Abstract
Alzheimer’s disease (AD) is the most common cause of dementia. Fingolimod has previously shown beneficial effects in different animal models of AD. However, it has shown contradictory effects when it has been applied at early disease stages. Our objective was to evaluate fingolimod in two different treatment paradigms. To address this aim, we treated male and female APP-transgenic mice for 50 days, starting either before plaque deposition at 50 days of age (early) or at 125 days of age (late). To evaluate the effects, we investigated the neuroinflammatory and glial markers, the Aβ load, and the concentration of the brain-derived neurotrophic factor (BDNF). We found a reduced Aβ load only in male animals in the late treatment paradigm. These animals also showed reduced microglia activation and reduced IL-1β. No other treatment group showed any difference in comparison to the controls. On the other hand, we detected a linear correlation between BDNF and the brain Aβ concentrations. The fingolimod treatment has shown beneficial effects in AD models, but the outcome depends on the neuroinflammatory state at the start of the treatment. Thus, according to our data, a fingolimod treatment would be effective after the onset of the first AD symptoms, mainly affecting the neuroinflammatory reaction to the ongoing Aβ deposition.
1. Introduction
Alzheimer’s disease (AD) is the most common cause of dementia and affects over 30 million people worldwide [1]. AD is characterized by the accumulation of different neurotoxic proteins, e.g., amyloid-β (Aβ) in plaques and tau as neurofibrillary tangles, leading to neurodegeneration, neuronal loss, and irreversible cognitive decline [2,3]. The causes responsible for the accumulation of Aβ and tau in the brain remain unclear. Even though no successful therapeutic strategies have been developed, several molecular mechanisms involved in the pathogenesis of AD have been suggested: Aβ overproduction and impaired Aβ clearance, dysregulated tau phosphorylation, altered glutamatergic neurotransmission, as well as astrocyte and microglia activation prior to the clinical onset [4,5,6]. All of these proposed mechanisms can be used to identify new treatment targets for AD as mono- or multi-targeted therapies.
Fingolimod
Fingolimod (FTY720, Gilenya; Novartis, Basel, Switzerland), a substrate of sphingosine kinases, targets several of the mechanisms mentioned above. The compound binds to sphingosine-1-phosphate (S1P) receptors in its phosphorylated state [7]. The main pharmacologic effect of fingolimod is immunomodulation by lymphocyte sequestration in the lymph nodes by inhibiting the function of S1P1 receptors in lymphocyte egression [8,9]. It was first clinically tested to modulate allograft rejection after a kidney transplantation [10]. However, the S1P1 receptor is also involved in diverse functions in the central nervous system (CNS), such as neurogenesis, astrocyte activation and proliferation, and the communication between astrocytes, neurons, and the blood–brain barrier (BBB) [11]. Furthermore, fingolimod regulates the biosynthesis of sphingolipids, which play important roles in neurodegenerative diseases [12]. For these reasons, fingolimod has been tested in different animal models of neurodegenerative and neuroinflammatory diseases (reviewed in [13]). In addition, fingolimod has shown promising inhibitory effects on Aβ toxicity and synthesis in vitro. Fingolimod ameliorated Aβ neurotoxicity in neuronal cultures, reducing the neuronal death probably by increasing the brain-derived neurotrophic factor (BDNF) concentration [14,15]. The multiple pathways targeted by fingolimod suggest that this drug may be a promising therapy for AD.
Fingolimod has been tested previously in animal models of AD. Various authors have reported on the beneficial effects of fingolimod treatments in mouse models of AD. More specifically, it decreased the Aβ production and/or deposition, induced neuroprotection, reduced neuroinflammation, reduced the occurrence of psychosis-like behaviour, and improved the cognitive function [16,17,18,19,20,21,22,23,24,25].
However, Takasugi and colleagues reported contradictory effects in an APP transgenic model (A7 model, human APP695 harbouring Swedish (K670N, M671L) and Austrian (T714I) mutations under control of Thy1.2 promoter of unknown genetic background [26]), showing a decrease in Aβ40, but an increase in Aβ42 [16]. This study is the only treatment that started before the manifestation of the first plaques, and therefore, also before onset of neuroinflammation.
In addition, fingolimod has been shown to reverse most of the Aβ-induced changes in the gene expression of 12-month-old APP transgenic mice with London (V642I) mutation (FVB-Tg[Thy1; APP695/Ld2] [27]), but it had no beneficial effect on the 3-month-old APP transgenic mice [28]. Thus, the adequate timing of fingolimod treatments still needs to be elucidated.
Here, we aim to evaluate the effect of commencing a fingolimod treatment at two relevant time points: (i) before or at the very early stages of plaque deposition and (ii) at advanced stages of plaque deposition when neuroinflammatory activation was already present in our animal model of AD.
2. Results
To evaluate the impact of the starting point on the efficacy of fingolimod treatment in AD, we investigated alterations in weight, behaviour, Aβ load, neuroinflammatory reaction, and BDNF in a mouse model of AD at two different ages.
2.1. Weight Development during Fingolimod Treatment
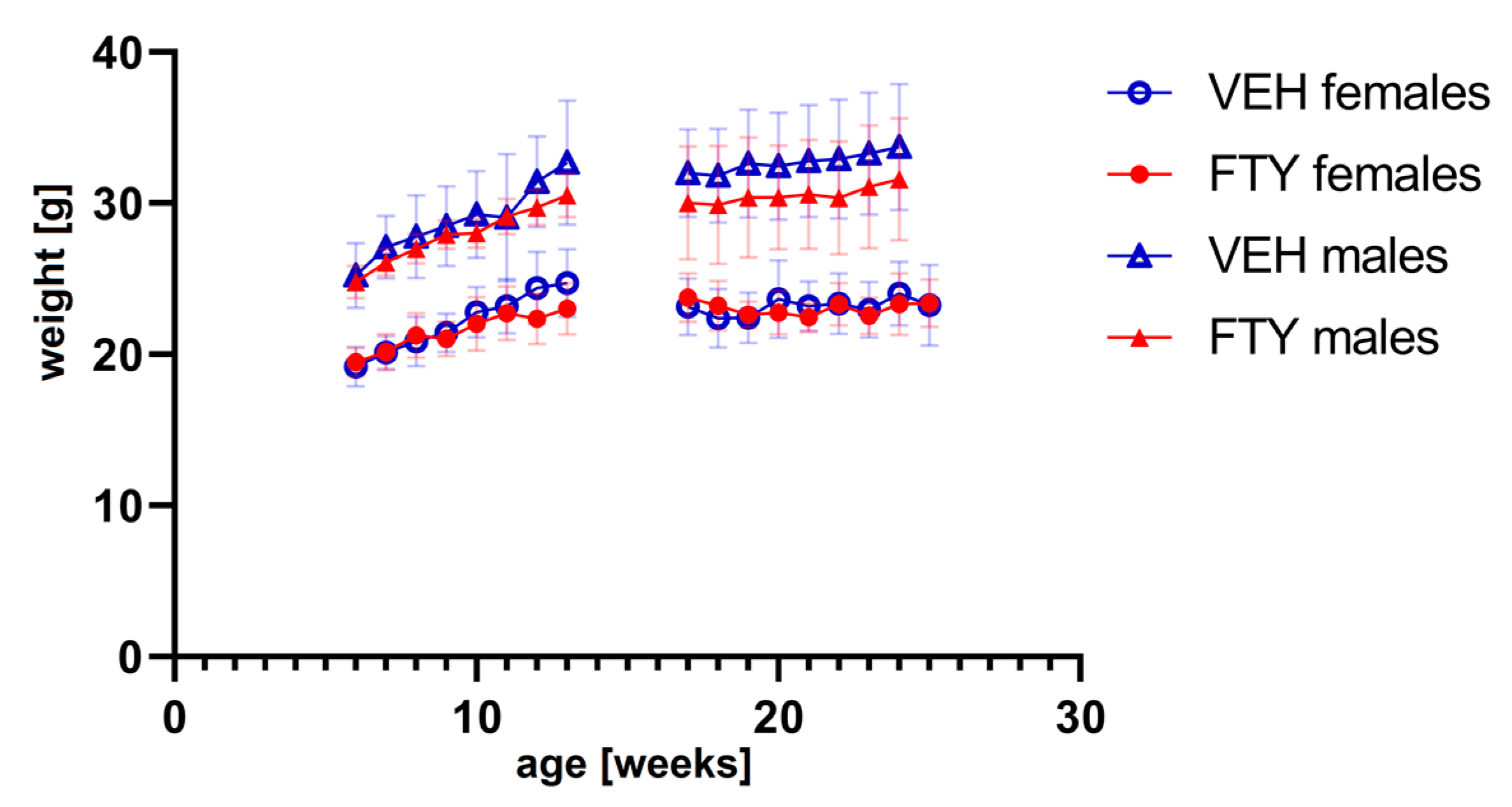
The fingolimod treatment in the drinking water did not affect the water consumption (data not shown) or body weight evolution of the animals at any of the ages tested (Figure 1). No significant differences were found.
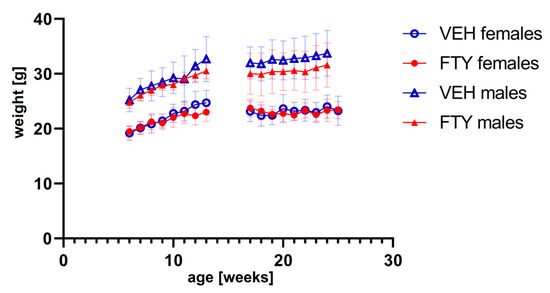
Figure 1.
The graph shows the weight progression of early treated (left) and late treated animals (right) for all experimental groups. FTY—fingolimod (FTY720)-treated animals; VEH—vehicle-treated control animals. Data are presented as mean ± SD; n = 8 in all groups, except for the late treatment males (n = 13). Statistical analysis was performed using two-way ANOVA (mixed-effects model). No significant differences were found.
2.2. Effects of Fingolimod on Activity or Spatial Orientation
The fingolimod treatment had no alternating effects on the overall daily activity of the mice neither in the light nor at the dark phase (Appendix A—Figure A1). The spatial orientation performance testing did not reveal significant differences (Appendix A—Figure A2).
2.3. Effects of Fingolimod on Aβ Load
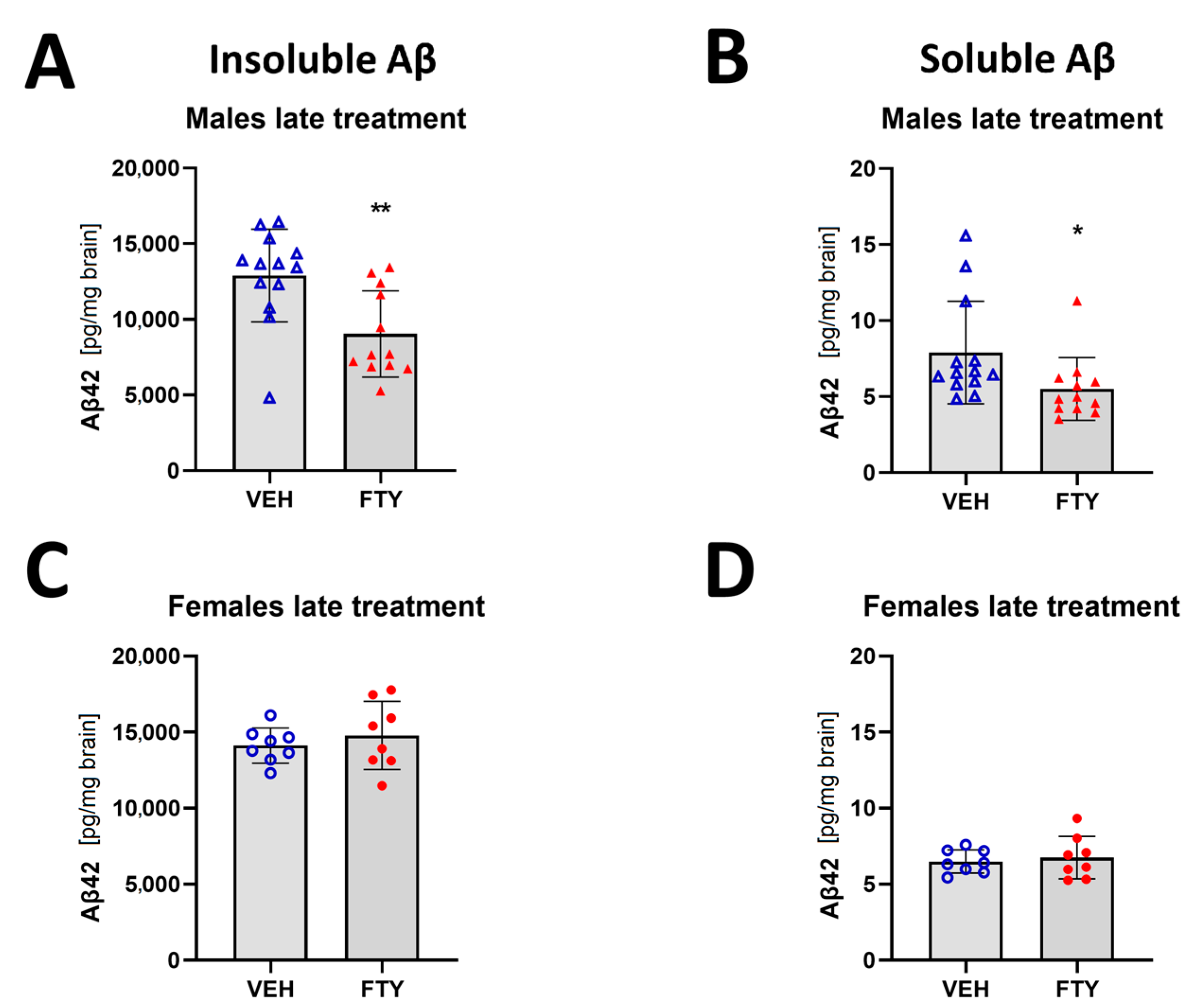
When the treatment was started at 125 days of age (late treatment), fingolimod reduced the deposition of insoluble Aβ (9.1 ± 2.6 vs. 12.9 ± 3.6 ng/mg brain, p = 0.003) and the accumulation of soluble Aβ (5.5 ± 2.6 vs. 7.9 ± 3.9 pg/mg brain, p = 0.006) in males. However, no differences were found in the female animals when we measured only insoluble Aβ load (14.8 ± 2.2 vs. 14.1 ± 1.2 pg/mg brain, p = 0.47) (Figure 2).
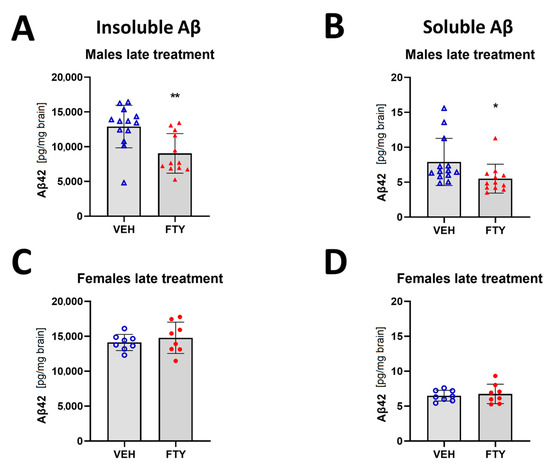
Figure 2.
Quantification of insoluble (A,C) and soluble (B,D) Aβ42 for late treated males (A,B) and females (C,D) APPtg mice. Data are presented as mean ± SD; n = 8 (except for males VEH, n = 13; FTY, n = 12). Significance was calculated using Wilcoxon–Mann–Whitney test, and it is given as *: p ≤ 0.05, **: p ≤ 0.01.
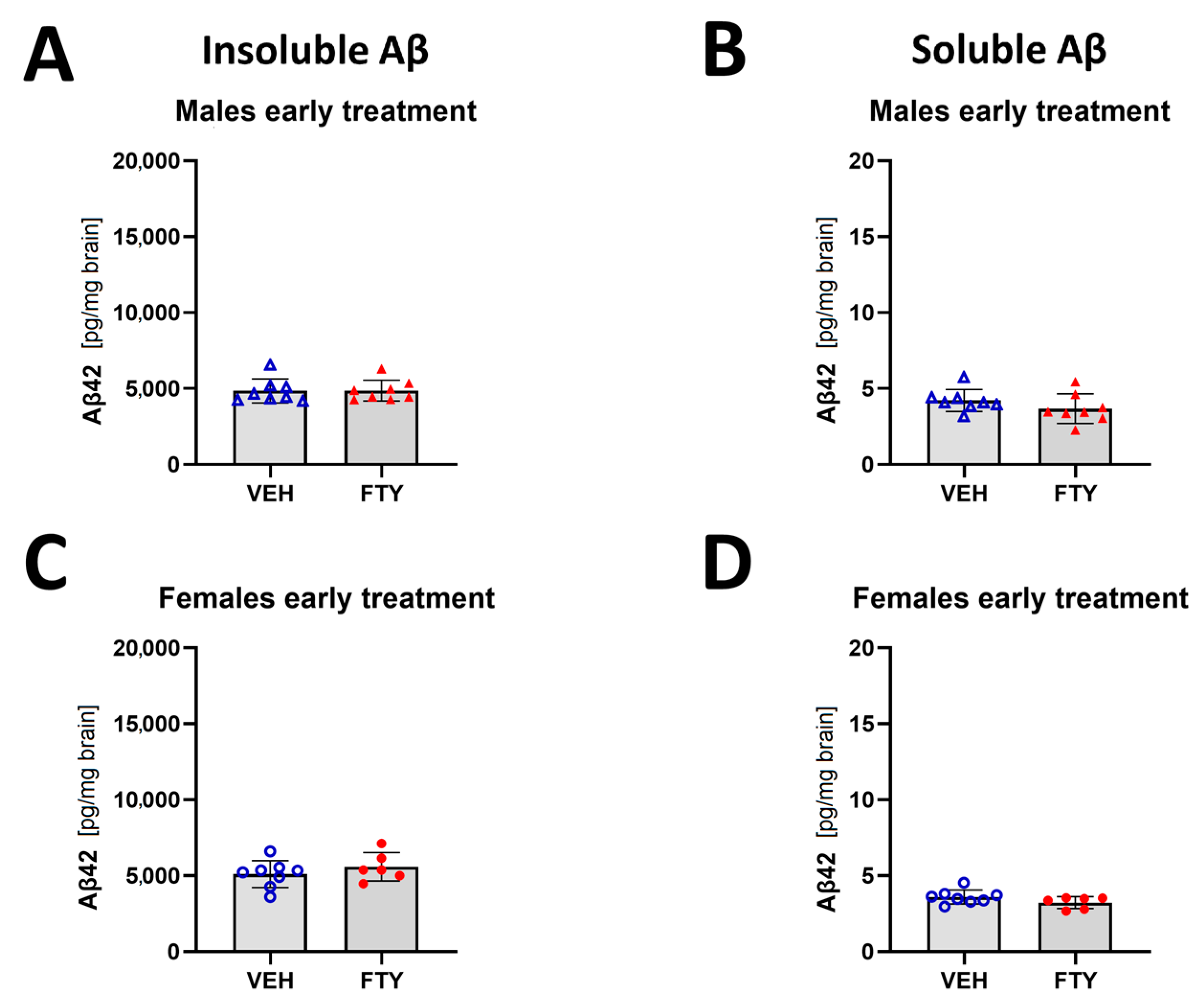
In addition, the early treated female and male mice did not show differences in insoluble Aβ compared to their respective control groups (starting treatment at 50 days of age; 5.6 ± 0.9 and 4.9 ± 0.7 pg/mg brain vs. 5.1 ± 0.9 and 4.9 ± 0.8 pg/mg brain, respectively) (Figure 3).
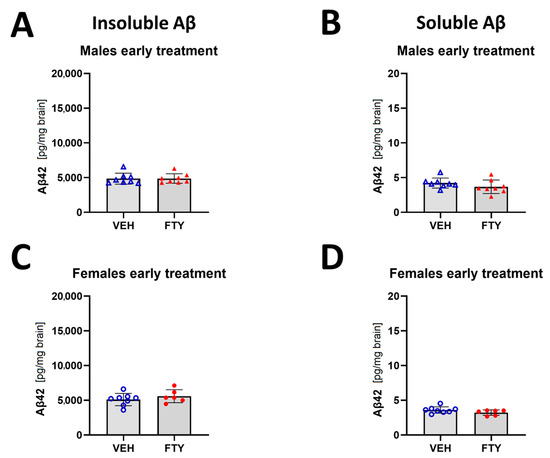
Figure 3.
Quantification of insoluble (A,C) and soluble (B,D) Aβ42 for early treated males (A,B) and females (C,D). Data are presented as mean ± SD; n = 8 (except for FTY treatment females, n = 6). Significance was calculated using Wilcoxon–Mann–Whitney test.
2.4. Effects of Fingolimod on the Reaction of Microglia and Astrocytes
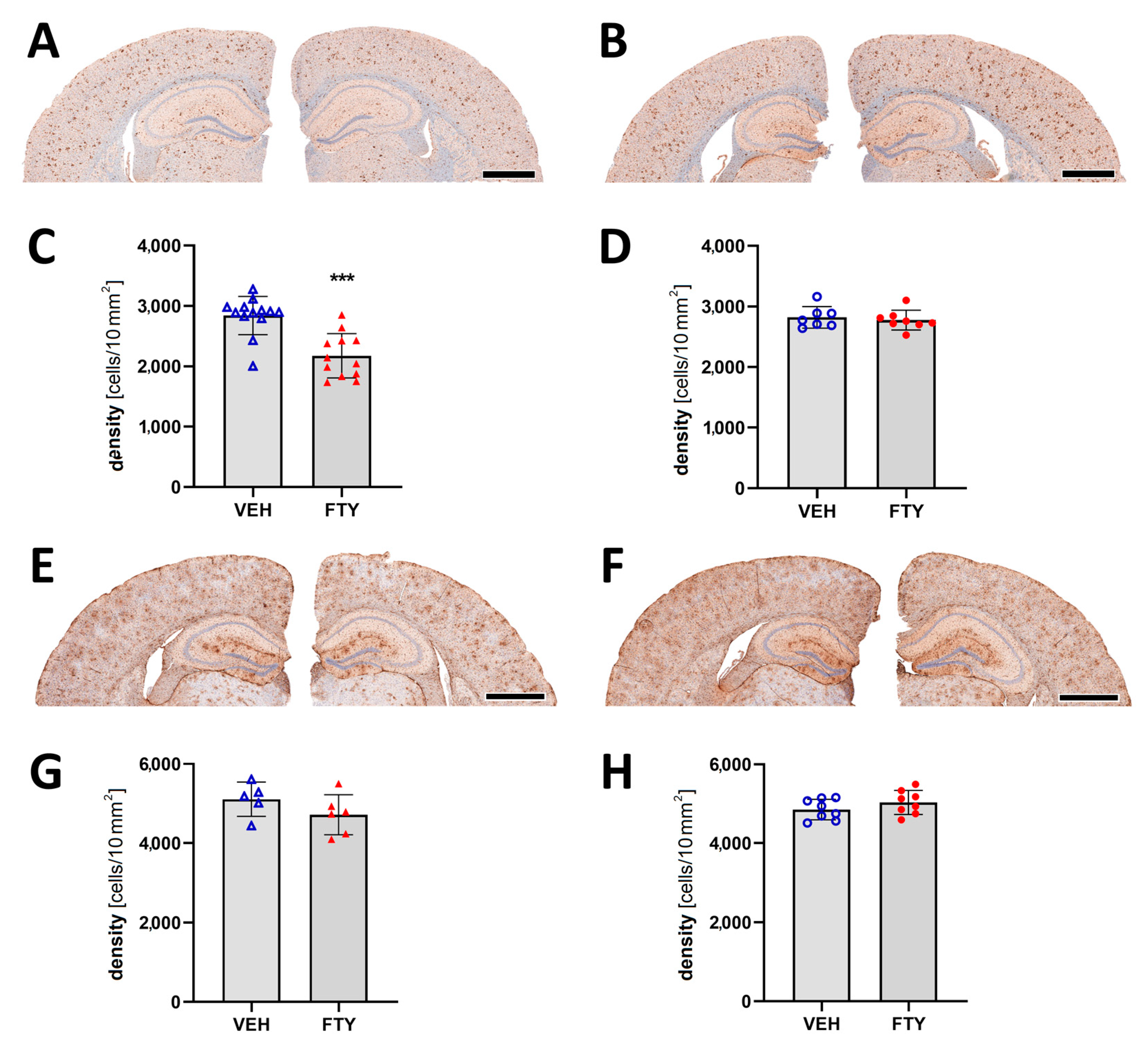
Assessment of microglia immunostaining against Iba1 revealed a decrease in the microglia coverage (total area covered by microglia; 7.12 ± 0.01% vs. 9.26 ± 0.01%; p = 0.004) and microglia density (number of cells per 10 mm2 area; 2175 ± 350 vs. 2832 ± 395 cells/10 mm2; p = 0.004; Figure 4A–D) in the late FTY-treated males compared to their VEH-treated controls. However, the fingolimod treatment had no effect on microglial activation in the female animals, which is similar to the unchanged Aβ levels.
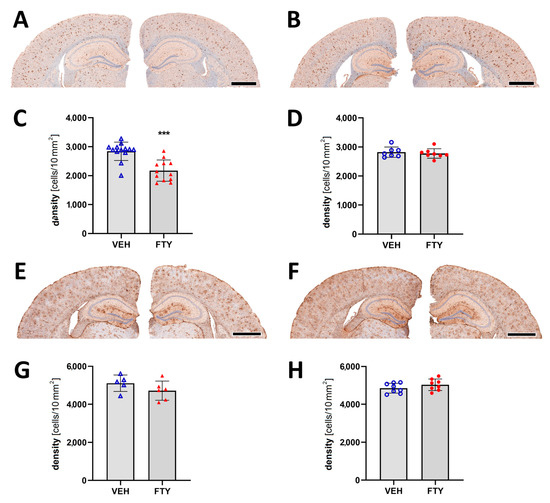
Figure 4.
Evaluation of micro- and astrogliosis in mice with late treatment paradigm (at 175 days of age). (A,B) Representative images of Iba1 immunostaining of male (A) and female (B) mice. Microglia activation was determined as density of IBA1-positive cells in males (C) and females (D). (E,F) Representative images of GFAP immunostaining in males (E) and females (F). Astrocyte density in males (G) and females (H). Data are presented as mean ± SD; VEH males n = 13 for IBA1, n = 5 for GFAP, FTY males n = 12 for IBA1, n = 6 for GFAP, females n = 8. Significance was calculated using Student’s t-test, and it is given as ***: p ≤ 0.001. Scale bars: 1 mm.
Astrocyte activation was measured as the astrocyte density in the cortex, and it was not significantly altered by the fingolimod treatment for any of the groups (Figure 4E–H).
2.5. Effects of Fingolimod on Brain Cytokine Levels
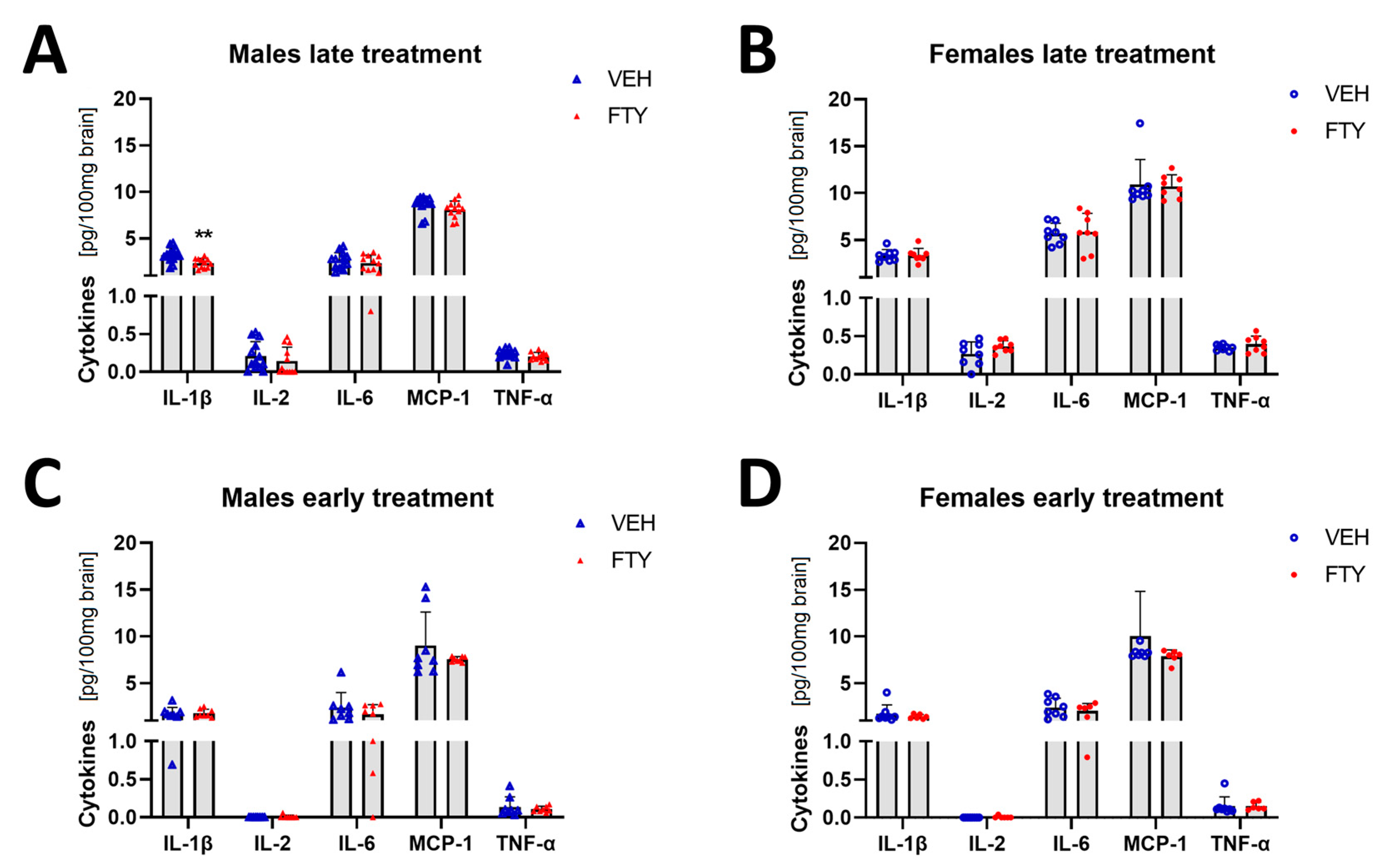
Microglial cells are important immune cells in the brain, and they are the cells that appear to be activated in the brain tissue when Aβ deposition starts. Therefore, we wanted to characterize the effect of fingolimod on the cytokine profile in the brain. Here, we found that the pro-inflammatory cytokine IL-1β level was reduced in the late treated males (2.34 ± 0.48 vs. 3.19 ± 0.76 pg/100 mg brain, p = 0.004) (Figure 5). No differences were observed in the other cytokines that were measured (IFN-g, IL-2, IL-4, IL-6, IL-10, MCP-1, and TNF-α) or in other experimental groups (females and younger animals).
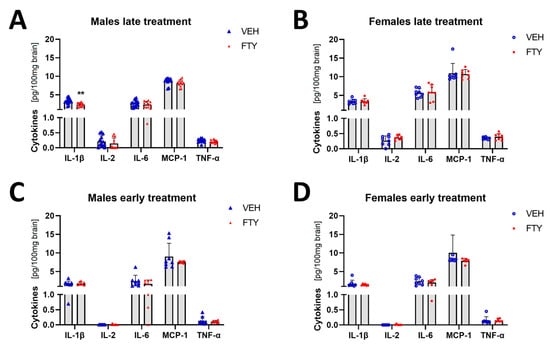
Figure 5.
Levels of selected cytokines in brain tissue in late and early treated animals. (A,B) Quantification of IL-1β, IL-1, IL-6, MCP-1, and TNFα in late treated males (A) and females (B). (C,D) Quantification of the cytokines in early treated males (C) and females (D). Data are presented as mean ± SD; n = 8 except late treatment males (VEH n = 13; FTY n = 12) and early FTY treatment females (n = 6). Significance was calculated using Student’s t-test, and it is given as **: p ≤ 0.01.
2.6. Effects of Fingolimod on Cerebral BDNF Levels
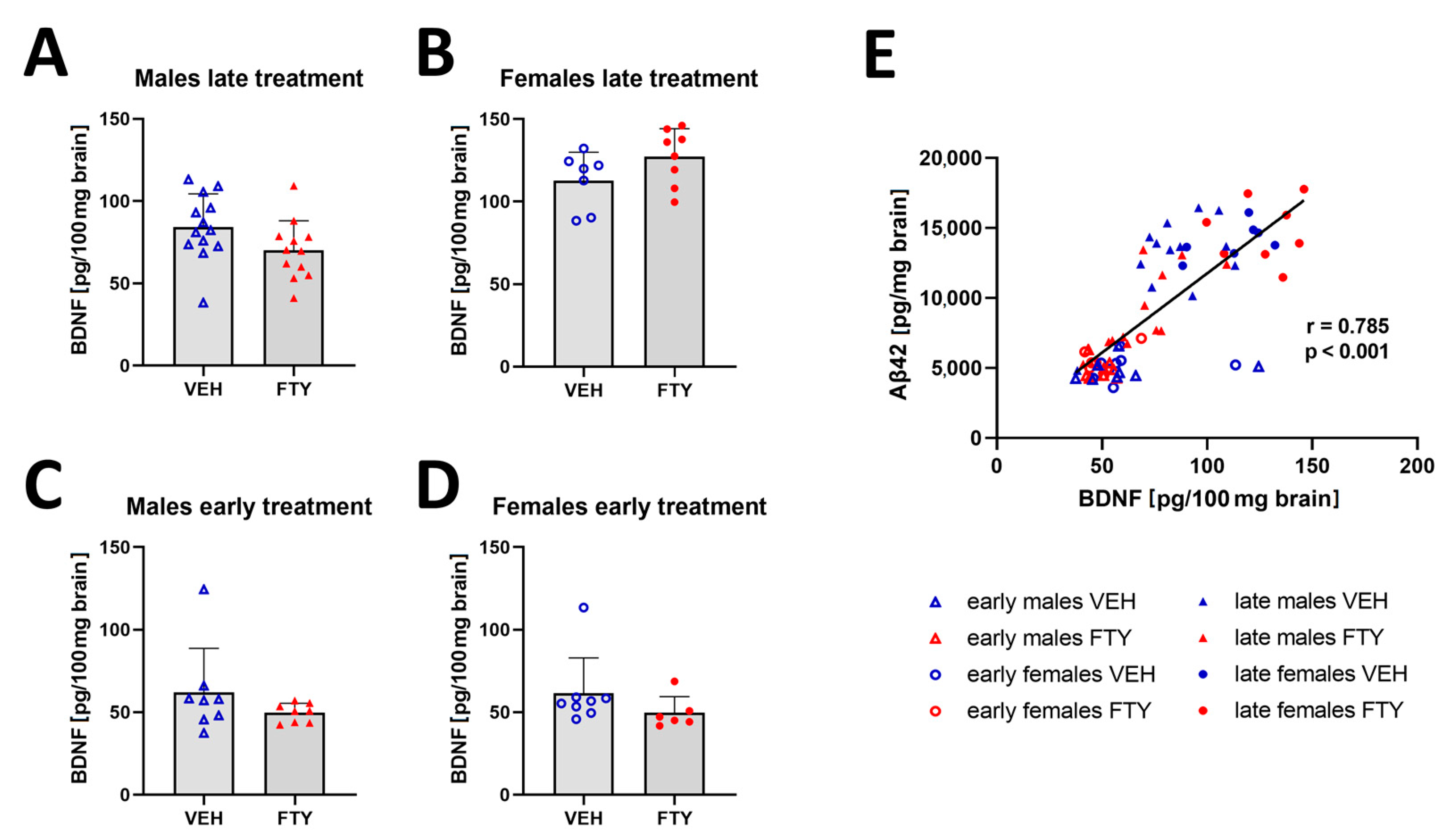
Previous studies have reported that fingolimod increased the BDNF concentration in the brain [20,29]. Thus, we quantified BDNF in the brain extracts in all of the experimental groups. We found a trend (non-significant) of decreased BDNF levels only in late treated males in comparison to that of their control group (70.1 ± 17.2 vs. 84.3 ± 19.3, p = 0.06). The remaining groups showed no significant difference in the BDNF levels (Figure 6). However, the BDNF concentration positively correlated to the Aβ levels in all of the groups (r = 0.785; p < 0.001).
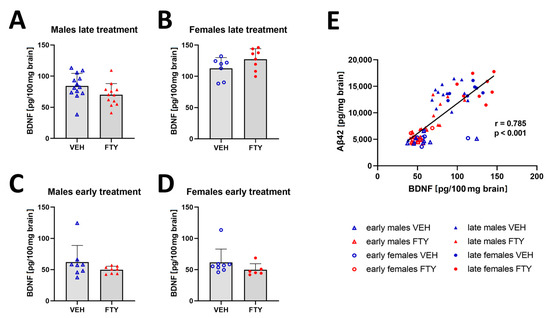
Figure 6.
Amount of BDNF in brain tissues. (A–D) Graphs show the quantification of BDNF in brain tissues of late treated male (A) and female (B) and early treated male (C) and female (D) APPtg mice. (E) Linear regression analysis between brain BDNF and Aβ concentrations show a positive correlation between both of the proteins. Data are presented as mean ± SD; n = 8, except for late treated males VEH, n = 13; FTY, n = 12) and early treated females (n = 6). Significance between experimental groups was calculated using the Wilcoxon–Mann–Whitney test. Correlation coefficient was analysed using Pearson’s correlation test.
3. Discussion
The present study shows that fingolimod has reducing effects on the Aβ load in our animal model of AD only when it is administered at a later stage of the disease. Fingolimod was reported previously to have beneficial effects on the animal models of AD [16,17,18,19,20,21,22,23,25]. However, two studies showed no effect of this treatment when it was administered at the early stages in contrast to the older animals [28,30], which is similar to our finding. Furthermore, the beneficial effect of our treatment was only apparent in male mice, while female mice showed no effect in any of the tested treatment paradigms. This sex-selective effect was not observed previously in any of the studies published, except for a study on patients, in which women were reported to have higher occurrence of adverse effects, mainly infections [31]. It is common that the treatment effects are different between male and female individuals. Interestingly, a recent review by the Norwegian Health Institute (FHI: Folkehelseinstituttet) of patient studies that were exclusively performed in Norway between 2017 and 2022 found that there is a substantial lack of systematically analysing the sex-related effects: 133 studies reported sex-related analyses and 178 did not [32]. Metabolically, male and female individuals are different also at different ages [33,34], and thus, the treatment response differences should be assumed as normal. In older age, increasing differences in anabolic hormone production and degradation adds to the apparent biochemical sex differences [35]. Not only biochemical differences, but also differences in the function of the immune system and its response towards Aβ, esp. microglia, could be influenced by sex (reviewed in [36]).
Fingolimod’s main pharmacologic effect is immunomodulation by lymphocyte sequestration, thus reducing the numbers of T and B cells in circulation [8]. It has been shown that effect of fingolimod depends on the inflammatory status of the animals [37]. Therefore, we hypothesized that in order to observe a positive treatment effect, the microglia must be activated before starting the treatment. We proposed to evaluate the effect of the immunomodulatory treatment with fingolimod in two groups of APPtg mice: those that were early on in the disease at 50–100 days (i.e., before extensive microglial activation) and those that were late in its progression at 125–175 days (when microglia are highly activated) [38]. Our study confirms that the fingolimod treatment is only effective in reducing both microglia activation and plaque load when it is administered at the late phase of Aβ deposition. Although S1P receptors are involved in different mechanisms in astrocytes [39,40,41,42], we did not find any alterations in the astrocyte activation of the APPtg mice with the fingolimod treatment.
As fingolimod’s main effect is immunomodulation, we also evaluated its effect on the brain cytokine levels in the APPtg mice. We found that the fingolimod treatment reduced the pro-inflammatory cytokine IL-1β level. The reduction of inflammatory markers has been described previously in other studies with AD models, but also, with other neurodegeneration models. This reduction might be related to the decrease in the activated microglia in the late-treated males, as observed in our study. On the other hand, several studies have found an increase in BDNF after the fingolimod treatment [14,20,29,43,44,45,46,47]. Contrarily, we found even a non-significant trend of decreased BDNF concentration in male mice treated with fingolimod in comparison to that of their vehicle-treated control group. Nevertheless, we observed a direct correlation between BDNF and Aβ levels in all of the groups. This increase in BDNF in APP+/PS1+ mice (Prp-APPswe/Prp-PS1ΔE9 [48]) has previously been reported [49]. Thus, the decrease observed in the males of the treated group might be sole due to the reduction in the Aβ load in these animals, and not because of a direct effect of the fingolimod treatment on the BDNF concentration.
In summary, we have shown that the fingolimod treatment has beneficial effects on an AD model, but its success depends on the neuroinflammatory state at the beginning of the treatment and the subject’s sex. Thus, according to our data and as previously described in [23], fingolimod treatments would be more effective after the onset of the first AD symptoms, mainly affecting the neuroinflammatory reaction towards Aβ deposits.
4. Material and Methods
4.1. Animal Models and Breeding Schemes
Heterozygous APP transgenic (APPtg) mice (APPPS1–21 [50]) were used in this study. APPPS1-21 mice have a combined APP (Swedish mutations) and PS1 (L166P mutation) transgene under control of the Thy1-promoter, leading primarily to pathological Aβ production in the frontocortical neurons and the first cortical Aβ plaques at 45–50 days of age, which occurs much later also in other brain regions, but to a significant lesser extend (e.g., hippocampus).
The transgenic breeding programs were maintained with male heterozygous, transgene-positive breeders and C57BL/6J transgene-negative females. The animals were housed in the animal care facility of the Department of Comparative Medicine at the Oslo University Hospital (Norway) with a 12 h/12 h light/dark cycle at a temperature of 22 °C with free access to irradiated food and autoclaved, acidified water. All of the experiments were approved by the competent authorities and conducted according to the European Union Directive and regional laws.
4.2. Experimental Design—Treatment Paradigms
Animals were distributed into eight experimental groups (see also Table A1):
- (a)
- Early vehicle-treated males;
- (b)
- Early FTY-treated males;
- (c)
- Early vehicle-treated females;
- (d)
- Early FTY-treated females;
- (e)
- Late vehicle-treated males;
- (f)
- Late FTY-treated males;
- (g)
- Late vehicle-treated females;
- (h)
- Late FTY-treated females.
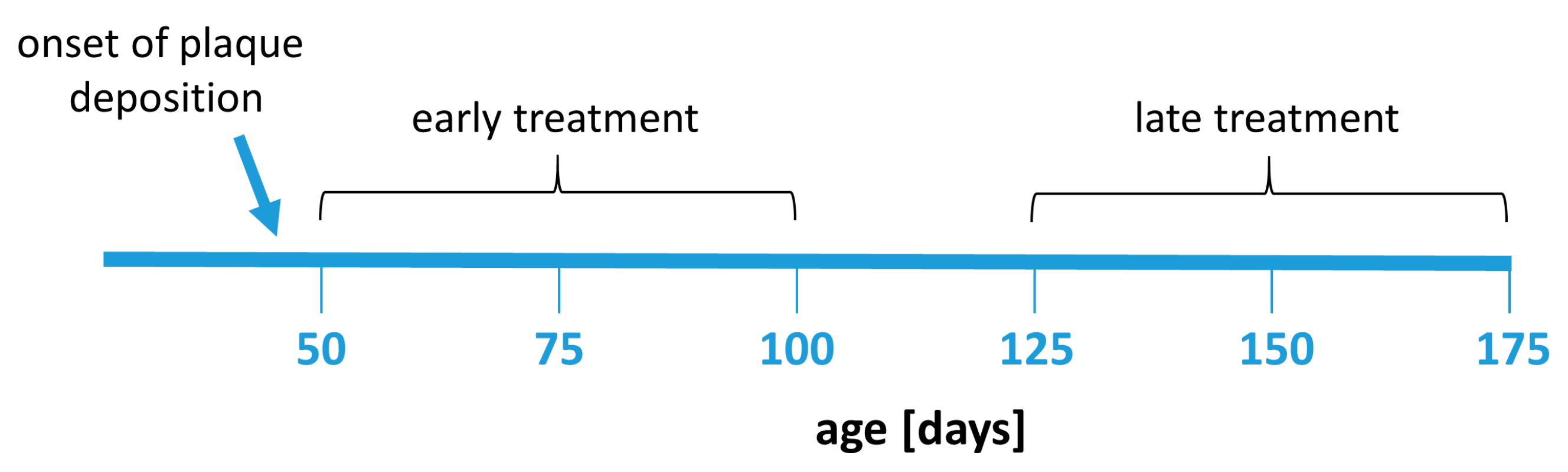
Fingolimod (FTY720, Sigma-Aldrich, Darmstadt, Germany) was given dissolved in drinking water at a dose of 1 mg/kg bodyweight per day. The animals had a mean uptake between 2.8 mL (late treated males) and 3.5 mL (early treated males) per day from the drinking water. The FTY720 dose in water was corrected according to water consumption in each group. The treatment was administered for 50 days starting at 50 or 125 days of age, respectively, depending on the experimental group (Figure 7). The body weight and water consumption rate of the animals was controlled weekly to monitor potential side effects and to adjust the dose.
Figure 7.
Experimental design. Animals were treated with fingolimod in two treatment paradigms: early treatment (between 50 and 100 days of age) or late treatment (between 125 and 175 days of age). The treatment duration (50 days) was the same in all treatment groups.
4.3. Assessment of Activity and Cognitive Performance
4.3.1. Assessment of Activity
The activity of the mice was recorded with infrared sensor-based technology (TSE Systems GmbH, Berlin, Germany) for a period of 48 h (plus 24 h for acclimatisation). Within this period, the mice were housed individually in small standard cages and a typical habitat. The treatment of the mice was performed daily between 9 a.m. and 10 a.m., which was excluded from the activity analysis. The movement of the mice was detected by infrared sensors located on top of the cages. The movements were integrated over 10 min time intervals. Subsequently, the data were analysed using the Phenomaster Software (TSE Systems GmbH, Berlin, Germany) and displayed cumulatively as a function of time (Figure A1).
4.3.2. Assessment of Spatial Orientation Performance
A modified version of our previously published protocol was applied [51,52,53,54]. Briefly, the mice were trained for six days, with four consecutive trials per day and a short recovery period of 60 s between the trials. On day 7, the mice were subjected to a probe trial, where the hidden platform was removed, and visual cue trials, where the platform was clearly marked with a visual cue to detect severe visual impairments. Data were acquired using EthoVision XT (version 15, Noldus Information Technology BV, Wageningen, The Netherlands). The animals were treated by gavage after behavioural testing each day to avoid interference due to potential acute effects.
4.4. Tissue Collection and Processing
The mice were sacrificed by ketamine/xylazine overdose (400 mg/kg ketamine, 40 mg/kg xylazine) and transcardially perfused with ice-cold 0.1 M phosphate-buffered saline (PBS). The brains were removed, and the cerebrums were divided into two hemispheres. One hemisphere was kept in 4% paraformaldehyde (PFA) in 0.1 M PBS for immunohistochemical processing. The other hemisphere was snap frozen in liquid nitrogen and stored in −80 °C until further protein extraction.
4.5. Immunohistochemistry Labelling and Morphological Quantification
Formalin-fixed hemispheres were embedded in paraffin and sliced into four-micrometer-thick coronal sections using a rotation microtome (HM355S, Leica Biosystems GmbH, Nussloch, Germany), as described previously [38,51,52,53,55,56,57,58,59,60,61]. The sections (bregma−2.0 mm) were stained for microglia (anti-IBA1, 1:1000, FUJIFILM Wako Chemicals Europe GmbH, 019–19741) or astrocytes (anti-GFAP, 1:500, Agilent, USA, Z033401-2) using a BOND-III® automated immunostaining system (Leica Biosystems GmbH, Nussloch, Germany) with a haematoxylin counterstain (provided with the staining kit, Bond Polymer Refine Detection, DS9800). The sections for anti-IBA1 staining were pre-treated with citric acid for 20 min before staining. For anti-GFAP staining, the Bond Enzyme Pre-Treatment Kit (AR9551, Leica Biosystems GmbH, Nussloch, Germany) was applied to the sections for 10 min.
After staining, the tissue sections were digitized at 230 nm resolution using a Pannoramic MIDI II slide scanner (3DHISTECH Ltd., Budapest, Hungary).
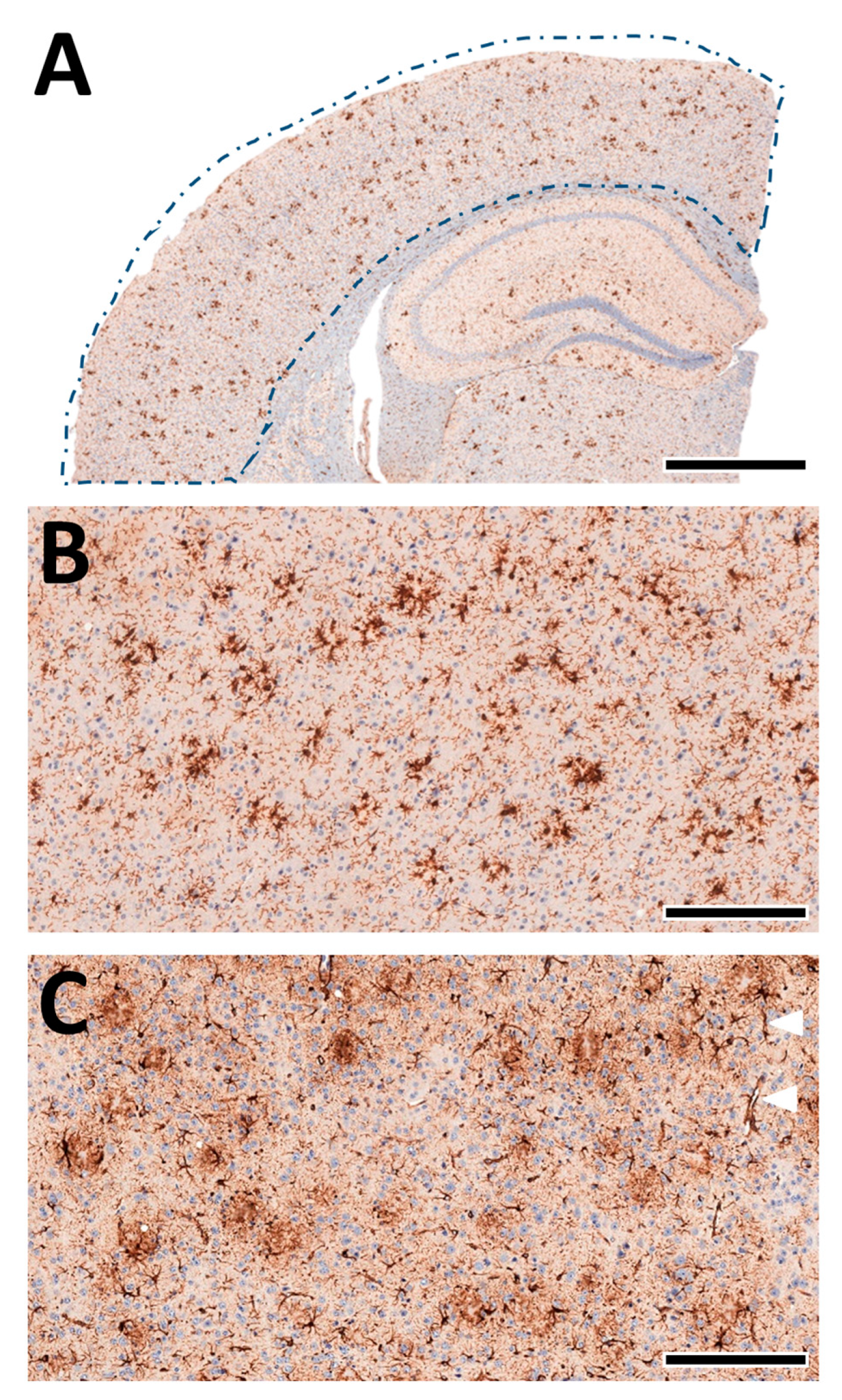
A quantitative analysis of the microglia and astrocytes in the cortex of each animal was performed automatically as previously described by us [38,55,56,61] using deep-learning algorithms generated with the DeePathology™ STUDIO (DeePathology Ltd., Ra’anana, Israel). We generated specific algorithms to identify IBA1+ cells or GFAP+ cells. The algorithms were applied for the ROIs (Figure 8A). The cell density (number per ROI) and relative areal coverage (% cell area per total ROI) were calculated for each animal.
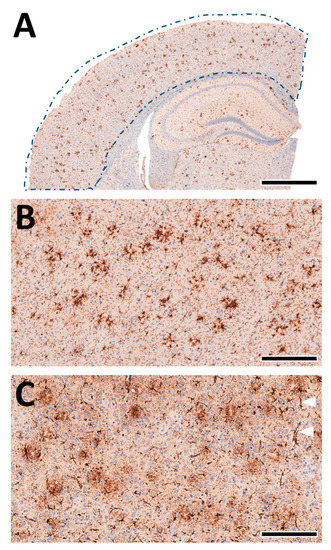
Figure 8.
Machine learning-assisted morphological analyses of cortex pathology. (A) Schematic presentation of the analysed cortex region (from Figure 4A, left). The brain tissue was automatically detected within the manually marked ROI. (B) Staining of IBA1+ cells in the cortex. The microglia nicely accumulate near-insoluble Aβ deposits and delineate amyloid plaques. (C) Staining of GFAP+ cells in the cortex. Astrocytes show a more diffuse pattern with localised perivascular pronunciation (white triangles). Scale bars: (A) 1 mm, (B,C) 50 µm.
4.6. Quantification of Aβ
We performed the quantification of the brain Aβ levels using an electrochemiluminescence immunoassay. Thus, we homogenized the brain hemispheres and soluble and insoluble Aβ fractions were extracted in TBS and guanidine buffer, respectively [51]. Immunoassays were performed using the V-PLEX Plus Aβ42 Peptide (4G8) Kit and an MESO QuickPlex SQ120 machine according to the manufacturer’s instructions (K150SLG, Meso Scale Diagnostics LLC, Rockville, MD, USA). The results were normalized to the sample weight. The brain Aβ42 content was calculated as pg/mg brain.
4.7. Quantification of BDNF and Cytokines
We performed a quantification of the BDNF and inflammatory cytokine levels in the brain by immunoassay. To achieve this aim, brain homogenates were extracted using lysis buffer (Phosphate buffer saline + 1% Triton X-100 + phosphatase inhibitor + protease inhibitor) at 2 µL/mg brain homogenate. The samples were homogenized for 30 s (SpeedMill PLUS, Analytik Jena GmbH, Jena, Germany) and centrifuged at 16,000 g for 15 min at 4 °C. Supernatants were diluted 4-fold using a working solution (Meso Scale Diagnostics LLC, Rockville, MD, USA), and immunoassays were performed using the U-PLEX Kit for BDNF and cytokines (IL-2, IL-4, IL-6; TNFα, IFNγ) and a MESO QuickPlex SQ120 machine following the manufacturer’s instructions.
4.8. Statistical Analyses
All of the statistical analyses were performed using GraphPad Prism 9 software (GraphPad Software, CA, USA). We verified the data for Gaussian normal distribution by using the Shapiro–Wilk normality test. The Student’s t-test or Mann–Whitney test were performed to determine the significant differences between the two groups. The correlation coefficient was analysed using Pearson’s correlation test. Data are presented as means ± standard deviation (SD). Differences were considered to be statistically significant when p < 0.05. The number of subjects (n) is reported in the figure legends and is summarized in Appendix B (Table A1). Detailed statistical methods are summarized in Appendix B (Table A2).
Author Contributions
Conceptualization, P.B. and J.P.; Methodology, P.B., M.B., L.M., J.W., T.B., I.E. and J.P.; Validation, P.B.; Formal Analysis, P.B. and M.B.; Investigation, P.B. and M.B.; Data Curation, P.B. and M.B.; Writing—Original Draft Preparation, P.B., M.B. and J.P.; Writing—Review and Editing, P.B., M.B., L.M., J.W., T.B., I.E., B.J. and J.P.; Visualization, P.B. and J.P.; Supervision, J.P.; Project Administration, P.B. and J.P.; Funding Acquisition, B.J. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
J.P. received funding from the German Research Foundation (DFG, Germany; #263024513), HelseSØ (Norway; #2019054, #2019055, and #2022046), Barnekreftforeningen (Norway; #19008), EEA and Norway grants Kappa programme [Iceland, Liechtenstein, Norway, Czech Republic; #TO01000078 (TAČR TARIMAD)], Norges forskningsråd [NFR, Norway; #295910 (NAPI), #327571 (PETABC)]. PETABC is an EU Joint programme—Neurodegenerative Disease Research (JPND) project. PETABC is supported through the following funding organizations under the aegis of JPND—www.jpnd.eu: NFR (Norway; #327571), FFG (Austria; #882717), BMBF (Germany; #01ED2106); MSMT (Czech Republic; #8F21002), Latvia; #ES RTD/2020/26, ANR (France; #20-JPW2-0002-04), SRC (Sweden; #2020-02905). L.M. appreciated the support by Nasjonalforeningen (Norway; #16154). B.J. appreciated the support by Latvia #ES RTD/2020/26 (PETABC).
Institutional Review Board Statement
The animal study protocol was approved by the local authorities (FOTS 7771, 24 Junuary 2015; FOTS 20540, 14 October 2019; IV2-2022 1 December 2022) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data files and figures can be downloaded from the ABCS1P project at http://www.doi.org/10.17605/OSF.IO/VWQ58 (accessed on 1 February 2023).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AD, Alzheimer’s disease; FTY720, fingolimod; GFAP, glial fibrillary acid protein; IBA1, ionized calcium-binding adaptor protein-1; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; PFA, paraformaldehyde; ROI, region of interest; SD, standard deviation; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Appendix A. Activity and Cognitive Performance Assessment
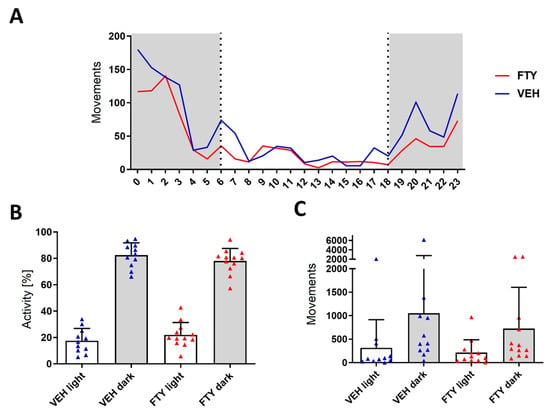
Figure A1.
Assessment of the circadian activity during a 24 h period revealed no differences between VEH-treated (blue triangles) and FTY-treated (red triangles) males. Animals were treated according to the early treatment paradigm. (A) Mean hourly count of movements of animal groups (grey indicates dark hours; white indicates light hours of the day). (B) Relative distribution of the activity and (C) number of detected movements for each experimental group during light and dark hours. Significance in activity between treatment groups was calculated using the Student’s t-test. Data are presented as mean ± SD, VEH n = 11, FTY n = 12.
Figure A1.
Assessment of the circadian activity during a 24 h period revealed no differences between VEH-treated (blue triangles) and FTY-treated (red triangles) males. Animals were treated according to the early treatment paradigm. (A) Mean hourly count of movements of animal groups (grey indicates dark hours; white indicates light hours of the day). (B) Relative distribution of the activity and (C) number of detected movements for each experimental group during light and dark hours. Significance in activity between treatment groups was calculated using the Student’s t-test. Data are presented as mean ± SD, VEH n = 11, FTY n = 12.
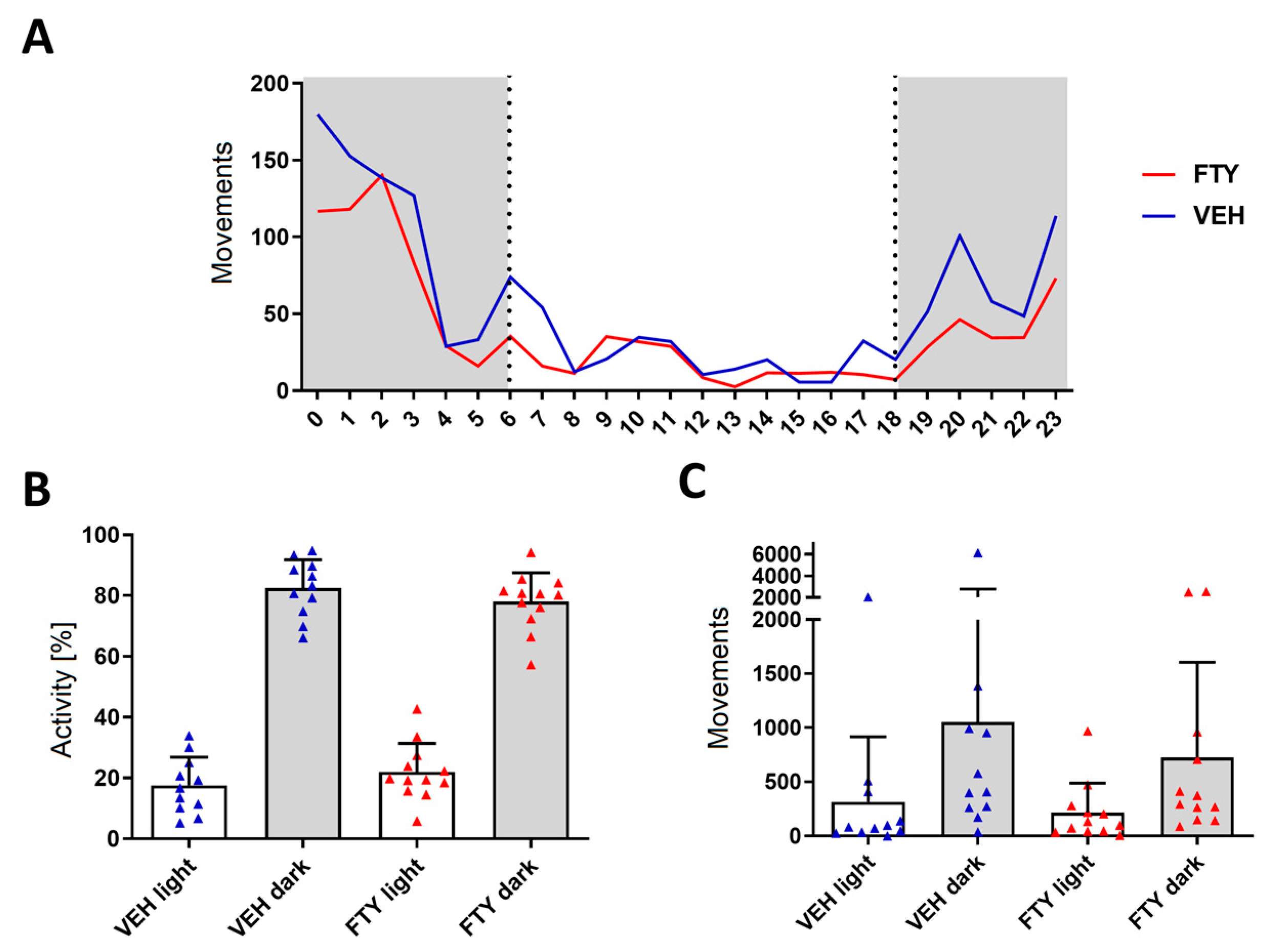
Figure A2.
Spatial orientation testing using water maze revealed no significant differences between VEH-treated (blue circles) and FTY-treated (red circles) females. Animals were treated according to the early treatment paradigm. (A) Time latency [s] average of each experimental group to find the platform during training trials. (B) Proximity average [cm] during training. (C) Proximity to target and (D) number of target crossings (n ∈ N) during the probe trial. Significance between treatment groups was calculated using the Student’s t-test (C) or Wilcoxon–Mann–Whitney test (D). Data are presented as mean (A,B) and mean ± SD (C,D), VEH n = 11, and FTY n = 11.
Figure A2.
Spatial orientation testing using water maze revealed no significant differences between VEH-treated (blue circles) and FTY-treated (red circles) females. Animals were treated according to the early treatment paradigm. (A) Time latency [s] average of each experimental group to find the platform during training trials. (B) Proximity average [cm] during training. (C) Proximity to target and (D) number of target crossings (n ∈ N) during the probe trial. Significance between treatment groups was calculated using the Student’s t-test (C) or Wilcoxon–Mann–Whitney test (D). Data are presented as mean (A,B) and mean ± SD (C,D), VEH n = 11, and FTY n = 11.
Appendix B. Statistics

Table A1.
Summary of experimental groups.
Table A1.
Summary of experimental groups.
| Paradigm | Experimental Group | Number of Animals |
|---|---|---|
| early (50–100 d) | VEH treatment males | 8, (11) 1 |
| FTY treatment males | 8, (12) 1 | |
| VEH treatment females | 8, (11) 1 | |
| FTY treatment females | 6 (of 8) 2, (11) 1 | |
| late (125–175 d) | VEH treatment males | 13 |
| FTY treatment males | 12 (of 13) 2 | |
| VEH treatment females | 8 | |
| FTY treatment females | 8 |
1 Numbers in parentheses correspond to animal used for spatial orientation assessment only. This extra group was treated with FTY or VEH using oral gavage. 2 Some animals died during the experiment.

Table A2.
Statistical tests used for the evaluation of differences between experimental groups for each variable studied.
Table A2.
Statistical tests used for the evaluation of differences between experimental groups for each variable studied.
| Variable | Statistical Test |
|---|---|
| animal weight | two-way ANOVA (mixed effects) |
| Aβ concentration | Wilcoxon–Mann–Whitney test |
| microglia density | Student’s t-test |
| astrocyte density | Student’s t-test |
| cytokine concentration | Student’s t-test |
| BDNF concentration | Wilcoxon–Mann–Whitney test |
| activity | Student’s t-test |
| spatial orientation (proximity and number of crossing) | Student’s t-test and Wilcoxon–Mann–Whitney test |
References
- Alzheimer Association Report. 2020 Alzheimer’s disease facts and figures. J. Alzheimers Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Thinakaran, G.; Koo, E.H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Pahnke, J.; Langer, O.; Krohn, M. Alzheimer’s and ABC transporters--new opportunities for diagnostics and treatment. Neurobiol. Dis. 2014, 72 Pt A, 54–60. [Google Scholar] [CrossRef]
- Pahnke, J.; Walker, L.C.; Scheffler, K.; Krohn, M. Alzheimer’s disease and blood-brain barrier function-Why have anti-beta-amyloid therapies failed to prevent dementia progression? Neurosci. Biobehav. Rev. 2009, 33, 1099–1108. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Mandala, S.; Hajdu, R.; Bergstrom, J.; Quackenbush, E.; Xie, J.; Milligan, J.; Thornton, R.; Shei, G.J.; Card, D.; Keohane, C.; et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002, 296, 346–349. [Google Scholar] [CrossRef]
- Chiba, K.; Yanagawa, Y.; Masubuchi, Y.; Kataoka, H.; Kawaguchi, T.; Ohtsuki, M.; Hoshino, Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol. 1998, 160, 5037–5044. [Google Scholar] [CrossRef]
- Pham, T.H.; Okada, T.; Matloubian, M.; Lo, C.G.; Cyster, J.G. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity 2008, 28, 122–133. [Google Scholar] [CrossRef]
- Budde, K.; Schutz, M.; Glander, P.; Peters, H.; Waiser, J.; Liefeldt, L.; Neumayer, H.H.; Bohler, T. FTY720 (fingolimod) in renal transplantation. Clin. Transpl. 2006, 20 (Suppl. S17), 17–24. [Google Scholar] [CrossRef]
- Brinkmann, V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007, 115, 84–105. [Google Scholar] [CrossRef]
- Czubowicz, K.; Jesko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef]
- Bascuñana, P.; Möhle, L.; Brackhan, M.; Pahnke, J. Fingolimod as a Treatment in Neurologic Disorders Beyond Multiple Sclerosis. Drugs RD 2020, 20, 197–207. [Google Scholar] [CrossRef]
- Doi, Y.; Takeuchi, H.; Horiuchi, H.; Hanyu, T.; Kawanokuchi, J.; Jin, S.; Parajuli, B.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Fingolimod phosphate attenuates oligomeric amyloid beta-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons. PLoS ONE 2013, 8, e61988. [Google Scholar] [CrossRef]
- Ruiz, A.; Joshi, P.; Mastrangelo, R.; Francolini, M.; Verderio, C.; Matteoli, M. Testing Abeta toxicity on primary CNS cultures using drug-screening microfluidic chips. Lab Chip 2014, 14, 2860–2866. [Google Scholar] [CrossRef]
- Takasugi, N.; Sasaki, T.; Ebinuma, I.; Osawa, S.; Isshiki, H.; Takeo, K.; Tomita, T.; Iwatsubo, T. FTY720/fingolimod, a sphingosine analogue, reduces amyloid betaamyloid beta production in neurons. PLoS ONE 2013, 8, e64050. [Google Scholar] [CrossRef]
- Asle-Rousta, M.; Kolahdooz, Z.; Dargahi, L.; Ahmadiani, A.; Nasoohi, S. Prominence of central sphingosine-1-phosphate receptor-1 in attenuating abeta-induced injury by fingolimod. J. Mol. Neurosci. 2014, 54, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, F.; Dargahi, L.; Nasoohi, S.; Omidbakhsh, R.; Mohamed, Z.; Chik, Z.; Naidu, M.; Ahmadiani, A. Neurorestorative effect of FTY720 in a rat model of Alzheimer’s disease: Comparison with memantine. Behav. Brain Res. 2013, 252, 415–421. [Google Scholar] [CrossRef]
- Asle-Rousta, M.; Kolahdooz, Z.; Oryan, S.; Ahmadiani, A.; Dargahi, L. FTY720 (fingolimod) attenuates beta-amyloid peptide (Abeta42)-induced impairment of spatial learning and memory in rats. J. Mol. Neurosci. 2013, 50, 524–532. [Google Scholar] [CrossRef]
- Fukumoto, K.; Mizoguchi, H.; Takeuchi, H.; Horiuchi, H.; Kawanokuchi, J.; Jin, S.; Mizuno, T.; Suzumura, A. Fingolimod increases brain-derived neurotrophic factor levels and ameliorates amyloid beta-induced memory impairment. Behav. Brain Res. 2014, 268, 88–93. [Google Scholar] [CrossRef]
- Aytan, N.; Choi, J.K.; Carreras, I.; Brinkmann, V.; Kowall, N.W.; Jenkins, B.G.; Dedeoglu, A. Fingolimod modulates multiple neuroinflammatory markers in a mouse model of Alzheimer’s disease. Sci. Rep. 2016, 6, 24939. [Google Scholar] [CrossRef]
- Carreras, I.; Aytan, N.; Choi, J.K.; Tognoni, C.M.; Kowall, N.W.; Jenkins, B.G.; Dedeoglu, A. Dual dose-dependent effects of fingolimod in a mouse model of Alzheimer’s disease. Sci. Rep. 2019, 9, 10972. [Google Scholar] [CrossRef]
- Kartalou, G.-I.; Salgueiro-Pereira, A.R.; Endres, T.; Lesnikova, A.; Casarotto, P.; Pousinha, P.; Delanoe, K.; Edelmann, E.; Castrén, E.; Gottmann, K.; et al. Anti-Inflammatory Treatment with FTY720 Starting after Onset of Symptoms Reverses Synaptic Deficits in an AD Mouse Model. Int. J. Mol. Sci. 2020, 21, 8957. [Google Scholar] [CrossRef]
- Krivinko, J.M.; Erickson, S.L.; MacDonald, M.L.; Garver, M.E.; Sweet, R.A. Fingolimod mitigates synaptic deficits and psychosis-like behavior in APP/PSEN1 mice. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12324. [Google Scholar] [CrossRef]
- Crivelli, S.M.; Luo, Q.; Kruining, D.V.; Giovagnoni, C.; Mane-Damas, M.; den Hoedt, S.; Berkes, D.; De Vries, H.E.; Mulder, M.T.; Walter, J.; et al. FTY720 decreases ceramides levels in the brain and prevents memory impairments in a mouse model of familial Alzheimer’s disease expressing APOE4. Biomed Pharm. 2022, 152, 113240. [Google Scholar] [CrossRef]
- Yamada, K.; Yabuki, C.; Seubert, P.; Schenk, D.; Hori, Y.; Ohtsuki, S.; Terasaki, T.; Hashimoto, T.; Iwatsubo, T. Abeta immunotherapy: Intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J. Neurosci. 2009, 29, 11393–11398. [Google Scholar] [CrossRef]
- Moechars, D.; Dewachter, I.; Lorent, K.; Reverse, D.; Baekelandt, V.; Naidu, A.; Tesseur, I.; Spittaels, K.; Haute, C.V.; Checler, F.; et al. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 1999, 274, 6483–6492. [Google Scholar] [CrossRef]
- Jesko, H.; Wieczorek, I.; Wencel, P.L.; Gassowska-Dobrowolska, M.; Lukiw, W.J.; Strosznajder, R.P. Age-Related Transcriptional Deregulation of Genes Coding Synaptic Proteins in Alzheimer’s Disease Murine Model: Potential Neuroprotective Effect of Fingolimod. Front. Mol. Neurosci. 2021, 14, 660104. [Google Scholar] [CrossRef] [PubMed]
- Deogracias, R.; Yazdani, M.; Dekkers, M.P.J.; Guy, J.; Ionescu, M.C.S.; Vogt, K.E.; Barde, Y.-A. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 14230–14235. [Google Scholar] [CrossRef]
- Fagan, S.G.; Bechet, S.; Dev, K.K. Fingolimod Rescues Memory and Improves Pathological Hallmarks in the 3xTg-AD Model of Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 1882–1895. [Google Scholar] [CrossRef]
- Manni, A.; Direnzo, V.; Iaffaldano, A.; Di Lecce, V.; Tortorella, C.; Zoccolella, S.; Iaffaldano, P.; Trojano, M.; Paolicelli, D. Gender differences in safety issues during Fingolimod therapy: Evidence from a real-life Relapsing Multiple Sclerosis cohort. Brain Behav. 2017, 7, e00804. [Google Scholar] [CrossRef]
- Østbø, N.M.; Vist, G.E.; Løchen, M.-L. Sex and Gender-based Analyses in Norwegian Treatment Studies: A Scoping Review. In Folkehelseinstituttet; Folkehelseinstituttet: Oslo, Norway, 2022; p. 44. [Google Scholar]
- Costanzo, M.; Caterino, M.; Sotgiu, G.; Ruoppolo, M.; Franconi, F.; Campesi, I. Sex differences in the human metabolome. Biol. Sex Differ. 2022, 13, 30. [Google Scholar] [CrossRef]
- Zhernakova, D.V.; Sinha, T.; Andreu-Sánchez, S.; Prins, J.R.; Kurilshikov, A.; Balder, J.-W.; Sanna, S.; Franke, L.; Kuivenhoven, J.A.; Zhernakova, A.; et al. Age-dependent sex differences in cardiometabolic risk factors. Nat. Cardiovasc. Res. 2022, 1, 844–854. [Google Scholar] [CrossRef]
- Pataky, M.W.; Young, W.F.; Nair, K.S. Hormonal and Metabolic Changes of Aging and the Influence of Lifestyle Modifications. Mayo Clin. Proc. 2021, 96, 788–814. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Diaz Diaz, A.C.; Malone, K.; Shearer, J.A.; Moore, A.C.; Waeber, C. Preclinical Evaluation of Fingolimod in Rodent Models of Stroke With Age or Atherosclerosis as Comorbidities. Front. Pharmacol. 2022, 13, 920449. [Google Scholar] [CrossRef]
- Bascuñana, P.; Brackhan, M.; Pahnke, J. Machine learning-supported analyses improve quantitative histologicl assessments of amyloid-β deposits and activated microglia. J. Alzheimer’s Dis. 2021, 79, 597–605. [Google Scholar] [CrossRef]
- Singh, S.K.; Kordula, T.; Spiegel, S. Neuronal contact upregulates astrocytic sphingosine-1-phosphate receptor 1 to coordinate astrocyte-neuron cross communication. Glia 2022, 70, 712–727. [Google Scholar] [CrossRef]
- Dusaban, S.S.; Chun, J.; Rosen, H.; Purcell, N.H.; Brown, J.H. Sphingosine 1-phosphate receptor 3 and RhoA signaling mediate inflammatory gene expression in astrocytes. J. Neuroinflammation 2017, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Obermeier, B.; Cotleur, A.; Love, A.; Takeshita, Y.; Sano, Y.; Kanda, T.; Ransohoff, R.M. Sphingosine 1 Phosphate at the Blood Brain Barrier: Can the Modulation of S1P Receptor 1 Influence the Response of Endothelial Cells and Astrocytes to Inflammatory Stimuli? PLoS ONE 2015, 10, e0133392. [Google Scholar] [CrossRef]
- O’Sullivan, S.A.; O’Sullivan, C.; Healy, L.M.; Dev, K.K.; Sheridan, G.K. Sphingosine 1-phosphate receptors regulate TLR4-induced CXCL5 release from astrocytes and microglia. J. Neurochem. 2018, 144, 736–747. [Google Scholar] [CrossRef]
- Di Pardo, A.; Amico, E.; Favellato, M.; Castrataro, R.; Fucile, S.; Squitieri, F.; Maglione, V. FTY720 (fingolimod) is a neuroprotective and disease-modifying agent in cellular and mouse models of Huntington disease. Hum. Mol. Genet. 2014, 23, 2251–2265. [Google Scholar] [CrossRef]
- Miguez, A.; Garcia-Diaz Barriga, G.; Brito, V.; Straccia, M.; Giralt, A.; Gines, S.; Canals, J.M.; Alberch, J. Fingolimod (FTY720) enhances hippocampal synaptic plasticity and memory in Huntington’s disease by preventing p75NTR up-regulation and astrocyte-mediated inflammation. Hum. Mol. Genet. 2015, 24, 4958–4970. [Google Scholar] [CrossRef]
- Vidal-Martinez, G.; Najera, K.; Miranda, J.D.; Gil-Tommee, C.; Yang, B.; Vargas-Medrano, J.; Diaz-Pacheco, V.; Perez, R.G. FTY720 Improves Behavior, Increases Brain Derived Neurotrophic Factor Levels and Reduces alpha-Synuclein Pathology in Parkinsonian GM2+/− Mice. Neuroscience 2019, 411, 1–10. [Google Scholar] [CrossRef]
- Vidal-Martinez, G.; Vargas-Medrano, J.; Gil-Tommee, C.; Medina, D.; Garza, N.T.; Yang, B.; Segura-Ulate, I.; Dominguez, S.J.; Perez, R.G. FTY720/Fingolimod Reduces Synucleinopathy and Improves Gut Motility in A53T Mice: CONTRIBUTIONS OF PRO-BRAIN-DERIVED NEUROTROPHIC FACTOR (PRO-BDNF) AND MATURE BDNF. J. Biol. Chem. 2016, 291, 20811–20821. [Google Scholar] [CrossRef]
- Ren, M.; Han, M.; Wei, X.; Guo, Y.; Shi, H.; Zhang, X.; Perez, R.G.; Lou, H. FTY720 Attenuates 6-OHDA-Associated Dopaminergic Degeneration in Cellular and Mouse Parkinsonian Models. Neurochem. Res. 2017, 42, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Borchelt, D.R.; Ratovitski, T.; van Lare, J.; Lee, M.K.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Price, D.L.; Sisodia, S.S. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 1997, 19, 939–945. [Google Scholar] [CrossRef]
- Szapacs, M.E.; Numis, A.L.; Andrews, A.M. Late onset loss of hippocampal 5-HT and NE is accompanied by increases in BDNF protein expression in mice co-expressing mutant APP and PS1. Neurobiol. Dis. 2004, 16, 572–580. [Google Scholar] [CrossRef]
- Radde, R.; Bolmont, T.; Kaeser, S.A.; Coomaraswamy, J.; Lindau, D.; Stoltze, L.; Calhoun, M.E.; Jaggi, F.; Wolburg, H.; Gengler, S.; et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006, 7, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Mohle, L.; Brackhan, M.; Bascunana, P.; Pahnke, J. Dimethyl fumarate does not mitigate cognitive decline and beta-amyloidosis in female APPPS1 mice. Brain Res. 2021, 1768, 147579. [Google Scholar] [CrossRef]
- Rai, S.P.; Krohn, M.; Pahnke, J. Early Cognitive Training Rescues Remote Spatial Memory but Reduces Cognitive Flexibility in Alzheimer’s Disease Mice. J. Alzheimers Dis. 2020, 75, 1301–1317. [Google Scholar] [CrossRef]
- Rai, S.P.; Bascunana, P.; Brackhan, M.; Krohn, M.; Mohle, L.; Paarmann, K.; Pahnke, J. Detection and Prediction of Mild Cognitive Impairment in Alzheimer’s Disease Mice. J. Alzheimers Dis. 2020, 77, 1209–1221. [Google Scholar] [CrossRef]
- Paarmann, K.; Prakash, S.R.; Krohn, M.; Mohle, L.; Brackhan, M.; Bruning, T.; Eiriz, I.; Pahnke, J. French maritime pine bark treatment decelerates plaque development and improves spatial memory in Alzheimer’s disease mice. Phytomed. Int. J. Phytother. Phytopharm. 2019, 57, 39–48. [Google Scholar] [CrossRef]
- Brackhan, M.; Calza, G.; Lundgren, K.; Bascunana, P.; Bruning, T.; Soliymani, R.; Kumar, R.; Abelein, A.; Baumann, M.; Lalowski, M.; et al. Isotope-labeled amyloid betaamyloid beta does not transmit to the brain in a prion-like manner after peripheral administration. EMBO Rep. 2022, 23, e54405. [Google Scholar] [CrossRef]
- Mohle, L.; Bascunana, P.; Brackhan, M.; Pahnke, J. Development of deep learning models for microglia analyses in brain tissue using DeePathology STUDIO. J. Neurosci. Methods 2021, 364, 109371. [Google Scholar] [CrossRef]
- Upite, J.; Bruning, T.; Mohle, L.; Brackhan, M.; Bascunana, P.; Jansone, B.; Pahnke, J. A New Tool for the Analysis of the Effect of Intracerebrally Injected Anti-Amyloid betaAmyloid beta Compounds. J. Alzheimers Dis. 2021, 84, 1677–1690. [Google Scholar] [CrossRef]
- Steffen, J.; Krohn, M.; Schwitlick, C.; Bruning, T.; Paarmann, K.; Pietrzik, C.U.; Biverstal, H.; Jansone, B.; Langer, O.; Pahnke, J. Expression of endogenous mouse APP modulates beta-amyloid deposition in hAPP transgenic mice. Acta Neuropathol. Commun. 2017, 5, 49. [Google Scholar] [CrossRef]
- Steffen, J.; Krohn, M.; Paarmann, K.; Schwitlick, C.; Bruning, T.; Marreiros, R.; Muller-Schiffmann, A.; Korth, C.; Braun, K.; Pahnke, J. Revisiting rodent models: Octodon degus as Alzheimer’s disease model? Acta Neuropathol. Commun. 2016, 4, 91. [Google Scholar] [CrossRef]
- Krohn, M.; Bracke, A.; Avchalumov, Y.; Schumacher, T.; Hofrichter, J.; Paarmann, K.; Frohlich, C.; Lange, C.; Bruning, T.; von Bohlen Und Halbach, O.; et al. Accumulation of murine amyloid betaamyloid beta mimics early Alzheimer’s disease. Brain 2015, 138 Pt 8, 2370–2382. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Möhle, L.; Brüning, T.; Eiriz, I.; Rafehi, M.; Stefan, K.; Stefan, S.M.; Pahnke, J. A Novel Huntington’s Disease Assessment Platform to Support Future Drug Discovery and Development. Int. J. Mol. Sci. 2022, 23, 14763. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).