Development of a Fermented Beverage with Chlorella vulgaris Powder on Soybean-Based Fermented Beverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
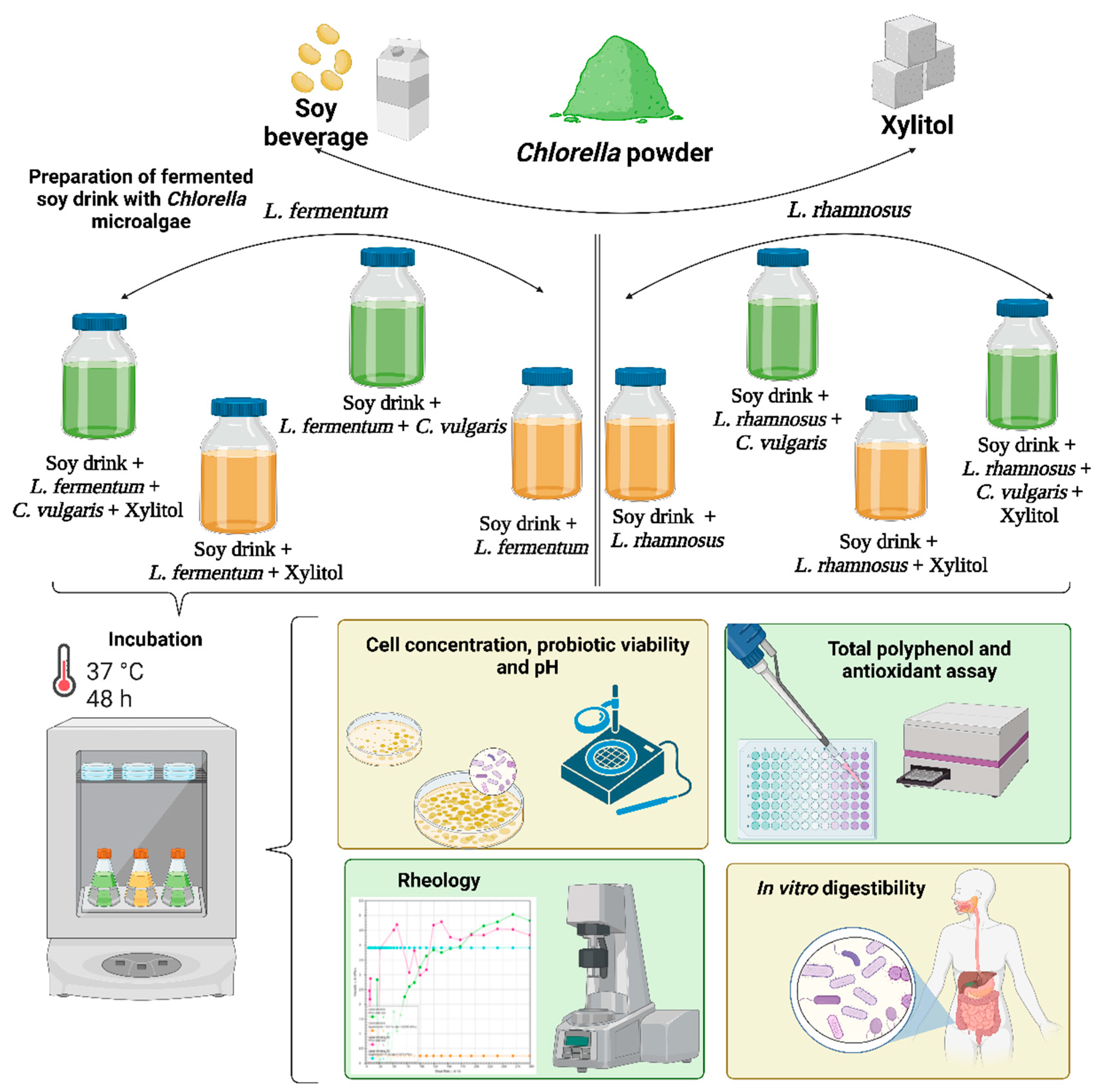
2.2. Preparation of Soy Drink with C. vulgaris Microalgae
2.3. Bacterial Cultures Preparation and Soy Drink Fermentation
2.4. Determination of Cell Concentration and Probiotic Viability and pH Level
2.5. Rheological Measurements
2.6. Determination of Total Polyphenols and Antioxidant Activity from Soy Beverages with C.vulgaris and Bacteria-Folin Ciocâlteu
2.7. Static In Vitro Simulation of Gastrointestinal Food Digestion
2.8. Statistical Analysis
3. Results and Discussions
3.1. Probiotic Viability and pH
3.2. Rheological Measurements
3.3. Total Polyphenols and Antioxidant Activity
3.4. Gastric Simulation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Regional Office for Europe. Plant-based diets and their impact on health, sustainability and the environment: A review of the evidence: WHO European Office for the Prevention and Control of Noncommunicable Diseases. 2021; World Health Organization. Regional Office for Europe: Copenhagen, Denmark. [Google Scholar]
- Bernaerts, T.M.M.; Panozzo, A.; Verhaegen, K.A.F.; Gheysen, L.; Foubert, I.; Moldenaers, P.; Hendrickx, M.E.; Van Loey, A.M. Impact of different sequences of mechanical and thermal processing on the rheological properties of Porphyridium cruentum and Chlorella vulgaris as functional food ingredients. Food Funct. 2018, 9, 2433–2446. [Google Scholar] [CrossRef]
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high β-glucosidase activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155. [Google Scholar] [CrossRef]
- Pop, O.L.; Dulf, F.V.; Cuibus, L.; Castro-Giráldez, M.; Fito, P.J.; Vodnar, D.C.; Coman, C.; Socaciu, C.; Suharoschi, R. Characterization of a Sea Buckthorn Extract and Its Effect on Free and Encapsulated Lactobacillus casei. Int. J. Mol. Sci. 2017, 18, 2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Res. Int. 2022, 156. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C.; Pop, O.L.; Socaciu, C. Monitoring Lactic Acid Fermentation in Media Containing Dandelion (Taraxacum officinale) by FTIR Spectroscopy. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Hotz, C.; Gibson, R.S. Traditional Food-Processing and Preparation Practices to Enhance the Bioavailability of Micronutrients in Plant-Based Diets. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egounlety, M.; Aworh, O. Effect of soaking, dehulling, cooking and fermentation with Rhizopus oligosporus on the oligosaccharides, trypsin inhibitor, phytic acid and tannins of soybean (Glycine max Merr.), cowpea (Vigna unguiculata L. Walp) and groundbean (Macrotyloma geocarpa Harms). J. Food Eng. 2003, 56, 249–254. [Google Scholar] [CrossRef]
- Kerezsi, A.D.; Jacquet, N.; Blecker, C. Advances on physical treatments for soy allergens reduction - A review. Trends Food Sci. Technol. 2022, 122, 24–39. [Google Scholar] [CrossRef]
- Kishida, T.; Ataki, H.; Takebe, M.; Ebihara, K. Soybean Meal Fermented by Aspergillus awamori Increases the Cytochrome P-450 Content of the Liver Microsomes of Mice. J. Agric. Food Chem. 2000, 48, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Song, Y.S.; Martínez-Villaluenga, C.; De Mejia, E.G.; Vidal-Valverde, C. Immunoreactivity and Amino Acid Content of Fermented Soybean Products. J. Agric. Food Chem. 2007, 56, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Pérez, V.; Pettigrew, J.; Martinez-Villaluenga, C.; DE Mejia, E. Fermentation of soybean meal and its inclusion in diets for newly weaned pigs reduced diarrhea and measures of immunoreactivity in the plasma. Anim. Feed. Sci. Technol. 2010, 159, 41–49. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.-S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- Pulikkan, J.; Mazumder, A.; Grace, T. Role of the Gut Microbiome in Autism Spectrum Disorders. Rev. Biomark. Stud. Psychiatr. Neurodegener. Disord. 2019, 1118, 253–269. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of Fermentation in Improving Nutritional Quality of Soybean Meal — A Review. Asian-Australasian J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Içier, F.; Gündüz, G.T.; Yılmaz, B.; Memeli, Z. Changes on some quality characteristics of fermented soy milk beverage with added apple juice. Lwt 2015, 63, 57–64. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, C.; Hajos, G.; Burbano, C.; Pedrosa, M.M.; Ayet, G.; Muzquiz, M.; Pusztai, A.; Gelencser, E. Effect of Natural Fermentation on the Lectin of Lentils Measured by Immunological Methods. Food Agric. Immunol. 2002, 14, 41–49. [Google Scholar] [CrossRef]
- Jang, C.; Oh, J.; Lim, J.; Kim, H.; Kim, J.-S. Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases. Foods 2021, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Yu, R.-C.; Chou, C.-C. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006, 23, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Y.; Thakur, K.; Feng, J.-Y.; Cai, J.-S.; Zhang, J.-G.; Hu, F.; Russo, P.; Spano, G.; Wei, Z.-J. Riboflavin-overproducing lactobacilli for the enrichment of fermented soymilk: Insights into improved nutritional and functional attributes. Appl. Microbiol. Biotechnol. 2020, 104, 5759–5772. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Calinoiu, L.F.; Vodnar, D.; Precup, G. A Review: The Probiotic Bacteria Viability under Different Conditions. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2016, 73, 55. [Google Scholar]
- Lv, K.; Yuan, Q.; Li, H.; Li, T.; Ma, H.; Gao, C.; Zhang, S.; Liu, Y.; Zhao, L. Chlorella pyrenoidosa Polysaccharides as a Prebiotic to Modulate Gut Microbiota: Physicochemical Properties and Fermentation Characteristics In Vitro. Foods 2022, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Ścieszka, S.; Gorzkiewicz, M.; Klewicka, E. Innovative fermented soya drink with the microalgae Chlorella vulgaris and the probiotic strain Levilactobacillus brevis ŁOCK 0944. Lwt 2021, 151, 112131. [Google Scholar] [CrossRef]
- Martelli, F.; Alinovi, M.; Bernini, V.; Gatti, M.; Bancalari, E. Arthrospira platensis as Natural Fermentation Booster for Milk and Soy Fermented Beverages. Foods 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona, R.; Murillo, M.C.; Lafarga, T.; Bermejo, R. Assessment of the potential of microalgae-derived phycoerythrin as a natural colorant in beverages. J. Appl. Phycol. 2022, 34, 3025–3034. [Google Scholar] [CrossRef]
- de Mello-Sampayo, C.; Corvo, M.L.; Mendes, R.; Duarte, D.; Lucas, J.; Pinto, R.; Batista, A.P.; Raymundo, A.; Silva-Lima, B.; Bandarra, N.M.; et al. Insights on the safety of carotenogenic Chlorella vulgaris in rodents. Algal Res. 2013, 2, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella vulgaris biomass used as colouring source in traditional butter cookies. Innov. Food Sci. Emerg. Technol. 2007, 8, 433–436. [Google Scholar] [CrossRef]
- Niccolai, A.; Shannon, E.; Abu-Ghannam, N.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (spirulina) biomass for probiotic-based products. J. Appl. Phycol. 2018, 31, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Shah, N.P. Lactic acid bacterial fermentation modified phenolic composition in tea extracts and enhanced their antioxidant activity and cellular uptake of phenolic compounds following in vitro digestion. J. Funct. Foods 2016, 20, 182–194. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation Into Innovative Food Products With Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. Lwt 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Mazinani, S.; Fadaei, V.; Khosravi-Darani, K. Impact of Spirulina platensison Physicochemical Properties and Viability of Lactobacillus acidophilus of Probiotic UF Feta Cheese. J. Food Process. Preserv. 2016, 40, 1318–1324. [Google Scholar] [CrossRef]
- Jeon, J.-K. Effect of Chlorella Addition on the Quality of Processed Cheese. J. Korean Soc. Food Sci. Nutr. 2006, 35, 373–377. [Google Scholar]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef]
- Pop, O.L.; Vodnar, D.C.; Suharoschi, R.; Mudura, E.; Socaciu, C. L. plantarum ATCC 8014 Entrapment with Prebiotics and Lucerne Green Juice and Their Behavior in Simulated Gastrointestinal Conditions. J. Food Process. Eng. 2015, 39, 433–441. [Google Scholar] [CrossRef]
- Pop, O.L.; Vodnar, D.C.; Suharoschi, R.; Socaciu, C. Stability Comparison of Free and Encapsulated Lactobacilus casei ATCC 393 in Yoghurt for Long Time Storage. Bull. Univ. Agric. Sci. Veter- Med. Cluj-Napoca. Food Sci. Technol. 2016, 73, 99. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Martău, G.A.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Biofunctional soy-based sourdough for improved rheological properties during storage. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Sakoui, S.; Derdak, R.; Pop, O.L.; Vodnar, D.C.; Addoum, B.; Teleky, B.-E.; Elemer, S.; Elmakssoudi, A.; Suharoschi, R.; Soukri, A.; et al. Effect of encapsulated probiotic in Inulin-Maltodextrin-Sodium alginate matrix on the viability of Enterococcus mundtii SRBG1 and the rheological parameters of fermented milk. Curr. Res. Food Sci. 2022, 5, 1713–1719. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef]
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara, M.; Pérez-Rodríguez, M.L. Evaluation of the Antioxidant Potential of Mixed Fruit-Based Beverages: A New Insight on the Folin-Ciocalteu Method. Food Anal. Methods 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Rabie, M.A.; Soliman, A.Z.; Diaconeasa, Z.S.; Constantin, B. Effect of Pasteurization and Shelf Life on the Physicochemical Properties of Physalis (P hysalis peruviana L.) Juice. J. Food Process. Preserv. 2014, 39, 1051–1060. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.E.; Ranga, F.; Simon, E.; Pop, O.L.; Babalau-Fuss, V.; Kapsalis, N.; Vodnar, D.C. Bioaccessibility of microencapsulated carotenoids, recovered from tomato processing industrial by-products, using in vitro digestion model. Lwt 2021, 152, 112285. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus spp. Bacteria. Foods 2020, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Khosravi-Darani, K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Guldas, M.; Irkin, R. Influence of Spirulina platensis powder on the microflora of yoghurt and acidophilus milk. Mljekarstvo: Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2010, 60, 237–243. [Google Scholar]
- Beheshtipour, H.; Mortazavian, A.M.; Mohammadi, R.; Sohrabvandi, S.; Khosravi-Darani, K. Supplementation of Spirulina platensis and Chlorella vulgaris Algae into Probiotic Fermented Milks. Compr. Rev. Food Sci. Food Saf. 2013, 12, 144–154. [Google Scholar] [CrossRef]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Solubility and aggregation behavior of protein fractions from the heterotrophically cultivated microalga Chlorella protothecoides. Food Res. Int. 2018, 116, 283–290. [Google Scholar] [CrossRef]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Solubility of extracted proteins from Chlorella sorokiniana, Phaeodactylum tricornutum, and Nannochloropsis oceanica: Impact of pH-value. Lwt 2019, 105, 408–416. [Google Scholar] [CrossRef]
- Grossmann, L.; Wörner, V.; Hinrichs, J.; Weiss, J. Sensory properties of aqueous dispersions of protein-rich extracts from Chlorella protothecoides at neutral and acidic pH. J. Sci. Food Agric. 2019, 100, 1344–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obadina, A.; Akinola, O.; Shittu, T.; Bakare, H. Effect of Natural Fermentation on the Chemical and Nutritional Composition of Fermented Soymilk Nono. Niger. Food J. 2013, 31, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Han, M.J. The fermentation characteristics of soy yogurt with different content of d-allulose and sucrose fermented by lactic acid bacteria from Kimchi. Food Sci. Biotechnol. 2019, 28, 1155–1161. [Google Scholar] [CrossRef]
- Precup, G.; Teleky, B.-E.; Ranga, F.; Vodnar, D.C. Assessment of Physicochemical and Rheological Properties of Xylo-Oligosaccharides and Glucose-Enriched Doughs Fermented with BB-12. Biology 2022, 11, 553. [Google Scholar] [CrossRef]
- De, B.; Shrivastav, A.; Das, T.; Goswami, T.K. Physicochemical and nutritional assessment of soy milk and soymilk products and comparative evaluation of their effects on blood gluco-lipid profile. Appl. Food Res. 2022, 2. [Google Scholar] [CrossRef]
- Zong, L.; Lu, M.; Wang, W.; Wa, Y.; Qu, H.; Chen, D.; Liu, Y.; Qian, Y.; Ji, Q.; Gu, R. The Quality and Flavor Changes of Different Soymilk and Milk Mixtures Fermented Products during Storage. Fermentation 2022, 8, 668. [Google Scholar] [CrossRef]
- Lee, N.-K.; Paik, H.-D. Bioconversion Using Lactic Acid Bacteria: Ginsenosides, GABA, and Phenolic Compounds. J. Microbiol. Biotechnol. 2017, 27, 869–877. [Google Scholar] [CrossRef]
- Okechukwu, Q.N.; Adadi, P.; Kovaleva, E.G. Production and Analysis of Beer Supplemented with Chlorella vulgaris Powder. Fermentation 2022, 8, 581. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Algal Nutrition—Mineral Nutrition. In Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Oxford, UK, 2003; pp. 95–115. [Google Scholar] [CrossRef]
- Marazza, J.A.; Nazareno, M.A.; de Giori, G.S.; Garro, M.S. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J. Funct. Foods 2012, 4, 594–601. [Google Scholar] [CrossRef]
- Horáčková, Š.; ŽALUDOVÁ, K.; Plocková, M. Stability of selected lactobacilli in the conditions simulating those in the gastrointestinal tract. Czech J. Food Sci. 2011, 29, S30–S35. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, A.; Guidone, A.; Ianniello, R.G.; Cioffi, S.; Aponte, M.; Pavlidis, D.; Tsakalidou, E.; Zotta, T.; Parente, E. A survey of non-starter lactic acid bacteria in traditional cheeses: Culture dependent identification and survival to simulated gastrointestinal transit. Int. Dairy J. 2015, 43, 42–50. [Google Scholar] [CrossRef]
- de Medeiros, V.P.B.; de Souza, E.L.; de Albuquerque, T.M.R.; Sassi, C.F.D.C.; Lima, M.D.S.; Sivieri, K.; Pimentel, T.C.; Magnani, M. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res. 2021, 60, 102547. [Google Scholar] [CrossRef]
- Das, S.; Mishra, B.K.; Hati, S. Effect of Nutritional Factors on Growth Behaviour, Proteolytic, β-Glucosidase and β-Galactosidase Activities of Lactobacillus Cultures during Soy-Drink Fermentation. Curr. Res. Nutr. Food Sci. J. 2020, 8, 877–888. [Google Scholar] [CrossRef]




| Beverages | TPC (µg GAE/mL) | DPPH (µM Trolox/g DW) |
|---|---|---|
| Soybean drink with C. vulgaris | 267.08 ±1.51 C | 301.76 ± 2.53 E |
| Soy drink with C. vulgaris and xylitol | 263.28 ± 1.31 C | 347.44 ± 1.17 D |
| Soy drink with C. vulgaris and L. rhamnosus | 327.26 ± 0.31 A | 450.13 ± 1.23 B |
| Soy drink with C. vulgaris and L. fermentum | 306.72 ± 1.33 B | 422.56 ± 0.65 C |
| Soy drink with C. vulgaris, xylitol, and L. rhamnosus | 321.40 ± 1.63 A | 497.43 ± 1.28 A |
| Soy drink with C. vulgaris, xylitol, and L. fermentum | 304.42 ± 1.72 B | 453.29 ± 1.21 B |
| Samples | Before Digestion (log10 CFU/mL) | After Digestion (log10 CFU/mL) |
|---|---|---|
| Soy drink + L. fermentum | 8.20 ± 0.60 b,A | 6.67 ± 0.64 b,B |
| Soy drink + L. fermentum + Xylitol | 7.30 ± 0.12 c,A | 6.62 ± 0.53 b,B |
| Soy drink + L. fermentum + C. vulgaris | 8.71 ± 0.64 a,A | 6.81 ± 0.45 a,B |
| Soy drink + L. fermentum + Xylitol + C. vulgaris | 8.62 ± 0.21 a,A | 6.72 ± 1.19 b,B |
| Soy drink + L. rhamnosus | 7.71 ± 0.44 b,A | 5.67 ± 0.69 c,B |
| Soy drink + L. rhamnosus + Xylitol | 7.87 ± 0.62 b,A | 5.54 ± 1.34 c,B |
| Soy drink + L. rhamnosus + C. vulgaris | 8.74 ± 0.49 a,A | 5.58 ± 0.58 c,B |
| Soy drink + L. rhamnosus + Xylitol + C. vulgaris | 8.68 ± 0.80 a,A | 5.51 ± 0.54 c,B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csatlos, N.-I.; Simon, E.; Teleky, B.-E.; Szabo, K.; Diaconeasa, Z.M.; Vodnar, D.-C.; Ciont, C.; Pop, O.-L. Development of a Fermented Beverage with Chlorella vulgaris Powder on Soybean-Based Fermented Beverage. Biomolecules 2023, 13, 245. https://doi.org/10.3390/biom13020245
Csatlos N-I, Simon E, Teleky B-E, Szabo K, Diaconeasa ZM, Vodnar D-C, Ciont C, Pop O-L. Development of a Fermented Beverage with Chlorella vulgaris Powder on Soybean-Based Fermented Beverage. Biomolecules. 2023; 13(2):245. https://doi.org/10.3390/biom13020245
Chicago/Turabian StyleCsatlos, Norbert-Istvan, Elemer Simon, Bernadette-Emőke Teleky, Katalin Szabo, Zorița Maria Diaconeasa, Dan-Cristian Vodnar, Călina Ciont (Nagy), and Oana-Lelia Pop. 2023. "Development of a Fermented Beverage with Chlorella vulgaris Powder on Soybean-Based Fermented Beverage" Biomolecules 13, no. 2: 245. https://doi.org/10.3390/biom13020245











