GABAB Receptor Activation Affects Eye Growth in Chickens with Visually Induced Refractive Errors
Abstract
:1. Introduction
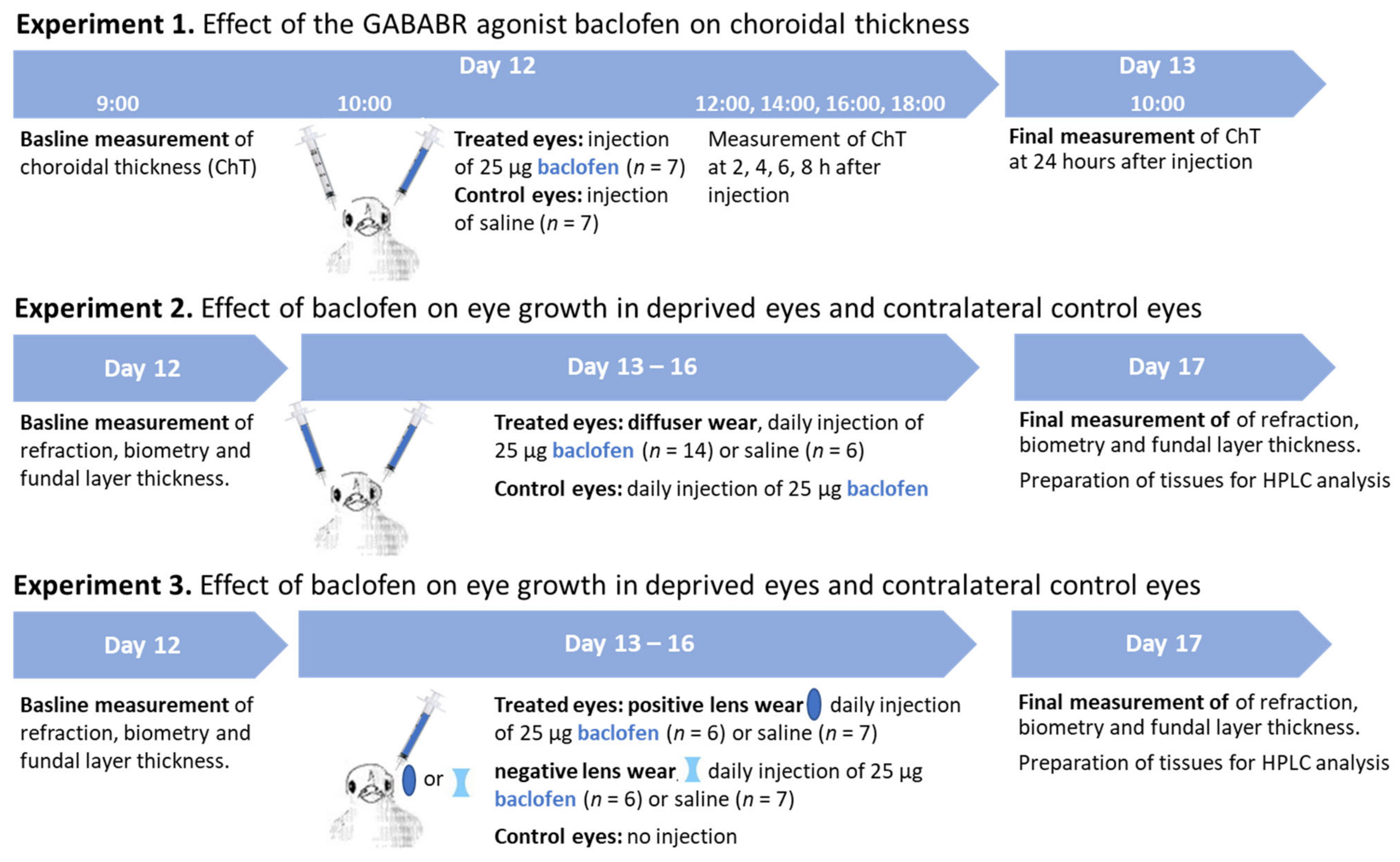
2. Materials and Methods
2.1. Animals
2.2. Treatments
2.3. Measurements
2.3.1. Refractive Error (RE) and Ocular Biometry
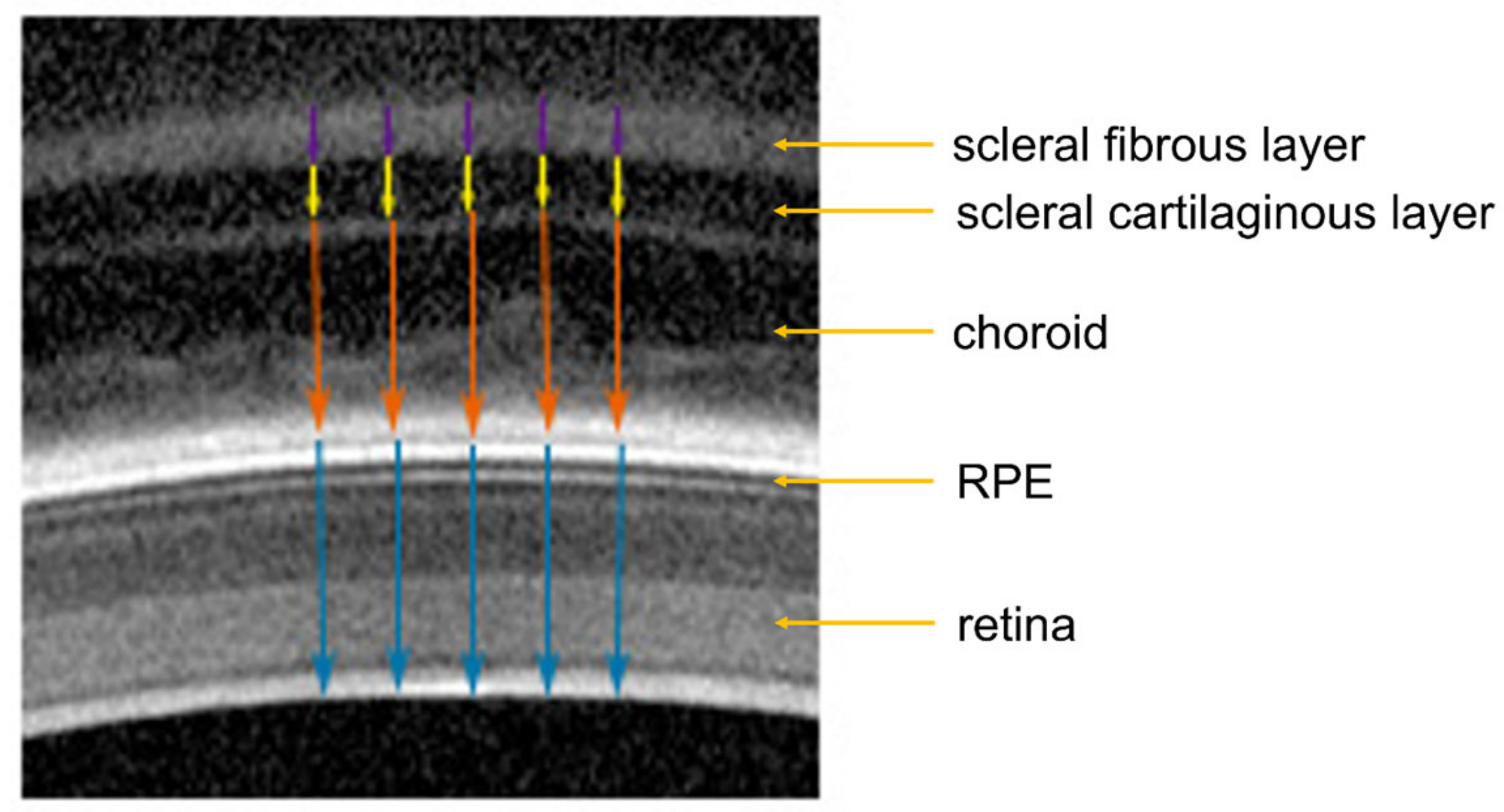
2.3.2. Optical Coherence Tomography (OCT)
2.3.3. Sample Preparation and Measurement of Dopamine and Metabolites via HPLC
2.4. Statistics
3. Results
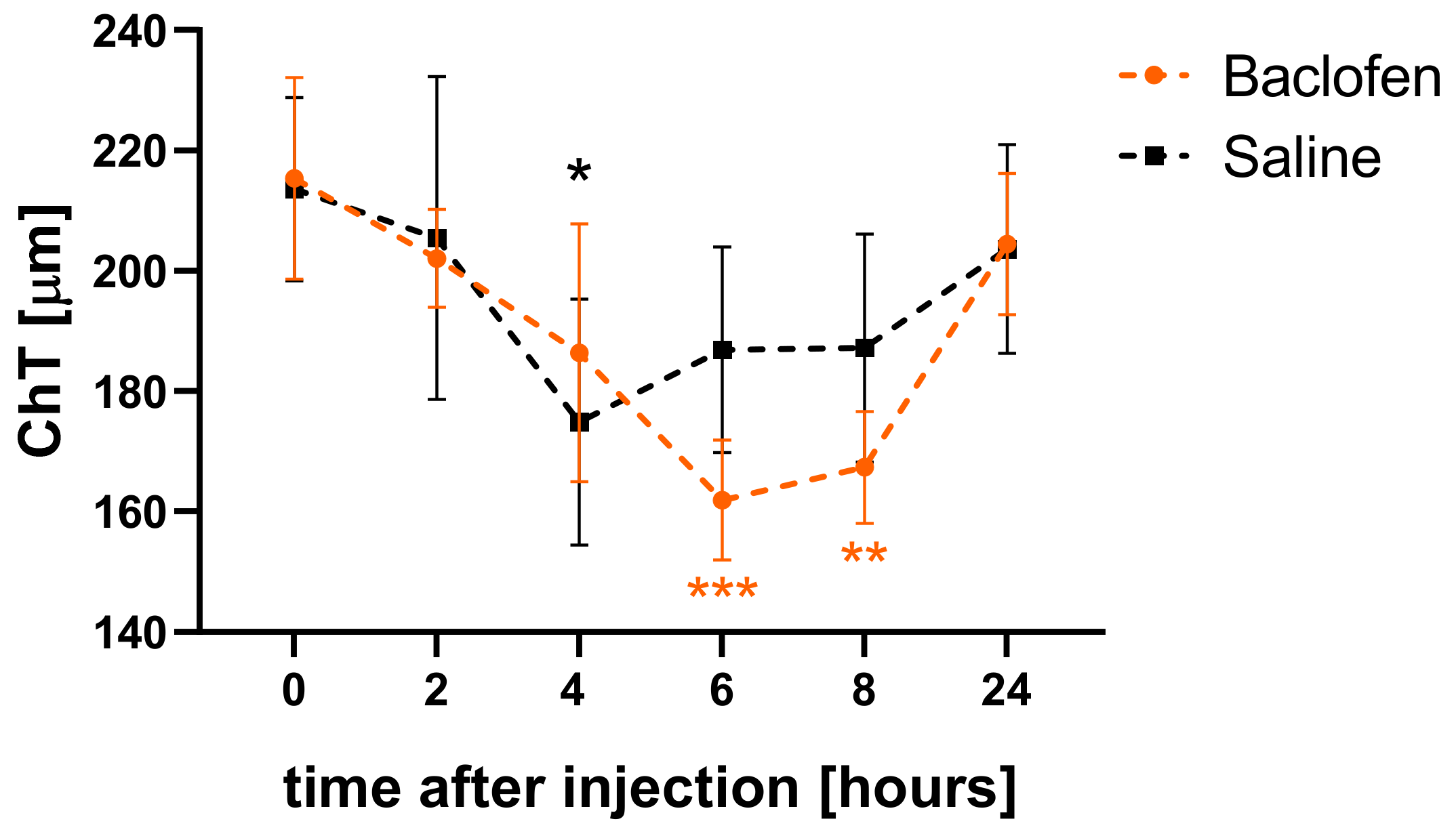
3.1. Baclofen Temporarily Reduced Choroidal Thickness in Eyes with Normal Visual Input
3.2. Baclofen Attenuates the Effects of Deprivation and Defocus on Eye Growth
3.3. Effect of Baclofen on Choroidal, Retinal and Scleral Thickness
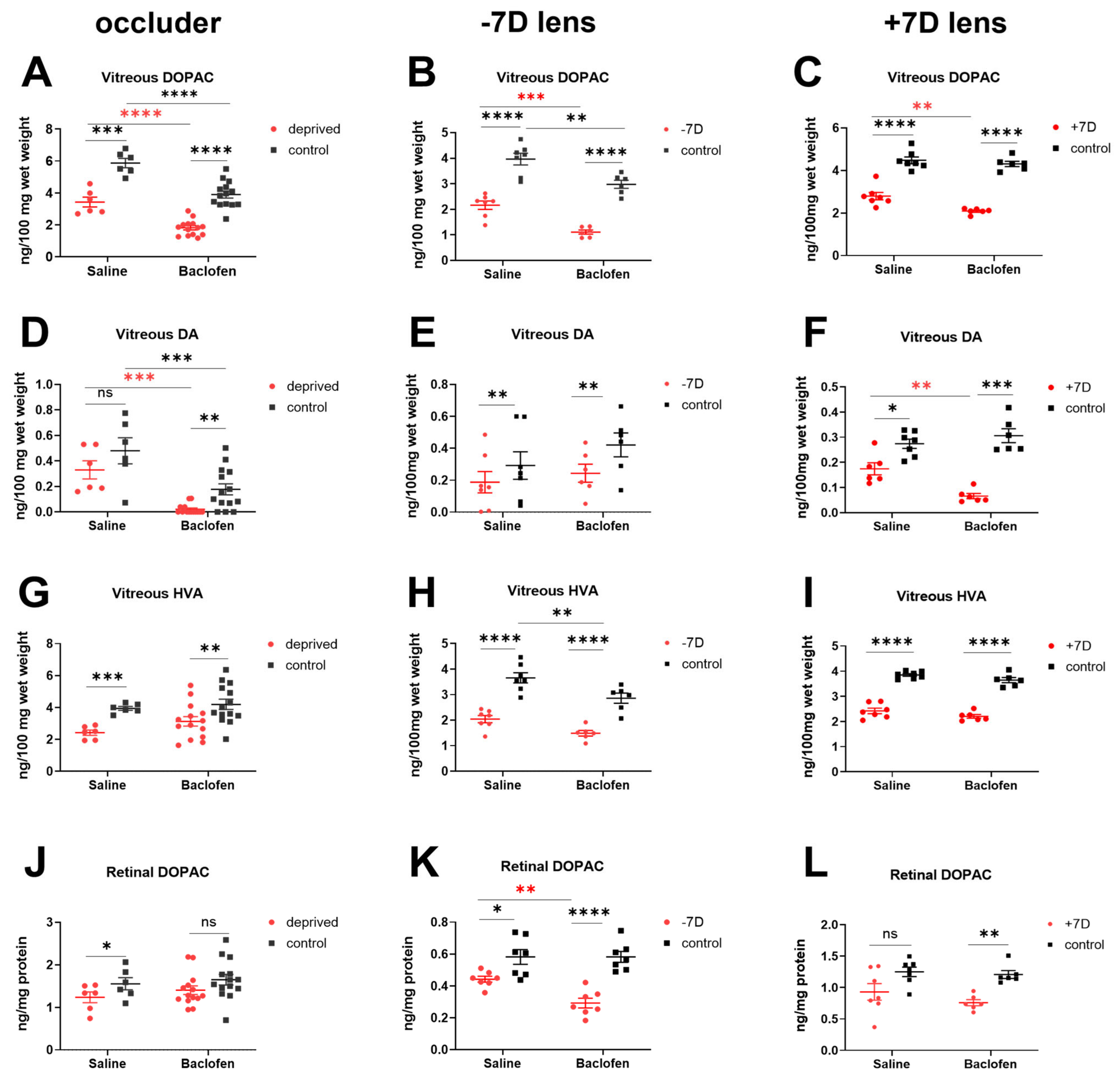
3.4. Baclofen Reduced Retinal Dopamine Metabolism in the Three Ametropia Models
4. Discussion
4.1. Effects of the GABABR Agonist Baclofen on Axial Length, Vitreous Chamber Depth and Refractive Development
4.2. Effect of the GABABR Agonist Baclofen on Dopamine Signaling
4.3. Effect of the GABABR Agonist Baclofen on Retinal and Choroidal THICKNESS
4.4. Association of Reduced Efficacy of Visual Stimuli, Especially Positive Lens Wear, with Baclofen Treatment
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.Y.; Hsu, N.W.; Yang, Y.C.; Chen, Y.L.; Shyong, M.P.; Tsai, D.C. Premyopia at Preschool Age: Population-based Evidence of Prevalence and Risk Factors from A Serial Survey in Taiwan. Ophthalmology 2022, 129, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaeffel, F.; Glasser, A.; Howland, H.C. Accommodation, refractive error and eye growth in chickens. Vis. Res. 1988, 28, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Wallman, J.; Gottlieb, M.D.; Rajaram, V.; Fugate-Wentzek, L.A. Local retinal regions control local eye growth and myopia. Science 1987, 237, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Troilo, D.; Smith, E.L., 3rd; Nickla, D.L.; Ashby, R.; Tkatchenko, A.V.; Ostrin, L.A.; Gawne, T.J.; Pardue, M.T.; Summers, J.A.; Kee, C.-S.; et al. IMI—Report on Experimental Models of Emmetropization and Myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, M31–M88. [Google Scholar] [CrossRef] [Green Version]
- Wildsoet, C.; Wallman, J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vis. Res. 1995, 35, 1175–1194. [Google Scholar] [CrossRef] [Green Version]
- Read, S.A.; Collins, M.J.; Sander, B.P. Human optical axial length and defocus. Investig. Opthalmology Vis. Sci. 2010, 51, 6262–6269. [Google Scholar] [CrossRef]
- Smith, E.L., 3rd; Hung, L.F.; Huang, J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Investig. Opthalmology Vis. Sci. 2012, 53, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.L., 3rd; Hung, L.F.; Arumugam, B.; Huang, J. Negative lens-induced myopia in infant monkeys: Effects of high ambient lighting. Investig. Opthalmology Vis. Sci. 2013, 54, 2959–2969. [Google Scholar] [CrossRef]
- She, Z.; Hung, L.F.; Arumugam, B.; Beach, K.M.; Smith, E.L., 3rd. The effects of reduced ambient lighting on lens compensation in infant rhesus monkeys. Vis. Res. 2021, 187, 14–26. [Google Scholar] [CrossRef]
- Spoerri, P.E. Neurotrophic effects of GABA in cultures of embryonic chick brain and retina. Synapse 1988, 2, 11–22. [Google Scholar] [CrossRef]
- Ikeda, H.; Hankins, M.W.; Kay, C.D. Actions of baclofen and phaclofen upon ON- and OFF-ganglion cells in the cat retina. Eur. J. Pharm. 1990, 190, 1–9. [Google Scholar]
- Agardh, E.; Bruun, A.; Ehinger, B.; Storm-Mathisen, J. GABA immunoreactivity in the retina. Investig. Opthalmology Vis. Sci. 1986, 27, 674–678. [Google Scholar]
- Guo, C.; Hirano, A.A.; Stella, S.L., Jr.; Bitzer, M.; Brecha, N.C. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD 65, and the GABA vesicular transporter. J. Comp. Neurol. 2010, 518, 1647–1669. [Google Scholar] [CrossRef] [Green Version]
- Popova, E. GABAergic neurotransmission and retinal ganglion cell function. J. Comp. Physiol. A 2015, 201, 261–283. [Google Scholar] [CrossRef]
- Yang, X.L. Characterization of receptors for glutamate and GABA in retinal neurons. Prog. Neurobiol. 2004, 73, 127–150. [Google Scholar] [CrossRef]
- Marshall, F.H.; Jones, K.A.; Kaupmann, K.; Bettler, B. GABAB receptors—The first 7TM heterodimers. Trends Pharmacol. Sci. 1999, 20, 396–399. [Google Scholar] [CrossRef]
- Sha, F.; Ye, X.; Zhao, W.; Xu, C.L.; Wang, L.; Ding, M.H.; Bi, A.L.; Wu, J.F.; Jiang, W.J.; Guo, D.D.; et al. Effects of electroacupuncture on the levels of retinal gamma-aminobutyric acid and its receptors in a guinea pig model of lens-induced myopia. Neuroscience 2015, 287, 164–174. [Google Scholar] [CrossRef]
- Guoping, L.; Xiang, Y.; Jianfeng, W.; Dadong, G.; Jie, H.; Wenjun, J.; Junguo, G. Alterations of Glutamate and gamma-Aminobutyric Acid Expressions in Normal and Myopic Eye Development in Guinea Pigs. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1256–1265. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Bi, A.L.; Xu, C.L.; Ye, X.; Chen, M.Q.; Wang, X.T.; Zhang, X.Y.; Guo, J.G.; Jiang, W.J.; Zhang, J.; et al. GABA and GABA receptors alterations in the primary visual cortex of concave lens-induced myopic model. Brain Res. Bull. 2017, 130, 173–179. [Google Scholar] [CrossRef]
- Stone, R.A.; Liu, J.; Sugimoto, R.; Capehart, C.; Zhu, X.; Pendrak, K. GABA, experimental myopia, and ocular growth in chick. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3933–3946. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.K.S.; Yeung, C.K.; Chiang, S.W.Y.; Chan, K.P.; Pang, C.P.; Lam, D.S.C. GABAA and GABAC (GABAA0r) Receptors Affect Ocular Growth and Form-Deprivation Myopia. Cutan. Ocul. Toxicol. 2005, 24, 187–196. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Wang, X.P.; Schmid, K.L.; Han, Y.F.; Han, X.G.; Tang, H.W.; Tang, X. GABAB Receptor Antagonist CGP46381 Inhibits Form-Deprivation Myopia Development in Guinea Pigs. Biomed. Res. Int. 2015, 2015, 207312. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.Y.; Wang, X.-P.; Schmid, K.L.; Han, X.G. Inhibition of form-deprivation myopia by a GABAAOr receptor antagonist, (1,2,5,6-tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA), in guinea pigs. Graefe’s Arch. Clin. Exp. 2014, 252, 1939–1946. [Google Scholar] [CrossRef]
- Srinivasalu, N.; McFadden, S.A.; Medcalf, C.; Fuchs, L.; Chung, J.; Philip, G.; Richardson, A.; Riaz, M.; Baird, P.N. Gene Expression and Pathways Underlying Form Deprivation Myopia in the Guinea Pig Sclera. Investig. Opthalmology Vis. Sci. 2018, 59, 1425–1434. [Google Scholar] [CrossRef] [Green Version]
- Barathi, V.A.; Chaurasia, S.S.; Poidinger, M.; Koh, S.K.; Tian, D.; Ho, C.; Iuvone, P.M.; Beuerman, R.W.; Zhou, L. Involvement of GABA transporters in atropine-treated myopic retina as revealed by iTRAQ quantitative proteomics. J. Proteome Res. 2014, 13, 4647–4658. [Google Scholar] [CrossRef] [Green Version]
- Christian, P.G.; Harkin, D.G.; Schmid, K.L. GABAergic agents modify the response of chick scleral fibroblasts to myopic and hyperopic eye cup tissues. Curr. Eye Res. 2014, 39, 172–187. [Google Scholar] [CrossRef]
- Hirasawa, H.; Betensky, R.A.; Raviola, E. Corelease of dopamine and GABA by a retinal dopaminergic neuron. J. Neurosci. 2012, 32, 13281–13291. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, H.; Contini, M.; Raviola, E. Extrasynaptic release of GABA and dopamine by retinal dopaminergic neurons. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140186. [Google Scholar] [CrossRef] [Green Version]
- Feldkaemper, M.; Diether, S.; Kleine, G.; Schaeffel, F. Interactions of spatial and luminance information in the retina of chickens during myopia development. Exp. Eye Res. 1999, 68, 105–115. [Google Scholar] [CrossRef]
- Feldkaemper, M.; Schaeffel, F. An updated view on the role of dopamine in myopia. Exp. Eye Res. 2013, 114, 106–119. [Google Scholar] [CrossRef]
- Zhou, X.; Pardue, M.T.; Iuvone, P.M.; Qu, J. Dopamine signaling and myopia development: What are the key challenges. Prog. Retin. Eye Res. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Santiago, M.; Machado, A.; Cano, J. In vivo release of dopamine from rat striatum, substantia nigra and prefrontal cortex: Differential modulation by baclofen. Br. J. Pharmacol. 1993, 109, 814–818. [Google Scholar] [CrossRef] [Green Version]
- Pitman, K.A.; Puil, E.; Borgland, S.L. GABA(B) modulation of dopamine release in the nucleus accumbens core. Eur. J. Neurosci. 2014, 40, 3472–3480. [Google Scholar] [CrossRef]
- Boatright, J.H.; Rubim, N.M.; Iuvone, P.M. Regulation of endogenous dopamine release in amphibian retina by gamma-aminobutyric acid and glycine. Vis. Neurosci. 1994, 11, 1003–1012. [Google Scholar] [CrossRef]
- Schaeffel, F.; Bartmann, M.; Hagel, G.; Zrenner, E. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vis. Res. 1995, 35, 1247–1264. [Google Scholar] [CrossRef] [Green Version]
- Kent, C.N.; Park, C.; Lindsley, C.W. Classics in Chemical Neuroscience: Baclofen. ACS Chem. Neurosci. 2020, 11, 1740–1755. [Google Scholar] [CrossRef]
- Schaeffel, F.; Hagel, G.; Eikermann, J.; Collett, T. Lower-field myopia and astigmatism in amphibians and chickens. J. Opt. Soc. Am. A 1994, 11, 487–495. [Google Scholar] [CrossRef]
- Schaeffel, F.; Howland, H.C. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vis. Res. 1991, 31, 717–734. [Google Scholar] [CrossRef]
- Mathis, U.; Feldkaemper, M.; Schaeffel, F. Effects of single and repeated intravitreal applications of atropine on choroidal thickness in alert chickens. Ophthalmic Res. 2021, 64, 664–674. [Google Scholar] [CrossRef]
- Wang, M.; Schaeffel, F.; Jiang, B.; Feldkaemper, M. Effects of Light of Different Spectral Composition on Refractive Development and Retinal Dopamine in Chicks. Investig. Opthalmology Vis. Sci. 2018, 59, 4413–4424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohngemach, S.; Hagel, G.; Schaeffel, F. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis. Neurosci. 1997, 14, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Megaw, P.; Morgan, I.; Boelen, M. Vitreal dihydroxyphenylacetic acid (DOPAC) as an index of retinal dopamine release. J. Neurochem. 2001, 76, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Schaeffel, F.; Trier, K.; Feldkaemper, M. Effects of 7-Methylxanthine on Deprivation Myopia and Retinal Dopamine Release in Chickens. Ophthalmic Res. 2019, 63, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Aleman, A.; Schaeffel, F. Probing the Potency of Artificial Dynamic ON or OFF Stimuli to Inhibit Myopia Development. Investig. Opthalmology Vis. Sci. 2019, 60, 2599–2611. [Google Scholar] [CrossRef] [Green Version]
- Schmid, K.L.; Strasberg, G.; Rayner, C.L.; Hartfield, P.J. The effects and interactions of GABAergic and dopaminergic agents in the prevention of form deprivation myopia by brief periods of normal vision. Exp. Eye Res. 2013, 110, 88–95. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Zhuang, J.; Yu, K. The Role of Retinal Dysfunction in Myopia Development. Cell. Mol. Neurobiol. 2022, 1–26. [Google Scholar] [CrossRef]
- Schaeffel, F.; Feldkaemper, M. Animal models in myopia research. Clin. Exp. Optom. 2015, 98, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Nickla, D.L.; Sarfare, S.; McGeehan, B.; Wei, W.; Elin-Calcador, J.; He, L.; Dhakal, S.; Dixon, J.; Maguire, M.G.; Stone, R.A.; et al. Visual conditions affecting eye growth alter diurnal levels of vitreous DOPAC. Exp. Eye Res. 2020, 200, 108226. [Google Scholar] [CrossRef]
- Ruan, G.X.; Allen, G.C.; Yamazaki, S.; McMahon, D.G. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008, 6, e249. [Google Scholar] [CrossRef]
- Chakraborty, R.; Ostrin, L.A.; Nickla, D.L.; Iuvone, P.M.; Pardue, M.T.; Stone, R.A. Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2018, 38, 217–245. [Google Scholar] [CrossRef]
- Lee, E.J.; Gibo, T.L.; Grzywacz, N.M. Dark-rearing-induced reduction of GABA and GAD and prevention of the effect by BDNF in the mouse retina. Eur J. Neurosci. 2006, 24, 2118–2134. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Völgyi, B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat. Rev. Neurosci. 2009, 10, 495–506. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Wang, X.P.; Schmid, K.L.; Liu, L. Identification of GABA receptors in chick retinal pigment epithelium. Neurosci. Lett. 2013, 539, 43–47. [Google Scholar] [CrossRef]
- Salehi, M.A.; Nowroozi, A.; Gouravani, M.; Mohammadi, S.; Arevalo, J.F. Associations of refractive errors and retinal changes measured by optical coherence tomography: A systematic review and meta-analysis. Surv. Ophthalmol. 2021, 67, 591–607. [Google Scholar] [CrossRef]
- Wu, H.; Chen, W.; Zhao, F.; Zhou, Q.; Reinach, P.S.; Deng, L.; Ma, L.; Luo, S.; Srinivasalu, N.; Pan, M.; et al. Scleral hypoxia is a target for myopia control. Proc. Natl. Acad. Sci. USA 2018, 115, E7091–E7100. [Google Scholar] [CrossRef] [Green Version]
- Mathis, U.; Feldkaemper, M.; Liu, H.; Schaeffel, F. Studies on the interactions of retinal dopamine with choroidal thickness in the chicken. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 261, 409–425. [Google Scholar]
- Fiszer de Plazas, S.; Alfie, J.; González, N.N. Light and dark adaptation influences GABA receptor sites in the chick retina. Neurochem. Res. 1986, 11, 973–981. [Google Scholar] [CrossRef]
- She, Z.; Hung, L.F.; Arumugam, B.; Beach, K.M.; Smith, E.L., 3rd. Effects of low intensity ambient lighting on refractive development in infant rhesus monkeys (Macaca mulatta). Vis. Res. 2020, 176, 48–59. [Google Scholar] [CrossRef]
- Crewther, S.G.; Murphy, M.; Crewther, D. Potassium channel and NKCC cotransporter involvement in ocular refractive control mechanisms. PLoS ONE 2008, 3, e2839. [Google Scholar] [CrossRef]
- Murphy, M.J.; Crewther, S. Ouabain inhibition of Na/K-ATPase across the retina prevents signed refractive compensation to lens-induced defocus, but not default ocular growth in young chicks. F1000Research 2013, 2, 97. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, A.; Huntsman, M.; Jones, E. GABA(B) receptor gene expression in monkey thalamus. J. Comp. Neurol. 1998, 394, 118–126. [Google Scholar] [CrossRef]
- Muñoz, A.; De Felipe, J.; Jones, E. Patterns of GABA(B)R1a,b receptor gene expression in monkey and human visual cortex. Cereb Cortex 2001, 11, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Binns, K.E.; Salt, T. The functional influence of nicotinic cholinergic receptors on the visual responses of neurones in the superficial superior colliculus. Vis. Neurosci. 2000, 17, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. Temporal integration of visual signals in lens compensation (a review). Exp. Eye Res. 2013, 114, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winawer, J.; Zhu, X.; Choi, J.; Wallman, J. Ocular compensation for alternating myopic and hyperopic defocus. Vis. Res. 2005, 45, 1667–1677. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Winawer, J.; Wallman, J. Potency of myopic defocus in spectacle lens compensation. Investig. Opthalmology Vis. Sci. 2003, 44, 2818–2827. [Google Scholar] [CrossRef]
- Zhu, X.; Kang, P.; Troilo, D.; Benavente-Perez, A. Temporal properties of positive and negative defocus on emmetropization. Sci. Rep. 2022, 12, 3582. [Google Scholar] [CrossRef]
- Feldkaemper, M.P.; Schaeffel, F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis. Neurosci. 2002, 19, 755–766. [Google Scholar] [CrossRef]
- Nickla, D.L.; Damyanova, P.; Lytle, G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp. Eye Res. 2009, 88, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Crewther, S.G.; Crewther, D. Inhibition of retinal ON/OFF systems differentially affects refractive compensation to defocus. Neuroreport 2003, 14, 1233–1237. [Google Scholar] [CrossRef]
- Fischer, K.F.; Lukasiewicz, P.; Wong, R. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J. Neurosci. 1998, 18, 3767–3778. [Google Scholar] [CrossRef] [Green Version]
- Kazula, A.; Nowak, J.; Iuvone, P. Regulation of melatonin and dopamine biosynthesis in chick retina: The role of GABA. Vis. Neurosci. 1993, 10, 621–629. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Schaeffel, F.; Yang, Z.; Feldkaemper, M.P. GABAB Receptor Activation Affects Eye Growth in Chickens with Visually Induced Refractive Errors. Biomolecules 2023, 13, 434. https://doi.org/10.3390/biom13030434
Liu H, Schaeffel F, Yang Z, Feldkaemper MP. GABAB Receptor Activation Affects Eye Growth in Chickens with Visually Induced Refractive Errors. Biomolecules. 2023; 13(3):434. https://doi.org/10.3390/biom13030434
Chicago/Turabian StyleLiu, Hong, Frank Schaeffel, Zhikuan Yang, and Marita Pauline Feldkaemper. 2023. "GABAB Receptor Activation Affects Eye Growth in Chickens with Visually Induced Refractive Errors" Biomolecules 13, no. 3: 434. https://doi.org/10.3390/biom13030434




