Exploring Genetic Interactions with Telomere Protection Gene pot1 in Fission Yeast
Abstract
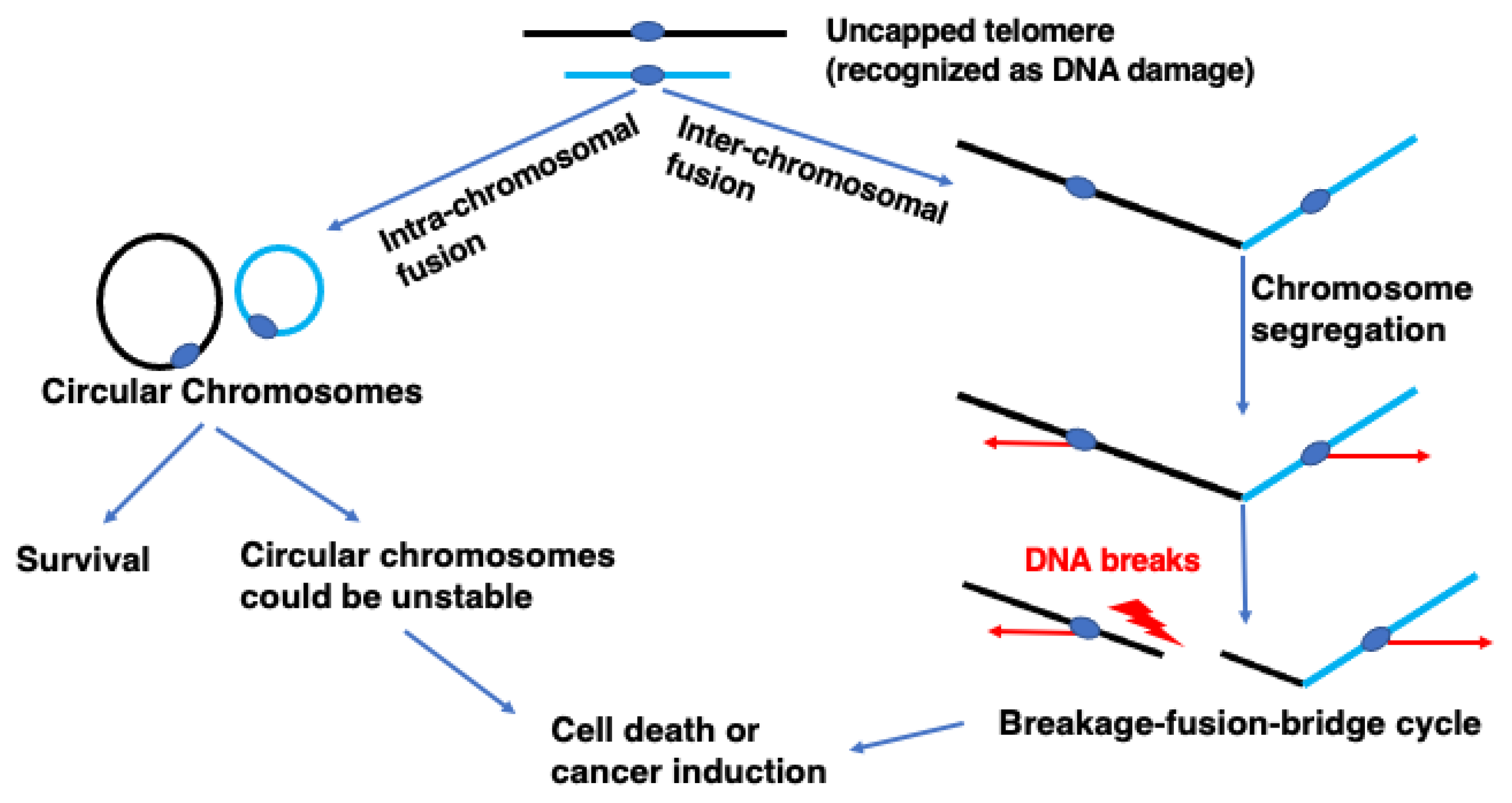
:1. Introduction
2. Telomere Protection by Pot1 in Human and in Fission Yeast Schizosaccharomyces pombe, and by Cdc13 in Budding Yeast Saccharomyces cerevisiae
3. Lethality of pot1 rqh1 Double Mutant Is Suppressed by Inactivation of Homologous Recombination
4. pot1 rqh1-hd Cells Maintain Telomeres by HR and Accumulate Recombination Intermediates at Telomeres
5. pot1 and Genes Encoding the Chromosomal Passenger Complex (CPC) Are Lethal
6. Histone H4 Acetylation and the Bromodomain Protein Bdf2 Are Required for the Growth of Cells with Circular Chromosomes
7. pot1 and Gene Encoding Phosphatidylinositol 4-Kinase Pik1 Is Lethal
8. The Effect of Chromosome Circularization in Torsional Constraint, DNA Damage, and Chromosome Organization
9. Biology of Yeast Carrying Ring Chromosomes and Comparison of the Difference between Six Types of S. pombe Mutants That Have Circular Chromosomes
10. Conclusions and Perspectives on Genes which Genetically Interact with the Telomere Protection Gene pot1 in Fission Yeast
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armanios, M. The Role of Telomeres in Human Disease. Annu. Rev. Genom. Hum. Genet. 2022, 23, 363–381. [Google Scholar] [CrossRef] [PubMed]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feijoo, P.; Dominguez, D.; Tusell, L.; Genesca, A. Telomere-dependent genomic integrity: Evolution of the fusion-bridge-breakage cycle concept. Curr. Pharm. Des. 2014, 20, 6375–6385. [Google Scholar] [CrossRef]
- Casari, E.; Gnugnoli, M.; Rinaldi, C.; Pizzul, P.; Colombo, C.V.; Bonetti, D.; Longhese, M.P. To Fix or Not to Fix: Maintenance of Chromosome Ends Versus Repair of DNA Double-Strand Breaks. Cells 2022, 11, 3224. [Google Scholar] [CrossRef]
- Reddel, R.R. Telomere maintenance mechanisms in cancer: Clinical implications. Curr. Pharm. Des. 2014, 20, 6361–6374. [Google Scholar] [CrossRef] [Green Version]
- Pristyazhnyuk, I.E.; Menzorov, A.G. Ring chromosomes: From formation to clinical potential. Protoplasma 2018, 255, 439–449. [Google Scholar] [CrossRef]
- Yip, M.Y. Autosomal ring chromosomes in human genetic disorders. Transl. Pediatr. 2015, 4, 164–174. [Google Scholar] [CrossRef]
- Gebhart, E. Ring chromosomes in human neoplasias. Cytogenet. Genome Res. 2008, 121, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Baumann, P.; Cech, T.R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 2001, 292, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Baumann, P.; Podell, E.; Cech, T.R. Human Pot1 (protection of telomeres) protein: Cytolocalization, gene structure, and alternative splicing. Mol. Cell. Biol. 2002, 22, 8079–8087. [Google Scholar] [CrossRef] [Green Version]
- Aramburu, T.; Plucinsky, S.; Skordalakes, E. POT1-TPP1 telomere length regulation and disease. Comput. Struct. Biotechnol. J. 2020, 18, 1939–1946. [Google Scholar] [CrossRef]
- Abreu, E.; Aritonovska, E.; Reichenbach, P.; Cristofari, G.; Culp, B.; Terns, R.M.; Lingner, J.; Terns, M.P. TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol. Cell. Biol. 2010, 30, 2971–2982. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Liu, D.; Wan, M.; Safari, A.; Kim, H.; Sun, W.; O’Connor, M.S.; Songyang, Z. TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature 2007, 445, 559–562. [Google Scholar] [CrossRef]
- Veldman, T.; Etheridge, K.T.; Counter, C.M. Loss of hPot1 function leads to telomere instability and a cut-like phenotype. Curr. Biol. 2004, 14, 2264–2270. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Stock, A.J.; Liu, Y. The enigma of excessively long telomeres in cancer: Lessons learned from rare human POT1 variants. Curr. Opin. Genet. Dev. 2020, 60, 48–55. [Google Scholar] [CrossRef]
- Wang, F.; Podell, E.R.; Zaug, A.J.; Yang, Y.; Baciu, P.; Cech, T.R.; Lei, M. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 2007, 445, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Robles-Espinoza, C.D.; Harland, M.; Ramsay, A.J.; Aoude, L.G.; Quesada, V.; Ding, Z.; Pooley, K.A.; Pritchard, A.L.; Tiffen, J.C.; Petljak, M.; et al. POT1 loss-of-function variants predispose to familial melanoma. Nat. Genet. 2014, 46, 478–481. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.T.; Hennick, K.; Johnson, J.; Finnerty, B.; Choo, S.; Short, S.B.; Drubin, C.; Forster, R.; McMaster, M.L.; Hockemeyer, D. Cancer-associated POT1 mutations lead to telomere elongation without induction of a DNA damage response. Embo J. 2021, 40, e107346. [Google Scholar] [CrossRef]
- Ramsay, A.J.; Quesada, V.; Foronda, M.; Conde, L.; Martínez-Trillos, A.; Villamor, N.; Rodríguez, D.; Kwarciak, A.; Garabaya, C.; Gallardo, M.; et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat. Genet. 2013, 45, 526–530. [Google Scholar] [CrossRef]
- Jain, D.; Cooper, J.P. Telomeric strategies: Means to an end. Annu. Rev. Genet. 2010, 44, 243–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, T.; Kanoh, J.; Saito, M.; Ishikawa, F. Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science 2008, 320, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Bunch, J.T.; Bae, N.S.; Leonardi, J.; Baumann, P. Distinct requirements for Pot1 in limiting telomere length and maintaining chromosome stability. Mol. Cell. Biol. 2005, 25, 5567–5578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.M.; Morin, G.B.; Chapman, K.B.; Weinrich, S.L.; Andrews, W.H.; Lingner, J.; Harley, C.B.; Cech, T.R. Telomerase catalytic subunit homologs from fission yeast and human. Science 1997, 277, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.M.; Cooper, J.P.; Cech, T.R. Two modes of survival of fission yeast without telomerase. Science 1998, 282, 493–496. [Google Scholar] [CrossRef] [Green Version]
- McClintock, B. A Correlation of Ring-Shaped Chromosomes with Variegation in Zea Mays. Proc. Natl. Acad. Sci. USA 1932, 18, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Li, Y.; Su, Y.; Shen, Y.; Tang, D.; Luo, Q.; Cheng, Z. A functional centromere lacking CentO sequences in a newly formed ring chromosome in rice. J. Genet. Genom. 2016, 43, 694–701. [Google Scholar] [CrossRef]
- Warmington, J.R.; Anwar, R.; Newlon, C.S.; Waring, R.B.; Davies, R.W.; Indge, K.J.; Oliver, S.G. A ‘hot-spot’ for Ty transposition on the left arm of yeast chromosome III. Nucleic Acids Res. 1986, 14, 3475–3485. [Google Scholar] [CrossRef] [Green Version]
- Haber, J.E.; Thorburn, P.C.; Rogers, D. Meiotic and mitotic behavior of dicentric chromosomes in Saccharomyces cerevisiae. Genetics 1984, 106, 185–205. [Google Scholar] [CrossRef]
- Wang, X.; Baumann, P. Chromosome fusions following telomere loss are mediated by single-strand annealing. Mol. Cell. 2008, 31, 463–473. [Google Scholar] [CrossRef]
- Naito, T.; Matsuura, A.; Ishikawa, F. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat. Genet. 1998, 20, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Nanbu, T.; Nguyễn, L.C.; Habib, A.G.; Hirata, N.; Ukimori, S.; Tanaka, D.; Masuda, K.; Takahashi, K.; Yukawa, M.; Tsuchiya, E.; et al. Fission Yeast Exo1 and Rqh1-Dna2 Redundantly Contribute to Resection of Uncapped Telomeres. PLoS ONE 2015, 10, e0140456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanbu, T.; Takahashi, K.; Murray, J.M.; Hirata, N.; Ukimori, S.; Kanke, M.; Masukata, H.; Yukawa, M.; Tsuchiya, E.; Ueno, M. Fission yeast RecQ helicase Rqh1 is required for the maintenance of circular chromosomes. Mol. Cell. Biol. 2013, 33, 1175–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mersaoui, S.Y.; Wellinger, R.J. Fine tuning the level of the Cdc13 telomere-capping protein for maximal chromosome stability performance. Curr. Genet. 2019, 65, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.; Skordalakes, E. Structure and function of the telomeric CST complex. Comput. Struct. Biotechnol. J. 2016, 14, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Garvik, B.; Carson, M.; Hartwell, L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol. Cell. Biol. 1995, 15, 6128–6138. [Google Scholar] [CrossRef] [Green Version]
- Dewar, J.M.; Lydall, D. Similarities and differences between “uncapped” telomeres and DNA double-strand breaks. Chromosoma 2012, 121, 117–130. [Google Scholar] [CrossRef]
- Gupta, S.V.; Schmidt, K.H. Maintenance of Yeast Genome Integrity by RecQ Family DNA Helicases. Genes 2020, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Stewart, E.; Chapman, C.R.; Al-Khodairy, F.; Carr, A.M.; Enoch, T. rqh1+, a fission yeast gene related to the Bloom’s and Werner’s syndrome genes, is required for reversible S phase arrest. Embo J. 1997, 16, 2682–2692. [Google Scholar] [CrossRef] [Green Version]
- Doe, C.L.; Dixon, J.; Osman, F.; Whitby, M.C. Partial suppression of the fission yeast rqh1(−) phenotype by expression of a bacterial Holliday junction resolvase. Embo J. 2000, 19, 2751–2762. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Huang, J. DNA End Resection: Facts and Mechanisms. Genom. Proteom. Bioinform. 2016, 14, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Tisi, R.; Vertemara, J.; Zampella, G.; Longhese, M.P. Functional and structural insights into the MRX/MRN complex, a key player in recognition and repair of DNA double-strand breaks. Comput. Struct. Biotechnol. J. 2020, 18, 1137–1152. [Google Scholar] [CrossRef]
- Bizard, A.H.; Hickson, I.D. The dissolution of double Holliday junctions. Cold Spring Harb. Perspect Biol. 2014, 6, a016477. [Google Scholar] [CrossRef]
- Ahmad, F.; Stewart, E. The N-terminal region of the Schizosaccharomyces pombe RecQ helicase, Rqh1p, physically interacts with Topoisomerase III and is required for Rqh1p function. Mol. Genet. Genom. 2005, 273, 102–114. [Google Scholar] [CrossRef]
- Takahashi, K.; Imano, R.; Kibe, T.; Seimiya, H.; Muramatsu, Y.; Kawabata, N.; Tanaka, G.; Matsumoto, Y.; Hiromoto, T.; Koizumi, Y.; et al. Fission yeast Pot1 and RecQ helicase are required for efficient chromosome segregation. Mol. Cell. Biol. 2011, 31, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Laursen, L.V.; Ampatzidou, E.; Andersen, A.H.; Murray, J.M. Role for the fission yeast RecQ helicase in DNA repair in G2. Mol. Cell. Biol. 2003, 23, 3692–3705. [Google Scholar] [CrossRef] [Green Version]
- Nakano, A.; Masuda, K.; Hiromoto, T.; Takahashi, K.; Matsumoto, Y.; Habib, A.G.; Darwish, A.G.; Yukawa, M.; Tsuchiya, E.; Ueno, M. Rad51-dependent aberrant chromosome structures at telomeres and ribosomal DNA activate the spindle assembly checkpoint. Mol. Cell. Biol. 2014, 34, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Habib, A.G.; Masuda, K.; Yukawa, M.; Tsuchiya, E.; Ueno, M. Long G2 accumulates recombination intermediates and disturbs chromosome segregation at dysfunction telomere in Schizosaccharomyces pombe. Biochem. Biophys. Res. Commun. 2015, 464, 140–146. [Google Scholar] [CrossRef]
- Kanoh, J. Unexpected roles of a shugoshin protein at subtelomeres. Genes Genet. Syst. 2018, 92, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S.; Osman, F.; Feeney, L.; Lorenz, A.; Bryer, C.; Whitby, M.C. MHF1-2/CENP-S-X performs distinct roles in centromere metabolism and genetic recombination. Open Biol. 2013, 3, 130102. [Google Scholar] [CrossRef] [Green Version]
- Ruchaud, S.; Carmena, M.; Earnshaw, W.C. Chromosomal passengers: Conducting cell division. Nat. Rev. Mol. Cell. Biol. 2007, 8, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Leverson, J.D.; Huang, H.K.; Forsburg, S.L.; Hunter, T. The Schizosaccharomyces pombe aurora-related kinase Ark1 interacts with the inner centromere protein Pic1 and mediates chromosome segregation and cytokinesis. Mol. Biol. Cell. 2002, 13, 1132–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, Y. Temporal and spatial regulation of targeting aurora B to the inner centromere. Cold Spring Harb. Symp. Quant. Biol. 2010, 75, 419–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, A.G.K.; Sugiura, K.; Ueno, M. Chromosome passenger complex is required for the survival of cells with ring chromosomes in fission yeast. PLoS ONE 2018, 13, e0190523. [Google Scholar] [CrossRef] [Green Version]
- Vanoosthuyse, V.; Prykhozhij, S.; Hardwick, K.G. Shugoshin 2 regulates localization of the chromosomal passenger proteins in fission yeast mitosis. Mol. Biol. Cell. 2007, 18, 1657–1669. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, N.; Mehrotra, R.; Ebe, M.; Yanagida, M. Condensin phosphorylated by the Aurora-B-like kinase Ark1 is continuously required until telophase in a mode distinct from Top2. J. Cell. Sci. 2011, 124, 1795–1807. [Google Scholar] [CrossRef] [Green Version]
- Kanoh, J.; Sadaie, M.; Urano, T.; Ishikawa, F. Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr. Biol. 2005, 15, 1808–1819. [Google Scholar] [CrossRef] [Green Version]
- Tadeo, X.; Wang, J.; Kallgren, S.P.; Liu, J.; Reddy, B.D.; Qiao, F.; Jia, S. Elimination of shelterin components bypasses RNAi for pericentric heterochromatin assembly. Genes Dev. 2013, 27, 2489–2499. [Google Scholar] [CrossRef] [Green Version]
- Gisselsson, D.; Höglund, M.; Mertens, F.; Johansson, B.; Dal Cin, P.; Van den Berghe, H.; Earnshaw, W.C.; Mitelman, F.; Mandahl, N. The structure and dynamics of ring chromosomes in human neoplastic and non-neoplastic cells. Hum. Genet. 1999, 104, 315–325. [Google Scholar] [CrossRef]
- Trombetta, D.; Mertens, F.; Lonoce, A.; D’Addabbo, P.; Rennstam, K.; Mandahl, N.; Storlazzi, C.T. Characterization of a hotspot region on chromosome 12 for amplification in ring chromosomes in atypical lipomatous tumors. Genes Chromosomes Cancer 2009, 48, 993–1001. [Google Scholar] [CrossRef]
- Yasuda, M.; Habib, A.G.K.; Sugiura, K.; Shamim, H.M.; Ueno, M. The fission yeast bromodomain protein Bdf2 is required for the growth of cells with circular chromosomes. Biosci. Biotechnol. Biochem. 2022, 86, 224–230. [Google Scholar] [CrossRef]
- Pérez-Salvia, M.; Esteller, M. Bromodomain inhibitors and cancer therapy: From structures to applications. Epigenetics 2017, 12, 323–339. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Chitsaz, F.; Abbasi, A.; Misteli, T.; Ozato, K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 2003, 100, 8758–8763. [Google Scholar] [CrossRef] [Green Version]
- Garabedian, M.V.; Noguchi, C.; Ziegler, M.A.; Das, M.M.; Singh, T.; Harper, L.J.; Leman, A.R.; Khair, L.; Moser, B.A.; Nakamura, T.M.; et al. The double-bromodomain proteins Bdf1 and Bdf2 modulate chromatin structure to regulate S-phase stress response in Schizosaccharomyces pombe. Genetics 2012, 190, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tadeo, X.; Hou, H.; Tu, P.G.; Thompson, J.; Yates, J.R., 3rd; Jia, S. Epe1 recruits BET family bromodomain protein Bdf2 to establish heterochromatin boundaries. Genes Dev. 2013, 27, 1886–1902. [Google Scholar] [CrossRef] [Green Version]
- Gómez, E.B.; Nugent, R.L.; Laria, S.; Forsburg, S.L. Schizosaccharomyces pombe histone acetyltransferase Mst1 (KAT5) is an essential protein required for damage response and chromosome segregation. Genetics 2008, 179, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, A.; Nguyen, L.C.; Shamim, H.M.; Iida, T.; Nakase, M.; Takegawa, K.; Senda, M.; Jida, S.; Ueno, M. Mutation in fission yeast phosphatidylinositol 4-kinase Pik1 is synthetically lethal with defect in telomere protection protein Pot1. Biochem. Biophys. Res. Commun. 2018, 496, 1284–1290. [Google Scholar] [CrossRef]
- Iida, N.; Yamao, F.; Nakamura, Y.; Iida, T. Mudi, a web tool for identifying mutations by bioinformatics analysis of whole-genome sequence. Genes Cells 2014, 19, 517–527. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, A.; Arai, R.; Yashiroda, Y.; Shirai, A.; Kamata, A.; Sekido, S.; Kobayashi, Y.; Hashimoto, A.; Hamamoto, M.; Hiraoka, Y.; et al. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 2006, 24, 841–847. [Google Scholar] [CrossRef]
- Fáberová, V.; Kalasová, I.; Krausová, A.; Hozák, P. Super-Resolution Localisation of Nuclear PI(4)P and Identification of Its Interacting Proteome. Cells 2020, 9, 1191. [Google Scholar] [CrossRef]
- Strahl, T.; Hama, H.; DeWald, D.B.; Thorner, J. Yeast phosphatidylinositol 4-kinase, Pik1, has essential roles at the Golgi and in the nucleus. J. Cell. Biol. 2005, 171, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Steinbach, S.K.; Desautels, M.; Hemmingsen, S.M. Essential role for Schizosaccharomyces pombe pik1 in septation. PLoS ONE 2009, 4, e6179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levens, D.; Baranello, L.; Kouzine, F. Controlling gene expression by DNA mechanics: Emerging insights and challenges. Biophys. Rev. 2016, 8, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, R.S.; Piña, B.; Roca, J. Positional dependence of transcriptional inhibition by DNA torsional stress in yeast chromosomes. Embo J. 2010, 29, 740–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takikawa, M.; Tarumoto, Y.; Ishikawa, F. Fission yeast Stn1 is crucial for semi-conservative replication at telomeres and subtelomeres. Nucleic Acids Res. 2017, 45, 1255–1269. [Google Scholar] [CrossRef] [Green Version]
- Shamim, H.M.; Minami, Y.; Tanaka, D.; Ukimori, S.; Murray, J.M.; Ueno, M. Fission yeast strains with circular chromosomes require the 9-1-1 checkpoint complex for the viability in response to the anti-cancer drug 5-fluorodeoxyuridine. PLoS ONE 2017, 12, e0187775. [Google Scholar] [CrossRef] [Green Version]
- Jain, D.; Hebden, A.K.; Nakamura, T.M.; Miller, K.M.; Cooper, J.P. HAATI survivors replace canonical telomeres with blocks of generic heterochromatin. Nature 2010, 467, 223–227. [Google Scholar] [CrossRef]
- Kai, M.; Wang, T.S. Checkpoint responses to replication stalling: Inducing tolerance and preventing mutagenesis. Mutat. Res. 2003, 532, 59–73. [Google Scholar] [CrossRef]
- Matsuda, A.; Asakawa, H.; Haraguchi, T.; Hiraoka, Y. Spatial organization of the Schizosaccharomyces pombe genome within the nucleus. Yeast 2017, 34, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Chikashige, Y.; Yamane, M.; Okamasa, K.; Tsutsumi, C.; Kojidani, T.; Sato, M.; Haraguchi, T.; Hiraoka, Y. Membrane proteins Bqt3 and -4 anchor telomeres to the nuclear envelope to ensure chromosomal bouquet formation. J. Cell. Biol. 2009, 187, 413–427. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Ye, T.; Zhang, X.R.; Nie, L.; Wang, H.; Li, W.; Lu, R.; Fu, C.; Du, L.L.; Zhou, J.Q. Single-chromosome fission yeast models reveal the configuration robustness of a functional genome. Cell. Rep. 2022, 40, 111237. [Google Scholar] [CrossRef]
- Martín, V.; Du, L.L.; Rozenzhak, S.; Russell, P. Protection of telomeres by a conserved Stn1-Ten1 complex. Proc. Natl. Acad. Sci. USA 2007, 104, 14038–14043. [Google Scholar] [CrossRef] [Green Version]
- Kibe, T.; Ono, Y.; Sato, K.; Ueno, M. Fission yeast Taz1 and RPA are synergistically required to prevent rapid telomere loss. Mol. Biol. Cell. 2007, 18, 2378–2387. [Google Scholar] [CrossRef] [Green Version]
- Sadaie, M.; Naito, T.; Ishikawa, F. Stable inheritance of telomere chromatin structure and function in the absence of telomeric repeats. Genes Dev. 2003, 17, 2271–2282. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, H.; Tarumoto, Y.; Ishikawa, F. Tel1(ATM) and Rad3(ATR) phosphorylate the telomere protein Ccq1 to recruit telomerase and elongate telomeres in fission yeast. Genes Dev. 2012, 26, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Ryan, C.J.; Roguev, A.; Patrick, K.; Xu, J.; Jahari, H.; Tong, Z.; Beltrao, P.; Shales, M.; Qu, H.; Collins, S.R.; et al. Hierarchical modularity and the evolution of genetic interactomes across species. Mol. Cell. 2012, 46, 691–704. [Google Scholar] [CrossRef] [Green Version]
- Sciochetti, S.A.; Piggot, P.J. A tale of two genomes: Resolution of dimeric chromosomes in Escherichia coli and Bacillus subtilis. Res. Microbiol. 2000, 151, 503–511. [Google Scholar] [CrossRef]
- Ishikawa, F.; Naito, T. Why do we have linear chromosomes? A matter of Adam and Eve. Mutat. Res. 1999, 434, 99–107. [Google Scholar] [CrossRef]

| Gene Name (Related Functions) | Phenotype (References) |
|---|---|
| Lethal genetic interaction with pot1 | |
| lig4, rad16, rad22, srs2 (Single strand annealing (SSA)) | No chromosome fusion by deletion of SSA genes [30] |
| rqh1, top3 (dissolution of double holiday junction) | Circular chromosome is not maintained by deletion of rqh1 or mutation in top3 [33] |
| ark1, bir1, pic1 (CPC) (chromosome segregation) | Circular chromosome is not maintained by mutation of CPC [54] |
| bdf2, mst1 (maintaining proper histone H4 acetylation level) | Circular chromosome causes growth defect by bdf2 or mst1 mutation [61] |
| pik1 (regulation of PI(4)P level in Golgi and possibly in nucleus?) | Circular chromosome causes growth defect by pik1 mutation? [67] |
| Suppresion of pot1, pot1 rqh1 double and pot1 rqh1-hd double mutant phenotype | |
| rqh1 (Helicase) | rqh1-hd mutation rescues telomere loss of pot1Δ cells [45] |
| chk1, wee1 mik1 double mutation, cdc2-3w (G2 length regulation) | chk1 or cdc2-3w mutation, or wee1 mik1 double mutation rescue TBZ sensitivity of pot1 rqh1-hd cells [47,48] |
| rad51, exo1 rqh1 double mutation (HR) | Lethality of pot1 rqh1 is suppressed by deletion of rad51 or exo1 [33] |
| exo1, rqh1 (processing of uncapped telomere) | Acute growth defect by pot1 shut-off is suppressed by exo1 and/or rqh1 mutation [32] |
| Enhancement of pot1 and pot1 rqh1 double mutant phenotype | |
| bub1, mad2 (Spindle checkpoint) | Growth of pot1 rqh1-hd cells becomes worse by bub1 or mad2 mutation [47] |
| hus1, rad1, rad9 (9-1-1 complex) (DNA damage checkpoint) | Mutation in 9-1-1 complex increases HU sensitivity of pot1Δ cells [76] |
| swi6 (Heterochromatin), sgo2 (Sister chromatid biorientation) | Mutation of swi6 or sgo2 increases chromosome segregation defect of pot1Δ cells [54] |
| Phenotype suppression by deletion of pot1 | |
| dcr1 (RNA interference machinery) | Pericentric silencing defect in dcr1Δ cells is suppressed by pot1 deletion [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, M. Exploring Genetic Interactions with Telomere Protection Gene pot1 in Fission Yeast. Biomolecules 2023, 13, 370. https://doi.org/10.3390/biom13020370
Ueno M. Exploring Genetic Interactions with Telomere Protection Gene pot1 in Fission Yeast. Biomolecules. 2023; 13(2):370. https://doi.org/10.3390/biom13020370
Chicago/Turabian StyleUeno, Masaru. 2023. "Exploring Genetic Interactions with Telomere Protection Gene pot1 in Fission Yeast" Biomolecules 13, no. 2: 370. https://doi.org/10.3390/biom13020370
APA StyleUeno, M. (2023). Exploring Genetic Interactions with Telomere Protection Gene pot1 in Fission Yeast. Biomolecules, 13(2), 370. https://doi.org/10.3390/biom13020370





