Quantitative Proteomic Characterization of Foreign Body Response towards Silicone Breast Implants Identifies Chronological Disease-Relevant Biomarker Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
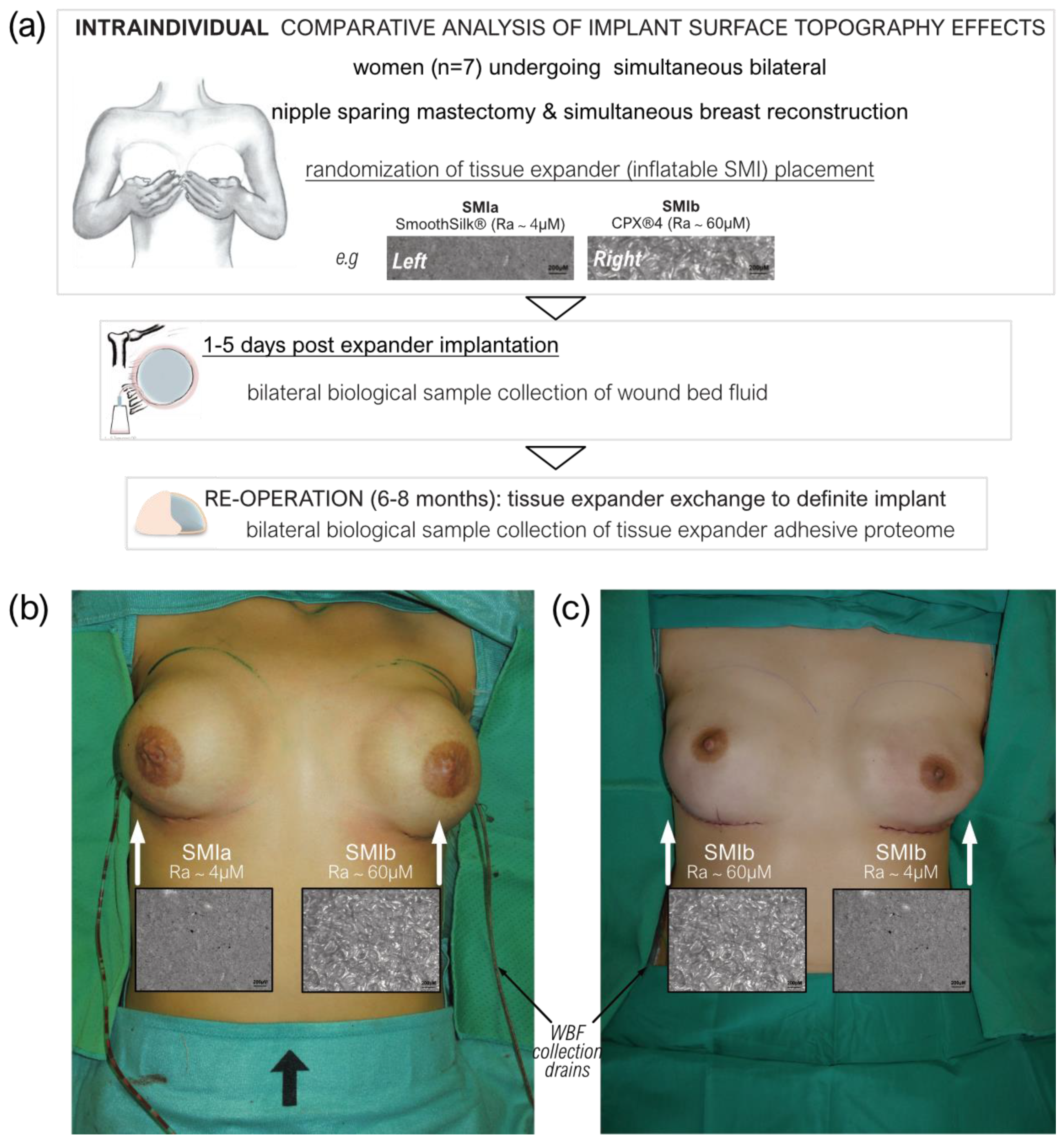
2.2. Study Design
2.3. Biological Sample Collection
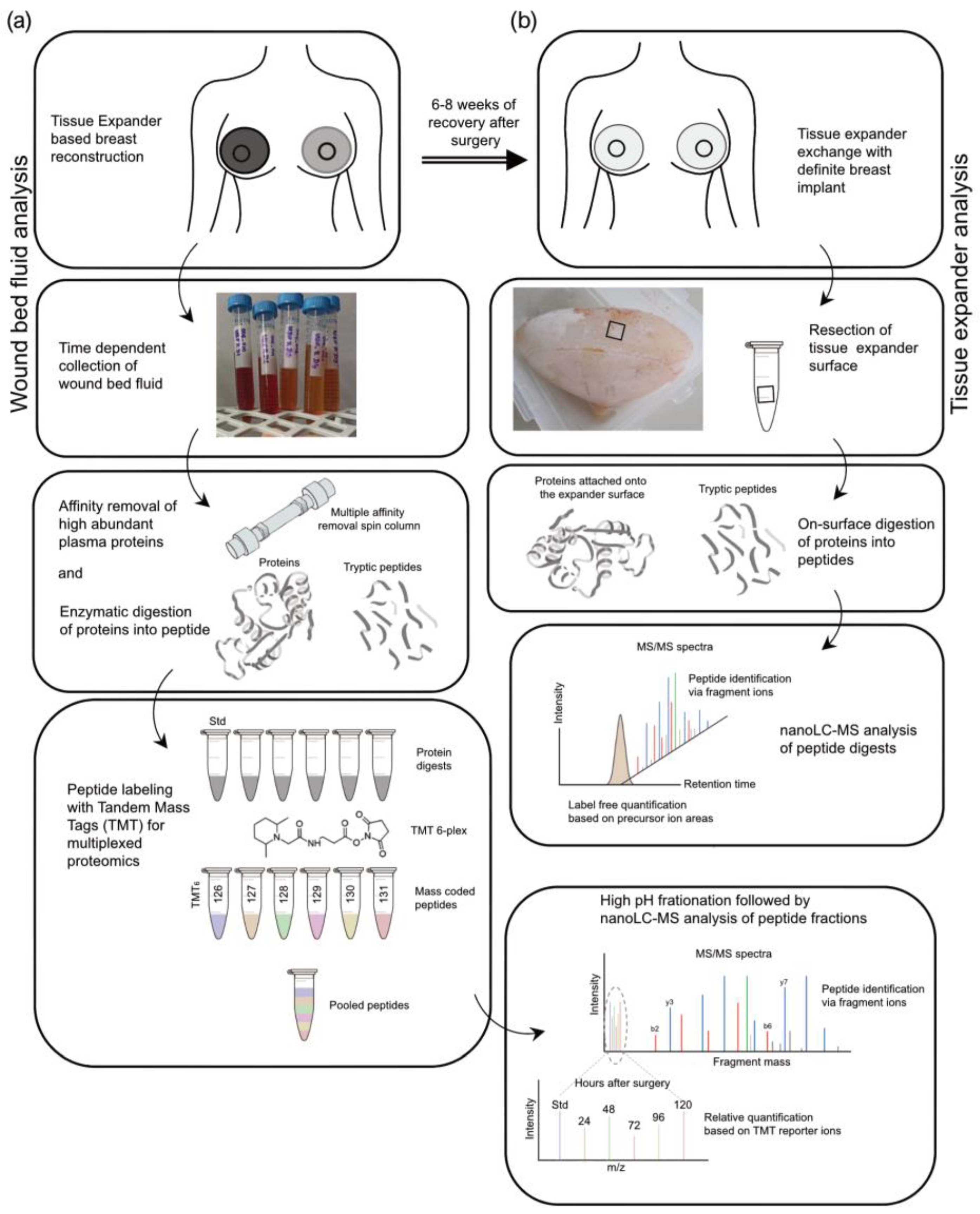
2.4. Sample Preparation of Wound Bed Fluid and Serum Samples for TMT-Based Quantitative Proteomic Approach
2.4.1. Immunoaffinity Depletion of Highly Abundant Plasma Proteins
2.4.2. Protein Reduction and Alkylation
2.4.3. Chloroform/Methanol Precipitation
2.4.4. In-Solution Protein Digestion and TMT-Labeling
2.5. Sample Preparation for Label-Free Quantitative Proteomic Analysis of Adsorbed Proteins to Tissue Expander Surface
2.5.1. Excision of Tissue Expander Slices
2.5.2. Protein Reduction, Digestion, and Alkylation
2.6. Liquid Chromatography Coupled to Tandem Mass Spectrometry (nanoLC-MS/MS)
2.7. Database Search
2.8. Quantification and Statistical Data Analysis
2.8.1. Identification, Characterization, and Quantification of Common Wound Bed Proteome
2.8.2. Identification and Characterization of Common Adsorbed Wound Bed Proteome on SMI Surface
3. Results
3.1. Patient Characteristics
3.2. The Workflow of Proteomics Analysis
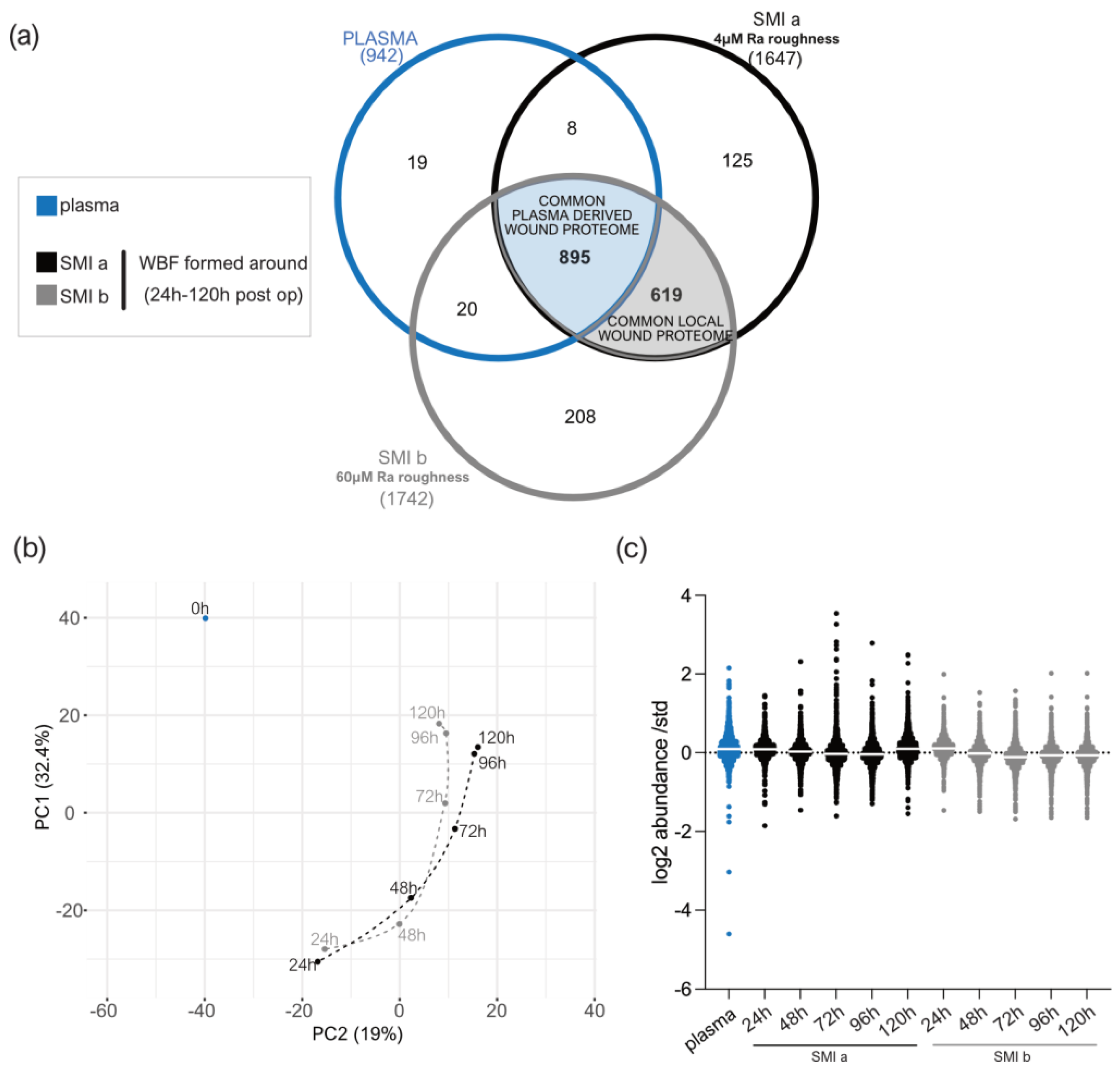
3.3. Quantification of Intraindividual Comparative Proteomic Profiling in Plasma, Wound, and SMI-Adhesive Proteome
3.4. Distribution of Wound Proteins Identified by Proteomic Analysis
3.5. Composition of Plasma-Derived Wound Proteome Formed around Silicon Tissue Expanders for the First Five Days after Implantation
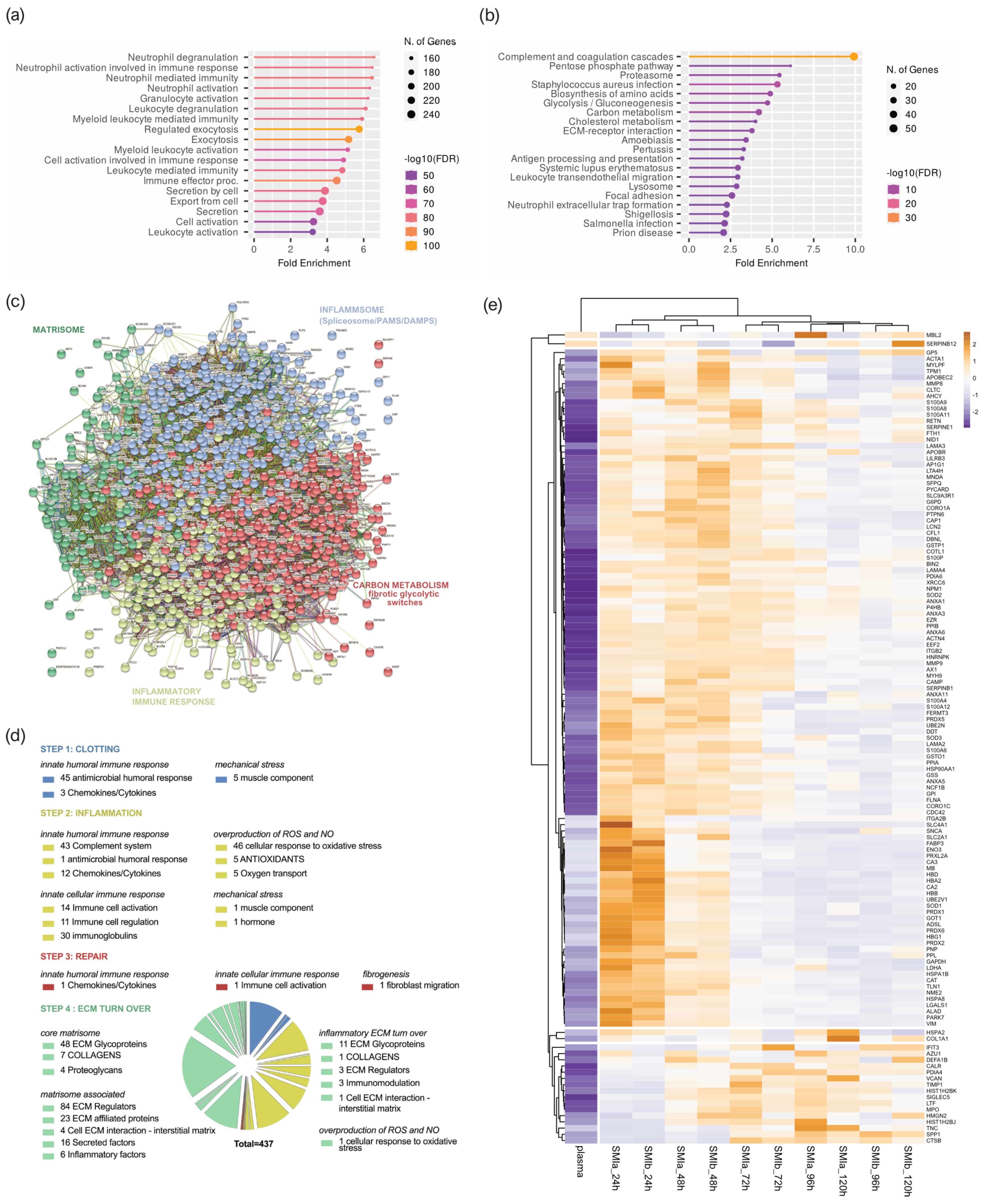
3.6. Proinflammatory Mediation in Local Wound Proteome Formed around Silicon Tissue Expanders for the First Five Days after Implantation
3.7. Wound Proteome Adsorption on SMI Surfaces in the First 8 Months Post-Implantation
3.8. From Wound to Early-Stage Fibrosis: Adhesion of Inflammatory Matrisome to Silicone Surfaces
4. Discussion
4.1. Intraindividual Comparative Proteomic Profiling in Plasma, Wound, and SMI-Adhesive Proteome
4.2. Immunomics: The Essence Lies in Sample Integrity
4.3. Immediate Inflammatory Rush in the Wound after SMI Implantation
4.4. Pathogen Binding and Activation of Inflammasome in the Wound
4.5. FBR and Inflammation: The Response in Inflammatory Matrisome
4.6. From Wound to Fibrosis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Wang, T.; Tian, L.; Song, H.; Gao, M. Roxatidine inhibits fibrosis by inhibiting NF-κB and MAPK signaling in macrophages sensing breast implant surface materials. Mol. Med. Rep. 2019, 21, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Kuehlmann, B.A.; Bonham, C.A.; Gurtner, G.C. Abstract 114. Plast. Reconstr. Surg. Glob. Open 2019, 7, 80. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Jou, I.-M.; Jeng, S.-F.; Chu, C.-H.; Huang, J.-S.; Hsu, T.-I.; Chang, L.-R.; Huang, P.-W.; Chen, J.-A.; Chou, T.-M. Hypoxia-induced epithelial-mesenchymal transition and fibrosis for the development of breast capsular contracture. Sci. Rep. 2019, 9, 10269. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Chaikuad, A.; Bullock, A.N. Structural Basis of Intracellular TGF-β Signaling: Receptors and Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022111. [Google Scholar] [CrossRef]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Lan, H.Y. Diverse Roles of TGF-β/Smads in Renal Fibrosis and Inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef]
- Margadant, C.; Sonnenberg, A. Integrin–TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Wick, G.; Grundtman, C.; Mayerl, C.; Wimpissinger, T.-F.; Feichtinger, J.; Zelger, B.; Sgonc, R.; Wolfram, D. The Immunology of Fibrosis. Annu. Rev. Immunol. 2013, 31, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Siggelkow, W.; Faridi, A.; Spiritus, K.; Klinge, U.; Rath, W.; Klosterhalfen, B. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials 2003, 24, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Handel, N.M.; Jensen, J.A.M.; Black, Q.B.; Waisman, J.R.M.; Silverstein, M.J.M. The Fate of Breast Implants. Plast. Reconstr. Surg. 1995, 96, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Siggelkow, W.; Gescher, D.; Klee, D.; Malik, E.; Rath, W.; Faridi, A. In Vitro Analysis of Modified Surfaces of Silicone Breast Implants. Int. J. Artif. Organs 2004, 27, 1100–1108. [Google Scholar] [CrossRef]
- Prantl, L.; Schreml, S.; Fichtner-Feigl, S.; Pöppl, N.; Eisenmann-Klein, M.; Schwarze, H.; Füchtmeier, B. Clinical and Morphological Conditions in Capsular Contracture Formed around Silicone Breast Implants. Plast. Reconstr. Surg. 2007, 120, 275–284. [Google Scholar] [CrossRef]
- Mempin, M.; Hu, H.; Chowdhury, D.; Deva, A.; Vickery, K. The A, B and C’s of Silicone Breast Implants: Anaplastic Large Cell Lymphoma, Biofilm and Capsular Contracture. Materials 2018, 11, 2393. [Google Scholar] [CrossRef]
- Bizjak, M.; Selmi, C.; Praprotnik, S.; Bruck, O.; Perricone, C.; Ehrenfeld, M.; Shoenfeld, Y. Silicone implants and lymphoma: The role of inflammation. J. Autoimmun. 2015, 65, 64–73. [Google Scholar] [CrossRef]
- Wolfram, D.; Oberreiter, B.; Mayerl, C.; Soelder, E.; Ulmer, H.; Piza-Katzer, H.; Wick, G.; Backovic, A. Altered systemic serologic parameters in patients with silicone mammary implants. Immunol. Lett. 2008, 118, 96–100. [Google Scholar] [CrossRef]
- Backovic, A.; Wolfram, D.; Del-Frari, B.; Piza, H.; Huber, L.A.; Wick, G. Simultaneous analysis of multiple serum proteins adhering to the surface of medical grade polydimethylsiloxane elastomers. J. Immunol. Methods 2007, 328, 118–127. [Google Scholar] [CrossRef]
- Dolores, W.; Christian, R.; Harald, N.; Hildegunde, P.; Georg, W. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J. Autoimmun. 2004, 23, 81–91. [Google Scholar] [CrossRef]
- Cappellano, G.; Ploner, C.; Lobenwein, S.; Sopper, S.; Hoertnagl, P.; Mayerl, C.; Wick, N.; Pierer, G.; Wick, G.; Wolfram, D. Immunophenotypic characterization of human T cells after in vitro exposure to different silicone breast implant surfaces. PLoS ONE 2018, 13, e0192108. [Google Scholar] [CrossRef]
- Wolfram, D.; Rabensteiner, E.; Grundtman, C.; Böck, G.; Mayerl, C.; Parson, W.; Almanzar, G.; Hasenöhrl, C.; Piza-Katzer, H.; Wick, G. T Regulatory Cells and TH17 Cells in Peri–Silicone Implant Capsular Fibrosis. Plast. Reconstr. Surg. 2012, 129, 327e–337e. [Google Scholar] [CrossRef]
- Borkner, C.B.; Wohlrab, S.; Möller, E.; Lang, G.; Scheibel, T. Surface Modification of Polymeric Biomaterials Using Recombinant Spider Silk Proteins. ACS Biomater. Sci. Eng. 2016, 3, 767–775. [Google Scholar] [CrossRef]
- Witherel, C.E.; Abebayehu, D.; Barker, T.H.; Spiller, K.L. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis. Adv. Health Mater. 2019, 8, e1801451. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.; Kim, S.; Wufuer, M.; Park, S.; Kim, Y.; Choi, D.; Jin, X.; Kim, Y.; Huang, Y.; et al. Efficient reduction of fibrous capsule formation around silicone breast implants densely grafted with 2-methacryloyloxyethyl phosphorylcholine (MPC) polymers by heat-induced polymerization. Biomater. Sci. 2020, 8, 1580–1591. [Google Scholar] [CrossRef]
- Swartzlander, M.D.; Barnes, C.A.; Blakney, A.K.; Kaar, J.L.; Kyriakides, T.R.; Bryant, S.J. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 2015, 41, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Zeplin, P.H.; Berninger, A.K.; Maksimovikj, N.C.; van Gelder, P.; Scheibel, T.; Walles, H. Improving the biocompatibility of silicone implants using spider silk coatings: Immunohistochemical analysis of capsule formation. Handchir. Mikrochir. Plast. Chir. Organ Dtsch. Arb. Handchir. Organ Dtsch. Arb. Mikrochir. Peripher. Nerven Gefasse Handchir Mikrochir Plast Chir. 2014, 46, 336–341. [Google Scholar] [CrossRef]
- Wick, G.; Backovic, A.; Rabensteiner, E.; Plank, N.; Schwentner, C.; Sgonc, R. The immunology of fibrosis: Innate and adaptive responses. Trends Immunol. 2010, 31, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, D.L.; Lefkowitz, S.S. Macrophage–neutrophil interaction: A paradigm for chronic inflammation revisited. Immunol. Cell Biol. 2001, 79, 502–506. [Google Scholar] [CrossRef]
- Duffield, J.; Lupher, M. PRM-151 (recombinant human serum amyloid P/pentraxin 2) for the treatment of fibrosis. Drug News Perspect. 2010, 23, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Vago, J.P.; Perretti, M.; Teixeira, M.M. Mediators of the Resolution of the Inflammatory Response. Trends Immunol. 2019, 40, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef]
- Sindrilaru, A.; Scharffetter-Kochanek, K. Disclosure of the Culprits: Macrophages—Versatile Regulators of Wound Healing. Adv. Wound Care 2013, 2, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Implants, Institute of Medicine (US) Committee on the Safety of Silicone Breast, Stuart Bondurant, Virginia Ernster, and Roger Herdman; Immunology of Silicone; National Academies Press: Washington, DC, USA, 1999.
- Greisler, H.P. Interactions at the Blood/Material Interface. Ann. Vasc. Surg. 1990, 4, 98–103. [Google Scholar] [CrossRef]
- Faruq, O.; Chien, P.N.; Dönmez, N.; Nam, S.-Y.; Heo, C.-Y. Functionalization of Silicone Surface with Drugs and Polymers for Regulation of Capsular Contracture. Polymers 2021, 13, 2731. [Google Scholar] [CrossRef]
- Naidu, S.H.; Beredjiklian, P.; Adler, L.; Bora, W.; Baker, D.G. In vivo inflammatory response to silicone elastomer particulate debris. J. Hand Surg. 1996, 21, 496–500. [Google Scholar] [CrossRef]
- Hallab, N.J.; Samelko, L.; Hammond, D. The Inflammatory Effects of Breast Implant Particulate Shedding: Comparison with Orthopedic Implants. Aesthetic Surg. J. 2019, 39, S36–S48. [Google Scholar] [CrossRef]
- Atiyeh, B.; Emsieh, S. Effects of Silicone Breast Implants on Human Cell Types In Vitro: A Closer Look on Host and Implant. Aesthetic Plast. Surg. 2022, 46, 2609–2611. [Google Scholar] [CrossRef]
- Xing, S.; Santerre, J.P.; Labow, R.S.; Boynton, E.L. The effect of polyethylene particle phagocytosis on the viability of mature human macrophages. J. Biomed. Mater. Res. 2002, 61, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Tavazzani, F.; Xing, S.; Waddell, J.E.; Smith, D.; Boynton, E.L. In vitro interaction between silicone gel and human monocyte-macrophages. J. Biomed. Mater. Res. Part A 2004, 72A, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.P.; Hill, E.W.; Bayat, A. Novel Proteomic Assay of Breast Implants Reveals Proteins with Significant Binding Differences: Implications for Surface Coating and Biocompatibility. Aesthetic Surg. J. 2018, 38, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Backovic, A.; Huang, H.-L.; Del Frari, B.; Piza, H.; Huber, L.A.; Wick, G. Identification and Dynamics of Proteins Adhering to the Surface of Medical Silicones in Vivo and in Vitro. J. Proteome Res. 2006, 6, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Staiano-Coico, L.; Higgins, P.J.; Schwartz, S.B.; Zimm, A.J.; Goncalves, J. Wound fluids: A reflection of the state of healing. J. Wound Ostomy Cont. Nurs. 2000, 46, 85S–93S. [Google Scholar]
- Harvey, J.; Mellody, K.T.; Cullum, N.; Watson, R.E.B.; Dumville, J. Wound fluid sampling methods for proteomic studies: A scoping review. Wound Repair Regen. 2022, 30, 317–333. [Google Scholar] [CrossRef]
- Hartman, E.; Wallblom, K.; van der Plas, M.J.A.; Petrlova, J.; Cai, J.; Saleh, K.; Kjellström, S.; Schmidtchen, A. Bioinformatic Analysis of the Wound Peptidome Reveals Potential Biomarkers and Antimicrobial Peptides. Front. Immunol. 2021, 11, 620707. [Google Scholar] [CrossRef]
- Kappel, R.M.; Klunder, A.J.H.; Pruijn, G.J.M. Silicon chemistry and silicone breast implants. Eur. J. Plast. Surg. 2013, 37, 123–128. [Google Scholar] [CrossRef]
- Doloff, J.C.; Veiseh, O.; de Mezerville, R.; Sforza, M.; Perry, T.A.; Haupt, J.; Jamiel, M.; Chambers, C.; Nash, A.; Aghlara-Fotovat, S.; et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat. Biomed. Eng. 2021, 5, 1115–1130. [Google Scholar] [CrossRef]
- Shahinuzzaman, A.D.A.; Chakrabarty, J.K.; Fang, Z.; Smith, D.; Kamal, A.H.M.; Chowdhury, S.M. Improved in-solution trypsin digestion method for methanol–chloroform precipitated cellular proteomics sample. J. Sep. Sci. 2020, 43, 2125–2132. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; Da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Anderson, J.M. Biocompatibility and Bioresponse to Biomaterials. Princ. Regen. Med. 2008, 39, 704–723. [Google Scholar] [CrossRef]
- He, Y.; Young, P.K.; Grinnell, F. Identification of Proteinase 3 as the Major Caseinolytic Activity in Acute Human Wound Fluid. J. Investig. Dermatol. 1998, 110, 67–71. [Google Scholar] [CrossRef]
- Hoffman, R.; Starkey, S.; Coad, J. Wound Fluid from Venous Leg Ulcers Degrades Plasminogen and Reduces Plasmin Generation by Keratinocytes. J. Investig. Dermatol. 1998, 111, 1140–1144. [Google Scholar] [CrossRef]
- Jerke, U.; Hernandez, D.P.; Beaudette, P.; Korkmaz, B.; Dittmar, G.; Kettritz, R. Neutrophil serine proteases exert proteolytic activity on endothelial cells. Kidney Int. 2015, 88, 764–775. [Google Scholar] [CrossRef]
- Schrader, M. Origins, Technological Development, and Applications of Peptidomics. Methods Mol. Biol. 2018, 1719, 3–39. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell. Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, C.; Hutzelmann, J. Plasminogen activator inhibitor-1 binds to fibrin and inhibits tissue-type plasminogen activator-mediated fibrin dissolution. J. Biol. Chem. 1992, 267, 17128–17135. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Liu, G.; Lundström, A.; Gelius, E.; Steiner, H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl. Acad. Sci. USA 1998, 95, 10078–10082. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution Structures of Human LL-37 Fragments and NMR-Based Identification of a Minimal Membrane-Targeting Antimicrobial and Anticancer Region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Structures of Human Host Defense Cathelicidin LL-37 and Its Smallest Antimicrobial Peptide KR-12 in Lipid Micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef]
- Woloszynek, J.C.; Hu, Y.; Pham, C.T.N. Cathepsin G-regulated Release of Formyl Peptide Receptor Agonists Modulate Neutrophil Effector Functions. J. Biol. Chem. 2012, 287, 34101–34109. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Liu, S.; Qin, Z. Extracellular S100A4 as a key player in fibrotic diseases. J. Cell. Mol. Med. 2020, 24, 5973–5983. [Google Scholar] [CrossRef]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Volk, S.W.; Wang, Y.; Mauldin, E.A.; Liechty, K.W.; Adams, S.L. Diminished Type III Collagen Promotes Myofibroblast Differentiation and Increases Scar Deposition in Cutaneous Wound Healing. Cells Tissues Organs 2011, 194, 25–37. [Google Scholar] [CrossRef]
- Tian, Y.; Li, H.; Gao, Y.; Liu, C.; Qiu, T.; Wu, H.; Cao, M.; Zhang, Y.; Ding, H.; Chen, J.; et al. Quantitative proteomic characterization of lung tissue in idiopathic pulmonary fibrosis. Clin. Proteom. 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bülow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Araújo-Gomes, N.; Romero-Gavilán, F.; Sánchez-Pérez, A.M.; Gurruchaga, M.; Azkargorta, M.; Elortza, F.; Martinez-Ibañez, M.; Iloro, I.; Suay, J.; Goñi, I. Characterization of serum proteins attached to distinct sol-gel hybrid surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Suto, T.; Karonitsch, T. The immunobiology of mTOR in autoimmunity. J. Autoimmun. 2019, 110, 102373. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y. Ribosomal Alteration-Derived Signals for Cytokine Induction in Mucosal and Systemic Inflammation: Noncanonical Pathways by Ribosomal Inactivation. Mediat. Inflamm. 2014, 2014, 708193. [Google Scholar] [CrossRef] [PubMed]
- Hadjicharalambous, M.R.; Lindsay, M.A. Idiopathic Pulmonary Fibrosis: Pathogenesis and the Emerging Role of Long Non-Coding RNAs. Int. J. Mol. Sci. 2020, 21, 524. [Google Scholar] [CrossRef]
- Rabhi, N.; Desevin, K.; Belkina, A.C.; Tilston-Lunel, A.; Varelas, X.; Layne, M.D.; Farmer, S.R. Obesity-induced senescent macrophages activate a fibrotic transcriptional program in adipocyte progenitors. Life Sci. Alliance 2022, 5, e202101286. [Google Scholar] [CrossRef]
- Akilbekova, D.; Bratlie, K.M. Quantitative Characterization of Collagen in the Fibrotic Capsule Surrounding Implanted Polymeric Microparticles through Second Harmonic Generation Imaging. PLoS ONE 2015, 10, e0130386. [Google Scholar] [CrossRef]
- Cheung, D.T.; Benya, P.D.; Perelman, N.; Dicesare, P.E.; Nimni, M.E. A Highly Specific and Quantitative Method for Determining Type III/I Collagen Ratios in Tissues. Matrix 1990, 10, 164–171. [Google Scholar] [CrossRef]
- Kuehlmann, B.; Zucal, I.; Bonham, C.A.; Joubert, L.-M.; Prantl, L. SEM and TEM for identification of capsular fibrosis and cellular behavior around breast implants—A descriptive analysis. BMC Cell Biol. 2021, 22, 25. [Google Scholar] [CrossRef]
- Chen, W.; Rock, J.B.; Yearsley, M.M.; Ferrell, L.D.; Frankel, W.L. Different collagen types show distinct rates of increase from early to late stages of hepatitis C–related liver fibrosis. Hum. Pathol. 2014, 45, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Kinoshita, R.; Tomonobu, N.; Gohara, Y.; Tomida, S.; Takahashi, Y.; Senoo, S.; Taniguchi, A.; Itano, J.; Yamamoto, K.-I.; et al. The heterodimer S100A8/A9 is a potent therapeutic target for idiopathic pulmonary fibrosis. J. Mol. Med. 2020, 99, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Anderton, S.M.; Van Der Zee, R.; Goodacre, J.A. Inflammation activates self hsp60-specific T cells. Eur. J. Immunol. 1993, 23, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Moré, S.H.; Breloer, M.; von Bonin, A. Eukaryotic heat shock proteins as molecular links in innate and adaptive immune responses: Hsp60-mediated activation of cytotoxic T cells. Int. Immunol. 2001, 13, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Carlier, T.D.B.; Badloe, F.M.S.; Ring, J.; Gutermuth, J.; Krohn, I.K. Autoreactive T cells and their role in atopic dermatitis. J. Autoimmun. 2021, 120, 102634. [Google Scholar] [CrossRef]
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensiv. Care 2015, 3, 54. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Kyle, D.J.; Oikonomou, A.; Hill, E.; Bayat, A. Development and functional evaluation of biomimetic silicone surfaces with hierarchical micro/nano-topographical features demonstrates favourable in vitro foreign body response of breast-derived fibroblasts. Biomaterials 2015, 52, 88–102. [Google Scholar] [CrossRef]
- Shin, B.H.; Kim, B.H.; Kim, S.; Lee, K.; Bin Choy, Y.; Heo, C.Y. Silicone breast implant modification review: Overcoming capsular contracture. Biomater. Res. 2018, 22, 37. [Google Scholar] [CrossRef] [Green Version]




| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Female sex | Sever coagulation disorder, representing a potential contraindication for the elective surgery |
| Age >18 years | Rheumatic disease accompanied by oblkigatory intake of immunomodulating therapeutic agents |
| High-risk family history for breast and/or ovarian cancer and/or BRCA1/2 gene mutation carrie | Severe renal functional disorder: renal insufficiency status IV or V (estimated glomerulary filtration rate (GFR) <30 mL/min) |
| Planned bilateral mastectomy with simultaneous breast reconstruction | Active hematological or oncological disease |
| Signed Informed consent form | HIV-Infection |
| Hepatitis-Infection | |
| Pregnancy or breast-feeding | |
| Intake of anti-inflammatory drugs | |
| Carrier of silicone implants (e.g., gastric banding, mammary implants) | |
| Subject is currently participating or intends to participate in another clinical trial that may interfere with the protocol of this study. | |
| Participants who have implanted devices that could be affected by a magnetic field (e.g., pacemakers, drug infusion devices, artificial sensing devices). When there is an alteration in hematologic and serum protein reference values post-chemotherapy. | |
| When there is a residual malignancy in the intended expansion site. | |
| Existing tissue at the intended expansion site is not adequate according to the surgeon’s criteria, because of previous radiation therapy, ulcerations, vascular compromise, history of compromised wound healing, or scar deformity. | |
| Radiation therapy before or after the expander placement can be associated with a higher rate of complications during the expansion and final implantation phases of the reconstructive process. | |
| Abscess or infection in the body in general. | |
| Participants with autoimmune diseases (e.g., lupus, scleroderma) or whose immune system is compromised (e.g., currently receiving immunosuppressive therapy such as steroids). | |
| Unsuitable tissue due to radiation damage on the chest wall, tight thoracic skin grafts or radical resection of the pectoralis major muscle. |
| SmoothSilk® | Mentor CPX4 | ||||
|---|---|---|---|---|---|
| Surface Roughness | Ra ~ 4 µM | Ra ~ 60 µM | |||
| Mean | (±std) | Mean | (±std) | p value | |
| age (y) | 35.2 | 11.4 | 35.2 | 11.4 | intraindividual comparison >0.9999 |
| weight (kg) | 71.4 | 24.5 | 71.4 | 24.5 | |
| size (cm) | 168.6 | 10.5 | 168.6 | 10.5 | |
| BMI | 25.1 | 6.7 | 25.1 | 6.7 | |
| Bilateral prophylactic NSME resection weight [g] | |||||
| left breast | 434.9 | 404.0 | 436.9 | 454.0 | 0.993196 |
| right breast | 334.2 | 257.5 | 337.9 | 174.4 | 0.975407 |
| Prepectoral reconstruction volume [cc] | |||||
| left breast | 405.5 | 156.3 | 392.6 | 151.8 | 0.877595 |
| right breast | 360.8 | 151.1 | 352.7 | 150.0 | 0.920737 |
| intaoperative filling | 254.7 | 169.4 | 254.7 | 169.4 | >0.9999 |
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
|---|---|---|---|---|
| RED | GREEN | YELLOW | BLUE | |
| CARBON METABOLISM in fibrolytic switches | MATRISOME | INFLAMMATORY IMMUNE CELL RESPONSE | INFLAMMSOME | |
| proteins | 281 | 120 | 159 | 308 |
| nodes | 281 | 120 | 159 | 308 |
| edges | 3592 | 503 | 1310 | 4526 |
| average node degree | 25.6 | 8.38 | 16.5 | 29.4 |
| local clustering coefficient | 0.51 | 0.52 | 0.54 | 0.49 |
| expected number of edges | 1284 | 18 | 163 | 479 |
| PPI enrichment p-value | ||||
| <1.0 × 10−16 | <1.0 × 10−16 | <1.0 × 10−16 | <1.0 × 10−16 | |
| Inflammatory Matrisome Class | Number of Common Plasma Derived Wound Proteins | Number of Differentially Expressed Plasma Derived Wound Proteins | Ratio [%] | |
|---|---|---|---|---|
| Innate humoral immune response | Antimicrobial humoral response | 46 | 15 | 33% |
| Chemokines/cytokines | 16 | 5 | 31% | |
| Complement system | 43 | 9 | 21% | |
| Innate cellular immune response | Immune cell activation | 15 | 5 | 33% |
| Immune cell regulation | 11 | 8 | 73% | |
| Immunoglobulins | 30 | 0 | 0% | |
| Overproduction of ROS and NO | Cellular response to oxidative stress | 47 | 24 | 51% |
| Oxygen transport | 5 | 5 | 100% | |
| Antioxidants | 5 | 5 | 100% | |
| Mechanical stress | Muscle component | 4 | 6 | 150% |
| Hormone | 1 | 1 | 100% | |
| Core matrisome | ECM glycoproteins | 59 | 13 | 22% |
| Collagens | 8 | 1 | 13% | |
| Proteoglycans | 4 | 0 | 0% | |
| Matrisome associated | ECM regulators | 87 | 12 | 14% |
| ECM affiliated proteins | 23 | 6 | 26% | |
| Cell ECM interaction—interstitial matrix | 5 | 2 | 40% | |
| Secreted factors | 16 | 9 | 56% | |
| Inflammatory factors | 6 | 2 | 33% | |
| Immunomodulation | 3 | 0 | 0% | |
| Fibroblast migration | 1 | 1 | 100% |
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
|---|---|---|---|---|
| RED | GREEN | YELLOW | BLUE | |
| CARBON METABOLISM in fibrolytic switches | MATRISOME | INFLAMMATORY IMMUNE CELL RESPONSE | INFLAMMSOME | |
| proteins | 150 | 221 | 117 | 123 |
| nodes | 150 | 221 | 117 | 123 |
| edges | 306 | 967 | 575 | 967 |
| average node degree | 4.08 | 0.17 | 9.83 | 15.7 |
| local clustering coefficient | 0.394 | 0.38 | 0.531 | 0.515 |
| expected number of edges | 49 | 307 | 123 | 242 |
| PPI enrichment p-value | ||||
| <1.0 × 10−16 | <1.0 × 10−16 | <1.0 × 10−16 | <1.0 × 10−16 | |
| Inflammatory Matrisome Class | Number of Common Local Wound Proteins | Number of Differentially Expressed Local Wound Proteins | Ratio [%] | |
|---|---|---|---|---|
| Innate humoral immune response | DAMP | 12 | 0 | 0% |
| PAMP | 1 | 0 | 0% | |
| Antimicrobial humoral response | 1 | 1 | 100% | |
| Chemokines/Cytokines | 1 | 0 | 0% | |
| Complement system | 13 | 2 | 15% | |
| Blood clotting pathway | 6 | 3 | 50% | |
| Proinflammatory mediators | 179 | 36 | 20% | |
| Inflammation-resolving | 2 | 0 | 0% | |
| Innate humoral immune response | Innate Immune Gene Expression | 1 | 0 | 0% |
| Immune cell activation | 2 | 0 | 0% | |
| Immune cell regulation | 11 | 3 | 27% | |
| Immunoglobulins | 20 | 0 | 0% | |
| Cellular response to oxidative stress | 2 | 0 | 0% | |
| Fibrosis mediator | 1 | 0 | 0% | |
| Core matrisome | ECM Glycoproteins | 15 | 1 | 7% |
| Keratins | 6 | 2 | 33% | |
| Collagens | 4 | 0 | 0% | |
| Proteoglycans | 5 | 1 | 20% | |
| Matrisome associated | ECM affiliated proteins | 13 | 5 | 38% |
| ECM Regulators | 16 | 1 | 6% | |
| ECM affiliated proteins | 11 | 0 | 0% | |
| Cell ECM interaction—interstitial matrix | 4 | 1 | 25% | |
| Secreted factors | 2 | 2 | 100% | |
| Inflammatory Signalling | 1 | 0 | 0% | |
| Oncologic marker | Mammaglobin A | 1 | 1 | 100% |
| Inflammatory Matrisome Class | Number of Plasma Derived Wound Proteins d1–d5 Post SMI Implantation | Number of Plasma Derived Wound Proteins Associated with SMI Surface 6–8 Months Post SMI Implantation | Ratio [%] | Number of Local Wound Proteins | Number of Local Wound Proteins Associated with SMI Surface 6–8 Months Post SMI Implantation | Ratio [%] | |
|---|---|---|---|---|---|---|---|
| Innate humoral immune response | DAMPs | 1 | 1 | 100% | |||
| Antimicrobial humoral response | 46 | 3 | 7% | ||||
| Chemokines/Cytokines | 16 | 1 | 6% | ||||
| Proinflammatory mediation (ribosomal proteins) | 179 | 8 | 4% | ||||
| Complement system | 43 | 11 | 26% | ||||
| Innate cellular immune response | Immune cell activation | 15 | 2 | 13% | |||
| Immune cell regulation | 11 | 6 | 55% | ||||
| Immunoglobulins | 30 | 5 | 17% | ||||
| Overproduction of ROS and NO | Cellular response to oxidative stress | 47 | 4 | 9% | |||
| Oxygen transport | 5 | 9 | 180% | ||||
| Antioxidants | 5 | 4 | 80% | ||||
| Mechanical stress | Muscle component | 4 | 1 | 25% | |||
| Hormone | 1 | 0 | 0% | ||||
| Inflammatory response | 223 | 46 | 21% | 180 | 9 | 5% | |
| Core matrisome | ECM glycoproteins | 59 | 13 | 22% | 15 | 2 | 13% |
| Collagens | 8 | 5 | 63% | 4 | 0 | 0% | |
| Proteoglycans | 4 | 3 | 75% | 5 | 1 | 20% | |
| Keratins | 6 | 0 | 0% | ||||
| Matrisome associated | ECM regulators | 87 | 15 | 17% | |||
| ECM affiliated proteins | 23 | 7 | 30% | ||||
| Cell ECM interaction—interstitial matrix | 5 | 2 | 40% | ||||
| Secreted factors | 16 | 11 | 69% | ||||
| Inflammatory factors | 6 | 1 | 17% | ||||
| Immunomodulation | 3 | 3 | 100% | ||||
| Fibroblast migration | 1 | 0 | 0% | ||||
| ECM Turn-Over | 212 | 60 | 28% | 30 | 3 | 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoberleitner, I.; Faserl, K.; Sarg, B.; Egle, D.; Brunner, C.; Wolfram, D. Quantitative Proteomic Characterization of Foreign Body Response towards Silicone Breast Implants Identifies Chronological Disease-Relevant Biomarker Dynamics. Biomolecules 2023, 13, 305. https://doi.org/10.3390/biom13020305
Schoberleitner I, Faserl K, Sarg B, Egle D, Brunner C, Wolfram D. Quantitative Proteomic Characterization of Foreign Body Response towards Silicone Breast Implants Identifies Chronological Disease-Relevant Biomarker Dynamics. Biomolecules. 2023; 13(2):305. https://doi.org/10.3390/biom13020305
Chicago/Turabian StyleSchoberleitner, Ines, Klaus Faserl, Bettina Sarg, Daniel Egle, Christine Brunner, and Dolores Wolfram. 2023. "Quantitative Proteomic Characterization of Foreign Body Response towards Silicone Breast Implants Identifies Chronological Disease-Relevant Biomarker Dynamics" Biomolecules 13, no. 2: 305. https://doi.org/10.3390/biom13020305
APA StyleSchoberleitner, I., Faserl, K., Sarg, B., Egle, D., Brunner, C., & Wolfram, D. (2023). Quantitative Proteomic Characterization of Foreign Body Response towards Silicone Breast Implants Identifies Chronological Disease-Relevant Biomarker Dynamics. Biomolecules, 13(2), 305. https://doi.org/10.3390/biom13020305








