Abstract
Systemic lupus erythematosus (SLE) is a disease of immune complex deposition; therefore, complement plays a vital role in the pathogenesis of SLE. In general, complement levels in blood and complement deposition in histological tests are used for the management of SLE. Thus, the evaluation of complement status can be useful in the diagnosis of SLE, assessment of disease activity, and prediction of treatment response and prognosis. In addition, novel complement biomarkers, such as split products and cell-bound complement activation products, are considered to be more sensitive than traditional complement markers, such as serum C3 and C4 levels and total complement activity (CH50), which become more widely used. In this review, we report the complement testing in the management of SLE over the last decade and summarize their utility.
1. Introduction
Systemic lupus erythematosus (SLE) is a typical systemic autoimmune disease characterized by the production of a variety of autoantibodies and the formation of immune complexes, with a chronic disease course and diverse organ involvement [1,2,3]. In 2014, besides the enhancement of SLE drugs, the treat-to-target concept was proposed for SLE [4], as previously proposed for rheumatoid arthritis. In addition, the basic framework of treatment strategies for SLE was established. In a treat-to-target strategy, treatment aiming at remission or where remission cannot be reached, the lowest possible disease activity must be conducted after periodically assessing overall SLE disease activity [4]. Furthermore, this strategy aims to ensure long-term survival, prevent organ damage, and optimize health-related quality of life by controlling disease activity and minimizing comorbidities and drug toxicity [4]. Therefore, these treatment strategies require strict management based on disease activity monitoring. Biomarkers can be used in this management, but they have not yet been established because SLE is associated with various organs with diverse immunological abnormalities [5,6,7].
SLE is a disease of immune complex deposition; thus, complements play a vital role in the pathogenesis of SLE [8,9,10]. In SLE, complements have a biphasic nature, which is known as the “lupus paradox [11].” That is, complement activation via immune complexes deposited in tissues causes tissue damage, whereas congenital deficiencies of the early components of complement activation pathways, such as C1q and C4, which are involved in the processing of apoptotic cells, frequently lead to the development of SLE [12]. In SLE, complement tests are usually abnormal, indicating complement-mediated pathologies. In general, complement levels in blood and complement deposition in histological tests are used for the management of SLE. Moreover, complement biomarkers can be useful in various settings, including the diagnosis of SLE, assessment of disease activity, and prediction of treatment response and prognosis.
This review outlines the reports of complement testing in the management of SLE over the last decade and summarizes its advantages. First, we discuss the advantages of serum C3 and C4 levels and total complement activity (CH50), the traditional complement markers, frequently used in routine clinical practice in various settings. Consequently, we also discuss novel biomarkers that are currently under research but are still expected to be applied and enhanced.
2. Overview on Complement
Complement is formed from more than 30 components found in blood and cell membranes, including plasma proteins, receptors on cell membranes, and regulatory factors. As shown in Figure 1, complement activation occurs through a series of chain reactions, which is known as a cascade. C3 activation is the most critical event. The complement response can be divided into two pathways: the “complement activation pathway” until C3 is degraded and the “late complement pathway” until the subsequent formation of the membrane attack complex. The complement activation pathway is divided into three: the classical pathway, the lectin pathway, and the alternative pathway. After C3 activation, the cascade to its deposition on a target as C3b and the formation of membrane attack complexes below C5 are common.
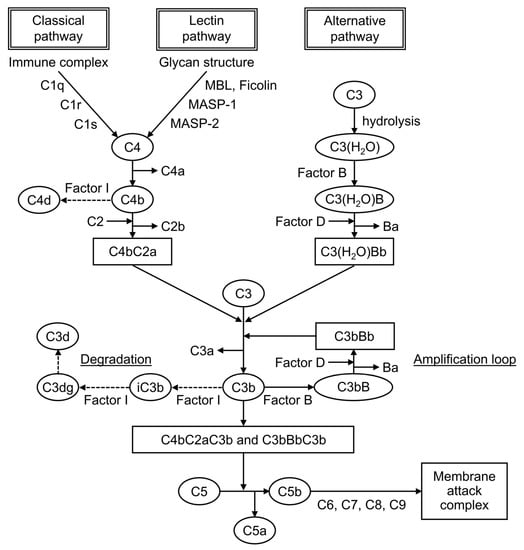
Figure 1.
The complement cascade.
The factors that trigger the complement activation pathway are distinct, and they mediate different complement molecules. In the classical pathway, when an antibody binds to an antigen to form an immune complex, C1q binds to this immune complex and immediately activates C1r, which activates C1s. Then, this activated C1s activates C4 and then C2, leading to form C4bC2a, which is a C3-converting enzyme that activates C3. When C3 is activated, it is cleaved into fragments C3a and C3b, and C3b associates with C4bC2a to form C4bC2aC3b, a C5-converting enzyme that activates C5. It mediates the cleavage of the α-chain of C3b, leading to the formation of iC3b, followed by its further degradation into C3dg and C3d. In the lectin pathway, when mannose-binding lectin (MBL) and ficolin recognize and bind to the characteristic glycan structure of the pathogen cell wall, MBL-associated serine protease, a serine protease that is bound to MBL, is activated. Then, it cleaves C4 and C2 to form the C3-converting enzyme, which is similar to the classical pathway. In the alternative pathway, strictly speaking, no recognition molecule is found. C3(H2O), a hydrolyzed C3, is present in the blood, and it is activated when it binds to polysaccharides and other substances on the surface of microorganisms, binding an activated factor B and forming the C3-converting enzyme under the action of factor D, a serine protease.
Three pathways are involved in SLE, although the classical pathway is particularly important. Complement is not only a useful biomarker, as described below, but is also considered an important target for therapeutic drugs [13,14]. Currently, no approved complement-targeted therapies are available for SLE, but clinical trials are ongoing. Drugs in phase 2 trials for lupus nephritis include ravulizumab, an anti-C5 monoclonal antibody; iptacopan, a complement factor B inhibitor; vemircopan, a complement factor D inhibitor; narsoplimab, an anti-MBL-associated serine protease 2 monoclonal antibody; and pegcetacoplan, a PEGylated C3 inhibitor. In addition, KP104, a bifunctional biologic designed to simultaneously block both the alternative pathway (factor H) and the late complement pathway (C5), is in a phase 2 trial for thrombotic microangiopathies secondary to SLE.
3. Complement Tests
In routine clinical practice, serum C3 and C4 levels and CH50 are usually measured in blood samples, and complement deposition is identified by fluorescent antibody assay of tissues.
C3 and C4 are proteins of the complement cascade. In general, C3 and C4 are measured in serum, but samples such as urine, pleural fluid, and spinal fluid can also be used. In SLE, hypocomplementemia, decreased levels either of C3 and C4 or both occurs as an immunological abnormality (Table 1). C3 is included in the common cascade after the convergence of the three pathways. By contrast, C4 is included in the classical and lectin pathways. Therefore, low levels of both proteins indicate the activation of these two pathways, whereas low C3 and normal C4 indicate the activation of the alternative pathway.

Table 1.
Complement markers.
Plasma C4 levels are strongly influenced by and correlated with C4 gene copy numbers [15,16,17,18]. Each copy of the C4 gene encodes for one of two isotypes, acidic C4A or basic C4B. Congenital deficiency or a low copy number of the C4A gene has been associated with the development of SLE [19]. By contrast, a low C4B gene copy number did not show this association, and a medium to high C4B copy number was associated with thrombosis and hypertension in patients with pediatric SLE [15]. The profiles of C4 and C3 protein levels are different in each patient with SLE depending on the C4 gene copy number; therefore, there is a group of patients with low C4 levels due to low C4 gene copy number and without reflecting disease activity [16]. If only C4 shows persistently low levels, then the possibility of a low copy number of the C4 genes should be considered in the evaluation [16,17,18].
CH50 measures the hemolytic activity of serum samples against sensitized sheep erythrocytes. It is indexed by its ability to form membrane attack complexes via the classical pathway, and it reflects immune complex formation. It helps in complement-screening tests because it simultaneously measures all the complement activities from C1 to C9. In active SLE, CH50 levels are low because of complement consumption caused by the increased activation of the classical pathway (Table 1). If they are extremely low, then the possibility of a congenital defect is considered. In the case of low CH50 levels with normal C3 and C4 levels, it is important to exclude artificial in vitro complement activation (cold activation) after the blood sample is taken.
Complements are also used in the immunological testing of tissues. In SLE, reactions to C1q, C3, and C4 are observed in the renal glomeruli and at the dermo–epidermal border of the skin by fluorescent antibody assay. It is useful in evaluating affected organs and in the differentiation of other diseases.
Other novel biomarkers used include C1q, which is essential for the activation of the classical pathway; split product, which is a degraded product of complement; and cell-bound complement activation product (CB-CAP), a cell surface binding of C4d.
4. Traditional Complement Markers in SLE Diagnosis
4.1. SLE Diagnosis
The diagnosis of SLE has no gold standard; thus, it is based on classification criteria that reflect the variety of immunological abnormalities and diverse organ involvement characteristics of SLE. Although the SLE classification criteria have been revised in recent years, the currently used 2019 European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) classification criteria for SLE emphasizes immunological abnormalities and nephritis [20]. Consequently, complement, which was not included in the 1997 ACR classification criteria, was added to the list of immune domains in the current classification criteria, along with SLE-specific and antiphospholipid antibodies [20,21,22]. Hypocomplementemia is defined as a decrease in C3, C4, or CH50 based on the 2012 Systemic Lupus International Collaborating Clinics classification criteria, or low C3 and/or C4 based on the 2019 EULAR/ACR classification criteria [20,22]. A decrease in both C3 and C4, which suggests the involvement of the classical pathway, was considered as a more important finding, which was given a higher weight. Regarding the validation studies of the 2019 EULAR/ACR classification criteria, the frequency of hypocomplementemia (low C3 or C4) at diagnosis of SLE is 50%–89%, which is not commonly observed in all cases [23,24,25].
Among nephritis, class III or IV findings on renal biopsy are strongly suggestive of SLE, and in the new classification criteria, such findings can be classified as SLE along with immunological abnormalities [20]. Complement such as C1q, C3, and C4 deposition by immunofluorescence is not included in the definition of the pathologic classification of lupus nephritis [26]. It largely plays an ancillary role, but it is an important reference finding in differentiating lupus nephritis from other diseases [26,27]. SLE is characterized by C1q deposition because of the activation of the classical pathway and it often presents a “full-house pattern” with multiple deposits of immunoglobulins and complements. In addition to IgA nephropathy characterized by IgA deposition and anti-neutrophil cytoplasmic antibody-associated nephritis without immunoglobulin or complement deposition, C3 nephropathy with positive fluorescent findings of C3 deposition and negative or weak C1q, C4, and immunoglobulin deposition has recently been proposed [28]. Complement deposition in the fluorescent antibody assay is also useful in differentiating these renal diseases [29].
4.2. Diagnosis and Prediction of Organ Involvement
After the diagnosis of SLE, proper evaluation of the affected organs is important to provide optimal treatment. SLE presents with a variety of organ involvement, and it may be classified into several subgroups based on the organs affected and immunological abnormalities. Studies investigating the relationship between complements and organ involvement have shown the association of hypocomplementemia with nephritis, hematologic disorders such as autoimmune hemolytic anemia and thrombocytopenia, skin rash, and arthritis [30,31,32]. In a study of patients with SLE comparing low C4 levels alone with low levels of both C3 and C4, the frequency of serositis and hematologic disorders was lower, and the severity of renal and hematologic disorders was milder in the cases with low C4 levels alone [33].
The measurement of complements is also useful in appropriately ruling out other diseases to confirm whether organ involvement is caused by SLE. In a report in which renal biopsies were performed on 48 patients with SLE without abnormal urinalysis and renal impairment, 36 patients had lupus nephritis (class I or II, 72%; class III or IV, 17%; and type V, 11%), and hypocomplementemia (low titers of CH50 and C3) and a high titer of anti-Sm antibodies were identified as predictive factors for silent lupus nephritis. Complements measured in samples other than serum are used to differentiate other diseases [34]. Elevated levels of C3 in cerebrospinal fluid are useful in diagnosing neuropsychiatric SLE [35] and decreased levels of C3 and C4 in pleural fluid are useful in discriminating lupus pleuritis from pleural effusion of other etiologies [36].
5. Traditional Complement Markers in the Assessment of SLE Activity
In a treat-to-target strategy, SLE treatment is aimed at remission or, at least, low disease activity after regular evaluation of the overall disease activity [4]. Therefore, disease activity assessment indicators and treatment goals must be established.
In general, the assessment of disease activity in SLE is based on a combination of the type and severity of organ involvement and immunological abnormalities. Complements, along with anti-dsDNA antibody titers, are used in routine clinical practice as a biomarker for immunological abnormalities in SLE, and hypocomplementemia reflects the activity of SLE. The systemic lupus erythematosus disease activity index (SLEDAI), the validated activity index, frequently used for assessing global SLE activity, includes the presence of low complements, that is the decrease in CH50, C3, or C4 in qualitative assessment [37]. On the contrary, the British Isles Lupus Assessment Group index, which evaluates disease activity by each organ-based system, does not include immunological abnormalities [38]. In addition, the newly proposed SLE disease activity score includes swollen joint counts, proteinuria, leucocyte counts, and platelet counts as values in the score, but it does not include quantitative serological immune markers such as serum complement levels or anti-dsDNA antibody titers [39].
A composite definition is also used for low disease activity and remission, which are treatment targets of SLE. Although no criteria have been established, the lupus low disease activity state (LLDAS) is used as a general low disease activity criterion, and the definitions of remission in SLE (DORIS) is used as a remission criterion [40,41]. These composite indices combine the disease activity measured using the SLEDAI, the exclusion of any current disease activity not included in the SLEDAI, physician global assessment, and medication use, including glucocorticoid dosage. The definition of LLDAS allows for a SLEDAI-2K score of 4 or less. LLDAS may also be met, although hypocomplementemia and elevated anti-dsDNA antibodies remain unchanged. On the contrary, DORIS initially proposed complete remission, which requires no serological activities [42]. However, in the 2021 definition, remission will be evaluated by clinical SLEDAI-2K, which excludes immunological abnormalities from SLEDAI-2K, and serological activity is no longer an issue in the definition of remission [41]. Thus, whether treatment goals should include the normalization of immunological abnormalities remains debatable. Cases in which hypocomplementemia and elevated anti-dsDNA antibodies persist after achieving treatment goals such as low disease activity and remission are often observed. These cases of clinical remission with persistent immunological abnormalities have been recognized as serologically active clinically quiescent (SACQ) SLE [38]. The clinical significance of the SACQ status remains unclear, but a treat-to-target strategy and treatment recommendations do not recommend that the treatment in clinically asymptomatic patients is escalated primarily on the basis of stable or persistent serological activity [4,43,44].
Levels of complements must be evaluated regularly in routine clinical practice, along with symptoms, physical examination, and blood laboratory tests. Complement levels are not completely linked to SLE activity, as normal complement levels in the active phase and persistent hypocomplementemia in remission are often observed. SLE evaluation is based on global assessment; thus, levels of complements should not be overestimated but should be used as a guide for decision-making.
6. Traditional Complement Markers in Predicting SLE Treatment Response
In recent years, biologic drugs such as belimumab, an anti-B-cell activating factor (BAFF) monoclonal antibody, and anifrolumab, an anti-interferon-α receptor monoclonal antibody, have become available for SLE. Compared with glucocorticoids and conventional immunosuppressive drugs, which act on a relatively broad range of immune cells, biologic drugs have a clear therapeutic target, and the mechanism of action of the drug may predict treatment response. Belimumab is known to be more effective at high BAFF levels and anifrolumab at high interferon signatures [45,46]. However, these markers cannot be measured in routine clinical practice. Thus, markers predicting response to biologic drugs must be established within routine clinical practice. In this regard, low C3 levels and high anti-dsDNA antibody titers have been reported to be associated with high BAFF levels and interferon signatures [47,48]. In addition, belimumab and anifrolumab are more effective in patients with immunological abnormalities and are more likely to be effective in patients with high anti-dsDNA antibody titers, low C3 levels, and low C4 levels [46,49].
7. Traditional Complement Markers in Predicting SLE Prognosis
The prevention of flares is an important goal in the management of SLE. Early detection of signs of flares through careful monitoring of the global and organ-specific disease activity is essential, but understanding the risk factors that predict flares is also important. The 2019 update of the EULAR recommendations for the management of SLE identifies persistent serological activity (low complement and/or high anti-dsDNA antibodies) as risk factors, along with younger age at disease onset, no use of antimalarials, and persistent generalized disease activity [43].
In routine clinical practice, the assessment of the disease activity often considers the fluctuation in complement levels and anti-dsDNA antibody titers, but many clinical studies have reported the presence of hypocomplementemia at a single point in time, such as at the achievement of remission or at the beginning of observation. Gensous et al. and Kostopoulou et al. conducted a systematic literature review on whether complement predicts SLE flares and reported that many studies have shown that hypocomplementemia (low serum C3 and C4 levels) is a predictor of flares [50,51]. However, given the effects of differences in study methods, patient backgrounds, organ systems involved, and treatment, no firm opinion has been achieved. Regarding the persistent immunological abnormalities, patients with SACQ-SLE have a higher risk of flares than patients without immunological abnormalities [52,53,54], although few reports have focused on persistent hypocomplementemia. In addition, analyses focusing on fluctuations in complement levels have reported that progressive reduction in complement levels may indicate future flares in patients with SACQ-SLE [55].
Some reports have examined factors that predict the outcome for each organ involved rather than for SLE as a whole. In lupus nephritis, low serum C3 levels at diagnosis or at the time of remission may predict renal flares [56,57]. Patients with lupus nephritis and persistent isolated C3 hypocomplementemia were more likely to progress to end-stage kidney disease [58]. In patients with end-stage kidney disease, low serum C4 levels at the time of induction of renal replacement therapy were associated with SLE flares [59]. Severe neuropsychiatric manifestations and low serum C4 levels at the time of SLE diagnosis predict the development of severe neuropsychiatric flares [60]. A low serum C3 level could indicate complications of lupus enteritis [61,62], and a lower CH50 level at the time of initial treatment could predict an inadequate treatment response in patients with lupus enteritis [63]. Low serum C3 levels and high SLEDAI-2K scores were reported as independent risk factors for the development of lupus myocarditis in patients with SLE [64].
8. Novel Complement Biomarkers in SLE Management
8.1. C1q
C1q is essential for activating the classical pathway, and a decrease in C1q levels, as with C3 and C4 levels, indicates high disease activity in SLE (Table 1). Although the utility of C1q deposition in renal tissue and anti-C1q antibodies in the management of SLE is well established [65], serum C1q can also be a useful biomarker (Table 2). Serum C1q levels were decreased in patients with SLE compared with healthy controls, and they were associated with disease activity as measured by SLEDAI [66]. Serum C1q levels were also decreased in patients with lupus nephritis compared with healthy controls and patients with SLE uncomplicated by nephritis, indicating the activity of nephritis and the histological activity score of renal tissue [66,67,68].
8.2. Split Products
Split products are the cleavage fragments of the complement components. Known cleavage fragments of C3 include iC3b, C3dg, and C3d, and C4d is a cleavage fragment of C4 (Figure 1). The split products are only produced by complement activation, unaffected by increased production by the acute-phase response. Therefore, the assay is characterized by its ability to reflect complement activation in vivo more sensitively than the currently used C3 and C4.
iC3b is the breakdown product of C3b, a cleavage fragment of C3. Serum iC3b levels were elevated in patients with SLE compared with healthy controls, and the serum iC3b/C3 ratio was more sensitive to changes in disease activity than serum C3 or C4 levels, making it more useful for detecting active SLE and predicting flares [69]. Furthermore, iC3b is degraded to C3dg and C3d. Plasma C3dg levels were higher in patients with SLE than in healthy controls, and its discriminative power at the time of SLE diagnosis was superior to that of serum C3 [70]. Plasma and urine C3d levels were elevated in patients with active lupus nephritis and correlated with disease activity, and a decrease in values after treatment predicted subsequent treatment response [71].
C4d is a cleavage product of C4b produced from the degradation of C4. Plasma C4d levels were elevated in patients with SLE compared with healthy controls, and they were particularly high in patients with nephritis [72,73]. Plasma C4d levels correlated with C4d deposition in renal tissue, indicating nephritis activity, and they could be used in predicting flares and treatment response [73]. C5a is a cleavage product of C5. Recently, avacopan, a C5a antagonist, has proven to be effective in anti-neutrophil cytoplasmic antibody-associated vasculitis [74]. Plasma and urine C5a levels were elevated in patients with active lupus nephritis, and plasma C5a levels correlated with disease activity in lupus nephritis and SLE [75]. Bb is a cleavage product of factor B associated with the alternative pathway. Plasma Bb levels were elevated in patients with active lupus nephritis, indicating the activity score of the renal tissue and predicting poor renal prognosis [76].
Split products are considered superior biomarkers compared with serum C3 and C4. However, the stable measurement of such products is difficult because of their short half-life, and a simple and reliable measurement assay has not been established [8], although a novel, stable and simple assay using the iC3b/C3dg-binding site of human complement receptor 2 was reported [77].


Table 2.
Summary of reports on novel complement biomarkers.
Table 2.
Summary of reports on novel complement biomarkers.
| Reference | Author, Year | Sample | Number of Subjects | Key Findings |
|---|---|---|---|---|
| [66] | Sandholm, 2019 | Plasma C1q | 69 LN 310 SLE without LN 322 HC | SLE patients had lower levels of C1q than matched HCs (median, 225 vs. 266 mg/L, p < 0.001). An association was found between the levels of C1q and the SLEDAI. Patients with nephritis had lower levels of C1q than those without nephritis (median, 194 vs. 228 mg/L, p < 0.01). |
| [67] | Tan, 2013 | Serum C1q | 218 LN HC | The serum C1q levels were significantly lower in LN than those in HCs (33.8 ± 20.4 vs. 62.0 ± 10.5 μg/mL, p < 0.001). Patients with lower serum C1q levels (<40.97 μg/mL) showed significantly higher levels of SLEDAI (p < 0.001). The serum C1q levels were associated with renal total activity indices scores (rs = −0.327, p < 0.001). |
| [68] | Xu, 2019 | Serum C1q | 905 SLE without LN 334 active LN 255 inactive LN 497 HC | Significantly decreased C1q levels were observed in the active LN and inactive LN groups, which was in contrast to their levels in the SLE and HC groups (153.2 ± 40.0 vs. 170.8 ± 36.2 vs. 170.6 ± 35.5 vs. 182.3 ± 29.0 mg/L, p < 0.05). |
| [69] | Kim, 2019 | Blood iC3b | 159 SLE 48 HC | Patients with SLE had elevated iC3b levels as compared to HCs (4.5 ± 2.8 vs. 2.1 ± 0.9 μg/mL, p < 0.001). Patients with active SLE had elevated iC3b:C3 ratio as compared to patients with inactive SLE and HCs (7.0 ± 7.8 vs. 4.3 ± 2.0 vs. 1.7 ± 0.6 μg/mg, p < 0.001). The iC3b:C3 ratio correlated with the extent of SLE disease activity and with clinically meaningful changes in disease activity in patients with SLE. The iC3b:C3 ratio outperformed C3 and C4 levels with regard to discriminating active SLE from inactive SLE, and major flares from no disease activity. |
| [70] | Troldborg, 2018 | Plasma C3dg | 169 SLE 170 HC | SLE patients had higher concentrations in plasma C3dg than HCs (Data were presented only in figures, p < 0.001). ROC analysis showed that C3dg (AUC 0.96) was superior to C3 (AUC 0.52) in differentiating between patients and HCs. |
| [71] | Ganguly, 2020 | Plasma C3d Urinary C3d | 28 active LN 4 inactive LN 10 HC | Urine and plasma levels of C3d for active LN were significantly different from inactive LN (urine, median, 388 vs. 9.9 ng/mg Cr, p < 0.001; plasma, median, 791 vs. 212 μg/mL, p < 0.001). There was a significant correlation of plasma C3d with SLEDAI (rs = 0.67, p < 0.001) and renal SLEDAI (rs = 0.44, p = 0.03). There was a significant correlation of urine C3d with SLEDAI (rs = 0.433, p < 0.001) and renal SLEDAI (rs = 0.35, p < 0.001). Treatment responders at 6 months showed a significant fall in urine C3d at 3 months. |
| [72] | Martin, 2017 | Plasma C4d | 69 SLE 97 HC | C4d levels were negligible in HC subjects and significantly increased in patients with SLE (p < 0.001). C4d levels discriminated between higher and lower disease activity according to ROC curve analysis (AUC 0.64, p < 0.001). At higher disease activity, C4d levels correlated with the modified SLEDAI (rs = 0.26, p = 0.011) and predominantly with LN (p = 0.003). |
| [73] | Martin, 2020 | Plasma C4d | 22 SLE without LN 71 LN 145 HC | In comparison to HCs, plasma C4d levels were significantly increased in SLE patients (0.33 mg/L vs. 0.94 mg/mL, p < 0.001) with significantly higher levels in LN patients (1.02 mg/L) than in non-renal SLE patients (0.57 mg/L, p = 0.004). C4d levels correlated significantly with urine-albumin to creatinine ratio (rs = 0.43, p = 0.011), renal activity index (rs = 0.37, p = 0.002) and glomerular deposits of C4d in kidney biopsies (rs = 0.7, p = 0.0002). Plasma C4d declined significantly after treatment in patients that experienced favourable clinical and histopathological response (p < 0.0001), while levels remained mainly unchanged in non-responders. |
| [75] | Ma, 2018 | Plasma C5a Urinary C5a | 66 SLE 40 HC | Plasma C5a levels were dramatically elevated in patients with active LN compared to those in remission and HCs. Urinary C5a were significantly elevated in LN patients compared to HCs (p < 0.001). Correlation analysis showed that the plasma C5a levels were positively correlated with 24h proteinuria in LN patients (rs = 0.47, p = 0.002) and SLEDAI scores in SLE patients (rs = 0.28, p = 0.02). |
| [76] | Song, 2017 | Plasma Bb, C1q, C4d, and C5a | 82 SLE without LN 222 active LN 34 LN in remission 39 HC | Plasma Bb levels were significantly higher in patients with active LN compared with patients in remission, active SLE without LN, and HCs (1.24 ± 0.75 vs. 0.78 ± 0.45 vs. 0.90 ± 0.49 vs. 0.69 ± 0.45 μg/mL, p < 0.001). Plasma Bb level was significantly correlated with some renal disease activity indices and was a risk factor for renal outcomes (Hazard ratio = 1.75; 95% confidence interval = 1.106−2.754; p = 0.017) in the LN group. |
LN, lupus nephritis; SLE, systemic lupus erythematosus; HC, healty control; SLEDAI, SLE disease activity index; ROC, receiver operating characteristic; AUC, area under the curve.
8.3. Cell-Bound Complement Activation Product (CB-CAP)
C4d is not only present in plasma, but it is also bound to the surface of blood cells. It is known as CB-CAP, which binds to erythrocytes, platelets, and B cells. C4d on each cell surface is measured using a flow cytometer. In 2004, erythrocyte C4d was measured and reported to be a biomarker with better sensitivity and specificity than traditional complement markers for the diagnosis of SLE [78]. It was later shown to be measurable on platelets, T cells, and B cells [79,80]. In the diagnosis of SLE, erythrocyte C4d and B-lymphocyte C4d could be used to observe elevated C4d on the surface of each cell, even in cases negative for anti-dsDNA antibodies [81]. Thus, a multianalyte assay panel, commercially known as the AVISE Lupus test (Exagen Inc., Vista, CA, USA), which combines erythrocyte C4d and B-lymphocyte C4d with eight lupus and non-lupus autoantibodies, has recently been used in distinguishing SLE from a variety of other rheumatic diseases [82,83]. In monitoring SLE activity, C4d on each cell surface was more sensitive and specific than serum C3 and C4, which acutely reflected activity even in cases with normal or no fluctuation in serum C3 and C4 levels [84,85]. In addition, platelet C4d was a useful marker for predicting thrombosis and vascular events [86,87,88].
C4d also deposits in renal tissue. Strong C4d deposition was consistently present in immune-complex glomerulonephritis, including lupus nephritis [89], and C4d deposition in renal peritubular capillaries is an important finding in the diagnosis of antibody-mediated rejection in kidney transplantation [90]. C4d deposition was common in the renal tissues of patients with lupus nephritis [91,92]. These deposits were localized in the glomeruli in almost all cases, followed by tubular basement membrane, arterioles, and peritubular capillaries [92]. Tubular basement membrane C4d deposition was related to the disease activity, and arteriolar C4d deposition was associated with worse renal outcomes [92].
9. Conclusions
This study summarized the usefulness of complement as a biomarker in the SLE clinical practice. Serum C3 and C4 levels, which are frequently used in routine clinical practice, are included in the classification criteria for SLE and disease activity indices. They are also considered useful SLE biomarkers. However, they are not absolute indicators that can be determined by complement alone. Therefore, the characteristics and limitations of the complement test must be fully understood and comprehensively evaluated in conjunction with clinical signs and anti-dsDNA antibody titers. The complement split products and CB-CAP might reflect SLE activity more sensitively than traditional complement markers and are expected to become more widely used.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update in the Diagnosis and Management of Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef]
- Pan, L.; Lu, M.P.; Wang, J.H.; Xu, M.; Yang, S.R. Immunological Pathogenesis and Treatment of Systemic Lupus Erythematosus. World J. Pediatr. 2020, 16, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Dörner, T.; Furie, R. Novel Paradigms in Systemic Lupus Erythematosus. Lancet 2019, 393, 2344–2358. [Google Scholar] [CrossRef] [PubMed]
- van Vollenhoven, R.F.; Mosca, M.; Bertsias, G.; Isenberg, D.; Kuhn, A.; Lerstrøm, K.; Aringer, M.; Bootsma, H.; Boumpas, D.; Bruce, I.N.; et al. Treat-to-Target in Systemic Lupus Erythematosus: Recommendations from an International Task Force. Ann. Rheum. Dis. 2014, 73, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, R.; Puxeddu, I.; Pratesi, F.; Migliorini, P. New Biomarkers in SLE: From Bench to Bedside. Rheumatology 2020, 59, V12–V18. [Google Scholar] [CrossRef]
- González, L.A.; Ugarte-Gil, M.F.; Alarcón, G.S. Systemic Lupus Erythematosus: The Search for the Ideal Biomarker. Lupus 2021, 30, 181–203. [Google Scholar] [CrossRef]
- Yu, H.; Nagafuchi, Y.; Fujio, K. Clinical and Immunological Biomarkers for Systemic Lupus Erythematosus. Biomolecules 2021, 11, 928. [Google Scholar] [CrossRef]
- Weinstein, A.; Alexander, R.V.; Zack, D.J. A Review of Complement Activation in SLE. Curr. Rheumatol. Rep. 2021, 23, 4–11. [Google Scholar] [CrossRef]
- Leffler, J.; Bengtsson, A.A.; Blom, A.M. The Complement System in Systemic Lupus Erythematosus: An Update. Ann. Rheum. Dis. 2014, 73, 1601–1606. [Google Scholar] [CrossRef]
- Sharma, M.; Vignesh, P.; Tiewsoh, K.; Rawat, A. Revisiting the Complement System in Systemic Lupus Erythematosus. Expert Rev. Clin. Immunol. 2020, 16, 397–408. [Google Scholar] [CrossRef]
- Carroll, M.C. The Lupus Paradox. Nat. Genet. 1998, 19, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.C.L.; Isaac, L. Systemic Lupus Erythematosus and Deficiencies of Early Components of the Complement Classical Pathway. Front. Immunol. 2016, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Felten, R.; Scherlinger, M.; Mertz, P.; Chasset, F.; Arnaud, L. New Biologics and Targeted Therapies in Systemic Lupus: From New Molecular Targets to New Indications. A Systematic Review. Jt. Bone Spine 2023, 90, 105523. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.M.; Yung, S.; Yap, D.Y.H. A Review of Advances in the Understanding of Lupus Nephritis Pathogenesis as a Basis for Emerging Therapies. F1000Research 2020, 9, 905. [Google Scholar] [CrossRef]
- Mulvihill, E.; Ardoin, S.; Thompson, S.D.; Zhou, B.; Yu, G.R.; King, E.; Singer, N.; Levy, D.M.; Brunner, H.; Wu, Y.L.; et al. Elevated Serum Complement Levels and Higher Gene Copy Number of Complement C4B Are Associated with Hypertension and Effective Response to Statin Therapy in Childhood-Onset Systemic Lupus Erythematosus (SLE). Lupus Sci. Med. 2019, 6, e000333. [Google Scholar] [CrossRef]
- Wu, Y.L.; Higgins, G.C.; Rennebohm, R.M.; Chung, E.K.; Yang, Y.; Zhou, B.; Nagaraja, H.N.; Birmingham, D.J.; Rovin, B.H.; Hebert, L.A.; et al. Three distinct profiles of serum complement C4 proteins in pediatric systemic lupus erythematosus (SLE) patients: Tight associations of complement C4 and C3 protein levels in SLE but not in healthy subjects. Adv. Exp. Med. Biol. 2006, 586, 227–247. [Google Scholar] [CrossRef]
- Margery-Muir, A.A.; Bundell, C.; Wetherall, J.D.; Whidborne, R.; Martinez, P.; Groth, D.M. Insights on the Relationship between Complement Component C4 Serum Concentrations and C4 Gene Copy Numbers in a Western Australian Systemic Lupus Erythematosus Cohort. Lupus 2018, 27, 1687–1696. [Google Scholar] [CrossRef]
- Lundtoft, C.; Pucholt, P.; Martin, M.; Bianchi, M.; Lundström, E.; Eloranta, M.L.; Sandling, J.K.; Sjöwall, C.; Jönsen, A.; Gunnarsson, I.; et al. Complement C4 Copy Number Variation Is Linked to SSA/Ro and SSB/La Autoantibodies in Systemic Inflammatory Autoimmune Diseases. Arthritis Rheumatol. 2022, 74, 1440–1450. [Google Scholar] [CrossRef]
- Savelli, S.L.; Roubey, R.A.S.; Kitzmiller, K.J.; Zhou, D.; Nagaraja, H.N.; Mulvihill, E.; Barbar-Smiley, F.; Ardoin, S.P.; Wu, Y.L.; Yu, C.Y. Opposite Profiles of Complement in Antiphospholipid Syndrome (APS) and Systemic Lupus Erythematosus (SLE) among Patients with Antiphospholipid Antibodies (APL). Front. Immunol. 2019, 10, 885. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Orbai, A.M.; Alarcõn, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and Validation of the Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Pons-Estel, G.J.; Ugarte-Gil, M.F.; Harvey, G.B.; Wojdyla, D.; Quintana, R.; Saurit, V.; Soriano, E.R.; Bonfa, E.; Massardo, L.; Cardiel, M.; et al. Applying the 2019 EULAR/ACR Lupus Criteria to Patients from an Established Cohort: A Latin American Perspective. RMD Open 2020, 6, e001097. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Brinks, R.; Costenbader, K.H.; Daikh, D.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. Performance of the 2019 EULAR/ACR Classification Criteria for Systemic Lupus Erythematosus in Early Disease, across Sexes and Ethnicities. Ann. Rheum. Dis. 2020, 79, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.K.; Ho, L.Y.; Lee, C.; To, C.H.; Mok, C.C. Validation of the 2019 EULAR/ACR Classification Criteria for Systemic Lupus Erythematosus in ANA-Positive Chinese Patients. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221100300. [Google Scholar] [CrossRef] [PubMed]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society Classification for Lupus Nephritis: Clarification of Definitions, and Modified National Institutes of Health Activity and Chronicity Indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. Kidney Int. 2004, 65, 521–530. [Google Scholar] [CrossRef]
- Pickering, M.C.; D’agati, V.D.; Nester, C.M.; Smith, R.J.; Haas, M.; Appel, G.B.; Alpers, C.E.; Bajema, I.M.; Bedrosian, C.; Braun, M.; et al. C3 Glomerulopathy: Consensus Report. Kidney Int. 2013, 84, 1079–1089. [Google Scholar] [CrossRef]
- Sethi, S.; Haas, M.; Markowitz, G.S.; D’Agati, V.D.; Rennke, H.G.; Jennette, J.C.; Bajema, I.M.; Alpers, C.E.; Chang, A.; Cornell, L.D.; et al. Mayo Clinic/Renal Pathology Society Consensus Report on Pathologic Classification, Diagnosis, and Reporting of GN. J. Am. Soc. Nephrol. 2016, 27, 1278–1287. [Google Scholar] [CrossRef]
- Reynolds, J.A.; McCarthy, E.M.; Haque, S.; Ngamjanyaporn, P.; Sergeant, J.C.; Lee, E.; Lee, E.; Kilfeather, S.A.; Parker, B.; Bruce, I.N. Cytokine Profiling in Active and Quiescent SLE Reveals Distinct Patient Subpopulations. Arthritis Res. Ther. 2018, 9, 173. [Google Scholar] [CrossRef]
- Iwasaki, T.; Doi, H.; Tsuji, H.; Tabuchi, Y.; Hashimoto, M.; Kitagori, K.; Akizuki, S.; Murakami, K.; Nakashima, R.; Yoshifuji, H.; et al. Phenotypic Landscape of Systemic Lupus Erythematosus: An Analysis of the Kyoto Lupus Cohort. Mod. Rheumatol. 2022, 32, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, H.Y.; Lee, E.; Kim, H.A.; Yoon, D.; Suh, C.H. Three Clinical Clusters Identified through Hierarchical Cluster Analysis Using Initial Laboratory Findings in Korean Patients with Systemic Lupus Erythematosus. J. Clin. Med. 2022, 11, 2406. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Xiao, Z.; Xu, J.; Hong, G.; Xu, X.; Zhang, Y.; Zhang, X. The Clinical Characteristics of Low C4 Alone in Patients with Systemic Lupus Erythematosus. Clin. Rheumatol. 2021, 40, 793–796. [Google Scholar] [CrossRef]
- Ishizaki, J.; Saito, K.; Nawata, M.; Mizuno, Y.; Tokunaga, M.; Sawamukai, N.; Tamura, M.; Hirata, S.; Yamaoka, K.; Hasegawa, H.; et al. Low Complements and High Titre of Anti-Sm Antibody as Predictors of Histopathologically Proven Silent Lupus Nephritis without Abnormal Urinalysis in Patients with Systemic Lupus Erythematosus. Rheumatology 2015, 54, 405–412. [Google Scholar] [CrossRef]
- Asano, T.; Ito, H.; Kariya, Y.; Hoshi, K.; Yoshihara, A.; Ugawa, Y.; Sekine, H.; Hirohata, S.; Yamaguchi, Y.; Sato, S.; et al. Evaluation of Blood-Brain Barrier Function by Quotient Alpha2 Macroglobulin and Its Relationship with Interleukin-6 and Complement Component 3 Levels in Neuropsychiatric Systemic Lupus Erythematosus. PLoS ONE 2017, 12, e0186414. [Google Scholar] [CrossRef]
- Chen, D.Y.; Huang, Y.H.; Chen, Y.M.; Chen, J.J.W.; Yang, T.Y.; Chang, G.C.; Tang, K.T. ANA Positivity and Complement Level in Pleural Fluid Are Potential Diagnostic Markers in Discriminating Lupus Pleuritis from Pleural Effusion of Other Aetiologies. Lupus Sci. Med. 2021, 8, e000562. [Google Scholar] [CrossRef]
- Touma, Z.; Urowitz, M.B.; Gladman, D.D. Systemic Lupus Erythematosus Disease Activity Index 2000 Responder Index-50 Website. J. Rheumatol. 2013, 40, 733. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, D.A.; Rahman, A.; Allen, E.; Farewell, V.; Akil, M.; Bruce, I.N.; D’Cruz, D.; Griffiths, B.; Khamashta, M.; Maddison, P.; et al. BILAG 2004. Development and Initial Validation of an Updated Version of the British Isles Lupus Assessment Group’s Disease Activity Index for Patients with Systemic Lupus Erythematosus. Rheumatology 2005, 44, 902–906. [Google Scholar] [CrossRef]
- Jesus, D.; Matos, A.; Henriques, C.; Zen, M.; Larosa, M.; Iaccarino, L.; Da Silva, J.A.P.; Doria, A.; Inês, L.S. Derivation and Validation of the SLE Disease Activity Score (SLE-DAS): A New SLE Continuous Measure with High Sensitivity for Changes in Disease Activity. Ann. Rheum. Dis. 2018, 2000, 365–371. [Google Scholar] [CrossRef]
- Franklyn, K.; Lau, C.S.; Navarra, S.V.; Louthrenoo, W.; Lateef, A.; Hamijoyo, L.; Wahono, C.S.; Chen, S.L.; Jin, O.; Morton, S.; et al. Definition and Initial Validation of a Lupus Low Disease Activity State (LLDAS). Ann. Rheum. Dis. 2016, 75, 1615–1621. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Bertsias, G.; Doria, A.; Isenberg, D.; Morand, E.; Petri, M.A.; Pons-Estel, B.A.; Rahman, A.; Ugarte-Gil, M.F.; Voskuyl, A.; et al. 2021 DORIS Definition of Remission in SLE: Final Recommendations from an International Task Force. Lupus Sci. Med. 2021, 8, e000538. [Google Scholar] [CrossRef] [PubMed]
- van Vollenhoven, R.F.; Voskuyl, A.; Bertsias, G.; Aranow, C.; Aringer, M.; Arnaud, L.; Askanase, A.; Balážová, P.; Bonfa, E.; Bootsma, H.; et al. A Framework for Remission in SLE: Consensus Findings from a Large International Task Force on Definitions of Remission in SLE (DORIS). Ann. Rheum. Dis. 2017, 76, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 Update of the EULAR Recommendations for the Management of Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C.; Hamijoyo, L.; Kasitanon, N.; Chen, D.Y.; Chen, S.; Yamaoka, K.; Oku, K.; Li, M.T.; Zamora, L.; Bae, S.C.; et al. The Asia-Pacific League of Associations for Rheumatology Consensus Statements on the Management of Systemic Lupus Erythematosus. Lancet Rheumatol. 2021, 3, e517–e531. [Google Scholar] [CrossRef]
- Wilkinson, C.; Henderson, R.B.; Jones-Leone, A.R.; Flint, S.M.; Lennon, M.; Levy, R.A.; Ji, B.; Bass, D.L.; Roth, D. The Role of Baseline BLyS Levels and Type 1 Interferon-Inducible Gene Signature Status in Determining Belimumab Response in Systemic Lupus Erythematosus: A Post Hoc Meta-Analysis. Arthritis Res. Ther. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Vital, E.M.; Merrill, J.T.; Morand, E.F.; Furie, R.A.; Bruce, I.N.; Tanaka, Y.; Manzi, S.; Kalunian, K.C.; Kalyani, R.N.; Streicher, K.; et al. Anifrolumab Efficacy and Safety by Type I Interferon Gene Signature and Clinical Subgroups in Patients with SLE: Post Hoc Analysis of Pooled Data from Two Phase III Trials. Ann. Rheum. Dis. 2022, 81, 951–961. [Google Scholar] [CrossRef]
- Roth, D.A.; Thompson, A.; Tang, Y.; Hammer, A.E.; Molta, C.T.; Gordon, D. Elevated BLyS Levels in Patients with Systemic Lupus Erythematosus: Associated Factors and Responses to Belimumab. Lupus 2016, 25, 346–354. [Google Scholar] [CrossRef]
- Hubbard, E.L.; Pisetsky, D.S.; Lipsky, P.E. Anti-RNP Antibodies Are Associated with the Interferon Gene Signature but Not Decreased Complement Levels in SLE. Ann. Rheum. Dis. 2022, 81, 632–643. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, J.; Su, Y.; Li, Y.; Li, X.; Xiong, C.; Cao, H.; Quasny, H.; Chu, M.; Curtis, P.; et al. Efficacy of Belimumab in Patients with Systemic Lupus Erythematosus from North East Asia: Results of Exploratory Subgroup Analyses. Mod. Rheumatol. 2022, roac076. [Google Scholar] [CrossRef]
- Gensous, N.; Marti, A.; Barnetche, T.; Blanco, P.; Lazaro, E.; Seneschal, J.; Truchetet, M.E.; Duffau, P.; Richez, C. Predictive Biological Markers of Systemic Lupus Erythematosus Flares: A Systematic Literature Review. Arthritis Res. Ther. 2017, 19, 238. [Google Scholar] [CrossRef]
- Kostopoulou, M.; Ugarte-Gil, M.F.; Pons-Estel, B.; van Vollenhoven, R.F.; Bertsias, G. The Association between Lupus Serology and Disease Outcomes: A Systematic Literature Review to Inform the Treat-to-Target Approach in Systemic Lupus Erythematosus. Lupus 2022, 31, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Steiman, A.J.; Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Prolonged Serologically Active Clinically Quiescent Systemic Lupus Erythematosus: Frequency and Outcome. J. Rheumatol. 2010, 37, 1822–1827. [Google Scholar] [CrossRef]
- Conti, F.; Ceccarelli, F.; Perricone, C.; Miranda, F.; Truglia, S.; Massaro, L.; Pacucci, V.A.; Conti, V.; Bartosiewicz, I.; Spinelli, F.R.; et al. Flare, Persistently Active Disease, and Serologically Active Clinically Quiescent Disease in Systemic Lupus Erythematosus: A 2-Year Follow-Up Study. PLoS ONE 2012, 7, e45934. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Mu, L.; Zhang, Z.; Gao, D.; Hao, Y.; Zhou, W. Treatments and Outcomes in Chinese Patients with Serologically Active Clinically Quiescent Systemic Lupus Erythematosus: A Retrospective Observational Study. Arthritis Res. Ther. 2021, 23, 275. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, Y.; Sada, K.; Asano, Y.; Hayashi, K.; Yamamura, Y.; Hiramatsu, S.; Ohashi, K.; Morishita, M.; Watanabe, H.; Matsumoto, Y.; et al. Progressive Reduction of Serum Complement Levels: A Risk Factor for Relapse in Patients with Hypocomplementemia in Systemic Lupus Erythematosus. Lupus 2018, 27, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, G.; Arends, S.; Stegeman, C.A.; De Leeuw, K. Predictors of Renal Flares and Long-Term Renal Outcome in Patients with Lupus Nephritis: Results from Daily Clinical Practice. Clin. Exp. Rheumatol. 2022, 40, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, S.; Chen, T.; Liang, D.; Yang, J.; Zeng, C.; Li, X.; Xie, G.; Liu, Z. Machine Learning for Prediction and Risk Stratification of Lupus Nephritis Renal Flare. Am. J. Nephrol. 2021, 52, 152–160. [Google Scholar] [CrossRef]
- Rossi, G.M.; Maggiore, U.; Peyronel, F.; Fenaroli, P.; Delsante, M.; Benigno, G.D.; Gianfreda, D.; Urban, M.L.; Manna, Z.; Arend, L.J.; et al. Persistent Isolated C3 Hypocomplementemia as a Strong Predictor of End-Stage Kidney Disease in Lupus Nephritis. Kidney Int. Rep. 2022, 7, 2647–2656. [Google Scholar] [CrossRef]
- Barrera-Vargas, A.; Quintanar-Martínez, M.; Merayo-Chalico, J.; Alcocer-Varela, J.; Gómez-Martín, D. Risk Factors for Systemic Lupus Erythematosus Flares in Patients with End-Stage Renal Disease: A Case-Control Study. Rheumatology 2016, 55, 429–435. [Google Scholar] [CrossRef]
- Aso, K.; Kono, M.; Kono, M.; Watanabe, T.; Shimizu, Y.; Ogata, Y.; Fujieda, Y.; Kato, M.; Oku, K.; Amengual, O.; et al. Low C4 as a Risk Factor for Severe Neuropsychiatric Flare in Patients with Systemic Lupus Erythematosus. Lupus 2020, 29, 1238–1247. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, G.; Lin, J.; Lin, Q.; Zheng, K.; Hu, S.; Zheng, S.; Du, G.; Matucci-Cerinic, M.; Furst, D.E.; et al. Predictive Model of Risk and Severity of Enteritis in Systemic Lupus Erythematosus. Lupus 2022, 31, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, Q.; Luo, M.; Gou, Y.; Jiang, D.; Zheng, X.; Yan, G.; He, F. Clinical Features of Lupus Enteritis: A Single-Center Retrospective Study. Orphanet J. Rare Dis. 2021, 16, 396. [Google Scholar] [CrossRef]
- Yoshida, Y.; Omoto, T.; Kohno, H.; Tokunaga, T.; Kuranobu, T.; Yukawa, K.; Watanabe, H.; Oi, K.; Sugimoto, T.; Mokuda, S.; et al. Lower CH50 as a Predictor for Intractable or Recurrent Lupus Enteritis: A Retrospective Observational Study. Mod. Rheumatol. 2021, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Zhao, L.; Liu, Y.; Wang, Y.; Tian, X.; Zhang, W.; Li, M.; Zhao, Y.; Leng, X.; et al. Clinical Characteristics and Outcomes of Lupus Myocarditis: A Retrospective Case Control Study in Chinese Patients. Clin. Exp. Rheumatol. 2022, 40, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, M. Autoantibodies against Complement Component C1q in Systemic Lupus Erythematosus. Clin. Transl. Immunol. 2021, 10, e1279. [Google Scholar] [CrossRef]
- Sandholm, K.; Persson, B.; Skattum, L.; Eggertsen, G.; Nyman, D.; Gunnarsson, I.; Svenungson, E.; Nilsson, B.; Ekdahl, K.N. Evaluation of a Novel Immunoassay for Quantification of C1q for Clinical Diagnostic Use. Front. Immunol. 2019, 10, 7. [Google Scholar] [CrossRef]
- Tan, Y.; Song, D.; Wu, L.H.; Yu, F.; Zhao, M.H. Serum Levels and Renal Deposition of C1q Complement Component and Its Antibodies Reflect Disease Activity of Lupus Nephritis. BMC Nephrol. 2013, 14, 63. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Y.M.; Yang, Y.W.; Liu, Y.S.; Feng, J.F. Diagnostic Performance of Serum Cystatin C and Complement Component 1q in Lupus Nephritis. Arthritis Res. Ther. 2019, 21, 267. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.J.; Strand, V.; Sen, D.P.; Fu, Q.; Mathis, N.L.; Schmidt, M.J.; Bruchas, R.R.; Staten, N.R.; Olson, P.K.; Stiening, C.M.; et al. Association of Blood Concentrations of Complement Split Product IC3b and Serum C3 with Systemic Lupus Erythematosus Disease Activity. Arthritis Rheumatol. 2019, 71, 420–430. [Google Scholar] [CrossRef]
- Troldborg, A.; Jensen, L.; Deleuran, B.; Stengaard-Pedersen, K.; Thiel, S.; Jensenius, J.C. The C3dg Fragment of Complement Is Superior to Conventional C3 as a Diagnostic Biomarker in Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 581. [Google Scholar] [CrossRef]
- Ganguly, S.; Majumder, S.; Kumar, S.; Gupta, R.; Muhammed, H.; Shobha, V.; Aggarwal, A.; Misra, R. Urinary C3d Is Elevated in Patients with Active Lupus Nephritis and a Fall in Its Level after 3 Months Predicts Response at 6 Months on Follow Up. Lupus 2020, 29, 1800–1806. [Google Scholar] [CrossRef]
- Martin, M.; Smolag, K.I.; Björk, A.; Gullstrand, B.; Okrój, M.; Leffler, J.; Jönsen, A.; Bengtsson, A.A.; Blom, A.M. Plasma C4d as Marker for Lupus Nephritis in Systemic Lupus Erythematosus. Arthritis Res. Ther. 2017, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Trattner, R.; Nilsson, S.C.; Björk, A.; Zickert, A.; Blom, A.M.; Gunnarsson, I. Plasma C4d Correlates with C4d Deposition in Kidneys and With Treatment Response in Lupus Nephritis Patients. Front. Immunol. 2020, 11, 582737. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, Y.; Horiuchi, T. The Complement System and ANCA Associated Vasculitis in the Era of Anti-Complement Drugs. Front. Immunol. 2022, 13, 926044. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, C.; Shi, B.; Zhang, Z.; Feng, R.; Guo, M.; Lu, L.; Shi, S.; Gao, X.; Chen, W.; et al. Mesenchymal Stem Cells Control Complement C5 Activation by Factor H in Lupus Nephritis. EBioMedicine 2018, 32, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Guo, W.Y.; Wang, F.M.; Li, Y.Z.; Song, Y.; Yu, F.; Zhao, M.H. Complement Alternative Pathway’s Activation in Patients with Lupus Nephritis. Am. J. Med. Sci. 2017, 353, 247–257. [Google Scholar] [CrossRef]
- Halkjær, L.; Troldborg, A.; Pedersen, H.; Jensen, L.; Hansen, A.G.; Hansen, T.K.; Bjerre, M.; Østergaard, J.A.; Thiel, S. Complement Receptor 2 Based Immunoassay Measuring Activation of the Complement System at C3-Level in Plasma Samples From Mice and Humans. Front. Immunol. 2020, 11, 774. [Google Scholar] [CrossRef]
- Manzi, S.; Navratil, J.S.; Ruffing, M.J.; Liu, C.C.; Danchenko, N.; Nilson, S.E.; Krishnaswami, S.; King, D.E.S.; Kao, A.H.; Ahearn, J.M. Measurement of Erythrocyte C4d and Complement Receptor 1 in Systemic Lupus Erythematosus. Arthritis Rheum. 2004, 50, 3596–3604. [Google Scholar] [CrossRef]
- Navratil, J.S.; Manzi, S.; Kao, A.H.; Krishnaswami, S.; Liu, C.C.; Ruffing, M.J.; Shaw, P.S.; Nilson, A.C.; Dryden, E.R.; Johnson, J.J.; et al. Platelet C4d Is Highly Specific for Systemic Lupus Erythematosus. Arthritis Rheum. 2006, 54, 670–674. [Google Scholar] [CrossRef]
- Liu, C.C.; Kao, A.H.; Hawkins, D.M.; Manzi, S.; Sattar, A.; Wilson, N.; Ahearn, J.M. Lymphocyte-Bound Complement Activation Products as Biomarkers for Diagnosis of Systemic Lupus Erythematosus. Clin. Transl. Sci. 2009, 2, 300–308. [Google Scholar] [CrossRef]
- Kalunian, K.C.; Chatham, W.W.; Massarotti, E.M.; Reyes-Thomas, J.; Harris, C.; Furie, R.A.; Chitkara, P.; Putterman, C.; Gross, R.L.; Somers, E.C.; et al. Measurement of Cell-Bound Complement Activation Products Enhances Diagnostic Performance in Systemic Lupus Erythematosus. Arthritis Rheum. 2012, 64, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Mossell, J.; Goldman, J.A.; Barken, D.; Alexander, R.V. The Avise Lupus Test and Cell-Bound Complement Activation Products Aid the Diagnosis of Systemic Lupus Erythematosus. Open Rheumatol. J. 2016, 10, 71–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, E.; Taylor, M.; McMahon, M. Utility of the AVISE Connective Tissue Disease Test in Predicting Lupus Diagnosis and Progression. Lupus Sci. Med. 2020, 7, e000345. [Google Scholar] [CrossRef] [PubMed]
- Arriens, C.; Alexander, R.V.; Narain, S.; Saxena, A.; Collins, C.E.; Wallace, D.J.; Massarotti, E.; Conklin, J.; Kalunian, K.C.; Putterman, C.; et al. Cell-Bound Complement Activation Products Associate with Lupus Severity in SLE. Lupus Sci. Med. 2020, 7, e000377. [Google Scholar] [CrossRef]
- Merrill, J.T.; Petri, M.A.; Buyon, J.; Ramsey-Goldman, R.; Kalunian, K.; Putterman, C.; Conklin, J.; Furie, R.A.; Dervieux, T. Erythrocyte-Bound C4d in Combination with Complement and Autoantibody Status for the Monitoring of SLE. Lupus Sci. Med. 2018, 5, e000263. [Google Scholar] [CrossRef]
- Svenungsson, E.; Gustafsson, J.T.; Grosso, G.; Rossides, M.; Gunnarsson, I.; Jensen-Urstad, K.; Larsson, A.; Ekdahl, K.N.; Nilsson, B.; Bengtsson, A.A.; et al. Complement Deposition, C4d, on Platelets Is Associated with Vascular Events in Systemic Lupus Erythematosus. Rheumatology 2020, 59, 3264–3274. [Google Scholar] [CrossRef]
- Gartshteyn, Y.; Mor, A.; Shimbo, D.; Khalili, L.; Kapoor, T.; Geraldino-Pardilla, L.; Alexander, R.V.; Conklin, J.; Dervieux, T.; Askanase, A.D. Platelet Bound Complement Split Product (PC4d) Is a Marker of Platelet Activation and Arterial Vascular Events in Systemic Lupus Erythematosus. Clin. Immunol. 2021, 228, 108755. [Google Scholar] [CrossRef]
- Petri, M.A.; Conklin, J.; O’Malley, T.; Dervieux, T. Platelet-Bound C4d, Low C3 and Lupus Anticoagulant Associate with Thrombosis in SLE. Lupus Sci. Med. 2019, 6, 6–11. [Google Scholar] [CrossRef]
- Drachenberg, C.B.; Papadimitriou, J.C.; Chandra, P.; Haririan, A.; Mendley, S.; Weir, M.R.; Rubin, M.F. Epidemiology and Pathophysiology of Glomerular C4d Staining in Native Kidney Biopsies. Kidney Int. Rep. 2019, 4, 1555–1567. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and Clarification of Criteria for T Cell– and Antibody-Mediated Rejection. Am. J. Transplant. 2020, 20, 2318–2331. [Google Scholar] [CrossRef]
- Allam, M.; Fathy, H.; Allah, D.A.; Salem, M.A.E. Lupus Nephritis: Correlation of Immunohistochemical Expression of C4d, CD163-Positive M2c-like Macrophages and Foxp3-Expressing Regulatory T Cells with Disease Activity and Chronicity. Lupus 2020, 29, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yu, X.; Wu, L.; Tan, Y.; Qu, Z.; Yu, F. The Spectrum of C4d Deposition in Renal Biopsies of Lupus Nephritis Patients. Front. Immunol. 2021, 12, 654652. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).