Y.; Torimoto, K.; Nishio, K.; Narisawa, M.; Tanaka, Y. Hypoglycemia induces vascular endothelial dysfunction in subjects with normal glucose tolerance. Sci. Rep. 2022, 12, 2598. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Xia, Z.; McNeill, J.H.; MacLeod, K.M. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2144–H2152. [Google Scholar] [CrossRef]
- Joy, N.G.; Tate, D.B.; Younk, L.M.; Davis, S.N. Effects of Acute and Antecedent Hypoglycemia on Endothelial Function and Markers of Atherothrombotic Balance in Healthy Humans. Diabetes 2015, 64, 2571–2580. [Google Scholar] [CrossRef]
- Khunti, K.; Davies, M.; Majeed, A.; Thorsted, B.L.; Wolden, M.L.; Paul, S.K. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: A cohort study. Diabetes Care 2015, 38, 316–322. [Google Scholar] [CrossRef]
- Iqbal, A.; Prince, L.R.; Novodvorsky, P.; Bernjak, A.; Thomas, M.R.; Birch, L.; Lambert, D.; Kay, L.J.; Wright, F.J.; Macdonald, I.A.; et al. Effect of Hypoglycemia on Inflammatory Responses and the Response to Low-Dose Endotoxemia in Humans. J. Clin. Endocrinol. Metab. 2019, 104, 1187–1199. [Google Scholar] [CrossRef]
- Goto, A.; Arah, O.A.; Goto, M.; Terauchi, Y.; Noda, M. Severe hypoglycaemia and cardiovascular disease: Systematic review and meta-analysis with bias analysis. BMJ Br. Med. J. 2013, 347, f4533. [Google Scholar] [CrossRef]
- Newby, A.C.; George, S.J.; Ismail, Y.; Johnson, J.L.; Sala-Newby, G.B.; Thomas, A.C. Vulnerable atherosclerotic plaque metalloproteinases and foam cell phenotypes. Thromb. Haemost. 2009, 101, 1006–1011. [Google Scholar] [PubMed]
- Zinman, B.; Marso, S.P.; Christiansen, E.; Calanna, S.; Rasmussen, S.; Buse, J.B. Hypoglycemia, Cardiovascular Outcomes, and Death: The LEADER Experience. Diabetes Care 2018, 41, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Pieber, T.R.; Marso, S.P.; McGuire, D.K.; Zinman, B.; Poulter, N.R.; Emerson, S.S.; Pratley, R.E.; Woo, V.; Heller, S.; Lange, M.; et al. DEVOTE 3: Temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia 2018, 61, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Mellbin, L.G.; Rydén, L.; Riddle, M.C.; Probstfield, J.; Rosenstock, J.; Díaz, R.; Yusuf, S.; Gerstein, H.C. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur. Heart J. 2013, 34, 3137–3144. [Google Scholar] [CrossRef]
- Festa, A.; Heller, S.R.; Seaquist, E.; Duan, R.; Hadjiyianni, I.; Fu, H. Association between mild and severe hypoglycemia in people with type 2 diabetes initiating insulin. J. Diabetes Complicat. 2017, 31, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.R.; Geybels, M.S.; Iqbal, A.; Liu, L.; Wagner, L.; Chow, E. A higher non-severe hypoglycaemia rate is associated with an increased risk of subsequent severe hypoglycaemia and major adverse cardiovascular events in individuals with type 2 diabetes in the LEADER study. Diabetologia 2022, 65, 55–64. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, S.; Fu, S.-L.; Yi, L.; Mao, H.; Tan, Q.; Xu, P.; Yang, G.-L. The Association of Hypoglycemia Assessed by Continuous Glucose Monitoring WITH Cardiovascular Outcomes and Mortality in Patients with Type 2 Diabetes. Front. Endocrinol. 2019, 10, 536. [Google Scholar] [CrossRef]
- Fährmann, E.R.; Adkins, L.; Loader, C.J.; Han, H.; Rice, K.M.; Denvir, J.; Driscoll, H.K. Severe hypoglycemia and coronary artery calcification during the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Res. Clin. Pract. 2015, 107, 280–289. [Google Scholar] [CrossRef]
- Desouza, C.; Salazar, H.; Cheong, B.; Murgo, J.; Fonseca, V. Association of hypoglycemia and cardiac ischemia: A study based on continuous monitoring. Diabetes Care 2003, 26, 1485–1489. [Google Scholar] [CrossRef]
- Wright, R.J.; Frier, B.M. Vascular disease and diabetes: Is hypoglycaemia an aggravating factor? Diabetes Metab. Res. Rev. 2008, 24, 353–363. [Google Scholar] [CrossRef]
- Giménez, M.; Gilabert, R.; Monteagudo, J.; Alonso, A.; Casamitjana, R.; Paré, C.; Conget, I. Repeated episodes of hypoglycemia as a potential aggravating factor for preclinical atherosclerosis in subjects with type 1 diabetes. Diabetes Care 2011, 34, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Cardiovascular disease in type 1 diabetes: A review of epidemiological data and underlying mechanisms. Diabetes Metab. 2020, 46, 442–449. [Google Scholar] [CrossRef]
- Donnelly, L.A.; Morris, A.D.; Frier, B.M.; Ellis, J.D.; Donnan, P.T.; Durrant, R.; Band, M.M.; Reekie, G.; Leese, G.P. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: A population-based study. Diabet. Med. 2005, 22, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Gruden, G.; Barutta, F.; Chaturvedi, N.; Schalkwijk, C.; Stehouwer, C.D.; Witte, D.R.; Fuller, J.H.; Perin, P.C.; Bruno, G. Severe hypoglycemia and cardiovascular disease incidence in type 1 diabetes: The EURODIAB Prospective Complications Study. Diabetes Care 2012, 35, 1598–1604. [Google Scholar] [CrossRef]
- Giménez, M.; López, J.J.; Castell, C.; Conget, I. Hypoglycaemia and cardiovascular disease in Type 1 Diabetes. Results from the Catalan National Public Health registry on insulin pump therapy. Diabetes Res. Clin. Pract. 2012, 96, e23–e25. [Google Scholar] [CrossRef]
- Gill, G.; Woodward, A.; Casson, I.; Weston, P. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes—The ‘dead in bed’ syndrome revisited. Diabetologia 2008, 52, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Janssen, A.W.M.; Jaeger, M.; Van de Wijer, L.; van der Heijden, W.; Ter Horst, R.; Vart, P.; van Gool, A.; Joosten, L.A.B.; Netea, M.G.; et al. Limited impact of impaired awareness of hypoglycaemia and severe hypoglycaemia on the inflammatory profile of people with type 1 diabetes. Diabetes Obes. Metab. 2020, 22, 2427–2436. [Google Scholar] [CrossRef]
- Jin, W.L.; Azuma, K.; Mita, T.; Goto, H.; Kanazawa, A.; Shimizu, T.; Ikeda, F.; Fujitani, Y.; Hirose, T.; Kawamori, R.; et al. Repetitive hypoglycaemia increases serum adrenaline and induces monocyte adhesion to the endothelium in rat thoracic aorta. Diabetologia 2011, 54, 1921–1929. [Google Scholar] [CrossRef]
- Kalra, S.; Mukherjee, J.J.; Venkataraman, S.; Bantwal, G.; Shaikh, S.; Saboo, B.; Das, A.K.; Ramachandran, A. Hypoglycemia: The neglected complication. Indian J. Endocrinol. Metab. 2013, 17, 819–834. [Google Scholar] [CrossRef]
- Chopra, S.; Kewal, A. Does hypoglycemia cause cardiovascular events? Indian J. Endocrinol. Metab. 2012, 16, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Goto, M.; Terauchi, Y.; Yamaguchi, N.; Noda, M. Association Between Severe Hypoglycemia and Cardiovascular Disease Risk in Japanese Patients with Type 2 Diabetes. J. Am. Heart. Assoc. 2016, 5, e002875. [Google Scholar] [CrossRef] [PubMed]
- Seaquist, E.R.; Anderson, J.; Childs, B.; Cryer, P.; Dagogo-Jack, S.; Fish, L.; Heller, S.R.; Rodriguez, H.; Rosenzweig, J.; Vigersky, R. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013, 36, 1384–1395. [Google Scholar] [CrossRef]
- Mannucci, E.; Dicembrini, I.; Lauria, A.; Pozzilli, P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care 2013, 36 (Suppl. S2), S259–S263. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.S.; Sung, S.H.; Huang, H.M.; Yang, H.L.; You, L.K.; Chuang, S.Y.; Huang, P.C.; Hsu, P.F.; Cheng, H.M.; Chen, C.H. Hypoglycemia and risk of vascular events and mortality: A systematic review and meta-analysis. Acta Diabetol. 2016, 53, 377–392. [Google Scholar] [CrossRef]
- Cryer, P.E. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 2013, 369, 362–372. [Google Scholar] [CrossRef]
- Lachin, J.M.; Nathan, D.M. Understanding Metabolic Memory: The Prolonged Influence of Glycemia During the Diabetes Control and Complications Trial (DCCT) on Future Risks of Complications During the Study of the Epidemiology of Diabetes Interventions and Complications (EDIC). Diabetes Care 2021, 44, 2216–2224. [Google Scholar] [CrossRef]
- Lash, R.W.; Lucas, D.O.; Illes, J. Preventing Hypoglycemia in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 1265–1268. [Google Scholar] [CrossRef]
- Silbert, R.; Salcido-Montenegro, A.; Rodriguez-Gutierrez, R.; Katabi, A.; McCoy, R.G. Hypoglycemia Among Patients with Type 2 Diabetes: Epidemiology, Risk Factors, and Prevention Strategies. Curr. Diab. Rep. 2018, 18, 53. [Google Scholar] [CrossRef]
- Leiter, L.A. Latest Evidence on Sulfonylureas: What’s New? Diabetes Ther. 2020, 11, 15–22. [Google Scholar] [CrossRef]
- Douros, A.; Dell’Aniello, S.; Yu, O.H.Y.; Filion, K.B.; Azoulay, L.; Suissa, S. Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: Population based cohort study. BMJ 2018, 362, k2693. [Google Scholar] [CrossRef]
- Azoulay, L.; Suissa, S. Sulfonylureas and the Risks of Cardiovascular Events and Death: A Methodological Meta-Regression Analysis of the Observational Studies. Diabetes Care 2017, 40, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Strong, J.; Urquhart, S. Insulin Initiation and Titration in Patients with Type 2 Diabetes. Diabetes Spectr. 2019, 32, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.S.; Crabtree, E.; McDonagh, M.S. Comparative effectiveness and harms of long-acting insulins for type 1 and type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 984–992. [Google Scholar] [CrossRef]
- Wysham, C.; Bhargava, A.; Chaykin, L.; de la Rosa, R.; Handelsman, Y.; Troelsen, L.N.; Kvist, K.; Norwood, P. Effect of Insulin Degludec vs Insulin Glargine U100 on Hypoglycemia in Patients with Type 2 Diabetes: The SWITCH 2 Randomized Clinical Trial. Jama 2017, 318, 45–56. [Google Scholar] [CrossRef]
- Vora, J.; Christensen, T.; Rana, A.; Bain, S.C. Insulin degludec versus insulin glargine in type 1 and type 2 diabetes mellitus: A meta-analysis of endpoints in phase 3a trials. Diabetes Ther. 2014, 5, 435–446. [Google Scholar] [CrossRef]
- Morris, A. Closed-loop insulin delivery has wide-ranging benefits. Nat. Rev. Endocrinol. 2018, 14, 688. [Google Scholar] [CrossRef]
- El-Khatib, F.H.; Balliro, C.; Hillard, M.A.; Magyar, K.L.; Ekhlaspour, L.; Sinha, M.; Mondesir, D.; Esmaeili, A.; Hartigan, C.; Thompson, M.J.; et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: A multicentre randomised crossover trial. Lancet 2017, 389, 369–380. [Google Scholar] [CrossRef]
- Berget, C.; Messer, L.H.; Forlenza, G.P. A Clinical Overview of Insulin Pump Therapy for the Management of Diabetes: Past, Present, and Future of Intensive Therapy. Diabetes Spectr. 2019, 32, 194–204. [Google Scholar] [CrossRef]
- NICE. Indicators for the NICE Menu for the QOF. Available online: https://www.nice.org.uk/Media/Default/Standards-and-indicators/QOF%20Indicator%20Key%20documents/NM141-diabetes-guidance.pdf (accessed on 4 July 2022).
- American Diabetes Association. 6 Glycemic Targets: Standards of Medical Care in Diabetes—2021. Diabetes Care 2020, 44, S73–S84. [Google Scholar] [CrossRef]
- Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; Grobbee, D.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009, 360, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; Perdue, L.H.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Calles-Escandón, J.; Lovato, L.C.; Simons-Morton, D.G.; Kendall, D.M.; Pop-Busui, R.; Cohen, R.M.; Bonds, D.E.; Fonseca, V.A.; Ismail-Beigi, F.; Banerji, M.A.; et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010, 33, 721–727. [Google Scholar] [CrossRef] [PubMed]


| Platelets | ||
|---|---|---|
| Initiators of Platelet Activation | Positive Primers | Negative Primers |
| Subendothelial collagen | Adrenaline | Nitric oxide |
| Von Willebrand Factor | Insulin-like Growth Factor 1 | Prostacyclin |
| Fibrinogen | Thrombopoietin | Adenosine |
| Laminin | GAS6 | Insulin |
| Thrombin | SCD40L | PGE2 |
| Study | Year | Sample Size | Follow-Up Period | Type of Diabetes | Definition of Hypoglycemia | Effect Size |
|---|---|---|---|---|---|---|
| DEVOTE 3 [66] | 2018 | 7637 | Median 2.0 years | Type 2 | Severe hypoglycemia | All-cause mortality HR 2.51(1.79–3.50) CVD HR 1.38(0.96–1.96) |
| ORIGIN [67] | 2013 | 12,537 | Median 6.2 years | Type 2 | Severe hypoglycemia | Primary outcomes HR 1.58(1.24–2.02) Mortality HR 1.74(1.39–2.19) CV Death HR1.71(1.27–2.30) Arrhythmic death HR 1.77(1.17–2.67) |
| ORIGIN [67] | 2013 | 12,537 | Median 6.2 years | Type 2 | Non-severe hypoglycemia | No association |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.A.G.; Niinuma, S.A.; Moin, A.S.M.; Atkin, S.L.; Butler, A.E. The Role of Platelets in Hypoglycemia-Induced Cardiovascular Disease: A Review of the Literature. Biomolecules 2023, 13, 241. https://doi.org/10.3390/biom13020241
Ali AAG, Niinuma SA, Moin ASM, Atkin SL, Butler AE. The Role of Platelets in Hypoglycemia-Induced Cardiovascular Disease: A Review of the Literature. Biomolecules. 2023; 13(2):241. https://doi.org/10.3390/biom13020241
Chicago/Turabian StyleAli, Ahmed Ali Gebril, Sara Anjum Niinuma, Abu Saleh Md Moin, Stephen L. Atkin, and Alexandra E. Butler. 2023. "The Role of Platelets in Hypoglycemia-Induced Cardiovascular Disease: A Review of the Literature" Biomolecules 13, no. 2: 241. https://doi.org/10.3390/biom13020241
APA StyleAli, A. A. G., Niinuma, S. A., Moin, A. S. M., Atkin, S. L., & Butler, A. E. (2023). The Role of Platelets in Hypoglycemia-Induced Cardiovascular Disease: A Review of the Literature. Biomolecules, 13(2), 241. https://doi.org/10.3390/biom13020241





