The Role of Oxytocin and Vasopressin in Drug-Induced Reward—Implications for Social and Non-Social Factors
Abstract
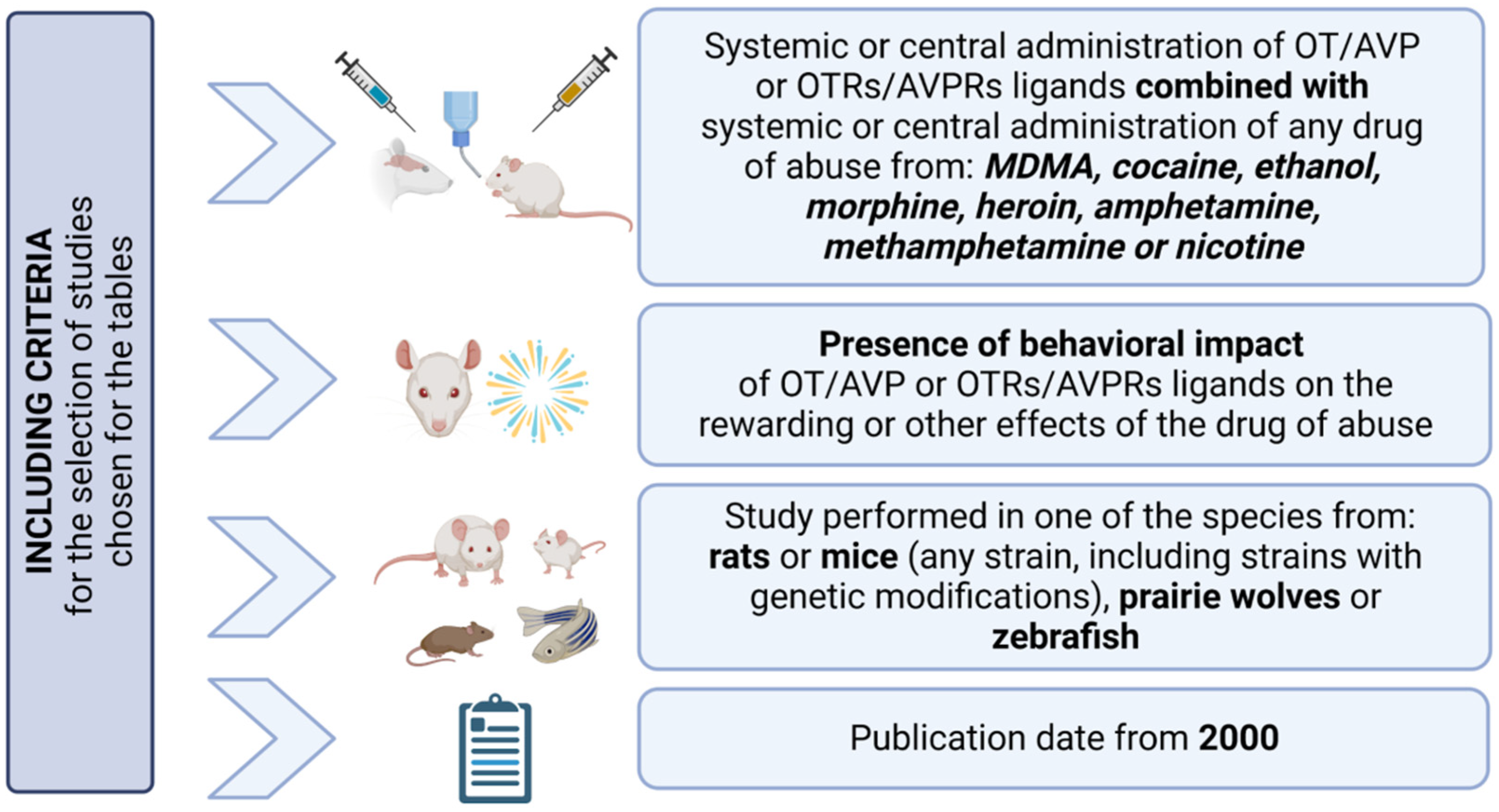
:1. Introduction
2. Neuromodulation of OT and AVP
3. The Impact of OT/AVP and OTRs/AVPRs Ligands on Behavioral and Rewarding Effects of Drugs of Abuse in Animal Models
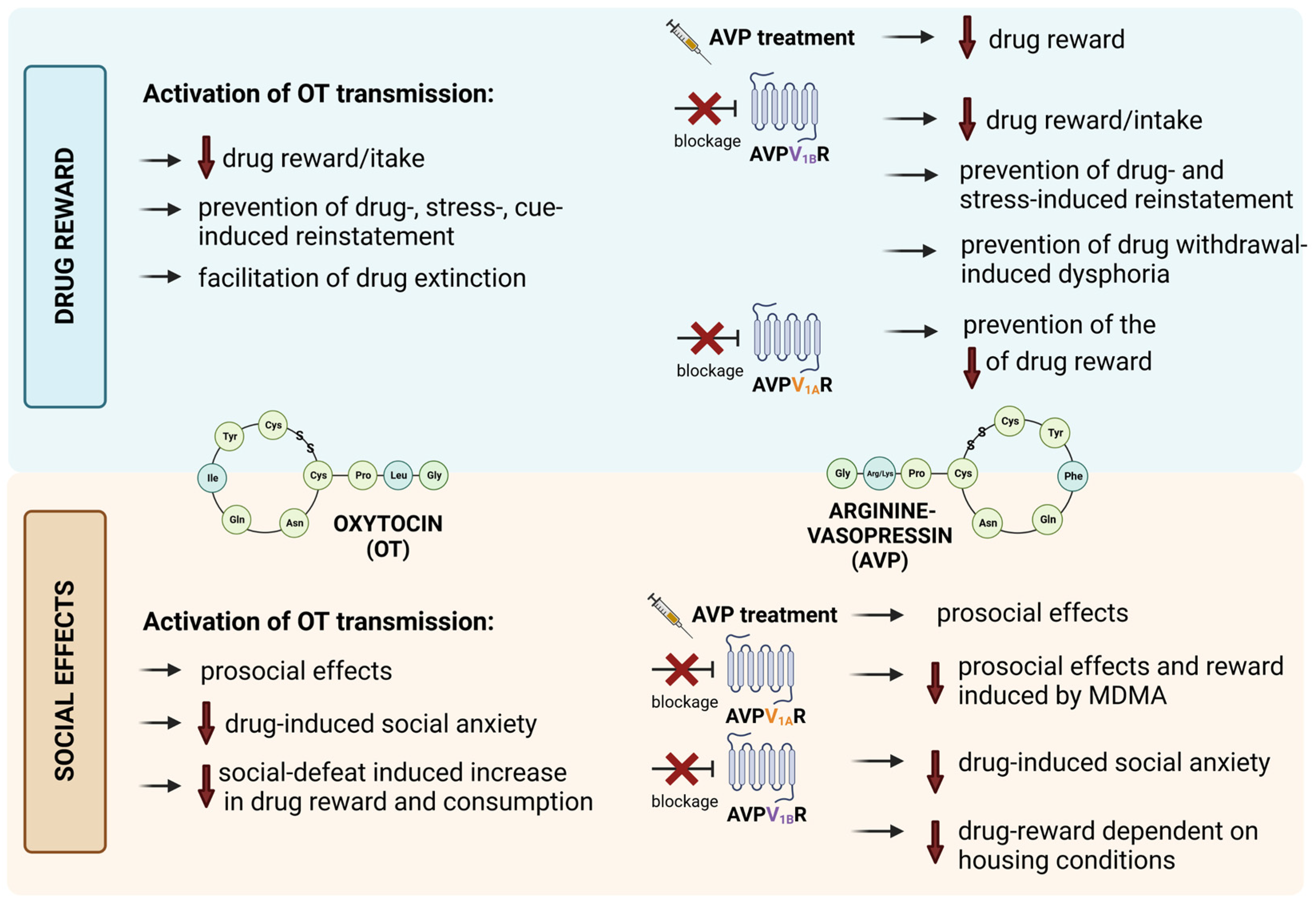
3.1. The Involvement of OT Transmission in Drug-Induced Reward
| Species and Sex | Drug of Abuse and Dose | OT Effective Dose | Behavioral Test | OT-Induced Effect | Ref. |
|---|---|---|---|---|---|
| Long–Evans rats ♂ | MDMA 2.5 mg/kg, ip | 0.25 mg/kg, ip | SI | ↑ in adjacent lying | [80] |
| Sprague Dawley rats ♂, ♀ | cocaine (♂) 0.2 mg/50 μL/bolus, iv (♀) 0.15 mg/50 μL/bolus, iv | 0.1, 0.3, 1, 3 mg/kg, ip | SA | ↓ cocaine intake (in ♀) | [41] |
| 1.0 mg/kg, ip | SA | ↓ cue-induced cocaine seeking following extinction (in ♀) | |||
| Sprague Dawley rats ♂, ♀ | cocaine 0.5 mg/kg/infusion, iv | 0.6 nmol/0.25 μL/side into NAc | SA | ↓ cue-induced reinstatement of cocaine seeking | [56] |
| 0.6 nmol/0.25 μL/side into PFC | SA | ↑ reinstatement to cocaine-associated cues | |||
| Sprague Dawley rats ♂ | cocaine 0.2 mg/50 μL/infusion, iv 10 mg/kg, ip, for cocaine- priming | 0.3, 1, 3 mg/kg, ip during SA | SA | ↓ cocaine intake | [42] |
| 0.3, 1 mg/kg, ip during reinstatement | SA | ↓ cocaine prime-induced (0.3 and 1 mg/kg) and cue-induced (1 mg/kg) reinstatement to cocaine seeking | |||
| Sprague Dawley rats ♂, ♀ | cocaine (♂) 0.2 mg/50 μL/infusion, iv (♀) 0.16 mg/50 μL/infusion, iv | 0.3, 1 mg/kg, ip | SA | ↓ cocaine-seeking during extinction and cue-induced reinstatement of cocaine-seeking | [58] |
| Sprague Dawley rats ♂, ♀ | cocaine (♂) 0.2 mg/50 μL/bolus, iv (♀) 0.15 mg /50 μL/bolus, iv | 1 mg/kg, ip | SA | ↓ cue-induced cocaine reinstatement | [55] |
| 3 μg/0.5 μL/side, icv | SA | ||||
| OF1 mice ♂ | cocaine 1, 10 mg/kg, ip | 1 mg/kg, ip | CPP | ↓ social defeat-induced increase of cocaine (1 mg/kg) rewarding effects; facilitation of the extinction of cocaine (10 mg/kg)-CPP; | [59] |
| cocaine 0.5 mg/kg/infusion, iv 10 mg/kg, ip for cocaine-priming | SA | facilitation of the extinction of cocaine-seeking behavior; ↓ of the cocaine-primed reinstatement of social defeat-induced cocaine-seeking | |||
| Sprague Dawley rats ♂ | cocaine 0.25 mg/0.1 mL.infusion, iv | 10 ng/10 μL, icv | SA | ↓ reinstatement of cue-induced cocaine seeking behavior | [60] |
| EPM | ↓ anxiety triggered by cue-induced reinstatement conditions and cocaine-paired conditioned locomotion | ||||
| Sprague Dawley rats ♂, ♀ | METH 0.1 mg/kg/50 μL/infusion, iv | 0.3, 1 mg/kg, ip | SA | ↓ cue-induced reinstatement (more in STs than in GTs) | [69] |
| Swiss mice ♂ | METH 2 mg/kg, ip | 0.5, 2.5 μg/μL, icv | locomotor activity test | ↓ METH-induced hyperactivity | [79] |
| Swiss mice ♂ | METH 2 mg/kg, ip | 0.5, 2.5 μg/μL into mPFC 2.5 μg/μL into DHC | CPP | ↓ stress-reinstained METH-induced CPP | [72] |
| Long–Evans rats ♀ | METH 0.06 mg/kg/infusion, iv; PR | 0.3 mg/kg, ip | SA | ↓ BP in individually- and socially-housed rats | [81] |
| Sprague Dawley rats ♂ | METH 2 mg/kg, ip | 2 mg/kg, ip | locomotor activity test | ↓ METH-induced hyperactivity | [82] |
| Sprague Dawley rats ♂ | METH 0.1 mg/kg/infusion, iv 1 mg/kg, ip for METH-priming | 1.5, 4.5 pmol (500 nL/side) into NAc core | SA | ↓ METH-primed reinstatement | [62] |
| Sprague Dawley rats ♂ | METH 0.1 mg/kg/infusion, iv 1 mg/kg, ip for METH-priming | 3.6 pmol (200 nL/side) into the STh | SA | ↓ METH-primed reinstatement | [61] |
| Sprague Dawley rats ♀ | METH 0.01, 0.03, 0.1, 0.3, 1 mg/kg, iv 1 mg/kg, ip for METH-priming | 1 mg/kg, ip (during adolescence) | SA | ↓ METH (0.03 mg/kg) self-administration (in PR and not FR); ↓ METH (1 mg/kg)-primed reinstatement | [44] |
| Sprague Dawley rats ♂ | METH 0.1 mg/kg/50 μL/infusion, iv 1 mg/kg, ip for METH-priming | 0.1 μg/side into the PrL | SA | ↓ cue-induced METH reinstatement | [68] |
| 1.0, 3.0 μg/side into the PrL | SA | ↓ METH-primed reinstatement | |||
| Sprague Dawley rats ♂ | METH 1 mg/kg, ip | 0.6 mg, ip | CPP | ↓ METH-induced CPP | [38] |
| 0.6 ng into the NAc core (0.5 μL/side) or into the STh (0.3 μL/side) | CPP | ||||
| Sprague Dawley rats ♂, ♀(with SDV) | METH 0.1 mg/kg/50 μL/infusion, iv 1 mg/kg, ip for METH-priming | 0.3, 1.0 mg/kg, ip | SA | ↓ METH intake in ♂ and ♀ (with SDV-prevention of this OT-induced suppressant effect); ↓ cue- and METH-primed reinstatement (with SDV-prevention of this OT-induced suppressant effect; only in ♂) | [45] |
| Sprague Dawley rats ♂ | METH 0.1 mg/kg, PR, iv 1 mg/kg, ip for METH-priming | 0.001, 0.01, 0.1, 0.3 and 1 mg/kg, ip, ascending (prior to self administration) or 1 mg/kg, ip (for reinstatement) | SA | ↓ METH intake; ↓ METH-induced hyperactivity; ↓ relapse to METH-seeking behavior | [43] |
| Sprague Dawley rats ♂, ♀ | METH (♀) 17.5 μg/50 μL/infusion, iv (♂) 20 μg/50 μL/infusion, iv | 1 mg/kg, ip | SA | ↓ cue-induced METH seeking in ♂ and ♀ | [63] |
| 0.6 nmol/0.25 μL/side into NaC core | SA | ||||
| C57BL/6 mice ♂, ♀ | METH 2 mg/kg, ip | 1.25 or 2.5 μg into hippocampus | CPP | ↓ context- and restraint stress-induced reinstatement of METH-CPP | [64] |
| Sprague Dawley rats ♂, ♀ | METH (♀) 17.5 μg/50 μL/infusion, iv (♂) 20 μg/50 μL/infusion, iv | 1 mg/kg ip | SA (with BE procedure) | ↓ METH-demand and ↓ reinstatement to METH-seeking in ♂ and ♀ | [66] |
| 0.6 μg/μL into NAc core | SA (with BE procedure) | ↓ METH-seeking | |||
| C57BL/6 mice ♂ | METH 2.0 mg/kg, ip | 2.5 μg, icv | MWM; NOR | ↓ METH-induced spatial memory enhancement ↓ METH-induced cognitive memory deficits | [83] |
| Sprague Dawley rats ♂, ♀ | METH 0.1 mg/kg/50 μL/infusion, iv (followed by ShA or LgA sessions) 1 mg/kg, ip for METH-priming | 1 mg/kg, ip (during METH abstinence) | SA EPM | ↓ incubation and METH-primed reinstatement in ♂ and ♀ ↓ of LgA-induced heightened anxiety phenotype effects | [70] |
| Sprague Dawley rats ♂ | METH 0.02 ug/50 μL/infusion, iv | 1 mg/kg, ip, before reinstatement | SA in rats pre-exposed to a predator odor threat (TMT) | ↓ METH-seeking in both saline- and TMT pre-exposed rats | [71] |
| 1 mg/kg, ip, prior to METH self-administration | ↓ stress-induced exacerbation of drug-seeking in TMT pre-exposed rats | ||||
| Long–Evans rats ♂ and/or ♀ | METH (♀) 17.5 μg/50 μL/infusion, iv (♂) 20 μg/50 μL/infusion, iv 1 mg/kg, ip for METH-priming | 1 mg/kg, ip | SA | ↓ METH seeking and ↓ PR responding for METH in ♀; ↓ cue-induced METH-reinstatement in ♀; ↓ METH-primed induced METH-seeking in ♂ and ♀ | [65] |
| Sprague Dawley rats ♂ | METH 0.1 mg /kg/50 μL/infusion, iv 1 mg/kg, ip for METH-priming | 1 mg/kg, ip | SA | ↓ METH-primed reinstatement | [67] |
| 3 pmol (500 nL/side) into the NAcc | SA | ||||
| Swiss mice ♂ | METH 2 mg/kg, ip | 0.1, 0.5, 2.5 μg/μL, icv | CPP | ↓ acquisition METH-CPP; facilitation of the extinction of METH-CPP; ↓ restraint stress-induced reinstatement to METH-CPP | [39] |
| Sprague Dawley rats ♂ (adolescent) | nicotine 25 μg/mL per bottle | 0.01 mg/kg, sc | two bottle free-choice paradigm | ↓ nicotine aversion after acclimation to nicotine solution | [78] |
| 1 mg/kg, sc | two bottle free-choice paradigm | ↑ nicotine intake | |||
| Wistar rats ♂ | nicotine 3.2 mg/kg/day, sc, in osmotic minipump | 0.06, 0.125, 0.25, 0.50, 0.75, or 1.0 mg/kg, ip | ICSS and somatic signs evaluation | ↓ withdrawal-induced elevations in somatic signs in nicotine-dependent rats with no effect on nicotine withdrawal-induced elevations in ICSS thresholds | [84] |
| Wistar rats ♂ | morphine 5 mg/kg, sc | 0.2 μg, icv | CPP | ↑ expression, but not acquisition, of morphine-induced CPP | [40] |
| Wistar rats ♂ | morphine 5 and 10 μg/site into the mPFC | 5 and 10 ng/site into the mPFC | MWM | ↓ morphine-induced decrease in memory related activities | [85] |
| Sprague Dawley rats ♂ | ethanol 10% and 15% | 0.1, 0.3, and 0.5mg/kg, ip | three-bottle choice (modified DID model) | ↓ ethanol consumption | [48] |
| ethanol 10% with gelatin | 0.3 mg/kg, ip | SA (oral) | |||
| C57BL/6J mice ♂, ♀ | ethanol 12%, 20 μL into the well | (a) 0.5, 1 mg/kg, ip (♀) and 1 mg/kg, ip (♂) (b) 1 mg/kg, ip (♂ and ♀) | SA (oral) | (a) ↓ of TMT-induced reinstatement of ethanol-seeking behavior; (b) ↓ of yohimbine-induced reinstatement of ethanol-seeking behavior | [74] |
| Wistar rats ♂ | ethanol 20% | 1 µg/5 µL, icv | two-bottle free-choice paradigm | ↓ ethanol consumption | [50] |
| C57BL/6J mice♂, ♀ | ethanol 3 and 6% | 3 mg/kg, ip | two-bottle free-choice paradigm with RFIDs | ↓ ethanol consumption on 3 out 4 treatment days | [51] |
| Wistar rats ♂ | ethanol 10%, 0.1 mL followed by exposition to ethanol vapor | 0.25, 0.5, and 1 mg/kg for FR; 0.125, and 0.25 mg/kg for PR | SA (oral) and alcohol vapor exposure | ↓ escalation of ethanol drinking (FR) ↓ enhanced motivation for ethanol (PR) | [52] |
| 0.25, 0.5 and 1 mg/kg/20 μL; intranasal for FR and 1 mg/kg/20 μL intranasal for PR | SA (oral) and ethanol vapor exposure | ||||
| 3, 10 and 30 μg, icv | SA (oral) and ethanolapor exposure | ↓ ethanol consumption in dependent rats | |||
| Sprague Dawley rats ♂ | ethanol 20% | 1 mg/kg, ip | SA (oral) | ↓ of yohimbine-induced reinstatement of ethanol-seeking behavior | [73] |
| 0.5 μg intra-CeA | SA (oral) | ||||
| Prairie voles ♂, ♀ | ethanol 15% | (a) 1, 3 and 10 mg/kg, ip (b) 3 mg/kg, ip | two-bottle free-choiceparadigm | (a) ↓ ethanol consumption (with restricted access to 15% ethanol) (b) ↓ ethanol consumption (with continuous access to 15% ethanol), depending on time of testing | [54] |
| Wistar rats ♂ | ethanol 15%, 1.5 g/kg, ip | 1 µg /5 μL, icv | OF; wire-hanging test;righting-reflex test | ↓ ethanol-induced motor impairment (sedation and ataxia) | [86] |
| C57BL/6N mice ♂ | ethanol2, 4, 6 and 8% (escalating) | 10 mg/kg, ip | two-bottle free-choice paradigm | ↓ ethanol consumption in control but not in CSC-triggered stressed mice | [49] |
| C57BL/6N mice ♂ | ethanol 20% | 1, 3, or 10 mg/kg, ip | binge-like DID | ↓ ethanol consumption | [46] |
| 1 mg/kg, ip | two-bottle free-choice paradigm | ↓ ethanol consumption | |||
| ethanol 12%, 20 μL to the well | 0.1, 0.3, or 1 mg/kg, ip (FR) and 0.3 mg/kg, ip (PR) | SA (oral) | ↓ ethanol consumption (FR) and ↓ motivation to seek ethanol reinforcement (PR) | ||
| Oxt-IRES-Cre mice without viral infusion ♂ | ethanol 20% | 1 mg/kg, ip | binge-like drinking (DID) | ↓ ethanol consumption | [47] |
| OF1 mice ♂ | ethanol 7.6%, 36 μLper nose poke | 1 mg/kg, ip | SA (oral) | ↓ (social-defeat)-induced increase in ethanol consumption | [53] |
| Species and Sex | Drug of Abuse and Dose | OTR Ligand and Dose | Behavioral Test | OTR Ligand-Induced Effect | Ref. |
|---|---|---|---|---|---|
| Sprague Dawley rats ♂ | cocaine 0.25 mg/0.1 mL/infusion, iv | Thr4,Gly7-oxytocin 10 nmol/10 μL, icv | SA | ↓ reinstatement of cue-induced cocaine seeking behavior | [60] |
| Wistar rats ♂ | ethanol 10%, 0.1 mL followed by exposition to ethanol vapor | PF-06655075 30 μg, icv | SA (oral) and ethanol vapor exposure | ↓ ethanol drinking in dependent rats | [52] |
| Sprague Dawley rats ♂, ♀ | ethanol adolescent intermittent exposure—4 g/kg every 48 h for a total of 11 exposure | WAY-267464 5 mg/kg, ip | SI | reversal of ethanol-induced social anxiety in ♂ | [87] |
| C57BL/6J mice ♂ | morphine 20–100 mg/kg/day—escalating, ip | carbetocin 6.4 mg/kg, ip | EPM, FST, sociability and social novelty test | ↓ withdrawal-induced negative emotional consequences (↓ of anxiety- and depressive-like and restoration of sociability behaviors) | [75] |
| morphine 10 mg/kg, sc | carbetocin 6.4 mg/kg, ip | CPP | prevention of stress-induced reinstatement to morphine-seeking | ||
| C57BL/6J mice ♂ | morphine 10 mg/kg, sc for conditioning; 2 mg/kg, ip for morphine-priming | carbetocin 6.4 mg/kg, ip | CPP | prevention of morphine priming-induced reinstatement to morphine CPP | [76] |
| C57BL/6 mice ♂ | ethanol 10%, 2 g/kg, ip | carbetocin 6.4 mg/kg, ip | CPP | ↓ acquisition of ethanol CPP; facilitation of extinction of ethanol-CPP; ↓ reinstatement induced by ethanol priming | [77] |
| Sprague Dawley rats♂, ♀ | MDMA 1.5 mg/kg, ip amphetamine 1 mg/kg, ip | carbetocin 2 and 20 mg/kg, ip | three-lever drug discrimination paradigm (MDMA/ amphetamine/saline) | ↑ MDMA lever presses—carbetocin generalized to the MDMA training cue; no effect on amphetamine lever selection | [88] |
| Swiss mice ♂ | ethanol 20%, 2 g/kg, ip | carbetocin 6.4 mg/kg, ip | CPP | mimicking of behavioral effects of EE on ethanol-CPP ↑ of ethanol-CPP | [89] |
| L,368,899 5 mg/kg, ip (during EE exposure but not during acquisition of ethanol CPP) | CPP | ↓ EE-induced ethanol CPP | |||
| C57BL/6J mice ♂ | MDMA 3 mg/kg, ip | L,368,899 10 mg/kg, ip | sociability test | ↓ prosocial effects of MDMA in highly sociable mice; no effect in low sociable mice | [90] |
| Oxt-IRES-Cre mice with intra-PVN infusion of active virus * ♂ | ethanol 20% | L,368,899 10 mg/kg, ip | binge-like drinking (DID) | reversal of (chemogenetic activation of PVN OT neurons)-induced ↓ of binge-like ethanol drinking | [47] |
| C57BL/6J mice ♂ | ethanol 20% | L,368,899 10 mg/kg, ip | binge-like drinking (DID) | ↓ of OT-induced reduction in binge-like ethanol consumption | [46] |
| Swiss mice ♂ | METH 2 mg/kg, ip | atosiban 2 μg/μL, icv | locomotor activity test | ↓ inhibitory effect of OT (0.5, 2.5 μg) on METH-induced hyperactivity in mice | [79] |
| Swiss mice ♂ | METH 2 mg/kg, ip | atosiban 10 μg/μL into mPFC | CPP | ↓ OT (2.5μg/μL into mPFC)-induced inhibition of stress-reinstained METH-induced CPP | [72] |
| Swiss mice ♂ | METH 2 mg/kg, ip | atosiban 2.0 μg/μL, icv | CPP | ↓ OT-induced effects (see Table 1) | [39] |
| OF1 mice ♂ | cocaine 1 mg/kg, ip | atosiban 1 mg/kg, ip | CPP | reversal of (positive social housing)-protective effect against increased cocaine reward | [91] |
| Sprague Dawley rats ♂, ♀ | MDMA 1.5 mg/kg, ip amphetamine 1 mg/kg, ip | atosiban 10 mg/kg, ip | three-lever drug discrimination paradigm (MDMA/ amphetamine/ saline) | disruption of MDMA- (but not AMP-) appropriate responding | [88] |
| Wistar rats ♂ | MDMA 5 mg/kg, ip | tocinoic acid 20 μg/μL, icv | SI | ↓ the facilitation of MDMA-induced social interactions | [92] |
3.2. The Involvement of AVP Transmission in Drug-Induced Reward
| Species and Sex | Drug of Abuse and Dose | AVP Effective Dose | Test | AVP-Induced Effect | Ref. |
|---|---|---|---|---|---|
| Long–Evans rats ♂ | MDMA | 0.0025 mg/kg, ip | SI | ↑ adjacent lying | [80] |
| 2.5 mg/kg, ip | |||||
| Wistar or Sprague Dawley rats ♂ | ethanol 4.5% (or CIE or modified CIE) | 4 μg or 0.4 μg/0.5 μL * into CEA | SI | ↑ ethanol withdrawal-induced social anxiety | [99] |
| Rats ** | amphetamine | 50 ng, icv | normal/abnormal behavior assessment *** | cross-sensitization of rats to amphetamine hyperlocomotion | [100] |
| Sprague Dawley rats ♂ | amphetamine | 0.2 ng/side into LS | CPP | ↓ expression of amphetamine-induced CPP | [93] |
| Species and Sex | Drug of Abuse and Dose | AVPR Ligand and Dose | Test | AVPR Ligand-Induced Effect | Ref. |
|---|---|---|---|---|---|
| adult zebrafish | MDMA 5 mg/kg im | SR49059 0.01, and 0.1 ng/kg, im | CPP | ↓ MDMA-induced CPP | [98] |
| SR49059 0.01, and 0.1 ng/kg, im | social preference test | ↓ MDMA-induced social preference | |||
| MDMA 10 mg/kg im | SR49059 0.1 and 1 ng/kg, im | novel tank diving test | ↓ MDMA-induced anxiolytic effect | ||
| MDMA 2.5 mg/kg im | SR49059 0.01 and 0.1 ng/kg, im | light-dark test | ↓ MDMA-induced anxiolytic effect | ||
| Sprague Dawley rats ♂ | METH 0.1 mg/kg/50 μL/ infusion, iv; 1 mg/kg, ip for METH-primed reinstatement | SR49059 1 mg/kg, ip | SA | ↓ OT-induced prevention of METH-primed reinstatement | [67] |
| Long–Evans rats ♂ | MDMA 5 mg/kg, ip | SR49059 1 mg/kg, ip | SI | ↓ MDMA-induced adjacent lying | [80] |
| Sprague Dawley rats ♂, ♀ | ethanol adolescent intermittent exposure—4 g/kg every 48 h for a total of 11 exposures | SSR149415 5, 10, 20 mg/kg, ip | SI | ↓ ethanol-induced social anxiety | [87] |
| Wistar rats ♂ | nicotine 0.4 mg/kg, sc | SSR149415 30 mg/kg, ip | locomotor activity test | ↓ expression of nicotine-induced sensitization | [101] |
| C57BL/6J mice ♂, ♀ | ethanol 15% | SSR149415 10 and 30 mg/kg ip | two-bottle choice paradigm with IA | ↓ ethanol intake and preference | [57] |
| SSR149415 1 and 3 mg/kg + naltrexone 1 mg/kg | |||||
| sP and sNP rats ♂ | ethanol 10% | SSR149415 30 mg/kg, ip | two-bottle choice paradigm | ↓ ethanol intake in sP rats | [95] |
| Wistar rats ♂ | nicotine 3.16 mg/kg/day in osmotic sc minipumps | SSR149415 0.1, 0.5, and 2 μg, icv (acute); 0.5 μg/day for 6 days, icv (chronic) | ICSS (mecamylamine-precipitated nicotine withdrawal) | chronic treatment: complete prevention of the elevations in brain reward thresholds linked with nicotine withdrawal (prevention of the nicotine withdrawal-caused dysphoria); acute treatment: partial prevention of nicotine withdrawal | [97] |
| C57BL/6N mice ♂ | morphine 10, 20 or 40 mg, sc (6 day progressive ratio) | SSR149415 10 mg/kg, ip | CPP | ↓ acquisition of morphine-CPP in the morphine only mice * (no effect on the acquisition of morphine CPP in the morphine cage-mate mice **) | [14] |
| Wistar rats ♂ | ethanol CIEV adjusted by controlling BALs | SSR149415 30 mg/kg, ip | SA | ↓ excessive levels of ethanol SA observed in dependent animals without affecting ethanol drinking in non-dependent animals | [94] |
| Fischer rats ♂ | heroin 0.05 mg/kg/infusion, iv 0.25 mg/kg, sc, for priming | SSR149415 30 mg/kg, ip | SA | ↓ foot shock-induced heroin reinstatement ↓ heroin-primed heroin reinstatement | [96] |
| Fischer rats ♂ | cocaine 45–90 mg/kg/day, ip (chronic binge pattern with EDC) | SSR149415 5 mg/kg, ip | chronic EDC binge cocaine with acute withdrawal paradigm | ↓ acute withdrawal-induced HPA axis activation (ACTH increase) after EDC | [102] |
| Wistar rats ♂ | ethanol 4.5% modified CIE or CIE | SSR149415 5 μg in 0.5 μL into CEA | SI | binary effect: ↓ of social interactions in control animals but reversal of ethanol withdrawal-induced decrease in social interactions | [99] |
3.3. The Relationship between Social Factor and OT/AVP Impact on the Effects of Drugs of Abuse
3.4. The Impact of OT/AVP and OTRs/AVPRs Ligands on Other Behavioral Effects of Drugs of Abuse
4. Interactions between OT/AVP Transmission, DA Release and Drug Reward System
5. Interactions between OT/AVP and Serotonergic Transmission
6. Clinical Trials of Intranasal OT in Drug Abuse
7. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report 2021: Trends and Developments; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Cole, S.L.; Hofford, R.S.; Evert, D.J.; Wellman, P.J.; Eitan, S. Social influences on morphine conditioned place preference in adolescent mice. Addict. Biol. 2013, 18, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.; Hicks, C.; Caminer, A.; Goodwin, J.; McGregor, I.S. Oxytocin and MDMA (‘Ecstasy’) enhance social reward in rats. Psychopharmacology 2015, 232, 2631–2641. [Google Scholar] [CrossRef]
- Thiel, K.J.; Sanabria, F.; Neisewander, J.L. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology 2009, 204, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Thiel, K.J.; Okun, A.C.; Neisewander, J.L. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008, 96, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Wronikowska, O.; Zykubek, M.; Kurach, Ł.; Michalak, A.; Boguszewska-Czubara, A.; Budzyńska, B. Vulnerability factors for mephedrone-induced conditioned place preference in rats-the impact of sex differences, social-conditioning and stress. Psychopharmacology 2021, 238, 2947–2961. [Google Scholar] [CrossRef]
- Bahr, S.J.; Hoffmann, J.P.; Yang, X. Parental and peer influences on the risk of adolescent drug use. J. Prim. Prev. 2005, 26, 529–551. [Google Scholar] [CrossRef]
- Simons-Morton, B.; Chen, R.S. Over time relationships between early adolescent and peer substance use. Addict. Behav. 2006, 31, 1211–1223. [Google Scholar] [CrossRef]
- Andrews, J.A.; Tildesley, E.; Hops, H.; Li, F. The influence of peers on young adult substance use. Health Psychol. 2002, 21, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Beloate, L.N.; Coolen, L.M. Influences of social reward experience on behavioral responses to drugs of abuse: Review of shared and divergent neural plasticity mechanisms for sexual reward and drugs of abuse. Neurosci. Biobehav. Rev. 2017, 83, 356–372. [Google Scholar] [CrossRef]
- Hosseinbor, M.; Yassini Ardekani, S.M.; Bakhshani, S.; Bakhshani, S. Emotional and social loneliness in individuals with and without substance dependence disorder. Int. J. High Risk Behav. Addict. 2014, 3, e22688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeinali, A.; Sharifi, H.; Enayati, M.; Asgari, P.; Pasha, G. The mediational pathway among parenting styles, attachment styles and self-regulation with addiction susceptibility of adolescents. J. Res. Med. Sci. 2011, 16, 1105–1121. [Google Scholar] [PubMed]
- Bachner-Melman, R.; Ebstein, R.P. The role of oxytocin and vasopressin in emotional and social behaviors. Handb. Clin. Neurol. 2014, 124, 53–68. [Google Scholar] [PubMed]
- Bates, M.L.S.; Hofford, R.S.; Emery, M.A.; Wellman, P.J.; Eitan, S. The role of the vasopressin system and dopamine D1 receptors in the effects of social housing condition on morphine reward. Drug Alcohol Depend. 2018, 188, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010, 65, 768–779. [Google Scholar] [CrossRef] [Green Version]
- Landgraf, R.; Neumann, I.D. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004, 25, 150–176. [Google Scholar] [CrossRef]
- Lozić, M.; Šarenac, O.; Murphy, D.; Japundžić-Žigon, N. Vasopressin, Central Autonomic Control and Blood Pressure Regulation. Curr. Hypertens. Rep. 2018, 20, 11. [Google Scholar] [CrossRef]
- Love, T.M. Oxytocin, motivation and the role of dopamine. Pharmacol. Biochem. Behav. 2014, 119, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Ross, H.E.; Young, L.J. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009, 30, 534–547. [Google Scholar] [CrossRef] [Green Version]
- Althammer, F.; Grinevich, V. Diversity of oxytocin neurons: Beyond magno- and parvocellular cell types? J. Neuroendocrinol. 2017, 30, e12549. [Google Scholar] [CrossRef]
- Grinevich, V.; Ludwig, M. The multiple faces of the oxytocin and vasopressin systems in the brain. J. Neuroendocrinol. 2021, 33, e13004. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitre, M.; Marlin, B.J.; Schiavo, J.K.; Morina, E.; Norden, S.E.; Hackett, T.A.; Aoki, C.J.; Chao, M.V.; Froemke, R.C. A distributed network for social cognition enriched for oxytocin receptors. J. Neurosci. 2016, 36, 2517–2535. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Qiu, L.; Xiao, W.; Ni, H.; Chen, L.; Wang, F.; Mai, W.; Wu, J.; Bao, A.; Hu, H.; et al. Reconstruction of the hypothalamoneurohypophysial system and functional dissection of magnocellular oxytocin neurons in the brain. Neuron 2021, 109, 331–346.e7. [Google Scholar] [CrossRef] [PubMed]
- Duque-Wilckens, N.; Torres, L.Y.; Yokoyama, S.; Minie, V.A.; Tran, A.M.; Petkova, S.P.; Hao, R.; Ramos-Maciel, S.; Rios, R.A.; Jackson, K.; et al. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc. Natl. Acad. Sci. USA 2020, 117, 26406–26413. [Google Scholar] [CrossRef]
- De Vries, G.J.; Buijs, R.M.; Van Leeuwen, F.W. Sex differences in vasopressin and other neurotransmitter systems in the brain. Prog. Brain Res. 1984, 61, 185–203. [Google Scholar]
- Zhang, L.; Hernandez, V.S.; Zetter, M.A.; Eiden, L.E. VGLUT-VGAT expression delineates functionally specialised populations of vasopressin-containing neurones including a glutamatergic perforant path-projecting cell group to the hippocampus in rat and mouse brain. J. Neuroendocrinol. 2020, 32, e12831. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Anagnostou, E. Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Tanizawa, O.; Mori, K.; Brownstein, M.J.; Okayama, H. Structure and expression of a human oxytocin receptor. Nature 1992, 356, 526–529. [Google Scholar] [CrossRef]
- Zingg, H.H.; Laporte, S.A. The oxytocin receptor. Trends Endocrinol. Metab. 2003, 14, 222–227. [Google Scholar] [CrossRef]
- De Keyzer, Y.; Auzan, C.; Lenne, F.; Beldjord, C.; Thibonnier, M.; Bertagna, X.; Clauser, E. Cloning and characterization of the human V3 pituitary vasopressin receptor. FEBS Lett. 1994, 356, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef] [PubMed]
- Thibonnier, M.; Coles, P.; Thibonnier, A.; Shoham, M. Molecular pharmacology and modeling of vasopressin receptors. Prog. Brain Res. 2002, 139, 179–196. [Google Scholar] [PubMed]
- Loup, F.; Tribollet, E.; Dubois-Dauphin, M.; Dreifuss, J.J. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991, 555, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.L.; Landry, D.W.; Granton, J.T. Science review: Vasopressin and the cardiovascular system part 1—Receptor physiology. Crit. Care 2003, 7, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, H.K. Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. Neuroscientist 2017, 23, 517–528. [Google Scholar] [CrossRef]
- Baracz, S.J.; Rourke, P.I.; Pardey, M.C.; Hunt, G.E.; McGregor, I.S.; Cornish, J.L. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behav. Brain Res. 2012, 228, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yang, J.Y.; Wang, F.; Zhao, Y.N.; Song, M.; Wu, C.F. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology 2009, 56, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Moaddab, M.; Hyland, B.I.; Brown, C.H. Oxytocin enhances the expression of morphine-induced conditioned place preference in rats. Psychoneuroendocrinology 2015, 53, 159–169. [Google Scholar] [CrossRef]
- Leong, K.C.; Zhou, L.; Ghee, S.M.; See, R.E.; Reichel, C.M. Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Exp. Clin. Psychopharmacol. 2016, 24, 55–64. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, W.L.; Young, A.B.; Lee, K.; McGinty, J.F.; See, R.E. Oxytocin reduces cocaine seeking and reverses chronic cocaine-induced changes in glutamate receptor function. Int. J. Neuropsychopharmacol. 2014, 18, pyu009. [Google Scholar] [CrossRef] [Green Version]
- Carson, D.S.; Cornish, J.L.; Guastella, A.J.; Hunt, G.E.; McGregor, I.S. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology 2010, 58, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.; Cornish, J.L.; Baracz, S.J.; Suraev, A.; McGregor, I.S. Adolescent pre-treatment with oxytocin protects against adult methamphetamine-seeking behavior in female rats. Addict. Biol. 2016, 21, 304–315. [Google Scholar] [CrossRef]
- Everett, N.A.; Turner, A.J.; Costa, P.A.; Baracz, S.J.; Cornish, J.L. The vagus nerve mediates the suppressing effects of peripherally administered oxytocin on methamphetamine self-administration and seeking in rats. Neuropsychopharmacology 2021, 46, 297–304. [Google Scholar] [CrossRef] [PubMed]
- King, C.E.; Griffin, W.C.; Luderman, L.N.; Kates, M.M.; McGinty, J.F.; Becker, H.C. Oxytocin Reduces Ethanol Self-Administration in Mice. Alcohol. Clin. Exp. Res. 2017, 41, 955–964. [Google Scholar] [CrossRef] [Green Version]
- King, C.E.; Griffin, W.C.; Lopez, M.F.; Becker, H.C. Activation of hypothalamic oxytocin neurons reduces binge-like alcohol drinking through signaling at central oxytocin receptors. Neuropsychopharmacology 2021, 46, 1950–1957. [Google Scholar] [CrossRef]
- MacFadyen, K.; Loveless, R.; DeLucca, B.; Wardley, K.; Deogan, S.; Thomas, C.; Peris, J. Peripheral oxytocin administration reduces ethanol consumption in rats. Pharm. Biochem. Behav. 2016, 140, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.; Slattery, D.A.; Flor, P.J.; Neumann, I.D.; Reber, S.O. Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addict. Biol. 2013, 18, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.T.; Bowen, M.T.; Bohrer, K.; McGregor, I.S.; Neumann, I.D. Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict. Biol. 2017, 22, 702–711. [Google Scholar] [CrossRef]
- Caruso, M.A.; Robins, M.T.; Fulenwider, H.D.; Ryabinin, A.E. Temporal analysis of individual ethanol consumption in socially housed mice and the effects of oxytocin. Psychopharmacology 2021, 238, 899–911. [Google Scholar] [CrossRef]
- Tunstall, B.J.; Kirson, D.; Zallar, L.J.; McConnell, S.A.; Vendruscolo, J.C.M.; Ho, C.P.; Oleata, C.S.; Khom, S.; Manning, M.; Lee, M.R.; et al. Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biol. 2019, 17, e2006421. [Google Scholar] [CrossRef] [PubMed]
- Reguilón, M.D.; Ferrer-Pérez, C.; Miñarro, J.; Rodríguez-Arias, M. Oxytocin reverses ethanol consumption and neuroinflammation induced by social defeat in male mice. Horm. Behav. 2021, 127, 104875. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.R.; Wenner, S.M.; Freestone, D.M.; Romaine, C.C.; Parian, M.C.; Christian, S.M.; Bohidar, A.E.; Ndem, J.R.; Vogel, I.R.; O’Kane, C.M. Oxytocin reduces alcohol consumption in prairie voles. Physiol. Behav. 2017, 179, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.C.; Freeman, L.R.; Berini, C.R.; Ghee, S.M.; See, R.E.; Reichel, C.M. Oxytocin Reduces Cocaine Cued Fos Activation in a Regionally Specific Manner. Int. J. Neuropsychopharmacol. 2017, 20, 844–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, R.A.; Logan, C.N.; Leong, K.C.; Peris, J.; Knackstedt, L.; Reichel, C.M. Regionally Specific Effects of Oxytocin on Reinstatement of Cocaine Seeking in Male and Female Rats. Int. J. Neuropsychopharmacol. 2018, 21, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Rubinstein, M.; Low, M.J.; Kreek, M.J. V1b Receptor Antagonist SSR149415 and Naltrexone Synergistically Decrease Excessive Alcohol Drinking in Male and Female Mice. Alcohol. Clin. Exp. Res. 2018, 42, 195–205. [Google Scholar] [CrossRef]
- Kohtz, A.S.; Lin, B.; Smith, M.E.; Aston-Jones, G. Attenuated cocaine-seeking after oxytocin administration in male and female rats. Psychopharmacology 2018, 235, 2051–2063. [Google Scholar] [CrossRef]
- Ferrer-Pérez, C.; Castro-Zavala, A.; Luján, M.Á.; Filarowska, J.; Ballestín, R.; Miñarro, J.; Valverde, O.; Rodríguez-Arias, M. Oxytocin prevents the increase of cocaine-related responses produced by social defeat. Neuropharmacology 2019, 146, 50–64. [Google Scholar] [CrossRef]
- Morales-Rivera, A.; Hernández-Burgos, M.M.; Martínez-Rivera, A.; Pérez-Colón, J.; Rivera, R.; Montalvo, J.; Rodríguez-Borrero, E.; Maldonado-Vlaar, C.S. Anxiolytic effects of oxytocin in cue-induced cocaine seeking behavior in rats. Psychopharmacology 2014, 231, 4145–4155. [Google Scholar] [CrossRef]
- Baracz, S.J.; Everett, N.A.; Cornish, J.L. The Involvement of Oxytocin in the Subthalamic Nucleus on Relapse to Methamphetamine-Seeking Behaviour. PLoS ONE 2015, 10, e0136132. [Google Scholar] [CrossRef] [Green Version]
- Baracz, S.J.; Everett, N.A.; McGregor, I.S.; Cornish, J.L. Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addict. Biol. 2016, 21, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Leong, K.C.; Berini, C.; Reichel, C.M. Antagonism of mGlu2/3 receptors in the nucleus accumbens prevents oxytocin from reducing cued methamphetamine seeking in male and female rats. Pharmacol. Biochem. Behav. 2017, 161, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Che, X.; Xu, T.; Luo, Y.; Yin, M.; Lu, X.; Wu, C.; Yang, J. Repeated oxytocin treatment during abstinence inhibited context- or restraint stress-induced reinstatement of methamphetamine-conditioned place preference and promoted adult hippocampal neurogenesis in mice. Exp. Neurol. 2022, 347, 113907. [Google Scholar] [CrossRef]
- Cox, B.M.; Young, A.B.; See, R.E.; Reichel, C.M. Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology 2013, 38, 2343–2353. [Google Scholar] [CrossRef] [Green Version]
- Cox, B.M.; Bentzley, B.S.; Regen-Tuero, H.; See, R.E.; Reichel, C.M.; Aston-Jones, G. Oxytocin Acts in Nucleus Accumbens to Attenuate Methamphetamine Seeking and Demand. Biol. Psychiatry 2017, 81, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Everett, N.A.; McGregor, I.S.; Baracz, S.J.; Cornish, J.L. The role of the vasopressin V1A receptor in oxytocin modulation of methamphetamine primed reinstatement. Neuropharmacology 2018, 133, 1–11. [Google Scholar] [CrossRef]
- Everett, N.; Baracz, S.; Cornish, J. Oxytocin treatment in the prelimbic cortex reduces relapse to methamphetamine-seeking and is associated with reduced activity in the rostral nucleus accumbens core. Pharmacol. Biochem. Behav. 2019, 183, 64–71. [Google Scholar] [CrossRef]
- Everett, N.A.; Carey, H.A.; Cornish, J.L.; Baracz, S.J. Sign tracking predicts cue-induced but not drug-primed reinstatement to methamphetamine seeking in rats: Effects of oxytocin treatment. J. Psychopharmacol. 2020, 34, 1271–1279. [Google Scholar] [CrossRef]
- Everett, N.A.; Baracz, S.J.; Cornish, J.L. The effect of chronic oxytocin treatment during abstinence from methamphetamine self-administration on incubation of craving, reinstatement, and anxiety. Neuropsychopharmacology 2020, 45, 597–605. [Google Scholar] [CrossRef]
- Ferland, C.L.; Reichel, C.M.; McGinty, J.F. Effects of oxytocin on methamphetamine-seeking exacerbated by predator odor pre-exposure in rats. Psychopharmacology 2016, 233, 1015–1024. [Google Scholar] [CrossRef] [Green Version]
- Han, W.Y.; Du, P.; Fu, S.Y.; Wang, F.; Song, M.; Wu, C.F.; Yang, J.Y. Oxytocin via its receptor affects restraint stress-induced methamphetamine CPP reinstatement in mice: Involvement of the medial prefrontal cortex and dorsal hippocampus glutamatergic system. Pharm. Biochem. Behav. 2014, 119, 80–87. [Google Scholar] [CrossRef]
- Ballas, H.S.; Wilfur, S.M.; Freker, N.A.; Leong, K.C. Oxytocin Attenuates the Stress-Induced Reinstatement of Alcohol-Seeking in Male Rats: Role of the Central Amygdala. Biomedicines 2021, 9, 1919. [Google Scholar] [CrossRef]
- King, C.E.; Becker, H.C. Oxytocin attenuates stress-induced reinstatement of alcohol seeking behavior in male and female mice. Psychopharmacology 2019, 236, 2613–2622. [Google Scholar] [CrossRef]
- Zanos, P.; Georgiou, P.; Wright, S.R.; Hourani, S.M.; Kitchen, I.; Winsky-Sommerer, R.; Bailey, A. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology 2014, 39, 855–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiou, P.; Zanos, P.; Garcia-Carmona, J.A.; Hourani, S.; Kitchen, I.; Kieffer, B.L.; Laorden, M.L.; Bailey, A. The oxytocin analogue carbetocin prevents priming-induced reinstatement of morphine-seeking: Involvement of dopaminergic, noradrenergic and MOPr systems. Eur. Neuropsychopharmacol. 2015, 25, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A. The oxytocin receptor impairs ethanol reward in mice. Physiol. Behav. 2015, 139, 321–327. [Google Scholar] [CrossRef]
- Lee, H.; Jang, M.; Noh, J. Oxytocin attenuates aversive response to nicotine and anxiety-like behavior in adolescent rats. Neurosci. Res. 2017, 115, 29–36. [Google Scholar] [CrossRef]
- Qi, J.; Yang, J.Y.; Song, M.; Li, Y.; Wang, F.; Wu, C.F. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2008, 376, 441–448. [Google Scholar] [CrossRef]
- Ramos, L.; Hicks, C.; Kevin, R.; Caminer, A.; Narlawar, R.; Kassiou, M.; McGregor, I.S. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: Involvement of the V1A receptor. Neuropsychopharmacology 2013, 38, 2249–2259. [Google Scholar] [CrossRef]
- Westenbroek, C.; Perry, A.N.; Jagannathan, L.; Becker, J.B. Effect of social housing and oxytocin on the motivation to self-administer methamphetamine in female rats. Physiol. Behav. 2019, 203, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.S.; Hunt, G.E.; Guastella, A.J.; Barber, L.; Cornish, J.L.; Arnold, J.C.; Boucher, A.A.; McGregor, I.S. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict. Biol. 2010, 15, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Y.; Yang, J.Y.; Dong, Y.X.; Hou, Y.; Liu, S.; Wu, C.F. Oxytocin inhibits methamphetamine-associated learning and memory alterations by regulating DNA methylation at the Synaptophysin promoter. Addict. Biol. 2020, 25, e12697. [Google Scholar] [CrossRef]
- Manbeck, K.E.; Shelley, D.; Schmidt, C.E.; Harris, A.C. Effects of oxytocin on nicotine withdrawal in rats. Pharmacol. Biochem. Behav. 2014, 116, 84–89. [Google Scholar] [CrossRef]
- Salighedar, R.; Erfanparast, A.; Tamaddonfard, E.; Soltanalinejad, F. Medial prefrontal cortex oxytocin-opioid receptors interaction in spatial memory processing in rats. Physiol. Behav. 2019, 209, 112599. [Google Scholar] [CrossRef]
- Bowen, M.T.; Peters, S.T.; Absalom, N.; Chebib, M.; Neumann, I.D.; McGregor, I.S. Oxytocin prevents ethanol actions at δ subunit-containing GABAA receptors and attenuates ethanol-induced motor impairment in rats. Proc. Natl. Acad. Sci. USA 2015, 112, 3104–3109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannenhoffer, C.A.; Kim, E.U.; Saalfield, J.; Werner, D.F.; Varlinskaya, E.I.; Spear, L.P. Oxytocin and vasopressin modulation of social anxiety following adolescent intermittent ethanol exposure. Psychopharmacology (Berl) 2018, 235, 3065–3077. [Google Scholar] [CrossRef]
- Broadbear, J.H.; Tunstall, B.; Beringer, K. Examining the role of oxytocin in the interoceptive effects of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) using a drug discrimination paradigm in the rat. Addict. Biol. 2011, 16, 202–214. [Google Scholar] [CrossRef]
- Rae, M.; Zanos, P.; Georgiou, P.; Chivers, P.; Bailey, A.; Camarini, R. Environmental enrichment enhances conditioned place preference to ethanol via an oxytocinergic-dependent mechanism in male mice. Neuropharmacology 2018, 138, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kuteykin-Teplyakov, K.; Maldonado, R. Looking for prosocial genes: ITRAQ analysis of proteins involved in MDMA-induced sociability in mice. Eur. Neuropsychopharmacol. 2014, 24, 1773–1783. [Google Scholar] [CrossRef]
- Ferrer-Pérez, C.; Reguilón, M.D.; Miñarro, J.; Rodríguez-Arias, M. Endogenous oxytocin is essential for the buffering effects of pair housing against the increase in cocaine reward induced by social stress. Physiol. Behav. 2020, 221, 112913. [Google Scholar] [CrossRef]
- Thompson, M.R.; Callaghan, P.D.; Hunt, G.E.; Cornish, J.L.; McGregor, I.S. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience 2007, 146, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Gárate-Pérez, M.F.; Méndez, A.; Bahamondes, C.; Sanhueza, C.; Guzmán, F.; Reyes-Parada, M.; Sotomayor-Zárate, R.; Renard, G.M. Vasopressin in the lateral septum decreases conditioned place preference to amphetamine and nucleus accumbens dopamine release. Addict. Biol. 2021, 26, e12851. [Google Scholar] [CrossRef]
- Edwards, S.; Guerrero, M.; Ghoneim, O.M.; Roberts, E.; Koob, G.F. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict. Biol. 2012, 17, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Litvin, Y.; Piras, A.P.; Pfaff, D.W.; Kreek, M.J. Persistent increase in hypothalamic arginine vasopressin gene expression during protracted withdrawal from chronic escalating-dose cocaine in rodents. Neuropsychopharmacology 2011, 36, 2062–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Leri, F.; Cummins, E.; Hoeschele, M.; Kreek, M.J. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology 2008, 33, 226–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Guzhva, L.; Ji, Y.; Bruijnzeel, A.W. Chronic treatment with the vasopressin 1b receptor antagonist SSR149415 prevents the dysphoria associated with nicotine withdrawal in rats. Behav. Brain Res. 2015, 292, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Ponzoni, L.; Braida, D.; Bondiolotti, G.; Sala, M. The Non-Peptide Arginine-Vasopressin v1a Selective Receptor Antagonist, SR49059, Blocks the Rewarding, Prosocial, and Anxiolytic Effects of 3,4-Methylenedioxymethamphetamine and Its Derivatives in Zebrafish. Front. Psychiatry 2017, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Harper, K.M.; Knapp, D.J.; Butler, R.K.; Cook, C.A.; Criswell, H.E.; Stuber, G.D.; Breese, G.R. Amygdala Arginine Vasopressin Modulates Chronic Ethanol Withdrawal Anxiety-Like Behavior in the Social Interaction Task. Alcohol. Clin. Exp. Res. 2019, 43, 2134–2143. [Google Scholar] [CrossRef]
- McBride, S.M.; Flynn, F.W. Centrally administered vasopressin cross-sensitizes rats to amphetamine and drinking hypertonic NaCl. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1452-8. [Google Scholar] [CrossRef]
- Goutier, W.; Kloeze, M.; McCreary, A.C. Nicotine-induced locomotor sensitization: Pharmacological analyses with candidate smoking cessation aids. Addict. Biol. 2016, 21, 234–241. [Google Scholar] [CrossRef]
- Zhou, Y.; Colombo, G.; Carai, M.A.; Ho, A.; Gessa, G.L.; Kreek, M.J. Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcohol. Clin. Exp. Res. 2011, 35, 1876–1883. [Google Scholar] [CrossRef]
- Bedi, G.; Hyman, D.; de Wit, H. Is ecstasy an “empathogen”? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol. Psychiatry 2010, 68, 1134–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrott, A.C. Human psychobiology of MDMA or ‘Ecstasy’: An overview of 25 years of empirical research. Hum. Psychopharmacol. 2013, 28, 289–307. [Google Scholar] [CrossRef]
- Pelloux, Y.; Giorla, E.; Montanari, C.; Baunez, C. Social modulation of drug use and drug addiction. Neuropharmacology 2019, 159, 107545. [Google Scholar] [CrossRef]
- Che, X.; Cai, J.; Liu, Y.; Xu, T.; Yang, J.; Wu, C. Oxytocin signaling in the treatment of drug addiction: Therapeutic opportunities and challenges. Pharmacol. Ther. 2021, 223, 107820. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.K.; Freels, T.G.; Prater, W.T.; Lester, D.B. Systemic oxytocin administration alters mesolimbic dopamine release in mice. Neuroscience 2019, 408, 226–238. [Google Scholar] [CrossRef]
- Young, K.A.; Liu, Y.; Gobrogge, K.L.; Wang, H.; Wang, Z. Oxytocin reverses amphetamine-induced deficits in social bonding: Evidence for an interaction with nucleus accumbens dopamine. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 8499–8506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.N.; Aldag, J.M.; Insel, T.R.; Young, L.J. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001, 21, 8278–8285. [Google Scholar] [CrossRef]
- Hysek, C.M.; Schmid, Y.; Simmler, L.D.; Domes, G.; Heinrichs, M.; Eisenegger, C.; Preller, K.H.; Quednow, B.B.; Liechti, M.E. MDMA enhances emotional empathy and prosocial behavior. Soc. Cogn. Affect. Neurosci. 2014, 9, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, M.G.; Francis, S.M.; Lee, R.; de Wit, H.; Jacob, S. Plasma oxytocin concentrations following MDMA or intranasal oxytocin in humans. Psychoneuroendocrinology 2014, 46, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Johns, J.M.; McMurray, M.S.; Joyner, P.W.; Jarrett, T.M.; Williams, S.K.; Cox, E.T.; Black, M.A.; Middleton, C.L.; Walker, C.H. Effects of chronic and intermittent cocaine treatment on dominance, aggression, and oxytocin levels in post-lactational rats. Psychopharmacology 2010, 211, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, M.S.; Joyner, P.W.; Middleton, C.W.; Jarrett, T.M.; Elliott, D.L.; Black, M.A.; Hofler, V.E.; Walker, C.H.; Johns, J.M. Intergenerational effects of cocaine on maternal aggressive behavior and brain oxytocin in rat dams. Stress 2008, 11, 398–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knobloch, H.S.; Grinevich, V. Evolution of oxytocin pathways in the brain of vertebrates. Front. Behav. Neurosci. 2014, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peris, J.; MacFadyen, K.; Smith, J.A.; de Kloet, A.D.; Wang, L.; Krause, E.G. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J. Comp. Neurol. 2017, 525, 1094–1108. [Google Scholar] [CrossRef]
- Li, J.; You, Z.; Chen, Z.; Song, C.; Lu, C. Chronic morphine treatment inhibits oxytocin release from the supraoptic nucleus slices of rats. Neurosci. Lett. 2001, 300, 54–58. [Google Scholar] [CrossRef]
- Bowen, M.T.; Neumann, I.D. Rebalancing the Addicted Brain: Oxytocin Interference with the Neural Substrates of Addiction. Trends Neurosci. 2017, 40, 691–708. [Google Scholar] [CrossRef]
- Light, K.C.; Grewen, K.M.; Amico, J.A.; Boccia, M.; Brownley, K.A.; Johns, J.M. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict. Behav. 2004, 29, 1541–1564. [Google Scholar] [CrossRef] [Green Version]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Allard, J.; Wayman, C.; Douglas, A.J. Dopamine-oxytocin interactions in penile erection. Eur. J. Neurosci. 2009, 30, 2151–2164. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Douglas, A.J. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci. Ther. 2010, 16, e92–e123. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Carlsson, J.; Ambrogini, P.; Narvaez, M.; Wydra, K.; Tarakanov, A.O.; Li, X.; Millón, C.; Ferraro, L.; Cuppini, R.; et al. Understanding the role of GPCR heteroreceptor complexes inmodulating the brain networks in health and disease. Front. Cell. Neurosci. 2017, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Mora, M.P.; Pérez-Carrera, D.; Crespo-Ramírez, M.; Tarakanov, A.; Fuxe, K.; Borroto-Escuela, D.O. Signaling in dopamine D2 receptor-oxytocin receptor heterocomplexes and its relevance for the anxiolytic effects of dopamine and oxytocin interactions in the amygdala of the rat. Biochim. Biophys. Acta 2016, 1862, 2075–2085. [Google Scholar] [CrossRef]
- Romero-Fernandez, W.; Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol. Psychiatry 2013, 18, 849–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Z.; Wang, H.; d’Oleire Uquillas, F.; Wang, X.; Ding, J.; Chen, H. Definition of Substance and Non-substance Addiction. Adv. Exp. Med. Biol. 2017, 1010, 21–41. [Google Scholar] [PubMed]
- Ahumada, C.; Bahamondes, C.; Cerda, C.A.; Silva, R.A.; Cruz, G.; Moya, P.R.; Sotomayor-Zárate, R.; Renard, G.M. Amphetamine treatment affects the extra-hypothalamic vasopressinergic system in a sex- and nucleus-dependent manner. J. Neuroendocrinol. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Borrero, E.; Rivera-Escalera, F.; Candelas, F.; Montalvo, J.; Muñoz-Miranda, W.J.; Walker, J.R.; Maldonado-Vlaar, C.S. Arginine vasopressin gene expression changes within the nucleus accumbens during environment elicited cocaine-conditioned response in rats. Neuropharmacology 2010, 58, 88–101. [Google Scholar] [CrossRef] [Green Version]
- Dölen, G.; Darvishzadeh, A.; Huang, K.W.; Malenka, R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013, 501, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Costa, G.; Gołembiowska, K. Neurotoxicity of MDMA: Main effects and mechanisms. Exp. Neurol. 2022, 347, 113894. [Google Scholar] [CrossRef]
- Yoshida, M.; Takayanagi, Y.; Inoue, K.; Kimura, T.; Young, L.J.; Onaka, T.; Nishimori, K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 2009, 29, 2259–2271. [Google Scholar] [CrossRef] [Green Version]
- Grieb, Z.A.; Lonstein, J.S. Oxytocin interactions with central dopamine and serotonin systems regulate different components of motherhood. Phil. Trans. R. Soc. B 2022, 377. [Google Scholar] [CrossRef] [PubMed]
- Dölen, G. Autism: Oxytocin, serotonin, and social reward. Soc. Neurosci. 2015, 10, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, H.; Wang, P.; Cui, W.; Xu, K.; Chen, D.; Hu, M.; Li, Z.; Geng, X.; Wei, S. Oxytocin and serotonin in the modulation of neural function: Neurobiological underpinnings of autism-related behavior. Front. Neurosci. 2022, 16, 919890. [Google Scholar] [CrossRef]
- Nagano, M.; Takumi, T.; Suzuki, H. Critical roles of serotonin-oxytocin interaction during the neonatal period in social behavior in 15q dup mice with autistic traits. Sci. Rep. 2018, 8, 13675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, D.S.; Lischke, A.; Grace, S.; Scheele, D.; Ma, Y.; Becker, B. Advances in the field of intranasal oxytocin research: Lessons learned and future directions for clinical research. Mol. Psychiatry 2021, 26, 80–91. [Google Scholar] [CrossRef]
- Moeini, M.; Omidi, A.; Sehat, M.; Banafshe, H.R. The effects of oxytocin on withdrawal, craving and stress response in heroin-dependent patients: A randomized, double-blind clinical trial. Eur. Addict. Res. 2019, 25, 41–47. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Arcuni, P.A.; Weinstein, D.; Woolley, J.D. Intranasal Oxytocin Selectively Modulates Social Perception, Craving, and Approach Behavior in Subjects With Alcohol Use Disorder. J. Addict. Med. 2016, 10, 182–189. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Smedley, K.L.; Leserman, J.; Jarskog, L.F.; Rau, S.W.; Kampov-Polevoi, A.; Garbutt, J.C. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol. Clin. Exp. Res. 2013, 37, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Stauffer, C.S.; Musinipally, V.; Suen, A.; Lynch, K.L.; Shapiro, B.; Woolley, J.D. A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addict. Res. Theory 2016, 24, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Van Hedger, K.; Kushner, M.J.; Lee, R.; de Wit, H. Oxytocin reduces cigarette consumption in daily smokers. Nicotine Tob. Res. 2019, 21, 799–804. [Google Scholar] [CrossRef]
- McRae-Clark, A.L.; Baker, N.L.; Maria, M.M.; Brady, K.T. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: A pilot study. Psychopharmacology 2013, 228, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melby, K.; Grawe, R.W.; Aamo, T.O.; Salvesen, O.; Spigset, O. Effect of intranasal oxytocin on alcohol withdrawal syndrome: A randomized placebo-controlled double-blind clinical trial. Drug Alcohol Depend. 2019, 197, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Glassman, M.; King-Casas, B.; Kelly, D.L.; Stein, E.A.; Schroeder, J.; Salmeron, B.J. Complexity of oxytocins effects in a chronic cocaine dependent population. Eur. Neuropsychopharmacol. 2014, 24, 1483–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wronikowska-Denysiuk, O.; Mrozek, W.; Budzyńska, B. The Role of Oxytocin and Vasopressin in Drug-Induced Reward—Implications for Social and Non-Social Factors. Biomolecules 2023, 13, 405. https://doi.org/10.3390/biom13030405
Wronikowska-Denysiuk O, Mrozek W, Budzyńska B. The Role of Oxytocin and Vasopressin in Drug-Induced Reward—Implications for Social and Non-Social Factors. Biomolecules. 2023; 13(3):405. https://doi.org/10.3390/biom13030405
Chicago/Turabian StyleWronikowska-Denysiuk, Olga, Weronika Mrozek, and Barbara Budzyńska. 2023. "The Role of Oxytocin and Vasopressin in Drug-Induced Reward—Implications for Social and Non-Social Factors" Biomolecules 13, no. 3: 405. https://doi.org/10.3390/biom13030405
APA StyleWronikowska-Denysiuk, O., Mrozek, W., & Budzyńska, B. (2023). The Role of Oxytocin and Vasopressin in Drug-Induced Reward—Implications for Social and Non-Social Factors. Biomolecules, 13(3), 405. https://doi.org/10.3390/biom13030405






