Abstract
Both cardiovascular disease and cancer continue to be causes of morbidity and mortality all over the world. Preventing and treating heart disease in patients undergoing cancer treatment remain an important and ongoing challenge for improving the lives of cancer patients, but also for their survival. Despite ongoing efforts to improve patient survival, minimal advances have been made in the early detection of cardiovascular disease in patients suffering from cancer. Understanding the communication between cancer and cardiovascular disease can be based on a deeper knowledge of the molecular mechanisms that define the profile of the bilateral network and establish disease-specific biomarkers and therapeutic targets. The role of exosomes, microvesicles, and apoptotic bodies, together defined as extracellular vesicles (EVs), in cross talk between cardiovascular disease and cancer is in an incipient form of research. Here, we will discuss the preclinical evidence on the bilateral connection between cancer and cardiovascular disease (especially early cardiac changes) through some specific mediators such as EVs. Investigating EV-based biomarkers and therapies may uncover the responsible mechanisms, detect the early stages of cardiovascular damage and elucidate novel therapeutic approaches. The ultimate goal is to reduce the burden of cardiovascular diseases by improving the standard of care in oncological patients treated with anticancer drugs or radiotherapy.
1. Introduction
Cardiovascular (CV) diseases and cancer are two of the most common causes of death worldwide. The World Health Organisation estimates that 17.9 million deaths per year are attributed to CV disease and nearly 10 million deaths are caused by cancer [1,2]. However, because of early detection and diagnosis and the discovery of novel therapeutic approaches, the mortality rate for these two types of pathologies, although still high, has reduced in recent years [3].
Cardiovascular disease prevention for cancer patients has become an increasingly important concern in the ongoing struggle to improve patients’ quality of life. This brings together experts in the fields of cardiology and oncology to provide timely and specialized CV care to oncological patients and survivors of all ages.
By addressing the intersection of the CV and oncologic fields, a comprehensive CV risk assessment for identification of cancer patients who are at high risk for suffering cardiac toxicities from cancer therapies can be pursued. Studies show that in some types of cancer, the mortality cause by other comorbidities, such as CV disease, is higher than the mortality of cancer itself [4]. This can be explained by the development of cardiotoxicity, which translates to the alteration of cardiac structure and function following chemotherapy, radiotherapy or immunotherapy, used either alone or in combination.
The long-term follow-up of cancer survivors who received cardiotoxic treatments must be taken into consideration for early identification and management of CV disease. More specifically, in addition to the use of advanced imaging for early identification of changes in cardiac function during cancer treatment, the identification of new biomarkers could yield important benefits in the survival of cancer patients. Recent studies have shown that the incidence of cancer in patients with CV disease is also increasing [5]. This means that one disease may influence the other, thus, leading to a vicious cycle.
Extracellular vesicles (EVs) could be among the most important biomarkers as they play a decisive role in early identification and determining the CV risk for cancer patients receiving specific treatment for this disease. In addition to their role as mediators in intercellular communication through the transfer of bioactive molecules from secretory cells of origin to recipient cells, modulating multiple pathophysiological processes and serving as biomarkers for doxorubicin (DOX)- induced cardiotoxicity, EVs can serve as both biomarkers for doxorubicin (DOX)-induced cardiotoxicity, and carriers of certain proteins, such as miRNA and lncRNA and certain chemotherapeutic drugs in decreasing the dosage of DOX and alleviating cardiotoxicity.
This review briefly describes some aspects of CV disease development in patients with cancer and highlights the potential role of EVs as diagnostic biomarkers and drug delivery vehicles. In order to establish the specific role of EVs in the dialogue between CV disease and cancer, fundamental, clinical and translational research has been recently encouraged and consolidated.
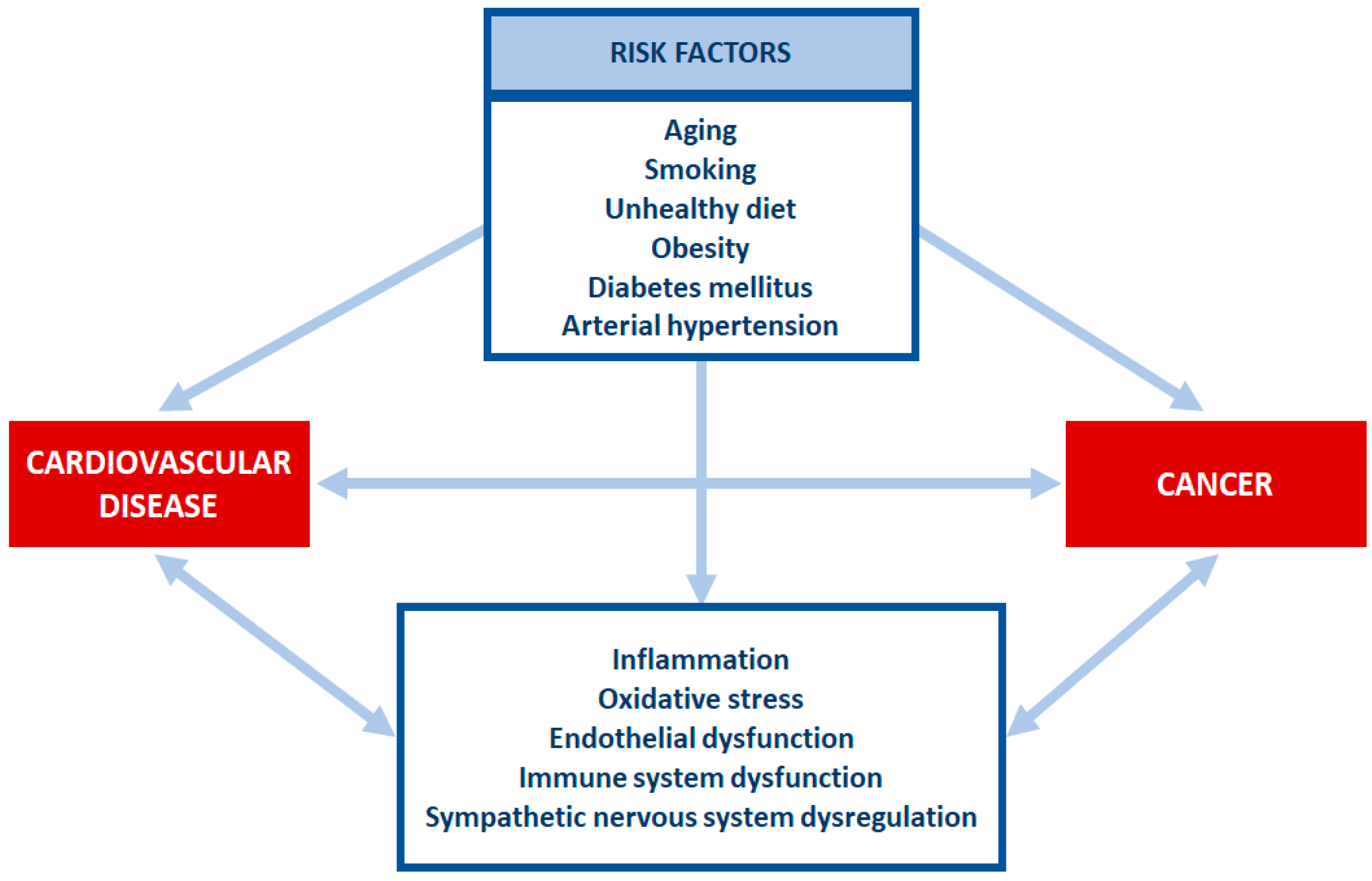
3. Cross Talk between Cardiovascular Disease and Cancer
Traditionally, the interest for cardio-oncology developed after the acknowledgement of CV side effects induced by chemotherapy drugs. In this view, CV disease and cancer were depicted as two separate entities and the link between them was unidirectional, represented by the intensely studied field of cancer therapy-related CV toxicity. Moreover, cancer itself may be predisposed to CV disease development through direct mechanisms. However, over the past years, the field of cardio-oncology has significantly evolved, with the consideration that CV disease may not only be a consequence of cancer, but it may itself predispose patients to cancer development. The term “reverse cardio-oncology” has emerged, as we began to understand that the systemic effects of CV disease can have pro-tumourigenic effects [35]. Data from major clinical trials indicated that cancer was the leading cause of non-CV mortality, accounting for more than 35% in both TOPCAT and PARADIGM-HF trials [36,37]. In the RE-LY trial, more than one third of deaths among patients with atrial fibrillation were caused by non-CV causes, with cancer being a leading cause [38]. Epidemiological data have reported an association between CV disease and an increased risk of developing cancer, suggesting that CV disease may enhance an oncogenic process through various pathophysiological pathways, including numerous factors secreted by the heart.
3.1. Myocardial Infarction
Combining two or more of the aforementioned risk factors may increase the risk to develop acute cardiac events, such as myocardial infarction (MI). A prospective cohort study included 28,763 patients without a previous history of MI that were followed from baseline to date of cancer, death, migration, or study end, for a mean period of 15.7 years [39]. During follow-up, 1747 patients suffered MI and 146 of them developed cancer. Patients with MI had an increased risk of 46% to develop cancer compared to those without MI. The incidence of cancer was the highest in the first 6 months after MI. Another prospective study that included 1081 patients with MI that were followed for almost 5 years demonstrated that the patients who subsequently developed heart failure (HF) had a higher incidence of cancer compared to those without HF [5]. Both studies stated that patients with a history of MI have a higher risk of cancer even after a significant period of time after MI [5,39]. The clarification comes from a study conducted on APCmin mice, which are prone to developing precancerous intestinal tumors, with HF induced by inflicting large anterior MI, where it was shown that myocardial ischemia and necrosis contribute to inflammation and angiogenesis and increase the levels of TNF, thus leading to the development of cancer [40].
Another study that began in 1996 included all Danish residents aged 30–99 (2,871,168 subjects) without prior MI or cancer, and they were followed up for 16 years [41]. Of all subjects, 122,275 suffered MI during follow-up, 11,375 developed subsequent cancer and 65,225 subjects died. In the reference population, 372,397 subjects developed cancer and 753,767 died [41]. MI was associated with an increased risk of cancer after adjusting for age, sex or chronic obstructive pulmonary disease, arterial hypertension, dyslipidemia, diabetes and socio-economic status, but not after further adjustment for the first 6 months after MI [41].
On the contrary to the abovementioned studies showing an increased risk of cancer in patients after MI, various types of cancer treated with antineoplastic pads containing sodium nitroprusside were associated with reduced mortality and improved outcome after MI (12.3% mortality with pads versus 29.9% without pads) [42].
3.2. Heart Failure
Heart failure is a clinical syndrome characterized by signs (peripheral oedema, pulmonary crackles, jugular venous distention) and symptoms (dyspnea, orthopnea, fatigue) but also by an abnormal structure and/or function of the heart. Patients with both preserved and reduced ejection fraction HF display a poor prognosis with an estimated 50% and 25% survival rate at 5 years from diagnosis and 10 years, respectively [43]. However, it seems that 20% and up to 50% of patients with HF die from non-CV causes [44].
A retrospective cohort study in Germany between 2000 and 2018 included 100,124 patients with HF and 100,124 patients without HF [45]. In total, 25.7% of patients with HF and 16.2% of patients without HF were diagnosed with cancer. HF is associated with an increased incidence of all cancer sites, the strongest association being for lip, oral cavity, pharynx and lung cancer [45].
Another study that took place between 2002 and 2018 included 103,421 patients with HF and 104,012 control subjects with no cancer within three years before baseline, who were followed for more than five years [46]. A total of 12,036 patients with HF were newly diagnosed with cancer compared to 7045 control subjects. HF patients showed increased incidence of lung, liver and nervous system cancer, with a similar spread in both men and women. Cancer mortality was also higher in HF patients, especially in those younger than 70 years old (a total of 103,608 deaths, 64,483 patients with HF versus 39,125 control subjects) [46]. The cause of death was known in 74.5% of all deaths. In total, 5946 of deaths were caused by cancer (4738 in patients with HF versus 1208 in the control group) and 12 575 deaths were caused by HF (9703 in patients with HF versus 2872 in the control group) [46]. All of these studies show that HF patients have a higher rate of cancer than healthy control populations. Going further with this idea, we could suggest that HF might actually cause cancer.
However, the vast majority of studies shows that cancer patients develop HF as a consequence of cancer treatment, whereas the studies showing that patients with HF may have an increased incidence of cancer during the course of the disease are quite few. This could be plausible from a biological point of view because both HF and cancer have common risk factors such as obesity and diabetes, but also inadequate lifestyle, including smoking, alcohol consumption and lack of physical activity. Moreover, the factors secreted by the failing heart could stimulate tumor growth. Therefore, because of the high incidence of both diseases and their impact on the lives of those affected, these patients deserve the maximum joint efforts of cardiologists and oncologists.
3.3. Cardiovascular Medication and Cancer
As discussed above, various risk factors, such as arterial hypertension, dyslipidemia or diabetes mellitus may promote the development and progression of cancer. However, studies have also shown that certain classes of medication used to treat CV disease may play a role in either promoting or inhibiting the development of cancer.
Antiplatelet drugs, such as aspirin, are used in the primary and secondary prevention of CV disease. As far as cancer is concerned, their use must be done with caution depending on the dose used and the age of the patients. There is rising evidence regarding the use of low-dose aspirin and cancer prevention. Thus, the studies have shown that the use of aspirin for more than five years may be correlated with a reduced risk of colorectal cancer, whereas the longer use (up to 20 years) is associated with even higher protection [47]. The mechanism of action of aspirin in preventing cancer remains to be elucidated. However, we could speculate that long-term administered aspirin could have a positive effect on cells with malignant potential and could suppress certain inflammatory processes. On the other hand, the use of aspirin in patients over 70 years old has been associated with cancer progression and/or mortality because of its risk of bleeding [48].
Diuretics, in general, do not influence the risk of cancer, but there is evidence that treatment with hydrochlorothiazide may increase the incidence of skin cancer beucase of its photosensitivity in a dose-dependent manner [49,50]. Thiazide diuretics are associated with insulin resistance, which is considered a risk factor for breast cancer [51]. The use of diuretics for more than ten years may increase the incidence of breast cancer by 16%, although other studies show the opposite, that long-term use of diuretics protects against breast cancer [52,53]. More studies are needed in order to elucidate the mechanism behind these statements and establish whether diuretics play a protective role or not against breast cancer.
Calcium channel blockers (CCBs) are a widely used class of medication for treating CV disease. CCBs inhibit apoptosis and interfere with cell differentiation via calcium triggering signals [54]. Studies evidenced that treatment for more than nine years with CCBs increases the risk of cancer, especially lung and prostate cancer in men and breast cancer in women (particularly invasive ductal carcinoma and invasive lobular carcinoma) [55,56]. However, one recent study showed no association between the use of CCBs and an increased risk of breast cancer compared with non-CCB antihypertensive drugs [57].
Selective beta-2 blockers may reduce the recurrence and metastasis of malignancies, improving overall survival in cancer, whereas beta-1 blockers showed no beneficial effect on cancer [58]. Non-selective beta-blockers reduced the incidence of hepatocellular carcinoma in patients with cirrhosis by inhibiting angiogenesis and decreasing bacterial translocation [59]. Both selective and non-selective beta-blockers, particularly propranolol, were associated with a reduction of mortality rate in pancreatic cancer, probably by inhibiting the damage induced by catecholamine stimulation of the adrenoreceptors [60]. Propranolol may reduce the expression of Ki67 and thus decrease tumour proliferation. It can also increase the phosphorylation of P53 in the early phases of breast cancer and induce cell apoptosis, thus showing promising effects in breast cancer [61]. Women taking propranolol years before breast cancer diagnosis showed a reduced rate of metastatic cancer and a lower mortality rate compared to women who were not on treatment with propranolol [58].
Drugs that inhibit the renin-angiotensin system (RAS), such as angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs), are some of the most widely used antihypertensive medications. Lately, there has been strong evidence that RAS is involved in inflammation, cell proliferation via active peptide angiotensin-II signaling, VEGF-mediated angiogenesis in malignancy and metastasis. Hence, drugs that inhibit RAS may slow down tumour growth, especially when used for a long time [62]. ACEIs may be correlated with a lower risk of prostate cancer, pancreatic cancer, colorectal, esophageal and gastric cancer [63,64,65,66]. However, there are data in the literature that try to demonstrate that the administration of ACEIs can increase the risk of lung cancer [67]. This can be explained by the existence of bradykinin and many related receptors in lung cancer tissue, thus releasing VEGF that stimulates angiogenesis. The risk of lung cancer is directly proportional with the dose and the duration of ACEIs use. One study showed that hypertensive patients taking ACEIs versus ARBs had a greater risk of pulmonary cancer [68].
Some experimental studies suggested that metformin, the most commonly prescribed drug for type II diabetes mellitus, displays antineoplastic effects, thus leading to a lower incidence of certain types of cancers in diabetic patients (liver, colon, stomach, breast cancer) [69,70]. Metformin may reduce the risk of cancer by 30 to 50% when compared to insulin or sulfonylureas [28].
The effects of CV drugs on various types of cancer are summarized in Table 1.

Table 1.
Effects of CV medication on different types of cancer.
3.4. Cardiac and Vascular Toxicity Caused by Antineoplastic Therapy
The increased number of cancer survivors is a direct consequence of the development of novel cancer therapies. These patients, though, are at high risk of developing CV disease because of the effect of cancer on the heart and, also, as a result of chemo- and/or radiotherapy. Risk factors for cardiac and vascular toxicity caused by cancer treatment include preexisting CV risk factors, combination of chemo- and radiotherapy, accumulating doses of drugs or left ventricular dysfunction prior to the initiation of therapy.
Cancer-related cardiac dysfunction can develop many years after the initial treatment, occurring with an incidence of 10% [71]. HF is the most concerning complication of cancer therapy, followed by myocarditis, coronary artery disease, valvular disease, arrhythmias, arterial hypertension and many other CV complications [72]. The development of CV toxicity significantly worsens the prognosis of oncological patients and may require dose-reduction or even discontinuation of chemotherapy. The pathophysiological classification of cardiotoxicity includes type 1, characterized by dose-dependence and irreversible myocardial damage, and type 2, characterized by dose-independence and reversible myocardial damage. Additionally, other mechanisms are involved in the development of cardiac dysfunction, such as inflammation, conduction abnormalities, arterial spasm and development of hypertension. Anthracyclines (e.g., doxorubicin—DOX) have shown well-established efficacy for the treatment of solid tumours (e.g., breast, gastric, ovarian cancer) and hematological malignancies, but they may also lead to irreversible cardiac damage, which is cumulative and dose-dependent. Anthracycline-induced cardiac dysfunction (AICD) is a result of the progressive decline of cardiac function, which may lead to dilated cardiomyopathy. However, a recent study showed that AICD was associated with hypokinetic non-dilated cardiomyopathy rather than typical dilated cardiomyopathy [73]. Women are less prone to develop AICD when compared to men. This may be a consequence of estrogens that are considered cardioprotective, as they enhance myocardial resistance to ischemia by attenuating abnormal oxidative stress and apoptosis [74].
Cardiac arrhythmias are also a consequence of antineoplastic drug use. The most common arrhythmias in cancer patients are prolonged QT interval, atrioventricular block, atrial fibrillation and ventricular arrhythmias. Bradycardia is a consequence of treatment with paclitaxel, thalidomide, palazonib and sunitinib, whereas atrial fibrillation is caused by anthracycline, ibrutinib, melphalan and paclitaxel [75,76]. Chemotherapy is also responsible for prolongation of the QT interval, a condition that resolves gradually as the drugs are being metabolized [77].
In addition to cardiotoxicity, chemotherapy is also responsible for vascular toxicity resulting in arterial toxicity (atherosclerosis, coronary artery disease) as well as venous toxicity (venous thromboembolism: deep vein thrombosis or pulmonary embolism). Treatment with paclitaxel can lead to coronary spasms and even MI [78]. Cisplatin has a dose-dependent effect and can cause coronary artery disease [79]. A recent study revealed an increased incidence of MI during treatment with 5-fluorouracil, whereas its use is associated with an increase in copeptin and rarely in cardiac troponin I [80]. Vascular complications are common in cancer, pulmonary cancer patients having the highest rate of mortality, whereas colon cancer patients have the highest bleeding risk [81]. Venous thromboembolism is the second cause of mortality in patients with cancer, after disease progression. Pulmonary embolism develops in about 50% of patients with untreated deep vein thrombosis [82]. The factors that predispose patients with cancer to venous thromboembolism are the type of cancer, radiotherapy, central venous catheter chemotherapy and medication side effects [83]. Arterial hypertension may appear as a result of VEGF signals inhibitors that lead to an imbalance between vasodilator and vasoconstrictor factors. The development of arterial hypertension in the early stages of treatment may be considered a predictor of the medication effect [84]. Therapy with platinum (e.g., oxaliplatin) may induce arterial hypertension and also myocardial ischemia [85].
The effects of chemo- and radiotherapy on CV disease are resumed in Table 2.

Table 2.
Effects of chemo- and radiotherapy on CV disease.
The general approach to prevent CV toxicity caused by chemotherapy includes pre-treatment estimation of patient-specific risk. Baseline risk assessment is recommended to all patients who will receive a cancer treatment with potential cardiotoxicity and includes, among others, cardiac history, CV risk factors, cardiac troponin, and B-type natriuretic peptide (BNP) or its N-terminal fragment (NT-proBNP), electrocardiogram and echocardiography, as well as cancer treatment history [94]. However, research efforts have lately focused on the identification of other biomarkers of cancer-related CV toxicity, which would predict more accurately the development of CV disease and would allow for intervention in the preclinical phase.
4. Mediators Connecting Cardiovascular Disease and Cancer
The pathophysiological overlap between cancer and CV disease is expressed at different levels, including inflammation, oxidative stress, neuro-hormonal activation, clonal hematopoiesis and circulating factors. Traditionally, the interest was to find predictors for CV toxicity associated with antineoplastic treatment and to identify these patients in whom chemotherapy would represent a heavy burden. Nowadays, the relationship between cancer and CV diseases is no longer unidirectional.
4.1. Circulating Factors
The association between HF and cancer was supported initially by experimental studies. In one such study, investigators studied whether certain biomarkers secreted by the failing hearts in mice would promote intestinal tumour proliferation. In this model, HF was the result of induced large anterior MI. The presence of HF resulted in a statistically significant increase in intestinal tumours, both for native and for heterotopically transplanted hearts in mice. Data from the experimental study were extrapolated to a human cohort, which included 180 healthy subjects, and also to approximately 100 patients with chronic HF [40]. The authors selected five proteins considered to be involved in the pathophysiological overlap between HF and cancer, of which SerpinA3, also known as α-1-antichymotrypsin, was increased in patients with HF and promoted cell proliferation in colon tumours. SerpinA3 belongs to the protein superfamily of serine protease inhibitors, which are involved in various inflammatory processes. Data from the literature suggest that levels of serpinA3 predict long-term mortality in patients with HF and are also associated with several cancer types, including colorectal, gastric, breast, liver and prostate cancer [95,96]. This association could have therapeutic implications, as treatment with mineralocorticoid receptor antagonists seems to downregulate the expression of serpinA3, but this needs to be further investigated [97]. Furthermore, the potential effects of serpinA3 on endothelial cell function or cardiac myocytes as contributing to HF and proliferation-associated genes (MCM6, FKBP10 and IGFBP2 in glioma) following activation of MAPK/ERK1/2, PI3K/AKT signalling in endometrial carcinoma are of interest and require further studies [95,98,99].
Other mediators that lie at the intersection of cancer and CV disease are tumour biomarkers, which have established roles in the early detection of different cancer types and for monitoring tumour progression. Moreover, certain tumour biomarkers, such as CA15-3, CEA and CA125 represent independent markers for CV disease and predict all-cause mortality in such patients, suggesting that there is still much to consider about the strong association between cancer and CV disease. One study showed that CA15-3 was strongly associated with CV mortality and HF, even after multivariable adjustment for common risk factors, whereas the correlation with coronary artery disease and stroke was not statistically significant. CEA was also associated with CV mortality, after adjustment for cofounders [100]. Inflammation has arisen as a viable cause both in CV disease and cancer, because correlation between tumour biomarkers and proinflammatory cytokines, such as TNF-α, IL-6 and IL-10, has been identified [101]. In addition, it was demonstrated that CA125 secretion could be enhanced by the inflammatory cytokines IL-1β, TNF-α and the lipopolysaccharide of Escherichia coli [102]. The proposed mechanism by which systemic inflammation affects CA125 concentrations involves JNK molecular pathways [103]. These data support the idea that CV disease and cancer share common ancillary mechanisms and pathways, which should be addressed in the future in order to ensure precise phenotyping of both diseases.
4.2. Inflammation
Chronic inflammation is involved in both CV disease and cancer. Inflammation is an important part of tumourigenesis, as it contributes to cancer initiation, proliferation and metastasis. The initial evidence for an association between inflammatory markers, CV disease and cancer originated from observational studies.
4.2.1. Cytokines
Elevated levels of C-reactive protein (CRP), interleukin-1 (IL-1) and interleukin-6 (IL-6) are associated with atherosclerosis and its complications, including plaque initiation, progression and rupture. Increased levels of CRP were also strongly associated with CV disease and cancer mortality [104]. Data from observational studies also revealed that increased levels of IL-6 were associated with both coronary heart disease and cancer [105,106]. However, these seem to be only observational associations, as data from genetic studies argues against a causative role of either CRP or IL-6 in the development of CV disease. IL-1 levels are increased in patients with CV disease, particularly chronic or decompensated heart failure, with increasing levels according to disease severity [107]. The most striking evidence for the role of IL-1 in the development of CV disease and cancer came from the CANTOS trial. In this randomized controlled trial, treatment with canakinumab, a monoclonal antibody targeting IL-1β, decreased the primary end-point (nonfatal MI, nonfatal stroke and CV death) by 15%. This drug was also associated with a decreased incidence and mortality of lung cancer, although this was not a primary outcome [108]. This study represented additional proof that inflammation links CV disease and cancer, acting as a central player in both diseases.
In contrast to cytokines, whose increased levels have been associated with both CV disease and cancer, there are some cytokines, such as IL-10, that play a role in the development of cancer, but with protective effects in CV diseases. Studies have shown that IL-10 has cardioprotective properties in diabetic mice with MI via regulation of heme clearance pathway, by reducing the size of infarction and improving cardiac function [109]. Furthermore, IL-10 improved capillary density, reduced apoptosis and decreased inflammation in the border zone of the infarcted hearts [109]. The anti-inflammatory action of IL-10 was reflected by inhibiting the secretion of other proinflammatory cytokines including TNF-α [104], nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs) [110]. In addition to its involvement in CV diseases, IL-10 could also serve as a biomarker for prognostic diseases or as a treatment target when taking into consideration the elevated concentration in cancer patients versus the healthy ones. High levels of IL-10 were observed in patients with Hodgkin’s lymphoma, gastric, pancreatic and pulmonary cancer, whereas IL-10 levels in patients with colon and renal cancer did not differ significantly from the controls [111,112,113]. Hence, IL-10 is considered an independent prognostic factor and correlates with poor survival.
Another interesting marker that is found at the intersection of hematologic malignancies and CV disease is clonal hematopoiesis of indeterminate potential (CHIP), defined as the presence of somatic mutations in hematopoietic cells, occurring among older persons and in the absence of other hematologic abnormalities. These somatic mutations, most frequently involving the genes DNMT3A and TET2, are involved in DNA methylation and have an established role in the development of myelodysplastic syndromes and acute myeloid leukemia. Moreover, CHIP emerged as a potent, independent risk factor for atherosclerotic CV disease, by regulating pro-inflammatory pathways in monocytes and macrophages, which are known to play important roles in the development of atherosclerosis. The presence of CHIP resulted in an almost two-fold increase in the risk of developing coronary heart disease, after adjustment for common risk factors. The association between CHIP and MI was even more remarkable, with a four-fold increase in the risk of developing early-onset MI for patients with CHIP. This causal association is supported by the results from a murine model, showing that mice engrafted with TET2 bone marrow had more significant atherosclerotic lesions than control mice [114]. Overall, the effect of promoting atherosclerosis is not explained by the opposing enzymatic activities of DNMT3A and TET2 on DNA methylation, but rather it is a marker of their non-enzymatic activity, which is yet to be further explored [115]. Interesting, the ERBB2 protein, or HER2/neu (transmembrane tyrosine kinase receptor), a protein that in humans is encoded by the ERBB2 gene, is implicated in the pathogenesis of multiple cancer types and intersection between cancer and chronic HF [116].
Recent studies have identified new factors called “cachexokines, which could be useful as biomarkers for the diagnosis of cancer-induced cardiac complications and might lead to the identification of new therapeutic targets. Shafer et al. developed a preclinical study and analyzed two types of mouse colon cancer cells that distinguish themselves by their capacity to induce cardiac cachexia [117]. The authors identified a group of seven secreted proteins, collectively called cachexokines (Bridging integrator 1, Syntaxin 7, Multiple inositol-polyphosphate phosphatase 1, Glucosidase alpha acid, Chemokine ligand 2, Adamts like 4 and Ataxin-10), which lead to cardiomyocyte atrophy and aberrant fatty acid metabolism in cardiomyocytes. The simultaneous downregulation of the seven cachexokines on colon cancer cells blocks their capacity to induce cardiac atrophy, whereas the blockage of the single proteins does not influence the cachectic phenotype. The same study showed that ataxin-10 correlates with cachexia, both in mouse models and in pancreatic cancer patients. These findings may pave the way for personalized predictive, diagnostic and therapeutic measures in cancer cachexia.
4.2.2. Immune Cells
Dysregulation of the immune system is involved in both cancer and CV disease. In cancer, an insufficient or inadequate immune response is responsible for tumour development and progression. The role of the immune system in CV disease is also crucial. Within hours following an acute MI, neutrophils gather in the infarcted area, followed by monocytes and macrophages in subsequent days. The role of cardiac macrophages is also prominent in HF with preserved ejection fraction, promoting cardiac remodelling and fibrosis. In a mouse model of breast cancer, it was showed that MI accelerated tumour cell proliferation, resulting in an approximately two-fold increase in tumour volume and weight at 20 days [118]. This proliferative effect was demonstrated by staining for Ki67+ tumour cells before and after inducing MI, resulting in a doubling of Ki67+ cells in the tumour border of mice with MI versus sham procedure. Further on, the authors attempted to discern the mechanisms by which MI promotes tumour proliferation. They found that MI determines an accumulation of CD11b+Ly6Chi monocytes in tumours, which share similar markers with monocytic myeloid derived suppressor cells, suggesting that MI promotes an immunosuppressive intratumoural response [118]. The authors concluded that MI induces complex epigenetic reprogramming of myeloid cells in hematopoietic reservoirs, resulting in an immunosuppressive phenotype in breast cancer that promotes tumour proliferation [118]. These preliminary results confirmed that dysregulation of the immune system, as a consequence of an acute CV event, influences tumour progression in an animal model. As stated previously, observational studies found an association between MI and cancer development in humans, but these data need further confirmation through rigorous, specifically designed trials.
4.3. Neuro-Hormonal Activation
Among the complex pathophysiological pathways of HF, renin–angiotensin–aldosterone system (RAAS) plays a central role, initially as a compensatory mechanism, and as the disease progresses, when it becomes maladaptive, leading to cardiac remodelling and sympathetic activation. The main mediator of RAAS, angiotensin II (AngII), which acts by binding to either type 1 Ang II receptor (AT1R) or type 2 Ang II receptor (AT2R), has been associated with detrimental effects, generally promoting cardiac hypertrophy, vasoconstriction and inflammation, or with beneficial effects, including anti-inflammatory and cardio-protective effects, respectively. Similarly, RAAS seems to be involved in cancer pathogenesis, with AngII/AT1R axis promoting tumour cell proliferation, inhibiting apoptosis and creating a pro-inflammatory and immunosuppressive tumour microenvironment. The involvement of RAAS in cancer pathogenesis would naturally raise the therapeutic question whether RAAS blockade with either ACEIs or ARBs would improve cancer survival. The first meta-analysis that investigated the effects of an RAAS blockade on cancer recurrence and survival demonstrated a statistically significant risk reduction of 40% and 25%, with the use of ACEI or ARB [119]. Another meta-analysis indicated a significant improvement in overall survival and cancer-specific survival with the use of RAAS blockers in patients with digestive system malignancies [120]. However, the involvement of RAAS in cancer pathogenesis seems to vary according to cancer type and several studies did not find a beneficial effect of treatment with RAAS inhibitors on cancer survival [119]. There is still much to be understood about the implications of RAAS in the pathophysiology of cancer.
5. Role of Extracellular Vesicles in Cross Talk between CV Disease and Cancer
In recent years, studies have shown that biological fluids display different types of vesicles released by the vast majority of cells, tissues and organs. These vesicles, with the classic general name of extracellular vesicles (EVs), have local or remote effects, participating in various physiological and pathological processes. Interestingly, they appear to maintain bidirectional communication between the heart and other organs and play a particularly important role in cardiac physiology and pathophysiology [121].
5.1. What Do We Know about Extracellular Vesicles?
The term EVs refers to all types of nanovesicles released into the extracellular space and subsequently into various biofluids including blood by apoptotic or activated cells, both in physiological and pathological conditions. Depending on the mechanism through which these vesicles are released, but also on their size, they have been classified into exosomes and microvesicles (also called microparticles or ectosomes) [122,123]. Additionally, some studies include apoptotic bodies (also called apoptosomes) in the large family of EVs [124]. Recent studies have adopted a new nomenclature for EVs, namely small and large vesicles [125]. The classification of EVs is constantly changing, but today it is accepted that EVs are represented by exosomes, microvesicles and possibly apoptotic bodies, based on their biogenesis, and small and large EVs based on their size. Current investigations consider several critical aspects of EVs, namely choosing the optimal separation method, finding the best storage conditions and establishing optimal characterization protocols and therapeutic strategies and dosages. Separation of large and small EV-enriched subpopulations from cardiac progenitor cells was efficiently achieved with two commonly purification methods: ultracentrifugation and size-exclusion chromatography [126]. As for their characterization, the small EVs (exosomes and microvesicles, with a diameter of ~100 nm), that sediment at 100,000× g, contain ALIX and CD63, and large EVs (apoptotic bodies, with a diameter >1000 nm), that sediment at 10,000× g, are abundant in calnexin [126]. In addition to these proteins specific to each subpopulation of EVs, they also contain a great variety of proteins, lipids and nucleic acids that reflect the cytosolic content of the source cell [127,128]. When comparatively investigating their functionality, no differences were found between small and large EVs [126]. The correct classification and characterization of EVs are important elements in completing the following steps, namely establishing their ability as biomarkers and therapeutic tools for various pathologies, including cancer and CV disease.
5.2. Extracellular Vesicles as Communication and Transport Entities
EVs are nanosized vesicles delimited by a lipid bilayer that contain various biologically active molecules (enzymes, proteins, transcription factors, lipids, and nucleic acids: DNA, mRNA, microRNA (miRNA), long non-coding RNA, circular RNA), and have the ability to reach all organs, tissues and cells of the body after their secretion into biological fluids. Almost any type of cells (endothelial cells, lymphocytes, T cells, macrophages, renal cells, cancer cells and stem cells) can release EVs in physiological state, and a number of these submicron vesicular membrane structures is increased during pathological processes [129]. Therefore, EVs can provide diagnostic and prognostic value in a variety of pathologies such as: chronic venous insufficiency, atherosclerosis and CV disease, ischemic stroke, diabetes, COVID-19, but also cancer [130,131,132,133,134,135,136,137,138,139]. In addition to their role as disease biomarkers or communication entities in different physiological ant pathological situations, EVs have long been studied for their influence as therapeutic tools, especially in atherosclerosis and CV disease [140,141,142,143]. Their potential as drug delivery systems has been recently noticed and disseminated in different medical applications: cardiac regeneration, cancer treatment and, also, in nuclear medicine as radionuclide carriers [144]. An opinion paper on EVs as theranostic agents discussed the benefits of radio-labeled EVs in diagnostic and interventional medicine [145]. The role of EVs in the development or regression of atherosclerotic lesions has been widely discussed, and the link between thrombosis and inflammation has been emphasized [146]. EVs from subcutaneous adipose tissue stem cells and bone marrow mesenchymal stem cells, loaded or not with Smad2/3-siRNA, ameliorated vascular dysfunction and reduced atherosclerosis-associated inflammation in an experimental animal model of atherosclerosis [142]. In addition, the same types of EVs significantly decreased specific hypertrophic markers and molecules involved in the inflammatory process associated with cardiac hypertrophy in an in vitro model of human-induced pluripotent stem cell-derived cardiomyocytes [143]. These effects were partially related to the miRNAs that EVs contain [142,143]. These findings suggest that cell-free therapy based on stem cell-derived EVs may provide new alternatives for therapeutic strategies targeting CV disease.
At present, there are quite a few studies that have researched and discussed the potential connection between CV disease and cancer.
5.3. Extracellular Vesicles as Nano Mediators in Cancer and Associated Cardiovascular Disease
EVs are considered potential mediators through which tumour cells communicate with each other and influence their microdomain promoting tumour progression and dissemination, by altering the immune response that subsequently generates cell proliferation, angiogenesis, matrix remodelling and finally metastasis [147,148]. EV contribution to tumour angiogenesis, neovascularization and hypoxia-dependent inter-tumour communication during cancer progression was demonstrated on metastatic brain tumour glioblastoma [149].
Generally, EVs were investigated as biomarkers for initial diagnosis, patient follow up, and, also, for evaluation of tumour response to therapy [150,151,152].
It is now well known that all cells, especially tumour cells, secrete heterogeneous EV subpopulations that vary in size, content, and function. EVs contain on their surface receptors specific to the tumour cells from which they come and specific to the type of cancer. Furthermore, their intravesical content depends on the type and stage of cancer. For instance, during hypoxia, EVs released from cancer cells are enriched in angiogenic factors, such as VEGF and hypoxia-inducible factor 1-alpha (HIF-1α) that promote angiogenesis and later tumour metastasis [153]. In ovarian cancer, EVs carrying VEGF contribute to angiogenesis and metastasis by crosstalk between cancer and endothelial cells [154]. In addition, glioblastoma-specific small EVs are demonstrated to contain the epidermal growth factor receptor variant III (EGFRvIII), the oncogenic mutant variant of EGFR frequently detected in glioblastoma tumours, which stimulates VEGF production and promotes angiogenesis [155]. Interestingly, the EGFRvIII mRNA levels were comparable in large and small EVs isolated from U87EGFRvIII cells, whereas large EVs had higher levels of EGFR protein (EGFRwt + EGFRvIII) compared to small EVs [125]. Thus, EGFRvIII mRNA and protein status can provide glioblastoma-specific liquid biopsy-based monitoring that can help not only diagnose but also evaluate tumour progression [155]. The role of EVs and their miRNA content as diagnostic, prognostic and disease monitoring biomarkers and as nanocarriers for gene therapy in glioblastoma progression and resistance to therapy have been widely debated in a recently published paper [139].
In breast cancer, EVs encompass epidermal growth factor-like repeats and discoidin I-like domains 3 (EDIL3), a glycoprotein that activates the integrin-FAK signaling cascade and plays a crucial role in cancer development [156,157,158]. EDIL3 is also known as a novel regulator of epithelial-mesenchymal transition in clear cell renal cell carcinoma [159]. EDIL3 on EVs also contribute to the enhancement of cell invasion and acceleration of lung metastasis in vivo [156]. Consequently, it was suggested that EDIL3 might be employed as a therapeutic target that limits disease progression.
There are a number of studies that have shown the primary role of EVs in the various stages of cancer and especially in tumour metastases. In addition, the role of EVs in CV pathology is well known and has been studied for a long time.
Very little data concern the involvement of EVs in cardio-oncological pathology, although intuitively, it is becoming increasingly clear that EVs could have a substantial effect.
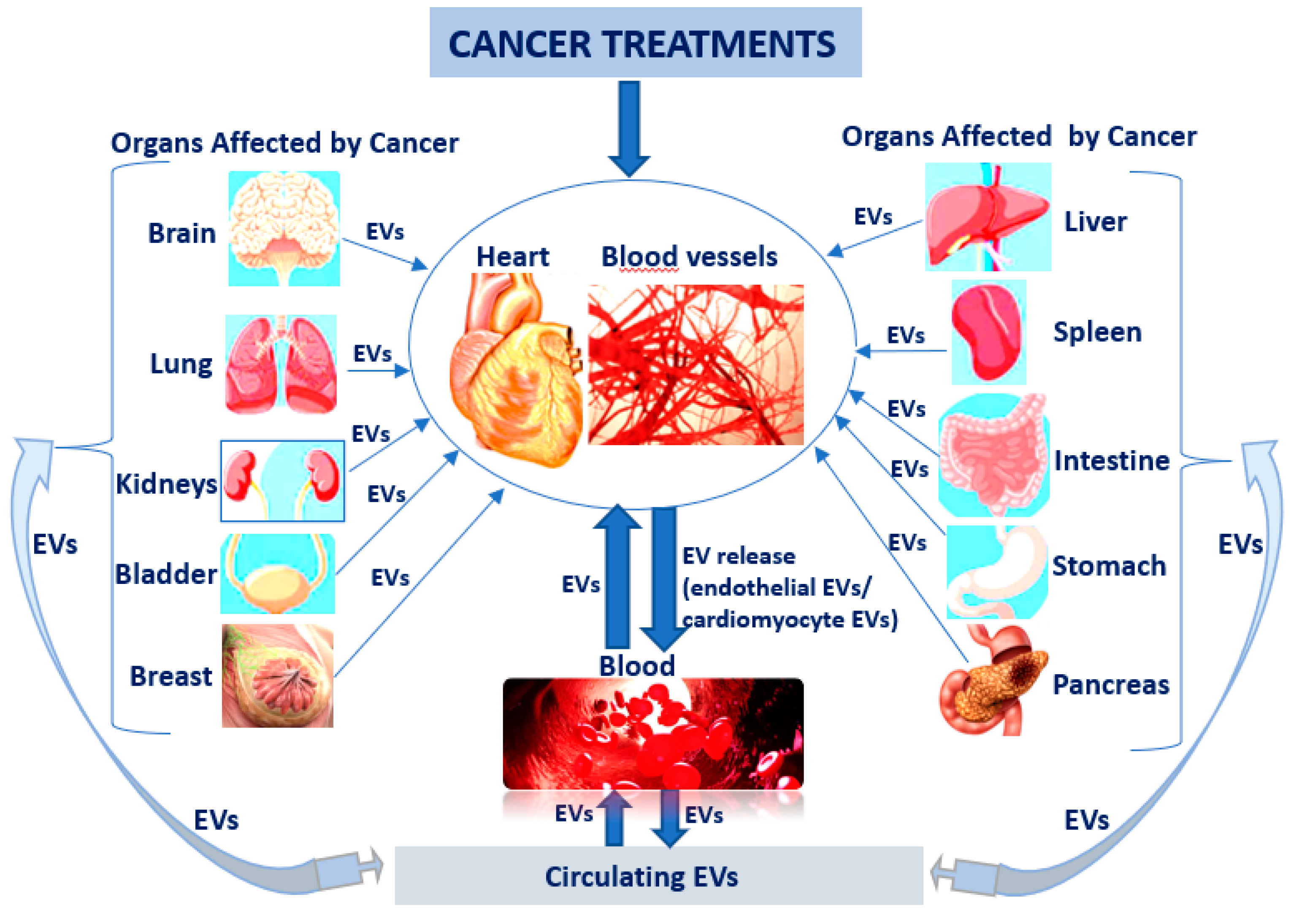
It is already known that hormone therapies used to treat breast and prostate cancers can raise the risk for a heart attack and stroke. In this case, patient follow-up is very important to identify the first cardiac changes manifested by a reduction of left ventricular ejection fraction, but finding early biomarkers could also have a decisive role in the early treatment of CV diseases. EVs could be one of the key biomarkers, especially since it is known that once released into circulation from apoptotic or activated cells (comprising tumoural cells) can potentially reach any organ, including heart and blood vessels. Furthermore, EVs can be mediators in reverse communication between heart and skeletal muscle, kidneys, bone marrow, lungs, liver, adipose tissue, and brain (Figure 2).

Figure 2.
EVs as key biomarkers in cardio-oncological pathology: cancer therapy affects the heart and blood vessels and generates the release of endothelial and cardiomyocyte EVs into circulation, which can subsequently disturb the other organs; EVs released into circulation from apoptotic, activated or tumour cells can reach any other organ, including heart and blood vessels.
In this research direction, the potential of exosomes (small EVs) as diagnostic biomarkers and therapeutic carriers for DOX-induced cardiotoxicity was discussed by Tian et al. in 2021 [160]. DOX, known as a cytotoxic anthracycline antibiotic used to treat several different types of cancer, kills not only tumour cells through circulation but also damages cardiomyocytes at the same time and induces senescence and various death forms of cardiac cells, such as apoptosis, necroptosis, autophagy, pyroptosis and ferroptosis [160].
5.3.1. EVs as Diagnosis Biomarkers in Cardio-Oncology
Regarding EVs’ biomarker capacity, in a DOX-induced cardiac injury mouse model, it was shown that cardiomyocytes release EVs that contain protein biomarkers (4-hydroxynonenal (HNE) and brain-type glycogen phosphorylase (PYGB)) of early cardiac injury, prior to cardiac troponin [161]. In addition, these EVs can be used to predict the antioxidant capacity against the chemotherapy of individual patients and to determine the dose of chemotherapy that will avoid normal tissue injury [161]. Thus, mice which develop antioxidant capacity after treatment with manganese superoxide dismutase (MnSOD) or cationic manganese (III) ortho N-substituted pyridylporphyrins (MnP)-pretreated mice have low levels of circulating EVs following DOX treatment. Furthermore, administration of dexrazoxane (DRZ), a clinically approved cardioprotective drug that has antioxidant effects, diminished EV release from heart tissue. However, the effect of DRZ on EV release was weaker than that of MnP, which might be explained by the high preference for the mitochondria of MnP.
Of note, miRNAs contained by EVs could have potential as biomarkers for early detection of DOX-induced cardiotoxicity [162]. Specifically, EVs-miR-502 found to be upregulated after single-DOX chemotherapy in dog model of sarcoma may be a damage marker for DOX-induced cardiotoxicity [162]. In addition, upregulation of miR-502 and downregulation of miR-107 and miR-146a were detected in EVs before any significant changes were seen in other established biomarkers, including cardiac troponin I (cTnI) and echocardiographic parameters (left ventricular ejection fraction and left ventricular internal dimension in diastole). It is worth mentioning that exosomal miRNAs were more persistent compared to cardiac troponin I (cTnI) in serum.
5.3.2. EVs as Therapeutic Delivery Tools in Cardio-Oncology
On the other hand, EVs themselves, because of their intravesical content could be important messengers for the regulation of various disturbed biological processes or ideal carriers for chemotherapeutic drugs to alleviate cardiotoxicity. Thus, large EVs from mesenchymal stem cells (MSCs) mitigated the negative effects induced by 1 μmol/L DOX for 24 h on induced pluripotent stem cell (iPSC)-derived cardiomyocytes [163]. These large EVs, found to be enriched in mitochondria, improved contractility, reactive oxygen species production, ATP production and mitochondrial biogenesis in cardiomyocytes [163]. Interestingly, inhibiting mitochondrial function with 1-methyl-4-phenylpyridinium attenuated the beneficial effects of EVs on cardiomyocyte injury induced by DOX treatment [163]. This exciting study was based on the SENECA Trial, a Phase 1 National Heart, Lung and Blood Institute-sponsored study that evaluated the role of MSCs in treating anthracycline-induced cardiomyopathy but without emphasizing the biological mechanisms involved.
ExosomesHypoxia isolated from human adipose-derived MSCs pretreated with hypoxia had a better cardioprotective effect then exosomes from untreated MSCs on human induced pluripotent stem cell (iPSC)-derived cardiomyocytes exposed to 0.5 μM DOX for 24 h [164]. LncRNA-MALAT1/miR-92a-3p/ATG4a partially mediated the cardioprotective roles of exosomeHypoxia in DOX-induced cardiac damage [164]. EVs collected from human iPSC-derived CV progenitor cells increased ATP levels and enhanced both mitochondrial respiration and anaerobic on iPSC-derived cardiomyocytes treated with 0.6 μM DOX (0.2 μM DOX every 48 h) [165]. The positive effects of intravenously delivered EVs on cardiac function have been demonstrated in male BALB/c mice with chemotherapy-induced cardiomyopathy (mice subjected to three weekly injections of DOX: total cumulated dose was of 12 mg/kg body weight) [165].
In addition, the intravenous administration of cardiac progenitor cell-derived exosomes protected neonatal rats against DOX/trastuzumab (TRZ)-induced cardiac toxicity through repressing inflammatory responses (attenuating macrophage infiltrates and iNOS expression), decreasing myocardial fibrosis and restoring cardiac function [166]. Particularly, exosomal miR-146-5p mediated some of the cardioprotective effects of exosomes (used at concentration of 3.5 × 106/cm2 for 1 h) by suppressing oxidative stress and target genes Traf6, Smad4, Irak1, Nox4 and Mpo in the neonatal rat ventricular myocytes exposed to 1 µM DOX for 3 h and 1 µM TRZ for a further 3 h [166].
Interestingly, exosomes derived from embryonic stem cells inhibited pyroptosis (cell death characterized by the release of vast pro-inflammatory factors) of H9c2 cardiomyoblasts induced by 2 µM DOX for 24 h. Expressions of TLR4, NLRP3, pyroptotic markers (caspase-1, IL-1ß, caspase-11, and gasdermin-D) and proinflammatory cytokines (TNF-α and IL-6) in H9c2 cells were decreased after 24 h of incubation with 10 µg exosomes [167]. The potential beneficial effects of embryonic stem cell-derived exosomes were also considered on DOX-induced pyroptosis and cardiac remodeling in C57BL/6J mice by three intraperitoneal (i.p) injections (4 mg/kg body weight), administrated on alternative days in a week time span (Monday, Wednesday, and Friday) for a cumulative dose of 12 mg/kg. Exosomes (400 µL/injection with 50 µg concentration of exosomes) injected on alternative days between DOX treatments (Tuesday, Thursday, and Saturday) decreased expressions of inflammasome markers (TLR4 and NLRP3), pyroptotic markers (caspase-1, IL1-β, and IL-18), cell signaling proteins (MyD88, p-P38, and p-JNK), pro-inflammatory M1 macrophages and TNF-α cytokine [168]. Moreover, exosomes increased M2 macrophages and the anti-inflammatory cytokine IL-10 inhibited cytoplasmic vacuolization, myofibril loss and hypertrophy, and improved heart function [168].
In addition to their abilities as important massagers, owing to their intravesicular content, EVs can also be carriers for drugs or other biological compounds. Relatively recently it has been shown that tumour cell-derived exosomes are home to their cells of origin and can be used as Trojan horses to deliver cancer drugs [169].
Specifically, exosomes from two cancer cell lines, HT1080 or Hela, engineered to carry DOX, systemically injected in nude mice bearing a subcutaneous HT1080 tumour for 15 days, fused preferentially with their parent cancer cells and eradicated tumour tissues more effectively than DOX alone (5 mgDOX/Kg of body weight) [169]. Similarly, in a mouse model of breast and ovarian cancer, it was proven that exosomes increased the therapeutic index of DOX without enhancing the cardiotoxicity of DOX. Thus, exosomes have become common delivery vehicles of DOX to target various cancers and receive better therapeutic effects than free DOX [170]. In conclusion, these exciting results indicated that exosomes derived from cancer patients might be a good weapon in the fight against cancer. This idea must be viewed with caution and studied more widely because of the metastatic role of cancer-derived exosomes.
However, rapid uptake of exosomes by the mononuclear phagocyte system (MPS) remains an obstacle for drug delivery into other targeted organs, including the heart. To solve this problem, Wan et al., hypothesized in 2020 that prior blocking of uptake of exosomes by the MPS would improve their delivery to the targeted organs [171]. They found that clathrin heavy chain (Cltc) plays a significant role in mediating endocytosis of the exosomes in the liver and spleen, and blocking it caused therapeutic exosomes (based miR-21a delivery) to be more efficiently distributed to targeted organs, such as the heart, and produced a much better therapeutic effect on cardiac function in the DOX-induced cardiotoxicity mouse model [171].
Furthermore, the immunogenicity and toxicity generated by EVs might complicate their use as transport vehicles. To reduce these negative effects, the mouse immature dendritic cells (DCs) were used for exosome production in a study conducted by Tian et al. in 2014 [172]. They used these exosomes engineered to express a well-characterized exosomal membrane protein (Lamp2b) fused to αv integrin-specific iRGD peptide (CRGDKGPDC) to deliver DOX to tumour tissue in BALB/c nude mice transplanted to human breast cancer cells (MDA-MB-231). Consequently, tumour growth was inhibited without overt toxicity [172].
In the treatment of cancer, the problem of adverse effects, which includes the occurrence of CV diseases, remains unsolved. In this sense, a recent study tried to propose another innovative treatment for cancer based on stimulation of cancer-specific T cells instead of the high-dose DOX treatment that generates cardiotoxicity. Thus, Phung et al. used DC-derived exosomes engineered with anti-CTLA-4 antibody anchored in their lipids as an anti-cancer strategy. In this way, they induced an increase in the response of T cells to the presence of cancer cells [173].
Table 3 summarizes some aspects regarding relationship between EVs and cardio-oncological pathology/therapy.

Table 3.
EVs as biomarkers and therapeutic carriers in cardio-oncological experimental models.
Recently, the impact of breast cancer therapies on the CV system with a focus on the endothelium and the problem of detecting the early asymptomatic stages of cancer therapy-related cardiac dysfunction by cardiac imaging alone has been highlighted. Unfortunately, the initiating mechanisms of CV changes in patients suffering from cancer are incompletely understood, but the status of circulating biomarkers has emerged as a possible explanation. It was pointed out that the investigation of biomarkers, including inflammatory markers, cardiac biomarkers, miRNAs and endothelial cell-derived EVs may uncover mechanisms of injury, detect early stages of CV damage and elucidate novel therapeutic approaches for cancer therapy-related cardiac dysfunction [174].
There are very few studies that show the diagnostic or prognostic role of these biomarkers for the occurrence of cardiomyopathy after chemotherapy, but the addition of other specific biomarkers could help. Some clinical studies highlighted that troponins can be used to assess the cardiotoxicity induced by chemotherapy, specially trastuzumab and several tyrosine kinase inhibitors [175,176,177]. Thus, the quantification of plasma troponin I and T concentrations, which are markers of myocardial cell damage and B type natriuretic peptides, which are cardiac neurohormones secreted specifically by the ventricles in response to increased wall stress, can also be used as indicators of cardiotoxicity [175]. The role of the main two serum biomarkers troponin and natriuretic peptides in cancer patients receiving cardiotoxic cancer therapies (anthracycline chemotherapy, trastuzumab, HER2-targeted therapies, vascular endothelial growth factor inhibitors, proteasome inhibitors, immune checkpoint inhibitors, cyclophosphamide and radiotherapy) was discussed extensively by a Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology [178].
Interestingly, positive associations were found between CV markers (high-sensitivity cardiac troponin T, NT-proBNP, myeloperoxidase, placental growth factor and growth differentiation factor 15), cardiac dysfunction and breast cancer therapy (particularly in sequential anthracycline and trastuzumab therapy) [179].
However, liquid biopsy for some relevant circulating biomarkers, including EVs, remains a promising non-invasive method for diagnosis and monitoring CV risk for cancer patients in the near future.
6. Conclusions and Future Directions
It is becoming more and more clear that patients suffering from cancer end up developing associated heart diseases over time as a result of the specific therapy administered. Because of the alarming increase in the number of patients with cardio-oncological diseases, clinicians and researchers alike are concerned with optimizing clinical results based especially on CV imaging, and finding relevant biomarkers for the early detection of cardiac dysfunction generated by cancer therapy. With reference to CV imaging, echocardiographic assessment of the left ventricular ejection fraction is limited in the sensitive detection of early, subclinical changes in cardiac function. As a result, the recently appearing large cardio-oncological research groups started to develop platforms and different algorithms to investigate novel mechanisms, biomarkers and therapies to improve CV outcomes in cancer patients.
Circulating biomarkers, including EVs (lipid-covered particles), could serve as a measure for identifying early cardiac changes caused by cancer therapy. A series of questions regarding EVs remain unsolved for the time being, one of them being related to the origin of tumour-specific EVs and their distinction from the rest of EVs originating from other activated but non-malignant cells.
It is increasingly clear that the investigation of EV-based biomarkers in cross talk between CV disease and cancer is in the beginning stages, but it is becoming a general challenge common to clinicians and researchers. The potential of proteins, miRNAs and other nucleic acids contained in EVs remains to be investigated and to be used later as biomarkers for cardiac toxicity.
In addition to their use as diagnostic biomarkers for early detection of cardiac disorders, EVs can be used as therapeutic vehicles for drugs or regulatory molecules (nucleic acids and proteins) to modulate chemo-resistance of cancer cells and decrease the cardiotoxicity induced by DOX. Furthermore, EVs can be engineered to stimulate immune cells to kill cancer cells, thus decreasing the dosage of DOX and attenuating cardiotoxicity. Because of the many benefits of EVs in cancer therapy and the cardiotoxicity generated by cancer-specific treatment, exploring EV-based therapies in cardio-oncological diseases remains an opportunity for the future.
The ultimate goal of multidisciplinary cardio-oncology teams will be to ensure that cancer patients receive appropriate anti-cancer therapy that minimizes any other CV complication, but also to receive specific treatment for associated heart disease assessed before, during and after cancer therapy.
Author Contributions
Conceptualization, A.G.; writing—original draft preparation, E.B., C.J., A.-M.V. and A.G.; writing—review and editing, E.B., C.J., A.-M.V., A.B. and A.G.; supervision, E.B. and A.G.; manuscript review, C.J., A.B. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Romanian Academy and EEA Grants 2014–2021: Project Contract no. 33/2021.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 20 May 2022).
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 May 2022).
- Wang, Y.; Wang, Y.; Han, X.; Sun, J.; Li, C.; Adhikari, B.K.; Zhang, J.; Miao, X.; Chen, Z. Cardio-Oncology: A Myriad of Relationships Between Cardiovascular Disease and Cancer. Front. Cardiovasc. Med. 2022, 9, 727487. [Google Scholar] [CrossRef]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Hasin, T.; Gerber, Y.; Weston, S.A.; Jiang, R.; Killian, J.M.; Manemann, S.M.; Cerhan, J.R.; Roger, V.L. Heart failure after myocardial infarction is associated with increased risk of cancer. J. Am. Coll. Cardiol. 2016, 68, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Mounce, L.T.A.; Campbell, J.L.; Henley, W.E.; Tejerina Arreal, M.C.; Porter, I.; Valderas, J.M. Predicting incident multimorbidity. Ann. Fam. Med. 2018, 16, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in age-related chronic inflammatory diseases. Aging Mech. Dis. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Hall, D.; Subramanian, M. Sympathetic nervous system as a target for aging and obesity-related cardiovascular diseases. GeroScience 2019, 41, 13–24. [Google Scholar] [CrossRef]
- Barbosa, A.L.A.; Vermeulen, S.H.H.M.; Aben, K.K.; Grotenhuis, A.J.; Vrieling, A.; Kiemeney, L.A. Smoking intensity and bladder cancer aggressiveness at diagnosis. PLoS ONE 2018, 13, e0194039. [Google Scholar] [CrossRef]
- Lee, J.; Cooke, J.P. Nicotine and pathological angiogenesis. Life Sci. 2012, 91, 1058–1064. [Google Scholar] [CrossRef]
- Lortet-Tieulent, J.; Goding Sauer, A.; Siegel, R.L.; Miller, K.D.; Islami, F.; Fedewa, S.A.; Jacobs, E.J.; Jemal, A. State-Level Cancer Mortality Attributable to Cigarette Smoking in the United States. JAMA Intern. Med. 2016, 176, 1792–1798. [Google Scholar] [CrossRef]
- Aykan, N.F. Red Meat and Colorectal Cancer. Oncol. Rev. 2015, 9, 288. [Google Scholar] [CrossRef]
- Wu, B.; Yang, D.; Yang, S.; Zhang, G. Dietary Salt Intake and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 801228. [Google Scholar] [CrossRef]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Hiu, Y.K. “Globesity” Epidemic. Ann. Obes. Disord. 2016, 1, 1007. [Google Scholar]
- World Obesity Day 2022—Accelerating Action to Stop Obesity. Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 20 May 2022).
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 2, 5887. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 2019, 93, 102673. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef]
- Gérard, C.; Brown, K.A. Obesity and breast cancer—Role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol. Cell. Endocrinol. 2018, 466, 15–30. [Google Scholar] [CrossRef]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P.A. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Ballotari, P.; Vicentini, M.; Manicardi, V.; Gallo, M.; Chiatamone Ranieri, S.; Greci, M.; Giorgi Rossi, P. Diabetes and risk of cancer incidence: Results from a population-based cohort study in northern Italy. BMC Cancer 2017, 17, 703. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and Cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Brahmkhatri, V.P.; Prasanna, C.; Atreya, H.S. Insulin-like growth factor system in cancer: Novel targeted therapies. BioMed Res. Int. 2015, 2015, 538019. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, B.; Aronis, K.N.; Vamvini, M.T.; Shields, K.; Mantzoros, C.S. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: A meta-analysis using primary data of published studies. Metabolism 2013, 62, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.S.; Park, Y.G.; Kim, B.K.; Han, S.Y.; Jee, Y.H.; Han, K.H.; Lee, M.H.; Song, H.K.; Cha, D.R.; Kang, S.W.; et al. Angiotensin II stimulates the synthesis of vascular endothelial growth factor through the p38 mitogen activated protein kinase pathway in cultured mouse podocytes. J. Mol. Endocrinol. 2006, 36, 377–388. [Google Scholar] [CrossRef]
- Vachhani, P.; George, S. VEGF inhibitors in renal cell carcinoma. Clin. Adv. Hematol. Oncol. 2016, 14, 1016–1028. [Google Scholar]
- Han, H.; Guo, W.; Shi, W.; Yu, Y.; Zhang, Y.; Ye, X.; He, J. Hypertension and breast cancer risk: A systematic review and meta-analysis. Sci. Rep. 2017, 7, srep44877. [Google Scholar] [CrossRef]
- Stocks, T.; Van Hemelrijck, M.; Manjer, J.; Bjorge, T.; Ulmer, H.; Hallmans, G.; Lindkvist, B.; Selmer, R.; Nagel, G.; Tretli, S.; et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012, 59, 802–810. [Google Scholar] [CrossRef]
- Abi Aad, S.; Pierce, M.; Barmaimon, G.; Farhat, F.S.; Benjo, A.; Mouhayar, E. Hypertension induced by chemotherapeutic and immunosuppresive agents: A new challenge. Crit. Rev. Oncol. Hematol. 2015, 93, 28–35. [Google Scholar] [CrossRef]
- Aboumsallem, J.P.; Moslehi, J.; de Boer, R.A. Reverse Cardio-Oncology: Cancer Development in Patients With Cardiovascular Disease. J. Am. Heart Assoc. 2020, 9, e013754. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Rinde, L.B.; Smabrekke, B.; Hald, E.M.; Brodin, E.E.; Njølstad, I.; Mathiesen, E.B.; Løchen, M.L.; Wilsgaard, T.; Brækkan, S.K.; Vik, A.; et al. Myocardial infarction and future risk of cancer in the general population—The Tromsø study. Eur. J. Epidemiol. 2017, 32, 193–201. [Google Scholar] [CrossRef]
- Meijers, W.C.; Maglione, M.; Bakker, S.J.L.; Oberhuber, R.; Kieneker, L.M.; de Jong, S.; Haubner, B.J.; Nagengast, W.B.; Lyon, A.R.; van der Vegt, B.; et al. Heart failure stimulates tumor growth by circulating factors. Circulation 2018, 138, 678–691. [Google Scholar] [CrossRef]
- Malmborg, M.; Christiansen, C.B.; Schmiegelow, M.D.; Torp-Pedersen, C.; Gislason, G.; Schou, M. Incidence of new onset cancer in patients with a myocardial infarction—A nationwide cohort study. BMC Cardiovasc. Disord. 2018, 18, 198. [Google Scholar] [CrossRef]
- Ghosh, R.; Ray, U.; Jana, P.; Bhattacharya, R.; Banerjee, D.; Sinha, A. Reduction of death rate due to acute myocardial infarction in subjects with cancers through systemic restoration of impaired nitric oxide. PLoS ONE 2014, 9, e88639. [Google Scholar] [CrossRef]
- Lam, C.S.; Gamble, G.D.; Ling, L.H.; Sim, D.; Leong, K.T.; Yeo, P.S.; Ong, H.Y.; Jaufeerally, F.; Ng, T.P.; Cameron, V.A.; et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur. Heart J. 2018, 39, 1770–1780. [Google Scholar] [CrossRef]
- Lund, L.H.; Donal, E.; Oger, E.; Hage, C.; Persson, H.; Haugen-Löfman, I.; Ennezat, P.V.; Sportouch-Dukhan, C.; Drouet, E.; Daubert, J.C.; et al. Association between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Loosen, S.H.; Jahn, J.K.; Gänsbacher, J.; Luedde, T.; Kostev, K.; Luedde, M. Heart failure is associated with an increased incidence of cancer diagnoses. ESC Heart Fail. 2021, 8, 3628–3633. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Robusto, F.; Rulli, E.; D’Ettorre, A.; Bisceglia, L.; Staszewsky, L.; Maack, C.; Lepore, V.; Latini, R.; Ameri, P. Cancer Incidence and Mortality According to Pre-Existing Heart Failure in a Community-Based Cohort. JACC CardioOncol. 2022, 4, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.P.; Moslein, G.; McRonald, F.; Bertario, L.; Evans, D.G.; et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Gibbs, P.; Orchard, S.G.; Lockery, J.E.; Bernstein, W.B.; Cao, Y.; Ford, L.; Haydon, A.; Kirpach, B.; Macrae, F.; et al. Effect of aspirin on cancer incidence and mortality in older adults. J. Natl. Cancer Inst. 2021, 113, 258–265. [Google Scholar] [CrossRef]
- Battistoni, A.; Volpe, M. Recent warnings about antihypertensive drugs and cancer risk: Where do they come from? Eur. Cardiol. 2020, 15, e21. [Google Scholar] [CrossRef]
- Adalsteinsson, J.A.; Muzumdar, S.; Waldman, R.; Hu, C.; Wu, R.; Ratner, D.; Ungar, J.; Silverberg, J.I.; Olafsdottir, G.H.; Kristjansson, A.K.; et al. Association between hydrochlorothiazide and the risk of in situ and invasive squamous cell skin carcinoma and basal cell carcinoma: A population-based case-control study. J. Am. Acad. Dermatol. 2021, 84, 669–675. [Google Scholar] [CrossRef]
- Li, C.I.; Malone, K.E.; Weiss, N.S.; Boudreau, D.M.; Cushing-Haugen, K.L.; Daling, J.R. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65-79 years. Cancer 2003, 98, 1504–1513. [Google Scholar] [CrossRef]
- Rizos, C.V.; Elisaf, M.S. Antihypertensive drugs and glucose metabolism. World J. Cardiol. 2014, 6, 517–530. [Google Scholar] [CrossRef]
- Alhanafy, A.M.; Labeeb, A.; Khalil, A. The role of diuretics in treatment of aromatase inhibitors induced musculoskeletal symptoms in women with non metastatic breast cancer. Asian Pac. J. Cancer Prev. 2018, 19, 3525. [Google Scholar] [CrossRef]
- Pahor, M.; Guralnik, J.M.; Ferrucci, L.; Corti, M.C.; Salive, M.E.; Cerhan, J.R.; Wallace, R.B.; Havlik, R.J. Calcium-channel blockade and incidence of cancer in aged populations. Lancet 1996, 348, 493–497. [Google Scholar] [CrossRef]
- Thakur, A.A.; Wang, X.; Garcia-Betancourt, M.M.; Forse, R.A. Calcium channel blockers and the incidence of breast and prostate cancer: A meta-analysis. J. Clin. Pharm. Ther. 2018, 43, 519–529. [Google Scholar] [CrossRef]
- Saltzman, B.S.; Weiss, N.S.; Sieh, W.; Fitzpatrick, A.L.; McTiernan, A.; Daling, J.R.; Li, C.I. Use of antihypertensive medications and breast cancer risk. Cancer Causes Control. 2013, 24, 365–371. [Google Scholar] [CrossRef]
- Rotshild, V.; Hirsh Raccah, B.; Gazawe, M.; Matok, I. Calcium Channel Blocker Use and the Risk for Breast Cancer: A Population-Based Nested Case-Control Study. Cancers 2022, 14, 2344. [Google Scholar] [CrossRef]
- Barron, T.I.; Connolly, R.M.; Sharp, L.; Bennett, K.; Visvanathan, K. Beta blockers and breast cancer mortality: A population-based study. J. Clin. Oncol. 2011, 29, 2635–2644. [Google Scholar] [CrossRef]
- Thiele, M.; Albillos, A.; Abazi, R.; Wiest, R.; Gluud, L.L.; Krag, A. Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: A meta-analysis of randomized trials. Liver Int. 2015, 35, 2009–2016. [Google Scholar] [CrossRef]
- Udumyan, R.; Montgomery, S.; Fang, F.; Almroth, H.; Valdimarsdottir, U.; Ekbom, A.; Smedby, K.E.; Fall, K. Beta-Blocker Drug Use and Survival among Patients with Pancreatic Adenocarcinoma. Cancer Res. 2017, 77, 3700–3707. [Google Scholar] [CrossRef]
- Montoya, A.; Amaya, C.N.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of non-selective beta-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 2017, 8, 6446–6460. [Google Scholar] [CrossRef]
- Rosenthal, T.; Gavras, I. Renin–Angiotensin Inhibition in Combating Malignancy: A Review. Anticancer Res. 2019, 39, 4597–4602. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, S.; Jia, C.M.; He, W.; Wu, L.T.; Li, Y.Q.; Wang, W.; Li, Z.; Ma, J. Antihypertensive drugs use and the risk of prostate cancer: A meta-analysis of 21 observational studies. BMC Urol. 2018, 18, 17. [Google Scholar] [CrossRef]
- Keith, S.W.; Maio, V.; Arafat, H.A.; Alcusky, M.; Karagiannis, T.; Rabinowits, C.; Lavu, H.; Louis, D.Z. Angiotensin blockade therapy and survival in pancreatic cancer: A population study. BMC Cancer 2022, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Chan, E.W.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. ACE (Angiotensin-Converting Enzyme) Inhibitors/Angiotensin Receptor Blockers Are Associated With Lower Colorectal Cancer Risk: A Territory-Wide Study With Propensity Score Analysis. Hypertension 2020, 76, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Huang, R.Y.; Yang, Y.C.; Hsieh, K.P.; Yang, Y.H. Prognostic impact of angiotensin-converting enzyme inhibitors and angiotensin receptors blockers in esophageal or gastric cancer patients with hypertension—A real-world study. BMC Cancer 2022, 22, 430. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.B.; Hicks, B.; Azoulay, L.; Pottegard, A. Use of ACE (Angiotensin-Converting Enzyme) Inhibitors and Risk of Lung Cancer. A Nationwide Nested Case-Control Study. Circ. Cardiovasc. Qual. Outcomes 2020, 14, 1. [Google Scholar]
- Lin, S.Y.; Lin, C.L.; Lin, C.C.; Hsu, W.H.; Lin, C.D.; Wang, I.K.; Hsu, C.I.; Kao, C.H. Association between angiotensin-converting enzyme inhibitors and lung cancer-a nationwide, population-based, propensity score-matched cohort study. Cancers 2020, 12, 747. [Google Scholar] [CrossRef]
- Luo, X.; Chen, X.; Wang, L.; Yang, B.; Cai, S. Metformin Adjunct With Antineoplastic Agents for the Treatment of Lung Cancer: A MetaAnalysis of Randomized Controlled Trials and Observational Cohort Studies. Front. Pharmacol. 2021, 12, 639016. [Google Scholar] [CrossRef]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef]
- Perez, I.E.; Taveras Alam, S.; Hernandez, G.A.; Sancassani, R. Cancer Therapy-Related Cardiac Dysfunction: An Overview for the Clinician. In Clinical Medicine Insights: Cardiology; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2019; Volume 13. [Google Scholar]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Kamphuis, J.A.M.; Linschoten, M.; Cramer, M.J.; Doevendans, P.A.; Asselbergs, F.W.; Teske, A. Early- and late anthracycline-induced cardiac dysfunction: Echocardiographic characterization and response to heart failure therapy. Cardio-Oncology 2020, 6, 23. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxidative Med. Cell Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Fazal, M.; Kapoor, R.; Cheng, P.; Rogers, A.J.; Narayan, S.M.; Wang, P.; Witteles, R.M.; Perino, A.C.; Baykaner, T.; Rhee, J.W. Arrhythmia Patterns in Patients on Ibrutinib. Front. Cardiovasc. Med. 2022, 8, 792310. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, J.; Liu, X.; Long, X.; Cao, L.; Wang, J. Atrial fibrillation following treatment with paclitaxel: A case report. Biomed. Rep. 2018, 9, 540–544. [Google Scholar] [CrossRef]
- Coppola, C.; Rienzo, A.; Piscopo, G.; Barbieri, A.; Arra, C.; Maurea, N. Management of QT prolongation induced by anti-cancer drugs: Target therapy and old agents. different algorithms for different drugs. Cancer Treat. Rev. 2018, 63, 135–143. [Google Scholar] [CrossRef]
- Santoro, C.; Esposito, R.; Lembo, M.; Sorrentino, R.; De Santo, I.; Luciano, F.; Casciano, O.; Giuliano, M.; De Placido, S.; Trimarco, B.; et al. Strain-oriented strategy for guiding cardioprotection initiation of breast cancer patients experiencing cardiac dysfunction. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1345–1352. [Google Scholar] [CrossRef]
- Hanchate, L.P.; Sharma, S.R.; Madyalkar, S. Cisplatin Induced Acute Myocardial Infarction and Dyslipidemia. J. Clin. Diagn. Res. 2017, 11, OD05–OD07. [Google Scholar] [CrossRef]
- Dyhl-Polk, A.; Schou, M.; Vistisen, K.K.; Sillesen, A.S.; Serup-Hansen, E.; Faber, J.; Klausen, T.W.; Bojesen, S.E.; Vaage-Nilsen, M.; Nielsen, D.L. Myocardial Ischemia Induced by 5-Fluorouracil: A Prospective Electrocardiographic and Cardiac Biomarker Study. Oncologist 2021, 26, e403–e413. [Google Scholar] [CrossRef]
- Herrmann, J. Vascular toxic effects of cancer therapies. Nat. Rev. Cardiol. 2020, 17, 503–522. [Google Scholar] [CrossRef]
- Khan, F.; Tritschler, T.; Kahn, S.R.; Rodger, M.A. Venous thromboembolism. Lancet 2021, 398, 64–77. [Google Scholar] [CrossRef]
- Farge, D.; Frere, C.; Connors, J.M.; Ay, C.; Khorana, A.A.; Munoz, A.; Brenner, B.; Kakkar, A.; Rafii, H.; Solymos, S.; et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019, 20, e566–e581. [Google Scholar] [CrossRef]
- Tanaka, H.; Takahashi, K.; Yamaguchi, K.; Kontani, K.; Motoki, T.; Asakura, M.; Kosaka, S.; Yokomise, H.; Houchi, H. Hypertension and proteinuria as predictive factors of effects of bevacizumab on advanced breast cancer in Japan. Biol. Pharm. Bull. 2018, 41, 644–648. [Google Scholar] [CrossRef]
- Guo, X.; Qian, X.; Jin, Y.; Kong, X.; Qi, Z.; Cai, T.; Zhang, L.; Wu, C.; Li, W. Hypertension Induced by Combination Therapy of Cancer: A Systematic Review and Meta-Analysis of Global Clinical Trials. Front. Pharmacol. 2021, 12, 712995. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.H.; Castellino, S.M.; Meléndez, G.C.; Klepin, H.D.; Ellis, L.R.; Lamar, Z.; Vasu, S.; Kitzman, D.W.; Ntim, W.O.; Brubaker, P.H.; et al. Left Ventricular Mass Change After Anthracycline Chemotherapy. Circ. Heart Fail. 2018, 11, e004560. [Google Scholar] [CrossRef] [PubMed]
- Weidner, K.; Behnes, M.; Haas, J.; Rusnak, J.; Fuerner, P.; Kuska, M.; Mukherji, A.; Borggrefe, M.; Hofheinz, R.D.; Akin, I. Oxaliplatin-Induced Acute ST Segment Elevation Mimicking Myocardial Infarction: A Case Report. Oncol. Res. Treat. 2018, 41, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, H.; Zhang, Z.; Xu, Y.; An, X.; Ai, X.; Li, L. Case Report: Oxaliplatin-Induced Third-Degree Atrioventricular Block: First Discovery of an Important Side-Effect. Front. Cardiovasc. Med. 2022, 9, 900406. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, I.; Cipolla, C.M.; Colombo, N.; Cardinale, D. Cardioncological Approach for Trastuzumab Therapy in Breast Cancer Patients With Cardiotoxicity: Impact on Adherence and Clinical Outcome. Front. Pharmacol. 2020, 11, 1190. [Google Scholar] [CrossRef]
- Rieg, A.D.; Bünting, N.A.; Cranen, C.; Suleiman, S.; Spillner, J.W.; Schnöring, H.; Schröder, T.; von Stillfried, S.; Braunschweig, T.; Manley, P.W.; et al. Tyrosine kinase inhibitors relax pulmonary arteries in human and murine precision-cut lung slices. Respir. Res. 2019, 20, 111. [Google Scholar] [CrossRef]
- Chakraborty, R.; Bin Riaz, I.; Malik, S.U.; Marneni, N.; Mejia Garcia, A.; Anwer, F.; Khorana, A.A.; Rajkumar, S.V.; Kumar, S.; Murad, M.H.; et al. Venous thromboembolism risk with contemporary lenalidomide-based regimens despite thromboprophylaxis in multiple myeloma: A systematic review and meta-analysis. Cancer 2020, 126, 1640–1650. [Google Scholar] [CrossRef]
- Rao, V.U.; Reeves, D.J.; Chugh, A.R.; O’Quinn, R.; Fradley, M.G.; Raghavendra, M.; Dent, S.; Barac, A.; Lenihan, D. Clinical approach to cardiovascular toxicity of oral antineoplastic agents: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2693–2716. [Google Scholar] [CrossRef]
- Fillon, M. Lung cancer radiation may increase the risk of major adverse cardiac events. CA Cancer J. Clin. 2019, 69, 435–437. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar]
- Delrue, L.; Vanderheyden, M.; Beles, M.; Paolisso, P.; di Gioia, G.; Dierckx, R.; Verstreken, S.; Goethals, M.; Heggermont, W.; Bartunek, J. Circulating SERPINA3 improves prognostic stratification in patients with a de novo or worsened heart failure. ESC Heart Fail. 2021, 8, 4780–4790. [Google Scholar] [CrossRef]
- Soman, A.; Asha Nair, S. Unfolding the cascade of SERPINA3: Inflammation to cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188760. [Google Scholar] [CrossRef]
- Chadwick, J.A.; Hauck, J.S.; Lowe, J.; Shaw, J.J.; Guttridge, D.C.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; Rafael-Fortney, J.A. Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target. FASEB J. 2015, 29, 4544–4554. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, S.Q.; Zhang, G.T.; He, J.; Liu, Z.D.; Wang, M.R.; Cai, H.Q.; Wan, J.H. Highly expressed of SERPINA3 indicated poor prognosis and involved in immune suppression in glioma. Immun. Inflamm. Dis. 2021, 9, 1618–1630. [Google Scholar] [CrossRef]
- Matthews, V.B.; Knight, B.; Tirnitz-Parker, J.E.; Boon, J.; Olynyk, J.K.; Yeoh, G.C. Oncostatin M induces an acute phase response but does not modulate the growth or maturation-status of liver progenitor (oval) cells in culture. Exp. Cell Res. 2005, 306, 252–263. [Google Scholar] [CrossRef]
- Bracun, V.; Suthahar, N.; Shi, C.; de Wit, S.; Meijers, W.C.; Klip, I.T.; de Boer, R.A.; Aboumsallem, J.P. Established Tumour Biomarkers Predict Cardiovascular Events and Mortality in the General Population. Front. Cardiovasc. Med. 2021, 8, 753885. [Google Scholar] [CrossRef]
- Kosar, F.; Aksoy, Y.; Ozguntekin, G.; Ozerol, I.; Varol, E. Relationship between cytokines and tumour markers in patients with chronic heart failure. Eur. J. Heart Fail. 2006, 8, 270–274. [Google Scholar] [CrossRef]
- Zeillemaker, A.M.; Verbrugh, H.A.; Hoynck van Papendrecht, A.A.; Leguit, P. CA125 secretion by peritoneal mesothelial cells. J. Clin. Pathol. 1994, 47, 263–265. [Google Scholar] [CrossRef]
- Ganda, A.; Onat, D.; Demmer, R.T.; Wan, E.; Vittorio, T.J.; Sabbah, H.N.; Colombo, P.C. Venous congestion and endothelial cell activation in acute decompensated heart failure. Curr. Heart Fail. Rep. 2010, 7, 66–74. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef]
- IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies IL6R Genetics Consortium and Emerging Risk Factors Collaboration. Lancet 2012, 379, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Il’yasova, D.; Colbert, L.H.; Harris, T.B.; Newman, A.B.; Bauer, D.C.; Satterfield, S.; Kritchevsky, S.B. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, A.; Damås, J.K.; Øie, E.; Ueland, T.; Gullestad, L.; Aukrust, P. Systemic inflammation in heart failure—The whys and wherefores. Heart Fail. Rev. 2006, 11, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Liu, L.; Zhang, X.; Fan, X.; Krishnamurthy, P.; Verma, S.; Tongers, J.; Misener, S.; Ashcherkin, N.; Sun, H.; et al. IL-10 provides cardioprotection in diabetic myocardial infarction via upregulation of Heme clearance pathways. JCI Insight 2020, 5, e133050. [Google Scholar] [CrossRef]
- Dokka, S.; Shi, X.; Leonard, S.; Wang, L.; Castranova, V.; Rojanasakul, Y. Interleukin-10-mediated inhibition of free radical generation in macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L1196–L1202. [Google Scholar] [CrossRef]
- Bohlen, H.; Kessler, M.; Sextro, M.; Diehl, V.; Tesch, H. Poor clinical outcome of patients with Hodgkin’s disease and elevated interleukin-10 serum levels. Clinical significance of interleukin-10 serum levels for Hodgkin’s disease. Ann. Hematol. 2000, 79, 110–113. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Hatada, T.; Yamamoto, M.; Miyake, T.; Matsunaga, T.; Fukumoto, Y.; Yamada, Y.; Fukuda, K.; Saito, H.; Tatebe, S. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer 2009, 12, 95–100. [Google Scholar] [CrossRef]
- Hsu, T.-I.; Wang, Y.-C.; Hung, C.-Y.; Yu, C.-H.; Su, W.-C.; Chang, W.-C.; Hung, J.-J. Positive feedback regulation between IL10 and EGFR promotes lung cancer formation. Oncotarget 2016, 7, 20840–20854. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Cobo, I.; Tanaka, T.; Glass, C.K.; Yeang, C. Clonal hematopoiesis driven by DNMT3A and TET2 mutations: Role in monocyte and macrophage biology and atherosclerotic cardiovascular disease. Curr. Opin. Hematol. 2022, 29, 1–7. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Ouwerkerk, W.; Santema, B.T.; van Veldhuisen, D.J.; Lang, C.C.; Ng, L.L.; Anker, S.D.; Dickstein, K.; Metra, M.; Cleland, J.G.F.; et al. Differences in biomarkers and molecular pathways according to age for patients with HFrEF. Cardiovasc. Res. 2021, 117, 2228–2236. [Google Scholar] [CrossRef]
- Schafer, M.; Oeing, C.U.; Rohm, M.; Baysal-Temel, E.; Lehmann, L.H.; Bauer, R.; Volz, H.C.; Boutros, M.; Sohn, D.; Sticht, C.; et al. Ataxin-10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia. Mol. Metab. 2016, 5, 67–78. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Newman, A.A.C.; Afonso, M.S.; van Solingen, C.; Corr, E.M.; Brown, E.J.; Albers, K.B.; Yamaguchi, N.; Narke, D.; Schlegel, M.; et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat. Med. 2020, 26, 1452–1458. [Google Scholar] [CrossRef]
- Song, T.; Choi, C.H.; Kim, M.K.; Kim, M.; Yun, B.S.; Seong, S.J. The effect of angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) on cancer recurrence and survival: A meta-analysis. Eur. J. Cancer Prev. 2017, 26, 78–85. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, D.S.; Xin, L.; Zhou, L.Q.; Zhang, H.T.; Liu, L.; Yuan, Y.W.; Li, S.H. The renin-angiotensin system blockers and survival in digestive system malignancies: A systematic review and meta-analysis. In Medicine; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2020; Volume 99, Issue 7. [Google Scholar]
- Gabisonia, K.; Khan, M.; Fabio, A. Recchia. Extracellular vesicle-mediated bidirectional communication between heart and other organs. Heart Circ. Physiol. 2022, 322, H769–H784. [Google Scholar] [CrossRef]
- Alexandru, N.; Costa, A.; Constantin, A.; Cochior, D.; Georgescu, A. Microparticles: From biogenesis to biomarkers and diagnostic tools in cardiovascular disease. Curr. Stem Cell Res. Ther. 2017, 12, 89–102. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Alexandru, N.; Magda, S.L.; Constantin, A.; Nemecz, M.; Filippi, A.; Ioghen, O.C.; Ceafalan, L.C.; Bojin, F.; Tanko, G.; et al. Extracellular Vesicles as Valuable Players in Diabetic Cardiovascular Diseases. In Extracellular Vesicles; De Bona, A.G., Ed.; IntechOpen: London, UK, 2019; pp. 1–25. [Google Scholar]
- Georgescu, A.; Simionescu, M. Extracellular Vesicles: Versatile nanomediators, potential biomarkers and therapeutic agents in atherosclerosis and COVID-19-related thrombosis. Int. J. Mol. Sci. 2021, 22, 5967. [Google Scholar] [CrossRef]
- Yekula, A.; Minciacchib, V.R.; Morellob, M.; Shaoc, H.; Parkc, Y.; Zhange, X.; Muralidharana, K.; Freemanb, M.R.; Weisslederc, R.; Leec, H.; et al. Large and small extracellular vesicles released by glioma cells in vitro and in vivo. J. Extracell. Vesicles 2019, 9, 1689784. [Google Scholar] [CrossRef]
- Saludas, L.; Garbayo, E.; Ruiz-Villalba, A.; Hernandez, S.; Vader, P.; Prosper, F.; Blanco-Prieto, M.J. Isolation methods of large and small extracellular vesicles derived from cardiovascular progenitors: A comparative study. Eur. J. Pharm. Biopharm. 2022, 170, 187–196. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, N.; Procopciuc, A.; Vîlcu, A.; Comariţa, I.K.; Bădilă, E.; Georgescu, A. Extracellular vesicles—Incorporated microRNA signature as biomarker and diagnosis of prediabetes state and its complications. Rev. Endocr. Metab. Disord. 2021, 23, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Constantin, A.; Filippi, A.; Alexandru, N.; Nemecz, M.; Georgescu, A. Extracellular Vesicles from Adipose Tissue Stem Cells in Diabetes and Associated Cardiovascular Disease; Pathobiological Impact and Therapeutic Potential. Int. J. Mol. Sci. 2020, 21, 9598. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, A.; Alexandru, N.; Popov, D.; Amuzescu, M.; Andrei, E.; Zamfir, C.; Maniu, H.; Badila, A. Chronic venous insufficiency is associated with elevated level of circulating microparticles. J. Thromb. Haemost. 2009, 7, 1566–1575. [Google Scholar] [CrossRef]
- Georgescu, A.; Alexandru, N.; Andrei, E.; Titorencu, I.; Dragan, E.; Tarziu, C.; Ghiorghe, S.; Badila, E.; Bartos, D.; Popov, D. Circulating microparticles and endothelial progenitor cells in atherosclerosis;pharmacological effects of irbesartan. J. Thromb. Haemost. 2012, 10, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, A.; Alexandru, N.; Andrei, E.; Dragan, E.; Cochior, D.; Dias, S. Effects of transplanted circulating endothelial progenitor cells and platelet microparticles in atherosclerosis development. Biol. Cell 2016, 108, 219–243. [Google Scholar] [CrossRef]
- Bădilă, E.; Daraban, A.M.; Ghiorghe, A.; Georgescu, A.; Alexandru, N.; Bartoş, D.; Tîrziu, C. Rethinking cardiovascular therapy—The effect of irbesartan on circulating microparticles and endothelial progenitor cells in patients with hypertension and dyslipidemia. Farmacia 2014, 62, 93–106. [Google Scholar]
- Mathiesen, A.; Hamilton, T.; Carter, N.; Brown, M.; McPheat, W.; Dobrian, A. Endothelial Extracellular Vesicles: From Keepers of Health to Messengers of Disease. Int. J. Mol. Sci. 2021, 22, 4640. [Google Scholar] [CrossRef]
- Orbe, J.; Alexandru, N.; Roncal, C.; Belzunce, M.; Bibiot, P.; Rodriguez, J.A.; Meijers, J.C.; Georgescu, A.; Paramo, J.A. Lack of TAFI increases brain damage and microparticle generation after thrombolytic therapy in ischemic stroke. Thromb. Res. 2015, 136, 445–450. [Google Scholar] [CrossRef]
- Alexandru, N.; Badila, E.; Weiss, E.; Cochior, D.; Stępień, E.; Georgescu, A. Vascular Complications in Diabetes: Microparticles and Microparticle Associated MicroRNAs as Active Players. Biochem. Biophys. Res. Commun. 2016, 472, 1–10. [Google Scholar] [CrossRef]
- Stępień, E.L.; Durak-Kozica, M.; Kamińska, A.; Targosz-Korecka, M.; Libera, M.; Tylko, G.; Opalińska, A.; Kapusta, M.; Solnica, B.; Georgescu, A.; et al. Circulating ectosomes: Determination of angiogenic microRNAs in type 2 diabetes. Theranostics 2018, 8, 3874–3890. [Google Scholar] [CrossRef]
- Rosell, A.; Havervall, S.; von Meijenfeldt, F.; Hisada, Y.; Aguilera, K.; Grover, S.P.; Lisman, T.; Mackman, N.; Thålin, C. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality—Brief report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 878–882. [Google Scholar] [CrossRef]
- Simionescu, N.; Zonda, R.; Petrovici, A.R.; Georgescu, A. The multifaceted role of extracellular vesicles in glioblastoma: microRNA nanocarriers for disease progression and gene therapy. Pharmaceutics 2021, 13, 988. [Google Scholar] [CrossRef]
- Alexandru, N.; Andrei, E.; Niculescu, L.; Dragan, E.; Ristoiu, V.; Georgescu, A. Microparticles of healthy origins improve endothelial progenitor cell dysfunction via microRNA transfer in an atherosclerotic hamster model. Acta Physiol. 2017, 221, 230–249. [Google Scholar] [CrossRef]
- Alexandru, N.; Andrei, E.; Safciuc, F.; Dragan, E.; Balahura, A.M.; Badila, E.; Georgescu, A. Intravenous administration of allogenic cell-derived microvesicles of healthy origins defends against atherosclerotic cardiovascular disease development by a direct action on endothelial progenitor cells. Cells 2020, 9, 423. [Google Scholar] [CrossRef]
- Comarița, I.K.; Vîlcu, A.; Constantin, A.; Procopciuc, A.; Safciuc, F.; Alexandru, N.; Dragan, E.; Nemecz, M.; Filippi, A.; Chitoiu, L.; et al. Therapeutic potential of stem cell-derived extracellular vesicles on atherosclerosis-induced vascular dysfunction and its key molecular players. Front. Cell Dev. Biol. 2022, 10, 817180. [Google Scholar] [CrossRef]
- Constantin, A.; Comarița, I.K.; Alexandru, N.; Filippi, A.; Bojin, F.; Gherghiceanu, M.; Vîlcu, A.; Nemecz, M.; Niculescu, L.S.; Păunescu, V.; et al. Stem Cell-Derived Extracellular Vesicles Reduce the Expression of Molecules Involved in Cardiac Hypertrophy—In a Model of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Front. Pharmacol. 2022, 13, 1003684. [Google Scholar] [CrossRef]
- Gasecka, A.; van der Pol, E.; Nieuwland, R.; Stepien, E. Extracellular vesicles in post-infarct ventricular remodelling. Kardiol. Pol. 2018, 76, 69–76. [Google Scholar] [CrossRef]
- Stepien, E.R.C.; Moskal, P. Novel biomarker and drug delivery systems for theranostics—Extracellular vesicles. Bio-Algorithms Med-Syst. 2021, 17, 301–309. [Google Scholar] [CrossRef]
- Suades, R.; Greco, M.F.; Padró, T.; Badimon, L. Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis. Cells 2022, 11, 1845. [Google Scholar] [CrossRef]
- Wang, J.; Bettegowda, C. Applications of DNA-based liquid biopsy for central nervous system neoplasms. J. Mol. Diagn. 2017, 19, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Balaj, L.; Stott, S.L.; Nahed, B.; Carter, B.S. Liquid biopsy for brain tumors. Expert Rev. Mol. Diagn. 2017, 17, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringnér, M.; Mörgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.O.; Widmark, A. Prostate cancer derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br. J. Cancer. 2009, 100, 1603–1607. [Google Scholar] [CrossRef]
- Logozzi, M.; De Milito, A.; Lugini, L.; Borghi, M.; Calabro, L.; Spada, M.; Perdicchio, M.; Marino, M.L.; Federici, C.; Iessi, E.; et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE 2009, 4, e5219. [Google Scholar] [CrossRef]
- Rabinowits, G.; Gerçel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal microRNA: A diagnostic marker for lung cancer. Clin. Lung Cancer 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Shao, C.; Yang, F.; Miao, S.; Liu, W.; Wang, C.; Shu, Y.; Shen, H. Role of hypoxia-induced exosomes in tumor biology. Mol. Cancer 2018, 17, 120. [Google Scholar] [CrossRef]
- Yi, H.; Ye, J.; Yang, X.-M.; Zhang, L.-W.; Zhang, Z.-G.; Chen, Y.-P. High-grade ovarian cancer secreting effective exosomes in tumor angiogenesis. Int. J. Clin. Exp. Pathol. 2015, 8, 5062–5070. [Google Scholar]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Lee, J.E.; Moon, P.G.; Cho, Y.E.; Kim, Y.B.; Kim, I.S.; Park, H.; Baek, M.C. Identification of EDIL3 on extracellular vesicles involved in breast cancer cell invasion. J. Proteom. 2016, 131, 17–28. [Google Scholar] [CrossRef]
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qui, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef]
- Gaspar, B.S.; Ionescu, R.F.; Enache, R.M.; Dobrică, E.C.; Crețoiu, S.M.; Crețoiu, D.; Voinea, S.C. Extracellular Vesicles as Intercellular Communication Vehicles in Regenerative Medicine. In Extracellular Vesicles; Manash, K.P., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Sağkan, R.I.; Akın Balı, D.F. Epidermal Growth Factor-Like Repeats and Discoidin I-Like Domains 3 is a Novel Regulator of Epithelial-Mesenchymal Transition in Clear Cell Renal Cell Carcinoma: In Silico Analysis. Erciyes Med. J. 2021, 43, 122–129. [Google Scholar]
- Tian, C.; Yang, Y.; Bai, B.; Wang, S.; Liu, M.; Sun, R.C.; Yu, T.; Chu, X.M. Potential of exosomes as diagnostic biomarkers and therapeutic carriers for doxorubicin-induced cardiotoxicity. Int. J. Biol. Sci. 2021, 17, 1328–1338. [Google Scholar] [CrossRef]
- Yarana, C.; Carroll, D.; Chen, J.; Chaiswing, L.; Zhao, Y.; Noel, T.; Alstott, M.; Bae, Y.; Dressler, E.V.; Moscow, J.A.; et al. Extracellular Vesicles Released by Cardiomyocytes in a Doxorubicin-Induced Cardiac Injury Mouse Model Contain Protein Biomarkers of Early Cardiac Injury. Clin. Cancer Res. 2017, 24, 1644–1653. [Google Scholar] [CrossRef]
- Beaumier, A.; Robinson, S.R.; Robinson, N.; Lopez, K.E.; Meola, D.M.; Barber, L.G.; Bulmer, B.J.; Calvalido, J.; Rush, J.E.; Yeri, A.; et al. Extracellular vesicular microRNAs as potential biomarker for early detection of doxorubicin-induced cardiotoxicity. J. Vet. Intern. Med. 2020, 34, 1260–1271. [Google Scholar] [CrossRef]
- O’Brien, C.G.; Ozen, M.O.; Ikeda, G.; Vaskova, E.; Jung, J.H.; Bayardo, N.; Santoso, M.R.; Shi, L.; Wahlquist, C.; Jiang, Z.; et al. Mitochondria-Rich Extracellular Vesicles Rescue Patient-Specific Cardiomyocytes From Doxorubicin Injury: Insights Into the SENECA Trial. J. Am. Coll. Cardiol. CardioOncol. 2021, 3, 428–440. [Google Scholar]
- Xia, W.; Chen, H.; Xie, C.; Hou, M. Long-noncoding RNA MALAT1 sponges microRNA-92a-3p to inhibit doxorubicin-induced cardiac senescence by targeting ATG4a. Aging 2020, 12, 8241–8260. [Google Scholar] [CrossRef]
- Desgres, M.; Autret, G.; Petrusca, L.; Correa, B.M.; Marigny, C.; Gac, L.L.; Guillas, C.; Tavitian, B.; Croisille, P.; Silvestre, J.S.; et al. Effects of Intravenously Delivered Extracellular Vesicles on Cardiac Function in Chemotherapy-Induced Cardiomyopathy. Circulation 2021, 144, A12755. [Google Scholar]
- Milano, G.; Biemmi, V.; Lazzarini, E.; Balbi, C.; Ciullo, A.; Bolis, S.; Ameri, P.; Di Silvestre, D.; Mauri, P.; Barile, L.; et al. Intravenous administration of cardiac progenitor cell-derived exosomes protects against doxorubicin/trastuzumab-induced cardiac toxicity. Cardiovasc. Res. 2020, 116, 383–392. [Google Scholar] [CrossRef]
- Dargani, Z.T.; Singla, D.K. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am. J. Physiol. Heart C 2019, 317, H460–H471. [Google Scholar] [CrossRef]
- Singla, D.K.; Johnson, T.A.; Tavakoli Dargani, Z. Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells 2019, 8, 1224. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Hu, S.; Huang, K.; Su, T.; Li, Z.; Vandergriff, A.; Cores, J.; Dinh, P.U.; Allen, T.; Shen, D.; et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 2020, 10, 3474–3487. [Google Scholar] [CrossRef] [PubMed]
- Hadla, M.; Palazzolo, S.; Corona, G.; Caligiuri, I.; Canzonieri, V.; Toffoli, G.; Rizzolio, F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine 2016, 11, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhao, L.; Lu, F.; Gao, X.; Dong, Y.; Zhao, Y.; Wei, M.; Yang, G.; Xing, C.; Liu, L. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics 2020, 10, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Phung, C.D.; Pham, T.T.; Nguyen, H.T.; Nguyen, T.T.; Ou, W.; Jeong, J.H.; Choi, H.G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Anti-CTLA-4 Antibody-Functionalized Dendritic Cell-derived Exosomes Targeting Tumor-draining Lymph Nodes for Effective Induction of Antitumor T-cell Responses. Acta Biomater. 2020, 115, 371–382. [Google Scholar] [CrossRef]
- Ching, C.; Gustafson, D.; Thavendiranathan, P.; Fish, J.E. Cancer therapy-related cardiac dysfunction: Is endothelial dysfunction at the heart of the matter? Rev. Clin. Sci. 2021, 135, 1487–1503. [Google Scholar] [CrossRef]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Colombo, N.; Boeri, M.; Lamantia, G.; Civelli, M.; Peccatori, F.; Martinelli, G.; Fiorentini, C.; et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004, 109, 2749–2754. [Google Scholar] [CrossRef]
- Morris, P.G.; Chen, C.; Steingart, R.; Fleisher, M.; Lin, N.; Moy, B.; Come, S.; Sugarman, S.; Abbruzzi, A.; Lehman, R.; et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin. Cancer Res. 2011, 17, 3490–3499. [Google Scholar] [CrossRef]
- Schmidinger, M.; Zielinski, C.C.; Vogl, U.M.; Bojic, A.; Bojic, M.; Schukro, C.; Ruhsam, M.; Hejna, M.; Schmidinger, H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2008, 26, 5204–5212. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Demissei, B.G.; Hubbard, R.A.; Zhang, L.; Smith, A.M.; Sheline, K.; McDonald, C.; Narayan, V.; Domchek, S.M.; DeMichele, A.; Shah, P.; et al. Changes in Cardiovascular Biomarkers With Breast Cancer Therapy and Associations With Cardiac Dysfunction. J. Am. Heart Assoc. 2020, 9, e014708. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).