Circulating Tumor Cell Detection by Liquid Biopsy during Early-Stage Endometrial Cancer Surgery: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
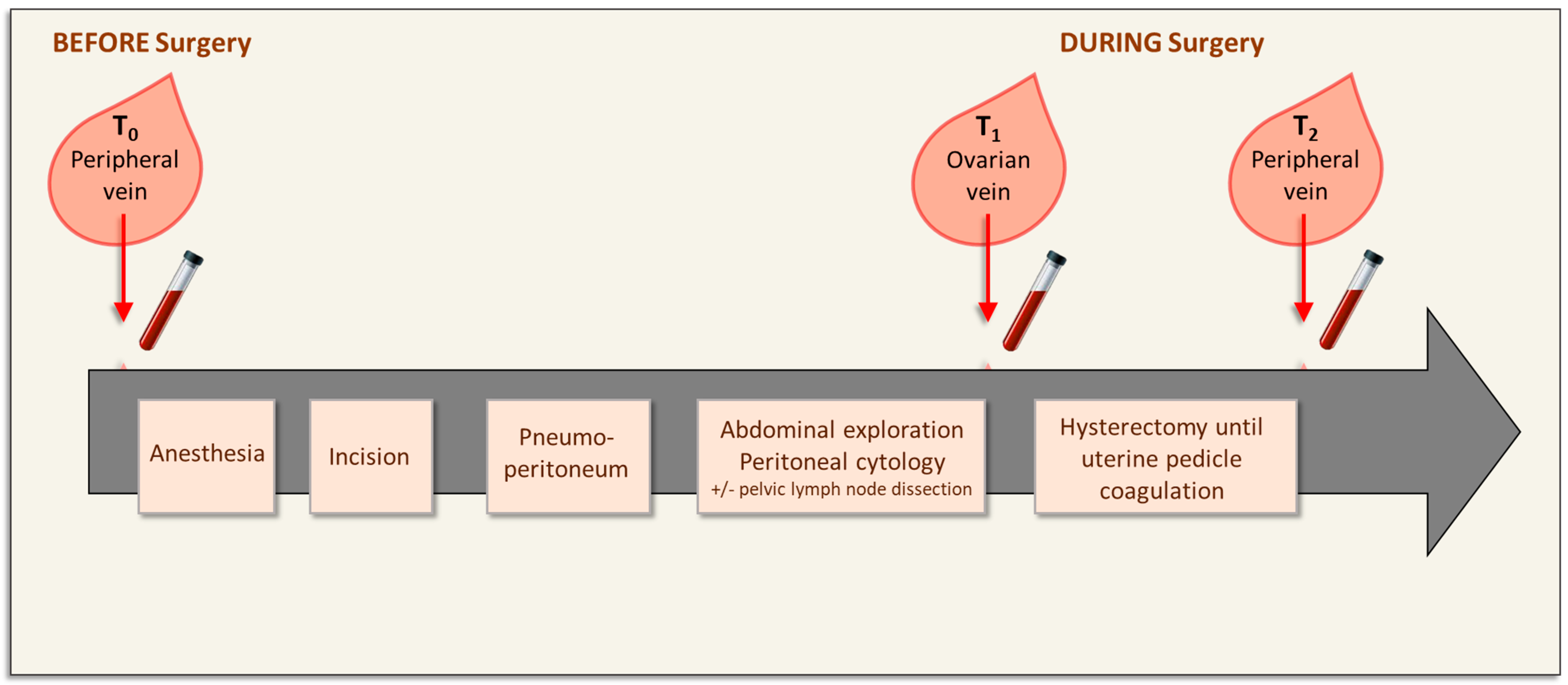
2.2. Surgical Procedure
2.3. Blood Sampling
2.4. CTC Enumeration and ER Expression Detection
2.5. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Surgical Procedure and Blood Collection
3.3. CTC Enumeration before, during, and after Surgery and ER Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AlHilli, M.M.; Podratz, K.C.; Dowdy, S.C.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Kumar, S.; Keeney, G.L.; Cliby, W.A.; Mariani, A. Preoperative biopsy and intraoperative tumor diameter predict lymph node dissemination in endometrial cancer. Gynecol. Oncol. 2013, 128, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Brakenhoff, R.H. Dissecting the metastatic cascade. Nat. Rev. Cancer 2004, 4, 448–456. [Google Scholar] [CrossRef]
- Lemech, C.R.; Ensell, L.; Paterson, J.C.; Eminowicz, G.; Lowe, H.; Arora, R.; Arkenau, H.T.; Widschwendter, M.; MacDonald, N.; Olaitan, A.; et al. Enumeration and Molecular Characterisation of Circulating Tumour Cells in Endometrial Cancer. Oncology 2016, 91, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Liu, M.C.; Dowdy, S.C.; Cliby, W.A.; Kerr, S.E.; Kalli, K.R.; Kipp, B.R.; Halling, K.C.; Campion, M.B.; Mariani, A. Detection of circulating tumor cells in high-risk endometrial cancer. Anticancer Res. 2015, 35, 683–687. [Google Scholar]
- Ji, X.Q.; Sato, H.; Tanaka, H.; Konishi, Y.; Fujimoto, T.; Takahashi, O.; Tanaka, T. Real-time quantitative RT-PCR detection of disseminated endometrial tumor cells in peripheral blood and lymph nodes using the LightCycler System. Gynecol. Oncol. 2006, 100, 355–360. [Google Scholar] [CrossRef]
- Joosse, S.A.; Beyer, B.; Gasch, C.; Nastały, P.; Kuske, A.; Isbarn, H.; Horst, L.J.; Hille, C.; Gorges, T.M.; Cayrefourcq, L.; et al. Tumor-Associated Release of Prostatic Cells into the Blood after Transrectal Ultrasound-Guided Biopsy in Patients with Histologically Confirmed Prostate Cancer. Clin. Chem. 2020, 66, 161–168. [Google Scholar] [CrossRef]
- Bidard, F.C.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, J.A.; Stebbing, J.; et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Bidard, F.C.; Jacot, W.; Kiavue, N.; Dureau, S.; Kadi, A.; Brain, E.; Bachelot, T.; Bourgeois, H.; Goncalves, A.; Ladoire, S.; et al. Efficacy of Circulating Tumor Cell Count-Driven vs Clinician-Driven First-line Therapy Choice in Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: The STIC CTC Randomized Clinical Trial. JAMA Oncol. 2021, 7, 34–41. [Google Scholar] [CrossRef]
- Lucci, A.; Hall, C.S.; Lodhi, A.K.; Bhattacharyya, A.; Anderson, A.E.; Xiao, L.; Bedrosian, I.; Kuerer, H.M.; Krishnamurthy, S. Circulating tumour cells in non-metastatic breast cancer: A prospective study. Lancet Oncol. 2012, 13, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F.; Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Miller, M.C.; Matera, J.; Allard, W.J.; Doyle, G.V.; Terstappen, L.W. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin. Cancer Res. 2006, 12, 4218–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, T.; Sun, X.; Shan, B.; Wang, J.; Liu, Y.; Gu, S.L.; Wang, Y.D. Detection of circulating tumour cells may add value in endometrial cancer management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Fishman, A.; Zemer, R.; Zimlichman, S.; Altaras, M.M. Detection of tumor circulating cells by cytokeratin 20 in the blood of patients with endometrial carcinoma. Gynecol. Oncol. 2000, 78, 352–355. [Google Scholar] [CrossRef]
- Alonso-Alconada, L.; Muinelo-Romay, L.; Madissoo, K.; Diaz-Lopez, A.; Krakstad, C.; Trovik, J.; Wik, E.; Hapangama, D.; Coenegrachts, L.; Cano, A.; et al. Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer. Mol. Cancer 2014, 13, 223. [Google Scholar] [CrossRef] [Green Version]
- Buscail, E.; Chiche, L.; Laurent, C.; Vendrely, V.; Denost, Q.; Denis, J.; Thumerel, M.; Lacorte, J.M.; Bedel, A.; Moreau-Gaudry, F.; et al. Tumor-proximal liquid biopsy to improve diagnostic and prognostic performances of circulating tumor cells. Mol. Oncol. 2019, 13, 1811–1826. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Jiao, L.R.; Apostolopoulos, C.; Jacob, J.; Szydlo, R.; Johnson, N.; Tsim, N.; Habib, N.A.; Coombes, R.C.; Stebbing, J. Unique localization of circulating tumor cells in patients with hepatic metastases. J. Clin. Oncol. 2009, 27, 6160–6165. [Google Scholar] [CrossRef]
- Tien, Y.W.; Kuo, H.C.; Ho, B.I.; Chang, M.C.; Chang, Y.T.; Cheng, M.F.; Chen, H.L.; Liang, T.Y.; Wang, C.F.; Huang, C.Y.; et al. A High Circulating Tumor Cell Count in Portal Vein Predicts Liver Metastasis From Periampullary or Pancreatic Cancer: A High Portal Venous CTC Count Predicts Liver Metastases. Medicine 2016, 95, e3407. [Google Scholar] [CrossRef]
- Kiss, I.; Kolostova, K.; Matkowski, R.; Jędryka, M.; Czekański, A.; Pavlasek, J.; Bobek, V. Correlation Between Disease Stage and the Presence of Viable Circulating Tumor Cells in Endometrial Cancer. Anticancer Res. 2018, 38, 2983–2987. [Google Scholar] [CrossRef]
- Deneve, E.; Riethdorf, S.; Ramos, J.; Nocca, D.; Coffy, A.; Daures, J.P.; Maudelonde, T.; Fabre, J.M.; Pantel, K.; Alix-Panabieres, C. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 2013, 59, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Buscail, E.; Maulat, C.; Muscari, F.; Chiche, L.; Cordelier, P.; Dabernat, S.; Alix-Panabieres, C.; Buscail, L. Liquid Biopsy Approach for Pancreatic Ductal Adenocarcinoma. Cancers 2019, 11, 852. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhu, D.; Tang, X.; Lu, D.; Qiu, X.; Li, B.; Lin, D.; Li, L.; Liu, J.; Zhou, Q. Circulating tumor cells in pulmonary vein and peripheral arterial provide a metric for PD-L1 diagnosis and prognosis of patients with non-small cell lung cancer. PLoS ONE 2019, 14, e0220306. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Tripathy, D.; Frenkel, E.P.; Shete, S.; Naftalis, E.Z.; Huth, J.F.; Beitsch, P.D.; Leitch, M.; Hoover, S.; Euhus, D.; et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 2004, 10, 8152–8162. [Google Scholar] [CrossRef] [Green Version]
- van Weelden, W.J.; Reijnen, C.; Küsters-Vandevelde, H.V.N.; Bulten, J.; Bult, P.; Leung, S.; Visser, N.C.M.; Santacana, M.; Bronsert, P.; Hirschfeld, M.; et al. The cutoff for estrogen and progesterone receptor expression in endometrial cancer revisited: A European Network for Individualized Treatment of Endometrial Cancer collaboration study. Hum. Pathol. 2021, 109, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Amintas, S.; Bedel, A.; Moreau-Gaudry, F.; Boutin, J.; Buscail, L.; Merlio, J.P.; Vendrely, V.; Dabernat, S.; Buscail, E. Circulating Tumor Cell Clusters: United We Stand Divided We Fall. Int. J. Mol. Sci. 2020, 21, 2653. [Google Scholar] [CrossRef] [Green Version]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Calori, G.; Podratz, K.C. Hematogenous dissemination in corpus cancer. Gynecol. Oncol. 2001, 80, 233–238. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Sawabata, N.; Nakamura, T.; Kawaguchi, T.; Watanabe, T.; Ouji, N.S.; Ito, T.; Taniguchi, S. Circulating tumor cells detected only after surgery for non-small cell lung cancer: Is it a predictor of recurrence? J. Thorac. Dis. 2020, 12, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Wind, J.; Tuynman, J.B.; Tibbe, A.G.; Swennenhuis, J.F.; Richel, D.J.; van Berge Henegouwen, M.I.; Bemelman, W.A. Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. Eur. J. Surg. Oncol. 2009, 35, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, E.C.; Lee, M.J.; Alarcon, S.V.; Lee, S.; Hoang, A.N.; Walton Diaz, A.; Chelluri, R.; Vourganti, S.; Trepel, J.B.; Pinto, P.A. Lack of Impact of Robotic Assisted Laparoscopic Radical Prostatectomy on Intraoperative Levels of Prostate Cancer Circulating Tumor Cells. J. Urol. 2016, 195, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Guo, Q.; Li, Y.H.; Sun, Z.Q.; Li, T.T.; Zhang, W.X.; Xiang, S.S.; Li, H.F. Effects of Minimally Invasive Esophagectomy and Open Esophagectomy on Circulating Tumor Cell Level in Elderly Patients with Esophageal Cancer. World J. Surg. 2016, 40, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.B.; Ge, M.J. The Effects of Different Surgical Approaches on the Perioperative Level of Circulating Tumor Cells in Patients with Non-Small Cell Lung Cancer. Thorac. Cardiovasc. Surg. 2016, 64, 515–519. [Google Scholar] [CrossRef]


| Characteristics | Values |
|---|---|
| Age, median (min; max) | 70 (46; 86) |
| FIGO stage, N (%) | |
| IA | 5 (50) |
| IB | 5 (50) |
| Biopsy grade, N (%) | |
| 1 | 5 (50) |
| 2 | 5 (50) |
| Tumor size (mm), median (min; max) | 28 (8; 37) |
| Pelvic lymph node dissection, N (%) | |
| No | 2 (20) |
| Sentinel lymph node biopsy | 6 (60) |
| Pelvic lymphadenectomy | 2 (20) |
| Characteristics | N | Median (Min–Max) | p |
|---|---|---|---|
| Age | 0.59 | ||
| <70 years | 4 | 37 (0–91) | |
| ≥70 years | 6 | 11 (0–65) | |
| FIGO stage | 0.08 | ||
| IA | 3 | 72 (27–91) | |
| IB | 5 | 2 (0–65) | |
| II | 2 | 3 (0–6) | |
| Biopsy grade | 0.97 | ||
| 1 | 4 | 15 (0–72) | |
| 2 | 5 | 15 (0–91) | |
| 3 | 1 | 6 (-) | |
| Tumor size (mm) | |||
| <30mm | 4 | 40 (2–72) | 0.39 |
| 30mm | 6 | 4 (0–91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francini, S.; Duraes, M.; Rathat, G.; Macioce, V.; Mollevi, C.; Pages, L.; Ferrer, C.; Cayrefourcq, L.; Alix-Panabières, C. Circulating Tumor Cell Detection by Liquid Biopsy during Early-Stage Endometrial Cancer Surgery: A Pilot Study. Biomolecules 2023, 13, 428. https://doi.org/10.3390/biom13030428
Francini S, Duraes M, Rathat G, Macioce V, Mollevi C, Pages L, Ferrer C, Cayrefourcq L, Alix-Panabières C. Circulating Tumor Cell Detection by Liquid Biopsy during Early-Stage Endometrial Cancer Surgery: A Pilot Study. Biomolecules. 2023; 13(3):428. https://doi.org/10.3390/biom13030428
Chicago/Turabian StyleFrancini, Sarah, Martha Duraes, Gauthier Rathat, Valérie Macioce, Caroline Mollevi, Laurence Pages, Catherine Ferrer, Laure Cayrefourcq, and Catherine Alix-Panabières. 2023. "Circulating Tumor Cell Detection by Liquid Biopsy during Early-Stage Endometrial Cancer Surgery: A Pilot Study" Biomolecules 13, no. 3: 428. https://doi.org/10.3390/biom13030428
APA StyleFrancini, S., Duraes, M., Rathat, G., Macioce, V., Mollevi, C., Pages, L., Ferrer, C., Cayrefourcq, L., & Alix-Panabières, C. (2023). Circulating Tumor Cell Detection by Liquid Biopsy during Early-Stage Endometrial Cancer Surgery: A Pilot Study. Biomolecules, 13(3), 428. https://doi.org/10.3390/biom13030428






