Preliminary Transcriptome Analysis of Long Noncoding RNA in Hypothalamic-Pituitary-Mammary Gland Axis of Dairy Cows under Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Animals and Sample Collection
2.3. Detection of Heat Shock Proteins (HSPs) and Antioxidant Enzyme Activity
2.4. Total RNA Extraction and Sequencing
2.5. Long Noncoding RNA Identification and Differential Expression Analysis
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Validation
2.7. Function Enrichment Analysis
2.8. lncRNA-miRNA-mRNA Network Association Analysis
3. Results
3.1. Comparison of Environmental Temperature and Humidity Index (THI) and Cow Information
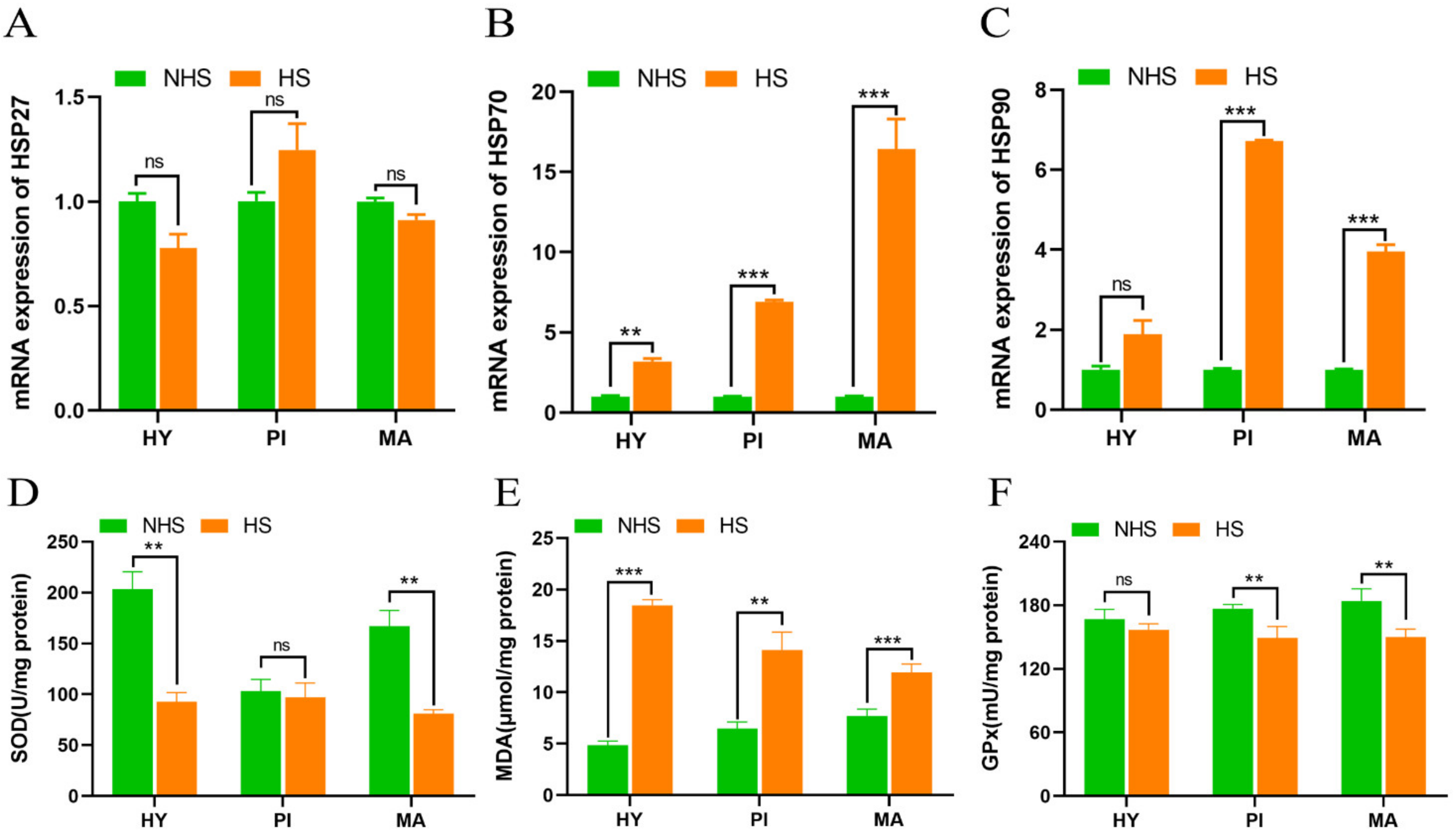
3.2. Effect of Heat Stress on Heat Shock Proteins and Antioxidant Enzymes in HPM-Axis-Related Tissues of Dairy Cows
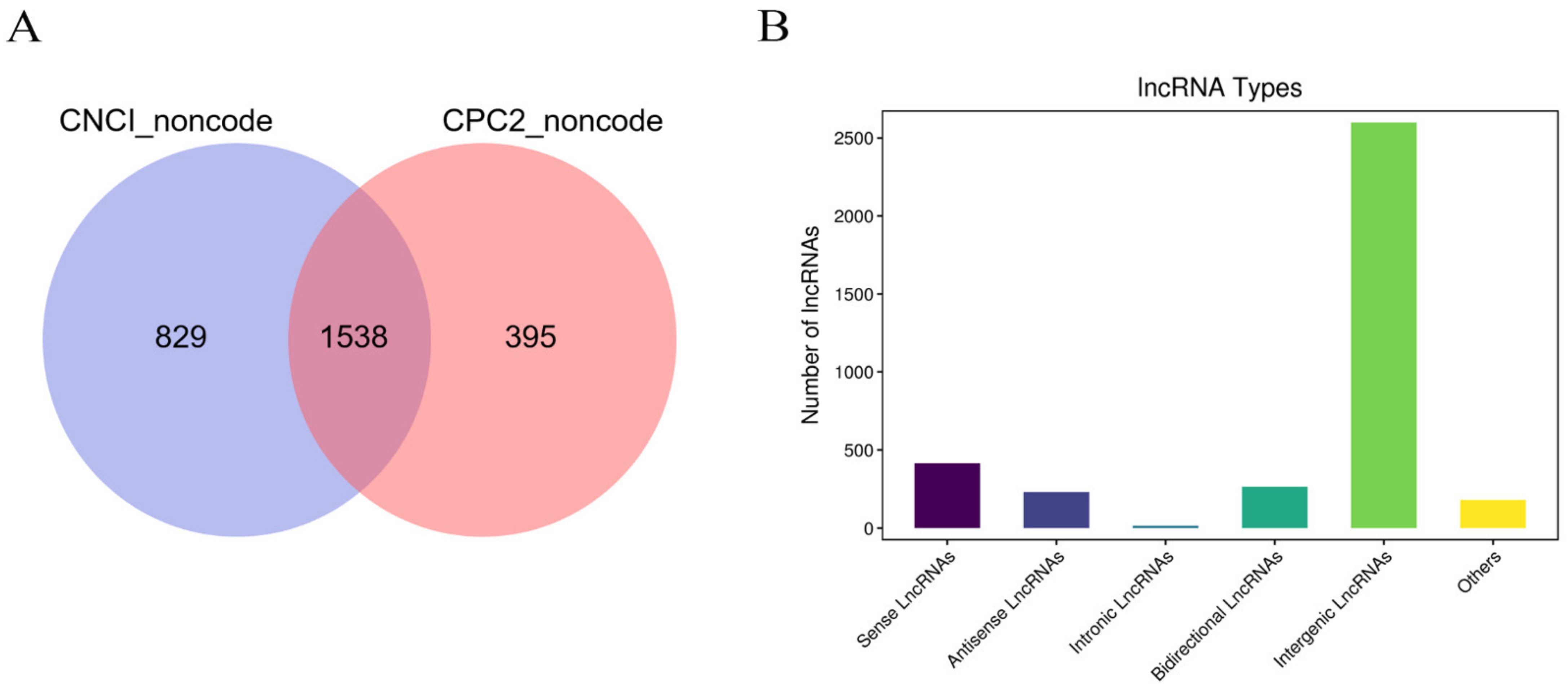
3.3. Identification of lncRNAs in HPM-Axis-Related Tissues of Dairy Cows under Heat Stress
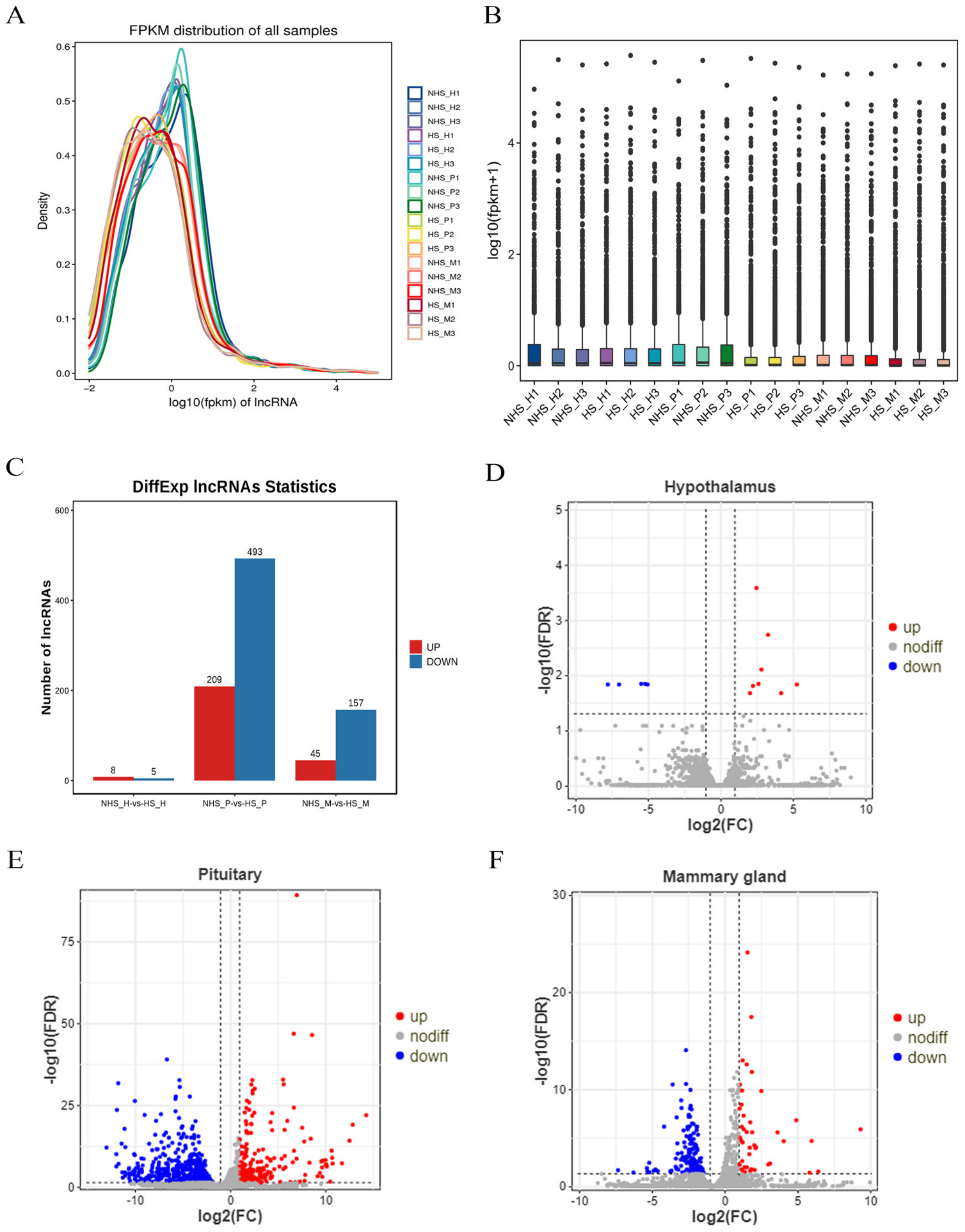
3.4. Differential Expression Analysis of lncRNAs
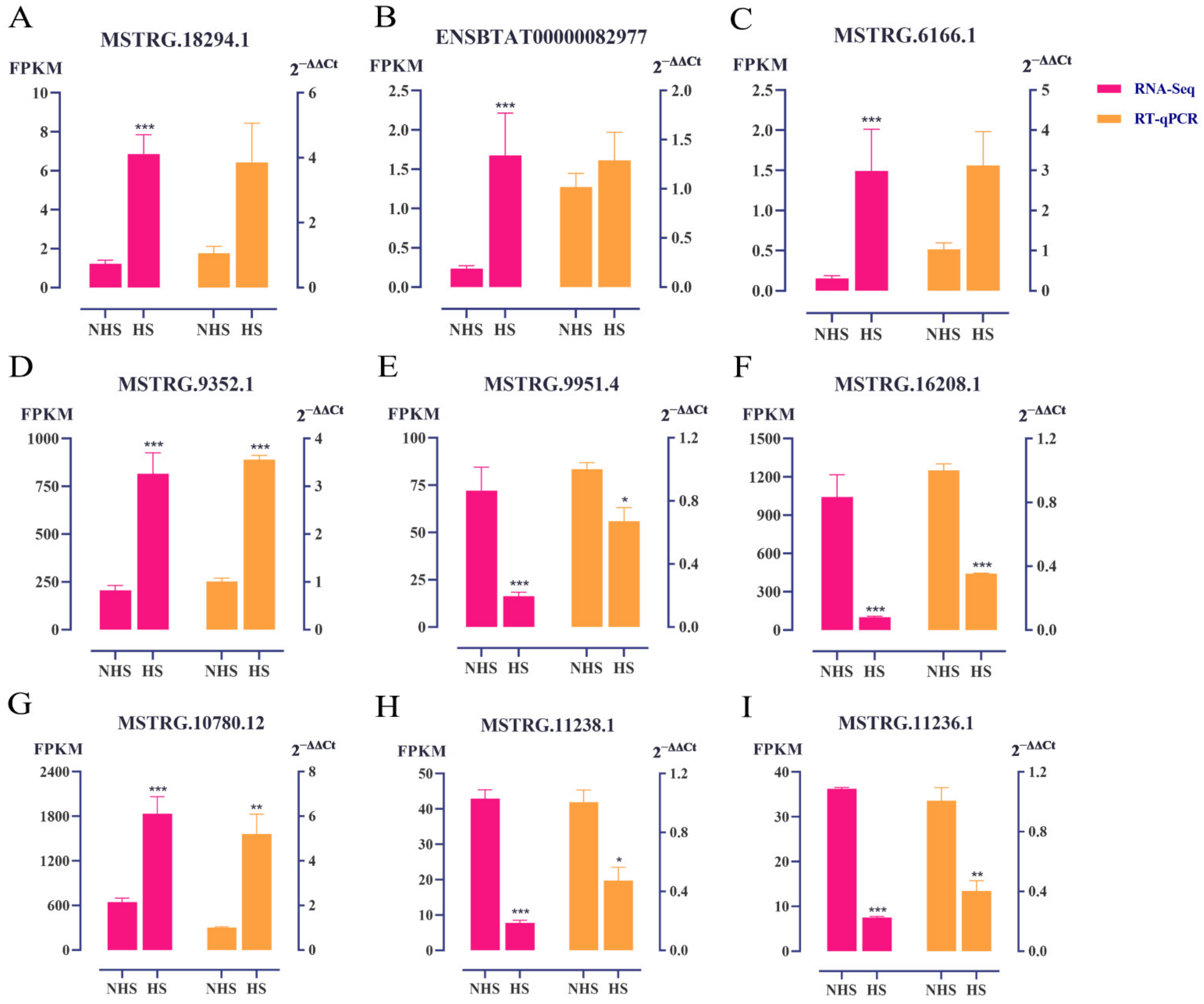
3.5. Validation of DE lncRNAs with qRT−PCR
3.6. Function Enrichment Analysis for Differentially Expressed lncRNAs
3.7. lncRNA–miRNA–mRNA Regulatory Network Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perano, K.M.; Usack, J.G.; Angenent, L.T.; Gebremedhin, K.G. Production and physiological responses of heat−stressed lactating dairy cattle to conductive cooling. J. Dairy Sci. 2015, 98, 5252–5261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wang, Y.; Li, L.; Han, Z.; Mao, S.; Wang, G. Betaine protects against heat exposure−induced oxidative stress and apoptosis in bovine mammary epithelial cells via regulation of ROS production. Cell Stress Chaperones 2019, 24, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Han, J.; Shao, J.; Chen, Q.; Sun, H.; Guan, L.; Li, Y.; Liu, J.; Liu, H. Transcriptional changes in the hypothalamus, pituitary, and mammary gland underlying decreased lactation performance in mice under heat stress. FASEB J. 2019, 33, 12588–12601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhu, G.K.; Milevskiy, M.J.G.; Wilson, W.; Shewan, A.M.; Brown, M.A. Non−coding RNAs in Mammary Gland Development and Disease. Adv. Exp. Med. Biol. 2016, 886, 121–153. [Google Scholar] [PubMed]
- Zheng, X.; Ning, C.; Zhao, P.; Feng, W.; Jin, Y.; Zhou, L.; Yu, Y.; Liu, J. Integrated analysis of long noncoding RNA and mRNA expression profiles reveals the potential role of long noncoding RNA in different bovine lactation stages. J. Dairy Sci. 2018, 101, 11061–11073. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Sun, B.; Zhang, F.; Zhong, Z.; Zhang, Y.; Li, F.; Zhang, T.; Khatib, H.; Wang, X. lncRNA MPFAST Promotes Proliferation and Fatty Acid Synthesis of Bovine Mammary Epithelial Cell by Sponging miR−103 Regulating PI3K−AKT Pathway. J. Agric. Food Chem. 2022, 70, 12004–12013. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, F.; Chen, Q.; Cai, J.; Hu, J.; Shen, J.; Zhang, J. Long noncoding RNA and mRNA profiling of hypothalamic−pituitary−mammary gland axis in lactating sows under heat stress. Genomics 2020, 112, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, L.; Deng, M.; Lian, Z.; Han, Y.; Sun, B.; Guo, Y.; Liu, G.; Liu, D. Heat Stress−Responsive Transcriptome Analysis in the Liver Tissue of Hu Sheep. Genes 2019, 10, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Qiao, J.; Zhang, Z.; Shang, X.; Chu, Z.; Fu, Y.; Chu, M. Identification and analysis of differentially expressed long non−coding RNAs of Chinese Holstein cattle responses to heat stress. Anim. Biotechnol. 2020, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.F.; Xia, H.B.; Wang, X.L.; Wang, Y.; Fang, J.; Li, S.J.; Zhai, Y.F.; Han, Z.Y. Comprehensive Profiling of ceRNA (circRNA−miRNA−mRNA) networks in hypothalamic−pituitary−mammary gland axis of dairy cows under heat stress. Int. J. Mol. Sci. 2023, 24, 888. [Google Scholar] [CrossRef]
- Pinto, S.; Hoffmann, G.; Ammon, C.; Amon, T. Critical THI thresholds based on the physiological parameters of lactating dairy cows. J. Therm. Biol. 2020, 88, 102523. [Google Scholar] [CrossRef]
- Ingraham, R.H.; Gillette, D.D.; Wagner, W.D. Relationship of temperature and humidity to conception rate of Holstein cows in subtropical climate. J. Dairy Sci. 1974, 57, 476–481. [Google Scholar] [CrossRef]
- Shan, Q.; Ma, F.; Wei, J.; Li, H.; Ma, H.; Sun, P. Physiological Functions of Heat Shock Proteins. Curr. Protein Pept. Sci. 2020, 21, 751–760. [Google Scholar] [CrossRef]
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, M.; Zhou, J.; Zhang, X. HSP27, 70 and 90, anti−apoptotic proteins, in clinical cancer therapy (Review). Int. J. Oncol. 2014, 45, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Gao, S.; Ouyang, J.; Ma, L.; Bu, D. Impacts of Heat Stress−Induced Oxidative Stress on the Milk Protein Biosynthesis of Dairy Cows. Animals 2021, 11, 726. [Google Scholar] [CrossRef]
- Zeng, H.; Xi, Y.; Li, Y.; Wang, Z.; Zhang, L.; Han, Z. Analysis of Astragalus Polysaccharide Intervention in Heat−Stressed Dairy Cows’ Serum Metabolomics. Animals 2020, 10, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, H.L.; Xing, G.D.; Qian, Y.; Zhong, J.F.; Chen, K.L. S−allyl cysteine ameliorates heat stress−induced oxidative stress by activating Nrf2/HO−1 signaling pathway in BMECs. Toxicol. Appl. Pharmacol. 2021, 416, 115469. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, L.; Kezer, F.L.; Ruff, F.; Szenci, O.; Bakony, M.; Jurkovich, V. Effect of artificial shade on saliva cortisol concentrations of heat−stressed dairy calves. Domest. Anim. Endocrinol. 2019, 66, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Follenius, M.; Brandenberger, G.; Oyono, S.; Candas, V. Cortisol as a sensitive index of heat−intolerance. Physiol. Behav. 1982, 29, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Christison, G.I.; Johnson, H.D. Cortisol turnover in heat−stressed cow. J. Anim. Sci. 1972, 35, 1005–1010. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Invest. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [Green Version]
- Magdub, A.; Johnson, H.D.; Belyea, R.L. Effect of environmental heat and dietary fiber on thyroid physiology of lactating cows. J. Dairy Sci. 1982, 65, 2323–2331. [Google Scholar] [CrossRef]
- Leyva−Corona, J.C.; Reyna−Granados, J.R.; Zamorano−Algandar, R.; Sanchez−Castro, M.A.; Thomas, M.G.; Enns, R.M.; Speidel, S.E.; Medrano, J.F.; Rincon, G.; Luna−Nevarez, P. Polymorphisms within the prolactin and growth hormone/insulin−like growth factor−1 functional pathways associated with fertility traits in Holstein cows raised in a hot−humid climate. Trop. Anim. Health Prod. 2018, 50, 1913–1920. [Google Scholar] [CrossRef]
- Chen, K.L.; Wang, H.L.; Jiang, L.Z.; Qian, Y.; Yang, C.X.; Chang, W.W.; Zhong, J.F.; Xing, G.D. Heat stress induces apoptosis through disruption of dynamic mitochondrial networks in dairy cow mammary epithelial cells. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 322–331. [Google Scholar] [CrossRef]
- Sun, X.C.; Wang, Y.; Zeng, H.F.; Xi, Y.M.; Lin, H.; Han, Z.Y.; Chen, K.L. SIRT3 protects bovine mammary epithelial cells from heat stress damage by activating the AMPK signaling pathway. Cell Death Discov. 2021, 7, 304. [Google Scholar] [CrossRef]
- Appuhamy, J.A.; Nayananjalie, W.A.; England, E.M.; Gerrard, D.E.; Akers, R.M.; Hanigan, M.D. Effects of AMP−activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. J. Dairy Sci. 2014, 97, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.C.; Zhao, S.G.; Wang, S.S.; Luo, C.C.; Gao, H.N.; Zheng, N.; Wang, J.Q. d−Glucose and amino acid deficiency inhibits casein synthesis through JAK2/STAT5 and AMPK/mTOR signaling pathways in mammary epithelial cells of dairy cows. J. Dairy Sci. 2018, 101, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Han, B.; Li, Q.; Yuan, Y.; Li, J.; Sun, D. Using RNA sequencing to identify putative competing endogenous RNAs (ceRNAs) potentially regulating fat metabolism in bovine liver. Sci. Rep. 2017, 7, 6396. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Gu, Z.; Li, L.; Liu, Y.; Wang, L.; Su, L. p38 MAPK−MK2 pathway regulates the heat−stress−induced accumulation of reactive oxygen species that mediates apoptotic cell death in glial cells. Oncol. Lett. 2018, 15, 775–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Cheng, G.; Xu, C.; Liu, H.; Wang, Y.; Li, N.; Zhu, C.; Xia, W. The role of miR−128−3p through MAPK14 activation in the apoptosis of GC2 spermatocyte cell line following heat stress. Andrology 2021, 9, 665–672. [Google Scholar] [CrossRef]
- Ghanbarian, H.; Yildiz, M.T.; Tutar, Y. MicroRNA Targeting. Methods Mol. Biol. 2022, 2257, 105–130. [Google Scholar]
- Alkan, A.H.; Akgul, B. Endogenous miRNA Sponges. Methods Mol. Biol. 2022, 2257, 91–104. [Google Scholar]
- Shetty, A.; Venkatesh, T.; Kabbekodu, S.P.; Tsutsumi, R.; Suresh, P.S. LncRNA−miRNA−mRNA regulatory axes in endometrial cancer: A comprehensive overview. Arch. Gynecol. Obstet. 2022, 306, 1431–1447. [Google Scholar] [CrossRef]
- Liang, T.; Zhou, B.; Shi, L.; Wang, H.; Chu, Q.; Xu, F.; Li, Y.; Chen, R.; Shen, C.; Schinckel, A.P. lncRNA AK017368 promotes proliferation and suppresses differentiation of myoblasts in skeletal muscle development by attenuating the function of miR−30c. FASEB J. 2018, 32, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Li, B.; Peng, W.; Ma, Y.; Huang, Y.; Lan, X.; Lei, C.; Qi, X.; Liu, G.E.; Chen, H. LncRNA−MEG3 promotes bovine myoblast differentiation by sponging miR−135. J. Cell Physiol. 2019, 234, 18361–18370. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, S.; Wang, L.; Zhang, W.; Chen, W.; Lv, X.; Li, Y.; Hussain, Z.; Sun, W. Long Noncoding RNA (lncRNA) CTTN−IT1 Elevates Skeletal Muscle Satellite Cell Proliferation and Differentiation by Acting as ceRNA for YAP1 Through Absorbing miR−29a in Hu Sheep. Front. Genet. 2020, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gan, L.; Wu, T.; Feng, F.; Luo, D.; Gu, H.; Liu, S.; Sun, C. Adiponectin reduces ER stress−induced apoptosis through PPARalpha transcriptional regulation of ATF2 in mouse adipose. Cell Death Dis. 2016, 7, e2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La, Y.; Ma, F.; Ma, X.; Bao, P.; Chu, M.; Liang, C.; Guo, X.; Yin, M.; Li, J.; Yan, P. Different expression of LHR, PRLR, GH and IGF1 during testicular development of yak. Reprod. Domest. Anim. 2022, 57, 221–227. [Google Scholar] [CrossRef]
- El−Shorbagy, H.M.; Abdel−Aal, E.S.; Mohamed, S.A.; El−Ghor, A.A. Association of PRLR, IGF1, and LEP genes polymorphism with milk production and litter size in Egyptian Zaraibi goat. Trop Anim. Health Prod. 2022, 54, 321. [Google Scholar] [CrossRef]
- Arun, S.J.; Thomson, P.C.; Sheehy, P.A.; Khatkar, M.S.; Raadsma, H.W.; Williamson, P. Targeted Analysis Reveals an Important Role of JAK−STAT−SOCS Genes for Milk Production Traits in Australian Dairy Cattle. Front. Genet. 2015, 6, 342. [Google Scholar] [CrossRef] [Green Version]




| RNAs | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| HSP27 | CCATTCCCGTCACCTTCCA | CGCTGGGCTAAGGGTCTTTAC |
| HSP70 | CTGAACCCGCAGAACACG | CCTTGGTCTCCCCTTTGT |
| HSP90 | TGACCAGCACCTACGG | CACCAGGTCCTTGACG |
| MSTRG.18294.1 | CTAAACAGCAGCAACAGTGAGC | GGAAAGATTGAGGGCAGGA |
| ENSBTAT00000082977 | AGGTCTTGGAGGCATCTTATC | CGCATCACCAGGACGAAT |
| MSTRG.6166.1 | GAAGACACCACCATCGGAGA | CACCTGCCACAACGAAGAGTT |
| MSTRG.9352.1 | CTTTAGCATCATTCCTTCCACC | GGGATTATGACTGAACGCCTC |
| MSTRG.9951.4 | TGGGTCTTGCTGGATGCTC | TGTGACGGGTTCCCTGTTG |
| MSTRG.16208.1 | GATTGTGAGGCATCGTCC | AGGGCTGTCAGATTCAAGG |
| MSTRG.10780.12 | CCATTGATCGCCAGGGTT | TTCGGGAGGGACGCACAT |
| MSTRG.11238.1 | GTTTGGGTCTTTCCCTACTGC | ATGTGCCAAGTAACAGAGAAGC |
| MSTRG.11236.1 | TGGGTCTTTCCCTACTGCTCT | ATAAGTGGCTGATGTGCCAAG |
| β−Actin | TCACCAACTGGGACGACA | GCATACAGGGACAGCACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Li, S.; Zhai, Y.; Chang, H.; Han, Z. Preliminary Transcriptome Analysis of Long Noncoding RNA in Hypothalamic-Pituitary-Mammary Gland Axis of Dairy Cows under Heat Stress. Biomolecules 2023, 13, 390. https://doi.org/10.3390/biom13020390
Zeng H, Li S, Zhai Y, Chang H, Han Z. Preliminary Transcriptome Analysis of Long Noncoding RNA in Hypothalamic-Pituitary-Mammary Gland Axis of Dairy Cows under Heat Stress. Biomolecules. 2023; 13(2):390. https://doi.org/10.3390/biom13020390
Chicago/Turabian StyleZeng, Hanfang, Shujie Li, Yunfei Zhai, Haomiao Chang, and Zhaoyu Han. 2023. "Preliminary Transcriptome Analysis of Long Noncoding RNA in Hypothalamic-Pituitary-Mammary Gland Axis of Dairy Cows under Heat Stress" Biomolecules 13, no. 2: 390. https://doi.org/10.3390/biom13020390
APA StyleZeng, H., Li, S., Zhai, Y., Chang, H., & Han, Z. (2023). Preliminary Transcriptome Analysis of Long Noncoding RNA in Hypothalamic-Pituitary-Mammary Gland Axis of Dairy Cows under Heat Stress. Biomolecules, 13(2), 390. https://doi.org/10.3390/biom13020390






