BOX38, a DNA Marker for Selection of Essential Oil Yield of Rosa × rugosa
Abstract
1. Introduction
2. Materials and Methods
2.1. The Rose Cultivars and Sampling
2.2. Hydrodistillation Extraction and Gas Chromatography with Mass Spectrometry (GC-MS) Analyses of Essential Oil
2.3. Mapping and Syntenic Analysis of NUDX1-1 Cluster
2.4. Distinguish of B38 Copy Numbers by Length of PCR-Products
3. Results
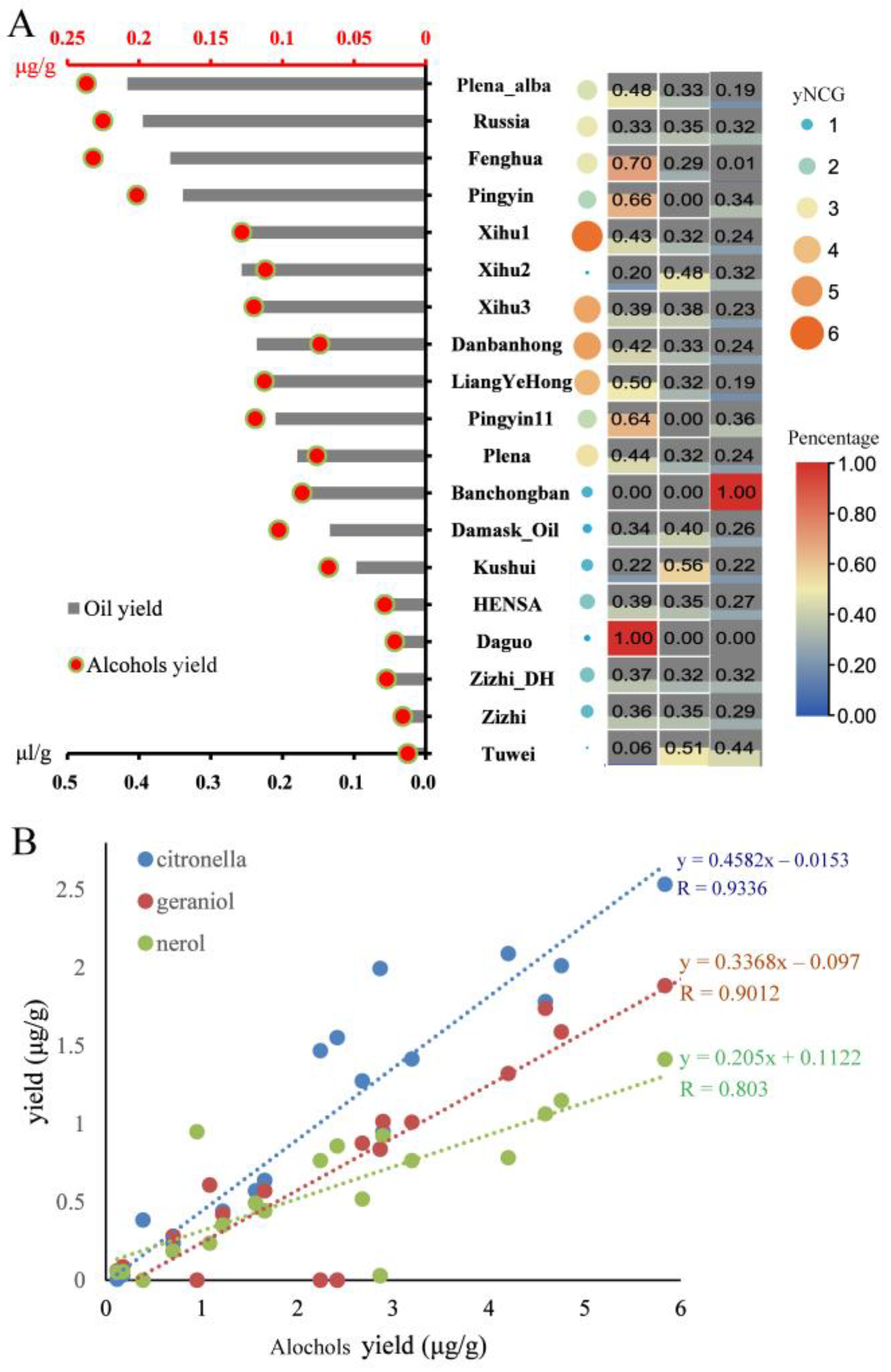
3.1. The Alternative Index of Essential Oil Yield
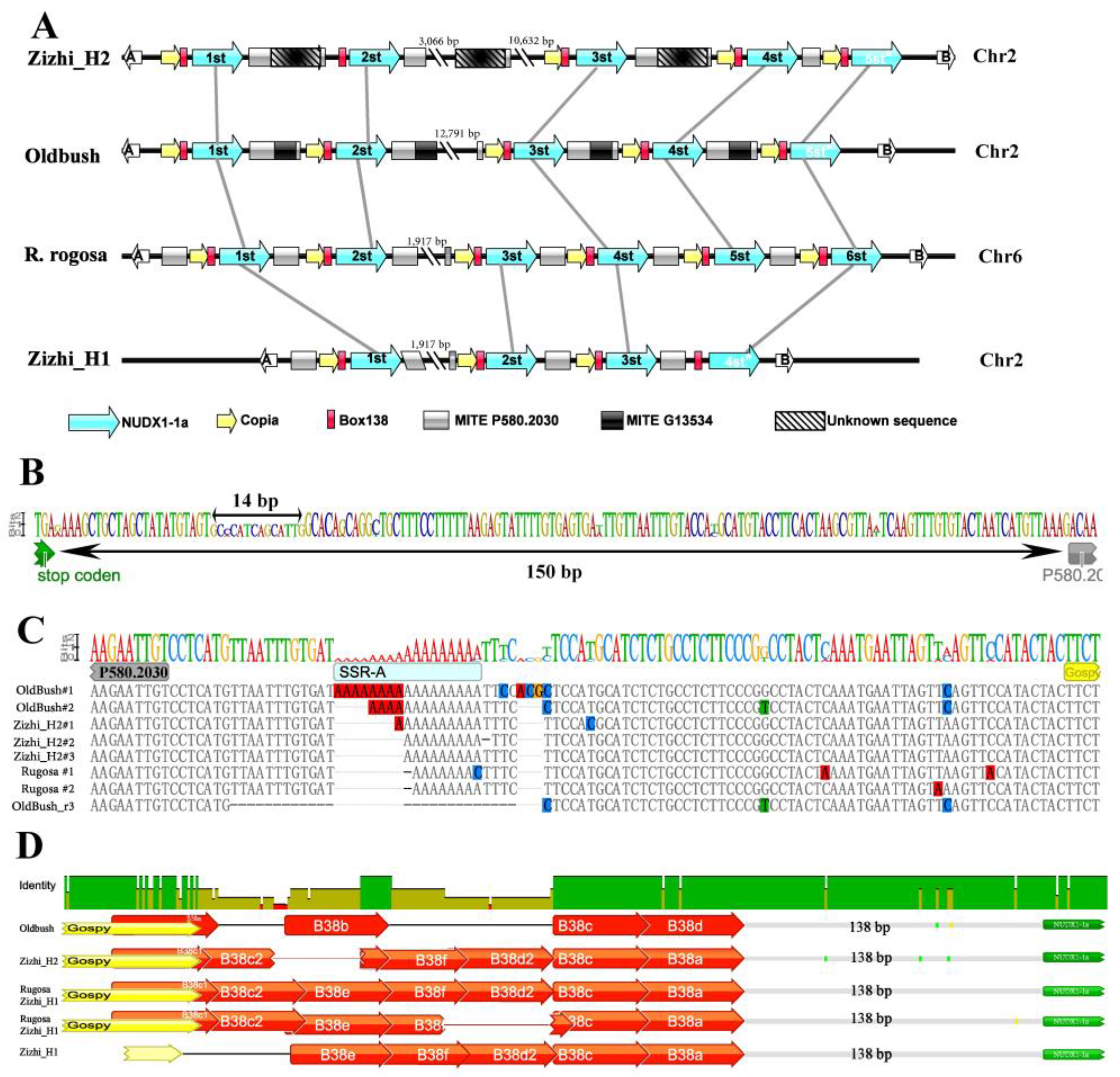
3.2. Cosserved NUDX1-1a Clusters and Variable B38
3.3. Positively Correlation of Repeat Number of B38 and Essential Oil Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ainane, T.; Abdoul-Latif, F.M.; El Montassir, Z.; Attahar, W.; Ainane, A.; Giuffrè, A.M. Essential oils rich in pulegone for insecticide purpose against legume bruchus species: Case of Ziziphora hispanica L. and Mentha pulegium L. AIMS Agric. Food 2023, 8, 105–118. [Google Scholar] [CrossRef]
- Luan, J.; Yang, M.; Zhao, Y.; Zang, Y.; Zhang, Z.; Chen, H. Aromatherapy with inhalation effectively alleviates the test anxiety of college students: A meta-analysis. Front. Psychol. 2023, 13, 1042553. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Liu, H.; Liu, H.; Xiang, S.; You, F.; Jiang, Y.; Lin, J.; Zhang, D.; Zheng, C. Pharmacologic effects approach of essential oils and their components on respiratory diseases. J. Ethnopharmacol. 2023, 304, 115962. [Google Scholar] [CrossRef] [PubMed]
- Dekić, M.; Radulović, N.; Antonijević, M.; Dekić, D.; Ličina, B. The essential oil of the condiment species Clinopodium thymifolium (Scop.) Kuntze: New natural products and seasonal variation. J. Sci. Food Agric. 2021, 102, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Raymond, O.; Gouzy, J.; Just, J.; Badouin, H.; Verdenaud, M.; Lemainque, A.; Vergne, P.; Moja, S.; Choisne, N.; Pont, C.; et al. The Rosa genome provides new insights into the domestication of modern roses. Nat. Genet. 2018, 50, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Wissemann, V. Conventional Taxonomy of Wild Roses. In Encyclopedia of Rose Science; Roberts, A., Debener, T., Gudin, S., Eds.; Elsevier: London, UK, 2003; p. 6. [Google Scholar]
- Guterman, I.; Shalit, M.; Menda, N.; Piestun, D.; Dafny-Yelin, M.; Shalev, G.; Bar, E.; Davydov, O.; Ovadis, M.; Emanuel, M.; et al. Rose scent genomics approach to discovering novel floral fragrance-related genes. Plant Cell 2002, 14, 2325–2338. [Google Scholar] [CrossRef]
- Kovacheva, N.; Rusanov, K.; Atanassov, I. Industrial Cultivation of Oil Bearing Rose and Rose Oil Production in Bulgaria during 21ST Century, Directions and Challenges. Biotechnol. Biotechnol. Equip. 2010, 24, 1793–1798. [Google Scholar] [CrossRef]
- Rusanov, K.; Kovacheva, N.; Vosman, B.; Zhang, L.; Rajapakse, S.; Atanassov, A.; Atanassov, I. Microsatellite analysis of Rosa damascena Mill. accessions reveals genetic similarity between genotypes used for rose oil production and old Damask rose varieties. Theor. Appl. Genet. 2005, 111, 804–809. [Google Scholar] [CrossRef]
- Cui, W.-H.; Du, X.-Y.; Zhong, M.-C.; Fang, W.; Suo, Z.-Q.; Wang, D.; Dong, X.; Jiang, X.-D.; Hu, J.-Y. Complex and reticulate origin of edible roses (Rosa, Rosaceae) in China. Hortic. Res. 2022, 9, uhab051. [Google Scholar] [CrossRef] [PubMed]
- Zang, F.; Ma, Y.; Tu, X.; Huang, P.; Wu, Q.; Li, Z.; Liu, T.; Lin, F.; Pei, S.; Zang, D.; et al. A high-quality chromosome-level genome of wild Rosa rugosa. DNA Res. 2021, 28, dsab017. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T. Geographical Impact on Essential Oil Composition of Endemic Kundmannia Anatolica Hub.-Mor.(Apiaceae). Afr. J. Tradit. Complement. Altern. Med. 2016, 14, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Gioffrè, G.; Ursino, D.; Labate, M.L.C.; Giuffrè, A.M. The peel essential oil composition of bergamot fruit (Citrus bergamia, Risso) of Reggio Calabria (Italy): A review. Emir. J. Food Agric. 2020, 32, 835–845. [Google Scholar] [CrossRef]
- Mendoza, J.D.S.; Correia, L.C.; Saad, J.C.C.; Siqueira, W.J.; Ming, L.C.; Campos, F.G.; Boaro, C.S.F.; Marques, M.O.M. Effect of irrigation depth on biomass production and metabolic profile of Lippia alba (linalool chemotype) essential oil. Agric. Water Manag. 2022, 262, 107393. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gupta, S.; Variyar, P.S. Comparison of Essential Oils Obtained from Different Extraction Techniques as an Aid in Identifying Aroma Significant Compounds of Nutmeg (Myristica Fragrans). Nat. Prod. Commun. 2015, 10, 144. [Google Scholar] [CrossRef]
- Liu, Y.; Zhi, D.; Wang, X.; Fei, D.; Zhang, Z.; Wu, Z.; Li, Y.; Chen, P.; Li, H. Kushui Rose (R. Setate x R. Rugosa) decoction exerts antitumor effects in C. elegans by downregulating Ras/MAPK pathway and resisting oxidative stress. Int. J. Mol. Med. 2018, 42, 1411–1417. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, J.; Niu, Y.; Wu, M. Characterization of key aroma compounds from different rose essential oils using gas chromatography-mass spectrometry, gas chromatography–olfactometry and partial least squares regression. Nat. Prod. Res. 2018, 32, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, K.E.; Kovacheva, N.M.; Atanassov, I.I. Comparative GC/MS Analysis of Rose Flower and Distilled Oil Volatiles of The Oil Bearing Rose Rosa damascena. Biotechnol. Biotechnol. Equip. 2011, 25, 2210–2216. [Google Scholar] [CrossRef]
- Spiller, M.; Berger, R.G.; Debener, T. Genetic dissection of scent metabolic profiles in diploid rose populations. Theor. Appl. Genet. 2010, 120, 1461–1471. [Google Scholar] [CrossRef]
- Venkatesha, K.; Gupta, A.; Rai, A.N.; Jambhulkar, S.; Bisht, R.; Padalia, R.C. Recent developments, challenges, and opportunities in genetic improvement of essential oil-bearing rose (Rosa damascena): A review. Ind. Crop. Prod. 2022, 184, 114984. [Google Scholar] [CrossRef]
- Sun, P.; Schuurink, R.C.; Caissard, J.-C.; Hugueney, P.; Baudino, S. My Way: Noncanonical Biosynthesis Pathways for Plant Volatiles. Trends Plant Sci. 2016, 21, 884–894. [Google Scholar] [CrossRef]
- Tholl, D.; Gershenzon, J. The flowering of a new scent pathway in rose. Science 2015, 349, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Magnard, J.-L.; Roccia, A.; Caissard, J.-C.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Hibrand-Saint Oyant, L.; Jullien, F.; Nicolè, F.; et al. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef]
- Conart, C.; Saclier, N.; Foucher, F.; Goubert, C.; Rius-Bony, A.; Paramita, S.N.; Moja, S.; Thouroude, T.; Douady, C.; Sun, P.; et al. Duplication and Specialization of NUDX1 in Rosaceae Led to Geraniol Production in Rose Petals. Mol. Biol. Evol. 2022, 39, msac002. [Google Scholar] [CrossRef]
- Sun, P.; Dégut, C.; Réty, S.; Caissard, J.; Hibrand-Saint Oyant, L.; Bony, A.; Paramita, S.N.; Conart, C.; Magnard, J.L.; Jeauffre, J.; et al. Functional diversification in the Nudix hydrolase gene family drives sesquiterpene biosynthesis in Rosa × wichurana. Plant J. 2020, 104, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, C.; Li, T.; Wang, M.; Tao, J.; Zhao, D.; Sheng, L. Flowery odor formation revealed by differential expression of monoterpene biosynthetic genes and monoterpene accumulation in rose (Rosa rugosa Thunb.). Plant Physiol. Biochem. 2014, 75, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Hibrand Saint-Oyant, L.; Ruttink, T.; Hamama, L.; Kirov, I.; Lakhwani, D.; Zhou, N.N.; Bourke, P.M.; Daccord, N.; Leus, L.; Schulz, D.; et al. A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. Nat. Plants 2018, 4, 473–484. [Google Scholar] [CrossRef]

| Cultivars | Observed PCR Products # | Length of B38 Repeats | Repeat Number of B38 | Yield (μg/g) |
|---|---|---|---|---|
| Xihu2 | 110 | 70 | 2 | 0.178 |
| Daguo | 184 | 144 | 4 | 0.387 |
| Damask_Oil | 184 | 144 | 4 | 0.701 |
| Kushui | 184 | 144 | 4 | 1.083 |
| HENSA | 184 | 144 | 4 | 1.656 |
| Russia | 184 | 144 | 4 | 2.892 |
| Tuwei | 202 | 162 | 5 | 0.117 |
| Banchongban | 202 | 162 | 5 | 0.951 |
| Zizhi | 235/240 | 195/200 | 6 | 1.218 |
| Zizhi_DH | 230/235 | 190/195 | 6 | 1.56 |
| Pingyin | 240 | 200 | 6 | 2.236 |
| Liangyehong | 265-270 | 225 | 7 | 2.414 |
| Pingyin11 | 265 | 225 | 7 | 2.675 |
| Plena_alba | 265 | 225 | 7 | 2.863 |
| Fenghua | 265 | 225 | 7 | 3.195 |
| Plena | 290 | 250 | 8 | 4.588 |
| Xihu3 | 290 | 250 | 8 | 4.753 |
| Danbanhong | 290 | 250 | 8 | 4.197 |
| Xihu1 | 315 | 275 | 9 | 5.835 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liang, Y.; Chu, Y.; Feng, L. BOX38, a DNA Marker for Selection of Essential Oil Yield of Rosa × rugosa. Biomolecules 2023, 13, 439. https://doi.org/10.3390/biom13030439
Wang J, Liang Y, Chu Y, Feng L. BOX38, a DNA Marker for Selection of Essential Oil Yield of Rosa × rugosa. Biomolecules. 2023; 13(3):439. https://doi.org/10.3390/biom13030439
Chicago/Turabian StyleWang, Jianwen, Yue Liang, Yadong Chu, and Liguo Feng. 2023. "BOX38, a DNA Marker for Selection of Essential Oil Yield of Rosa × rugosa" Biomolecules 13, no. 3: 439. https://doi.org/10.3390/biom13030439
APA StyleWang, J., Liang, Y., Chu, Y., & Feng, L. (2023). BOX38, a DNA Marker for Selection of Essential Oil Yield of Rosa × rugosa. Biomolecules, 13(3), 439. https://doi.org/10.3390/biom13030439






