Increased Enzymatic Activity of Acetylcholinesterase Indicates the Severity of the Sterile Inflammation and Predicts Patient Outcome following Traumatic Injury
Abstract
:1. Introduction
2. Materials and Methods
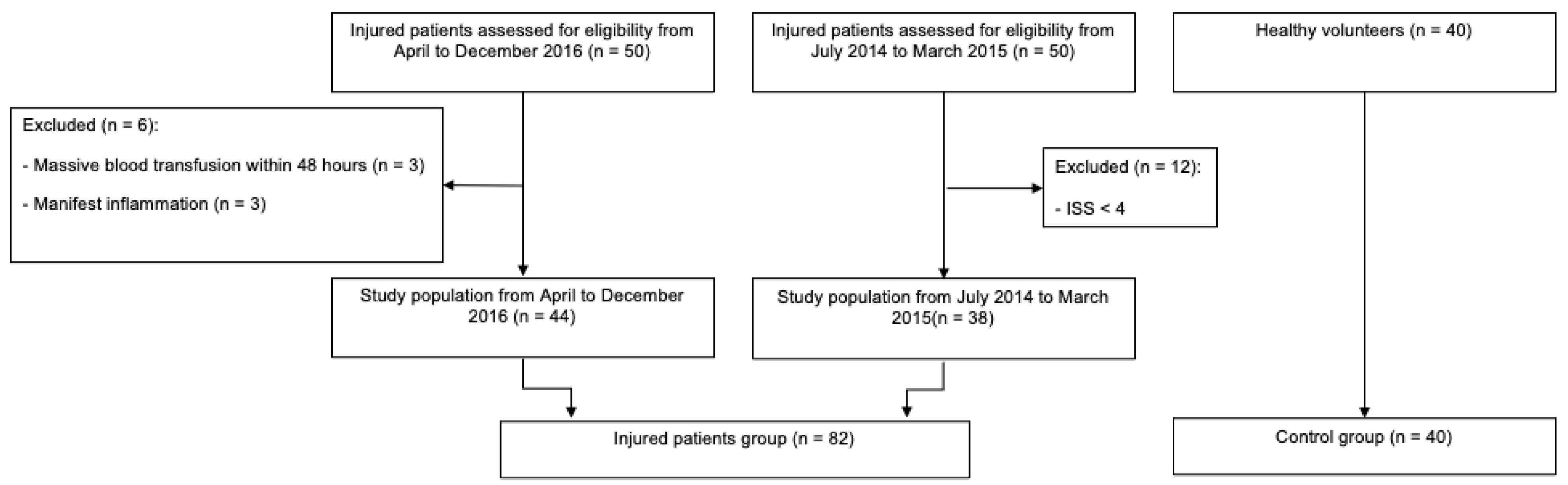
2.1. Study Design
2.2. Subjects and Measurements
2.3. Statistical Analysis
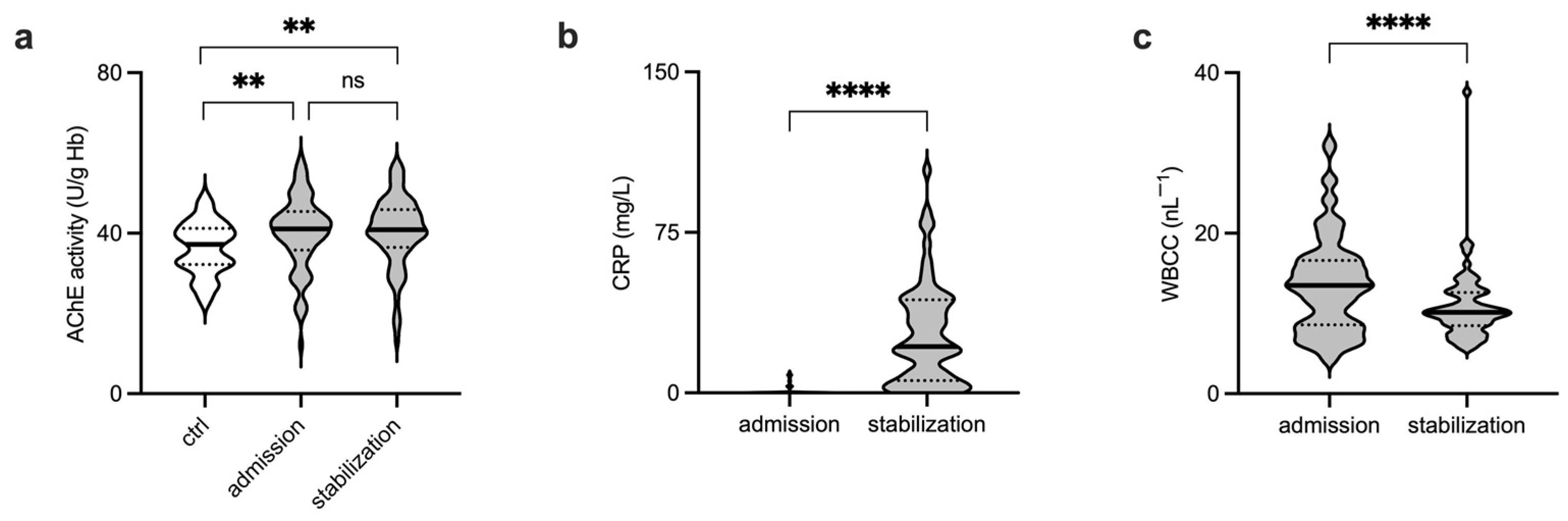
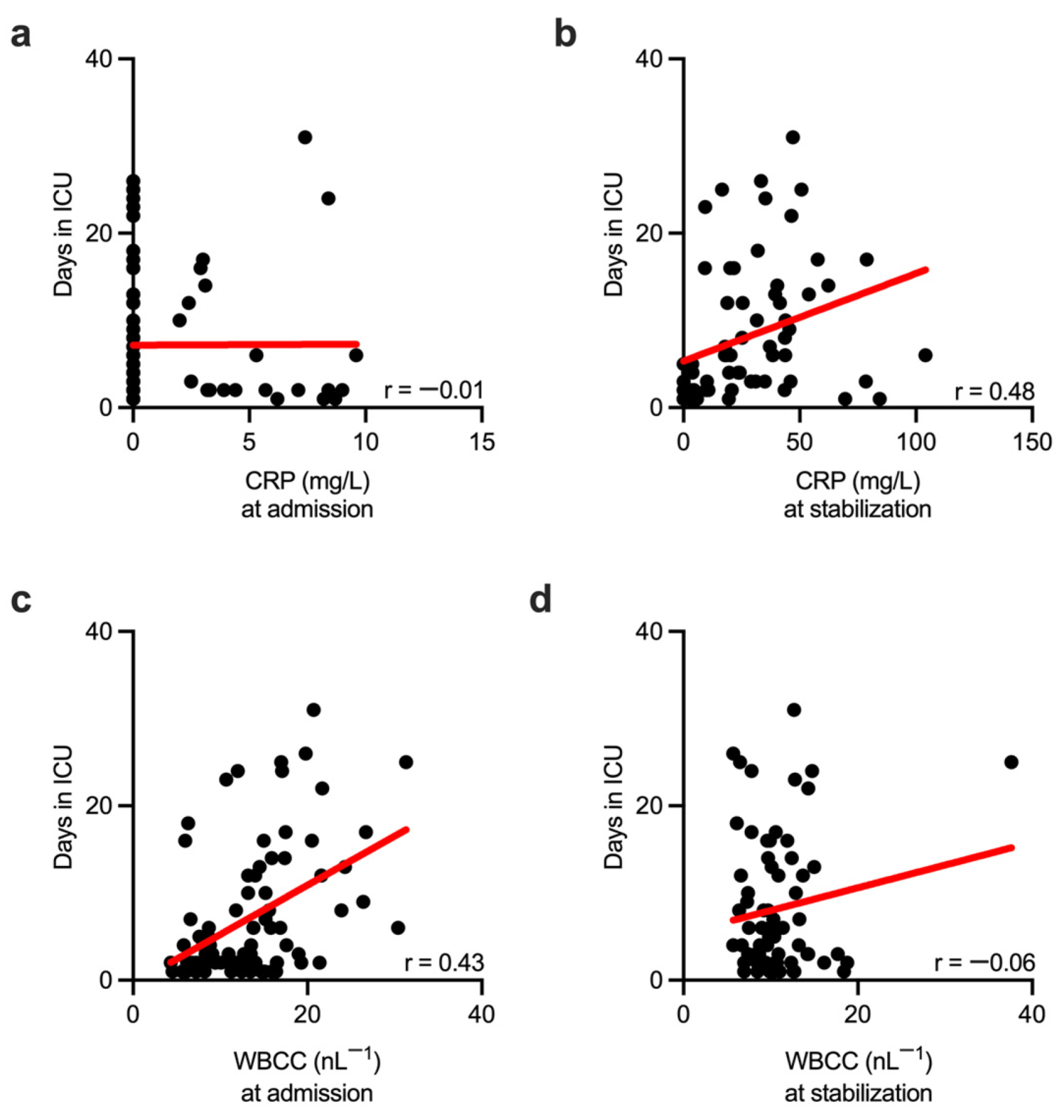
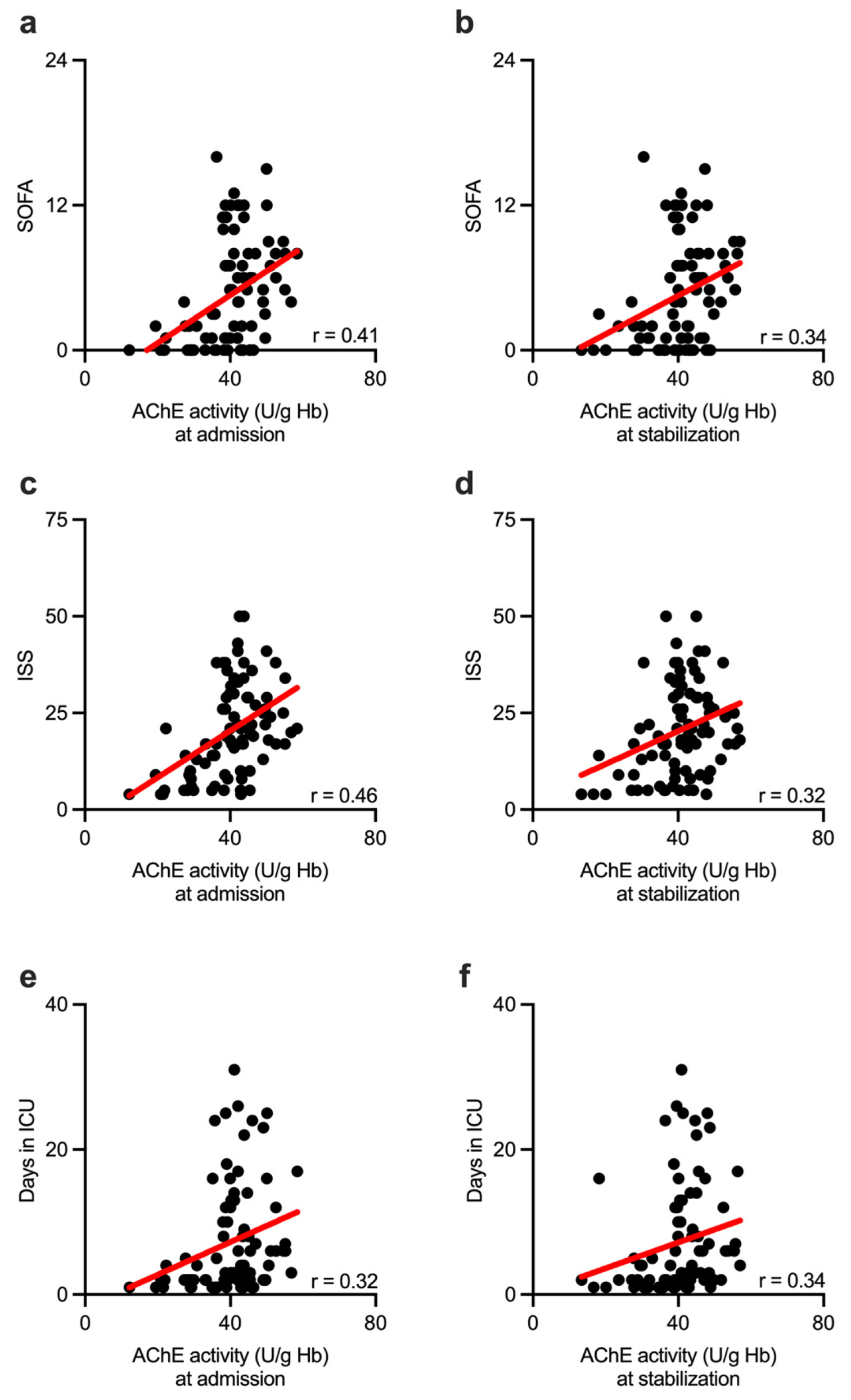
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauaia, A.; Moore, E.E.; Johnson, J.L.; Chin, T.L.; Banerjee, A.; Sperry, J.L.; Maier, R.V.; Burlew, C.C. Temporal Trends of Postinjury Multiple-Organ Failure. J. Trauma Acute Care 2014, 76, 582–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoover, D.B. Cholinergic Modulation of the Immune System Presents New Approaches for Treating Inflammation. Pharmacol. Therapeut. 2017, 179, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Bassi, C.; Saunders, M.E.; Nechanitzky, R.; Morgado-Palacin, I.; Zheng, C.; Mak, T.W. Beyond Neurotransmission: Acetylcholine in Immunity and Inflammation. J. Intern. Med. 2020, 287, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.M.; Raehl, C.L.; Bond, C.A.; Abbruscato, T.J.; Stenhouse, A.C. Methods for Assessing Drug-Related Anticholinergic Activity. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2005, 25, 1592–1601. [Google Scholar] [CrossRef]
- Bella, R.; Cantone, M.; Lanza, G.; Ferri, R.; Vinciguerra, L.; Puglisi, V.; Pennisi, M.; Ricceri, R.; Lazzaro, V.D.; Pennisi, G. Cholinergic Circuitry Functioning in Patients with Vascular Cognitive Impairment—No Dementia. Brain Stimul. 2016, 9, 225–233. [Google Scholar] [CrossRef]
- Tiepolt, S.; Meyer, P.M.; Patt, M.; Deuther-Conrad, W.; Hesse, S.; Barthel, H.; Sabri, O. PET Imaging of Cholinergic Neurotransmission in Neurodegenerative Disorders. J. Nucl. Med. 2022, 63, 33S–44S. [Google Scholar] [CrossRef]
- Worek, F.; Schilha, M.; Neumaier, K.; Aurbek, N.; Wille, T.; Thiermann, H.; Kehe, K. On-Site Analysis of Acetylcholinesterase and Butyrylcholinesterase Activity with the ChE Check Mobile Test Kit—Determination of Reference Values and Their Relevance for Diagnosis of Exposure to Organophosphorus Compounds. Toxicol. Lett. 2016, 249, 22–28. [Google Scholar] [CrossRef]
- Shihana, F.; Worek, F.; Dassanayake, G.A.; Rathgamage, S.H.; Dhanarisi, J.; Buckley, N.A. Evaluation of the Accuracy of “ChE Check Mobile” in Measurement of Acetylcholinesterase in Pesticide Poisoning. Clin. Toxicol. 2019, 57, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.; Olbert, M.; Heymann, A.; Zahn, P.K.; Plaschke, K.; von Dossow, V.; Bitzinger, D.; Barth, E.; Meister, M.; Kranke, P.; et al. Relevance of Peripheral Cholinesterase Activity on Postoperative Delirium in Adult Surgical Patients (CESARO). Eur. J. Anaesth. 2019, 36, 114–122. [Google Scholar] [CrossRef]
- Zujalovic, B.; Mayer, B.; Hafner, S.; Balling, F.; Barth, E. AChE-Activity in Critically Ill Patients with Suspected Septic Encephalopathy: A Prospective, Single-Centre Study. BMC Anesthesiol. 2020, 20, 287. [Google Scholar] [CrossRef]
- Zivkovic, A.R.; Schmidt, K.; Sigl, A.; Decker, S.O.; Brenner, T.; Hofer, S. Reduced Serum Butyrylcholinesterase Activity Indicates Severe Systemic Inflammation in Critically Ill Patients. Mediat. Inflamm. 2015, 2015, 274607. [Google Scholar] [CrossRef]
- Zivkovic, A.R.; Decker, S.O.; Zirnstein, A.C.; Sigl, A.; Schmidt, K.; Weigand, M.A.; Hofer, S.; Brenner, T. A Sustained Reduction in Serum Cholinesterase Enzyme Activity Predicts Patient Outcome Following Sepsis. Mediat. Inflamm. 2018, 2018, 1942193. [Google Scholar] [CrossRef] [Green Version]
- Espeter, F.; Künne, D.; Garczarek, L.; Kuhlmann, H.; Skarabis, A.; Zivkovic, A.R.; Brenner, T.; Schmidt, K. Critically Ill COVID-19 Patients Show Reduced Point of Care-Measured Butyrylcholinesterase Activity—A Prospective, Monocentric Observational Study. Diagnostics 2022, 12, 2150. [Google Scholar] [CrossRef]
- Kamolz, L.-P.; Andel, H.; Greher, M.; Ploner, M.; Meissl, G.; Frey, M. Serum Cholinesterase Activity Reflects Morbidity in Burned Patients. Burns 2002, 28, 147–150. [Google Scholar] [CrossRef]
- Shenhar-Tsarfaty, S.; Berliner, S.; Bornstein, N.M.; Soreq, H. Cholinesterases as Biomarkers for Parasympathetic Dysfunction and Inflammation-Related Disease. J. Mol. Neurosci. 2014, 53, 298–305. [Google Scholar] [CrossRef]
- Ba, L.; Wu, D.; Qian, A.; Zhang, M.; Xiong, B. Dynamic Changes of Serum Cholinesterase Activity after Severe Trauma. J. Zhejiang Univ. Sci. B 2014, 15, 1023–1031. [Google Scholar] [CrossRef] [Green Version]
- Zivkovic, A.R.; Bender, J.; Brenner, T.; Hofer, S.; Schmidt, K. Reduced Butyrylcholinesterase Activity Is an Early Indicator of Trauma-Induced Acute Systemic Inflammatory Response. J. Inflamm Res. 2016, 9, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Zivkovic, A.R.; Tourelle, K.M.; Brenner, T.; Weigand, M.A.; Hofer, S.; Schmidt, K. Reduced Serum Cholinesterase Activity Indicates Splenic Modulation of the Sterile Inflammation. J. Surg. Res. 2017, 220, 275–283. [Google Scholar] [CrossRef]
- Zivkovic, A.R.; Schmidt, K.; Stein, T.; Münzberg, M.; Brenner, T.; Weigand, M.A.; Kleinschmidt, S.; Hofer, S. Bedside-Measurement of Serum Cholinesterase Activity Predicts Patient Morbidity and Length of the Intensive Care Unit Stay Following Major Traumatic Injury. Sci. Rep. 2019, 9, 10437. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; Zivkovic, A.R.; Thiele, M.; Horter, J.; Brenner, T.; Weigand, M.A.; Kleinschmidt, S.; Hofer, S. Point-of-Care Measured Serum Cholinesterase Activity Predicts Patient Outcome Following Severe Burns. Burns 2021, 47, 863–872. [Google Scholar] [CrossRef]
- Decker, S.O.; Krüger, A.; Wilk, H.; Uhle, F.; Bruckner, T.; Hofer, S.; Weigand, M.A.; Brenner, T.; Zivkovic, A.R. Concurrent Change in Serum Cholinesterase Activity and Midregional-Proadrennomedullin Level Could Predict Patient Outcome Following Liver Transplantation. Biomolecules 2022, 12, 989. [Google Scholar] [CrossRef] [PubMed]
- Leuzinger, W. Structure and Function of Acetylcholinesterase*. Prog. Brain Res. 1969, 31, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Massoulié, J.; Legay, C.; Anselmet, A.; Krejci, E.; Coussen, F.; Bon, S. Biosynthesis and Integration of Acetylcholinesterase in the Cholinergic Synapse. Prog. Brain Res. 1996, 109, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Acetylcholinesterase and Butyrylcholinesterase as Possible Markers of Low-Grade Systemic Inflammation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2007, 13, RA214-21. [Google Scholar]
- Silva-Herdade, A.S.; Saldanha, C. Effects of Acetylcholine on an Animal Model of Inflammation. Clin. Hemorheol. Micro 2013, 53, 209–216. [Google Scholar] [CrossRef]
- Leal, J.K.F.; Adjobo-Hermans, M.J.W.; Brock, R.; Bosman, G.J.C.G.M. Acetylcholinesterase Provides New Insights into Red Blood Cell Ageing in Vivo and in Vitro. Blood Transfus Trasfus. Sangue 2016, 15, 232–238. [Google Scholar] [CrossRef]
- Saldanha, C. Human Erythrocyte Acetylcholinesterase in Health and Disease. Molecules 2017, 22, 1499. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological Functions of the Cholinergic System in Immune Cells. J. Pharmacol. Sci. 2017, 134, 1–21. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Moreno, R.; Takala, J.; Willatts, S.; Mendonça, A.D.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Baker, S.P.; O’Neill, B.; Haddon, W.; Long, W.B. The Injury Severity Score: A Method for Describing Patients With Multiple Injuries And Evaluating Emergency Care. J. Trauma INJ Infect. Critical Care 1974, 14, 187–196. [Google Scholar] [CrossRef]
- Bouillon, B.; Pieper, D.; Flohé, S.; Eikermann, M.; Prengel, P.; Ruchholtz, S.; Stürmer, K.M.; Waydhas, C.; Trentzsch, H.; Lendemans, S.; et al. Level 3 Guideline on the Treatment of Patients with Severe/Multiple Injuries. Eur. J. Trauma Emerg. S 2018, 44, 3–271. [Google Scholar] [CrossRef]
- Huston, J.M.; Rosas-Ballina, M.; Xue, X.; Dowling, O.; Ochani, K.; Ochani, M.; Yeboah, M.M.; Chatterjee, P.K.; Tracey, K.J.; Metz, C.N. Cholinergic Neural Signals to the Spleen Down-Regulate Leukocyte Trafficking via CD11b. J. Immunol. 2009, 183, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New Roles for an Old Actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef]
- Nizri, E.; Hamra-Amitay, Y.; Sicsic, C.; Lavon, I.; Brenner, T. Anti-Inflammatory Properties of Cholinergic up-Regulation: A New Role for Acetylcholinesterase Inhibitors. Neuropharmacology 2006, 50, 540–547. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Parrish, W.R.; Rosas-Ballina, M.; Ochani, M.; Puerta, M.; Ochani, K.; Chavan, S.; Al-Abed, Y.; Tracey, K.J. Brain Acetylcholinesterase Activity Controls Systemic Cytokine Levels through the Cholinergic Anti-Inflammatory Pathway. Brain Behav. Immun. 2009, 23, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Vaknine, S.; Soreq, H. Central and Peripheral Anti-Inflammatory Effects of Acetylcholinesterase Inhibitors. Neuropharmacology 2020, 168, 108020. [Google Scholar] [CrossRef]
- Hshieh, T.T.; Fong, T.G.; Marcantonio, E.R.; Inouye, S.K. Cholinergic Deficiency Hypothesis in Delirium: A Synthesis of Current Evidence. J. Gerontol. Ser. 2008, 63, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Oldenbeuving, A.W.; de Kort, P.L.; Jansen, B.P.; Kappelle, L.J.; Roks, G. A Pilot Study of Rivastigmine in the Treatment of Delirium after Stroke: A Safe Alternative. BMC Neurol. 2008, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Gamberini, M.; Bolliger, D.; Buse, G.A.L.; Burkhart, C.S.; Grapow, M.; Gagneux, A.; Filipovic, M.; Seeberger, M.D.; Pargger, H.; Siegemund, M.; et al. Rivastigmine for the Prevention of Postoperative Delirium in Elderly Patients Undergoing Elective Cardiac Surgery—A Randomized Controlled Trial* Crit. Care Med. 2009, 37, 1762–1768. [Google Scholar] [CrossRef]
- van Eijk, M.M.; Roes, K.C.; Honing, M.L.; Kuiper, M.A.; Karakus, A.; van der Jagt, M.; Spronk, P.E.; van Gool, W.A.; van der Mast, R.C.; Kesecioglu, J.; et al. Effect of Rivastigmine as an Adjunct to Usual Care with Haloperidol on Duration of Delirium and Mortality in Critically Ill Patients: A Multicentre, Double-Blind, Placebo-Controlled Randomised Trial. Lancet 2010, 376, 1829–1837. [Google Scholar] [CrossRef] [Green Version]
- Wolters, A.E.; Zaal, I.J.; Veldhuijzen, D.S.; Cremer, O.L.; Devlin, J.W.; van Dijk, D.; Slooter, A.J.C. Anticholinergic Medication Use and Transition to Delirium in Critically Ill Patients. Crit. Care Med. 2015, 43, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.R.; Sedlaczek, O.; von Haken, R.; Schmidt, K.; Brenner, T.; Weigand, M.A.; Bading, H.; Bengtson, C.P.; Hofer, S. Muscarinic M1 Receptors Modulate Endotoxemia-Induced Loss of Synaptic Plasticity. Acta Neuropathol. Commun. 2015, 3, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, M.; Ely, E.W.; Halfkann, D.; Schoen, J.; Sedemund-Adib, B.; Klotz, S.; Radtke, F.; Stehr, S.; Hueppe, M. Acetylcholinesterase and Butyrylcholinesterase in Cardiosurgical Patients with Postoperative Delirium. J. Intensive Care 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.G.; Boncyk, C.S.; Fedeles, B.; Pandharipande, P.P.; Chen, W.; Patel, M.B.; Brummel, N.E.; Jackson, J.C.; Raman, R.; Ely, E.W.; et al. Association between Cholinesterase Activity and Critical Illness Brain Dysfunction. Crit. Care 2022, 26, 377. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.A.; Sauaia, A.; Moore, E.E.; Haenel, J.B.; Burch, J.M.; Lezotte, D.C. Postinjury Multiple Organ Failure. J. Trauma INJ Infect. Critical Care 1996, 40, 501–512. [Google Scholar] [CrossRef]
- Profita, M.; Bonanno, A.; Siena, L.; Ferraro, M.; Montalbano, A.M.; Pompeo, F.; Riccobono, L.; Pieper, M.P.; Gjomarkaj, M. Acetylcholine Mediates the Release of IL-8 in Human Bronchial Epithelial Cells by a NFkB/ERK-Dependent Mechanism. Eur. J. Pharmacol. 2008, 582, 145–153. [Google Scholar] [CrossRef]
- Sato, E.; Koyama, S.; Okubo, Y.; Kubo, K.; Sekiguchi, M. Acetylcholine Stimulates Alveolar Macrophages to Release Inflammatory Cell Chemotactic Activity. Am. J. Physiol. Lung C 1998, 274, L970–L979. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Smith, R.M.; Banks, R.E.; Windsor, A.C.J.; Dickson, R.A.; Guillou, P.J. Stimulation of Inflammatory Markers after Blunt Trauma. Brit. J. Surg. 1998, 85, 986–990. [Google Scholar] [CrossRef]
- Akkose, S.; Ozgurer, A.; Bulut, M.; Koksal, O.; Ozdemír, F.; Ozguç, H. Relationships between Markers of Inflammation, Severity of Injury, and Clinical Outcomes in Hemorrhagic Shock. Adv. Ther. 2007, 24, 955–962. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [Green Version]
- Crockson, R.A.; Payne, C.J.; Ratcliff, A.P.; Soothtll, J.F. Time Sequence of Acute Phase Reactive Proteins Following Surgical Trauma. Clin. Chim Acta 1966, 14, 435–441. [Google Scholar] [CrossRef]
- Shakespeare, P.G.; Ball, A.J.; Spurr, E.D. Serum Protein Changes after Abdominal Surgery. Ann. Clin. Biochem. 1988, 26, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Karakaya, C.; Noyan, T.; Ekin, S.; Babayev, E. Serum IL-6 and CRP Levels in Patients with Trauma Involving Low-Extremity Bone Fractures. East. J. Med. 2013, 18, 176. [Google Scholar]
- Meisner, M.; Adina, H.; Schmidt, J. Correlation of Procalcitonin and C-Reactive Protein to Inflammation, Complications, and Outcome during the Intensive Care Unit Course of Multiple-Trauma Patients. Crit. Care 2005, 10, R1. [Google Scholar] [CrossRef] [Green Version]
- Eklund, C.M. Proinflammatory Cytokines in CRP Baseline Regulation. Adv. Clin. Chem. 2009, 48, 111–136. [Google Scholar] [CrossRef]
- Richter, K.; Sagawe, S.; Hecker, A.; Küllmar, M.; Askevold, I.; Damm, J.; Heldmann, S.; Pöhlmann, M.; Ruhrmann, S.; Sander, M.; et al. C-Reactive Protein Stimulates Nicotinic Acetylcholine Receptors to Control ATP-Mediated Monocytic Inflammasome Activation. Front. Immunol. 2018, 9, 1604. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Tajima, S.; Yamada, S.; Watanabe, Y.; Sato, K.Z.; Matsui, M.; Misawa, H.; Kasahara, T.; Kawashima, K. Constitutive Expression of MRNA for the Same Choline Acetyltransferase as That in the Nervous System, an Acetylcholine-Synthesizing Enzyme, in Human Leukemic T-Cell Lines. Neurosci. Lett. 1999, 259, 71–74. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. The Lymphocytic Cholinergic System and Its Biological Function. Life Sci. 2003, 72, 2101–2109. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H.; Horiguchi, K. Non-Neuronal Cholinergic System in Regulation of Immune Function with a Focus on A7 NAChRs. Int. Immunopharmacol. 2015, 29, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.C.; Cornwell, E.E.; Phillips, J.; Paradise, J.; Campbell, K. Early Leukocytosis in Trauma Patients: What Difference Does It Make? Curr. Surg. 2003, 60, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.A.; Purcell, T.B.; Mejia, C. Leukocytosis as a Predictor of Severe Injury in Blunt Trauma. West. J. Emerg. Med. 2007, 9, 81–85. [Google Scholar]
- Lam, S.W.; Leenen, L.P.H.; van Solinge, W.W.; Hietbrink, F.; Huisman, A. Evaluation of Hematological Parameters on Admission for the Prediction of 7-Day in-Hospital Mortality in a Large Trauma Cohort. Clin. Chem. Lab. Med. 2011, 49, 493–499. [Google Scholar] [CrossRef]
- Abramson, N.; Melton, B. Leukocytosis: Basics of Clinical Assessment. Am. Fam. Physician 2000, 62, 2053–2060. [Google Scholar]
- Paydar, S.; Bazrafkan, H.; Golestani, N.; Roozbeh, J.; Akrami, A.; Moradi, A.M. Effects of Intravenous Fluid Therapy on Clinical and Biochemical Parameters of Trauma Patients. Emerg. Tehran Iran 2014, 2, 90–95. [Google Scholar]


| Volunteers | |
| Number of volunteers | 40 |
| Age of volunteers (years) * | 38 (29–54) |
| Gender of volunteers (m/f) | 23/17 (58%/42%) |
| Trauma Patients | |
| Number of trauma patients | 82 |
| Age of trauma patients (years) * | 53 (33–69) |
| Gender of trauma patients (m/f) | 55/27 (67%/33%) |
| SOFA * | 4 (0–8) |
| Days in ICU * | 4 (2–12) |
| Traumatic injury | |
| ISS * | 19 (10–29) |
| ISS 4–15 (number of patients) | 29 |
| ISS 16–24 (number of patients) | 24 |
| ISS > 25 (number of patients) | 29 |
| ISS body regions | |
| Head or neck (number of patients) | 48 |
| Face (number of patients) | 16 |
| Chest (number of patients) | 55 |
| Abdomen or pelvic contents (number of patients) | 23 |
| Extremities or pelvic girdle (number of patients) | 43 |
| External (number of patients) | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zivkovic, A.R.; Paul, G.M.; Hofer, S.; Schmidt, K.; Brenner, T.; Weigand, M.A.; Decker, S.O. Increased Enzymatic Activity of Acetylcholinesterase Indicates the Severity of the Sterile Inflammation and Predicts Patient Outcome following Traumatic Injury. Biomolecules 2023, 13, 267. https://doi.org/10.3390/biom13020267
Zivkovic AR, Paul GM, Hofer S, Schmidt K, Brenner T, Weigand MA, Decker SO. Increased Enzymatic Activity of Acetylcholinesterase Indicates the Severity of the Sterile Inflammation and Predicts Patient Outcome following Traumatic Injury. Biomolecules. 2023; 13(2):267. https://doi.org/10.3390/biom13020267
Chicago/Turabian StyleZivkovic, Aleksandar R., Georgina M. Paul, Stefan Hofer, Karsten Schmidt, Thorsten Brenner, Markus A. Weigand, and Sebastian O. Decker. 2023. "Increased Enzymatic Activity of Acetylcholinesterase Indicates the Severity of the Sterile Inflammation and Predicts Patient Outcome following Traumatic Injury" Biomolecules 13, no. 2: 267. https://doi.org/10.3390/biom13020267
APA StyleZivkovic, A. R., Paul, G. M., Hofer, S., Schmidt, K., Brenner, T., Weigand, M. A., & Decker, S. O. (2023). Increased Enzymatic Activity of Acetylcholinesterase Indicates the Severity of the Sterile Inflammation and Predicts Patient Outcome following Traumatic Injury. Biomolecules, 13(2), 267. https://doi.org/10.3390/biom13020267









