Evidence for Natural Products as Alternative Wound-Healing Therapies
Abstract
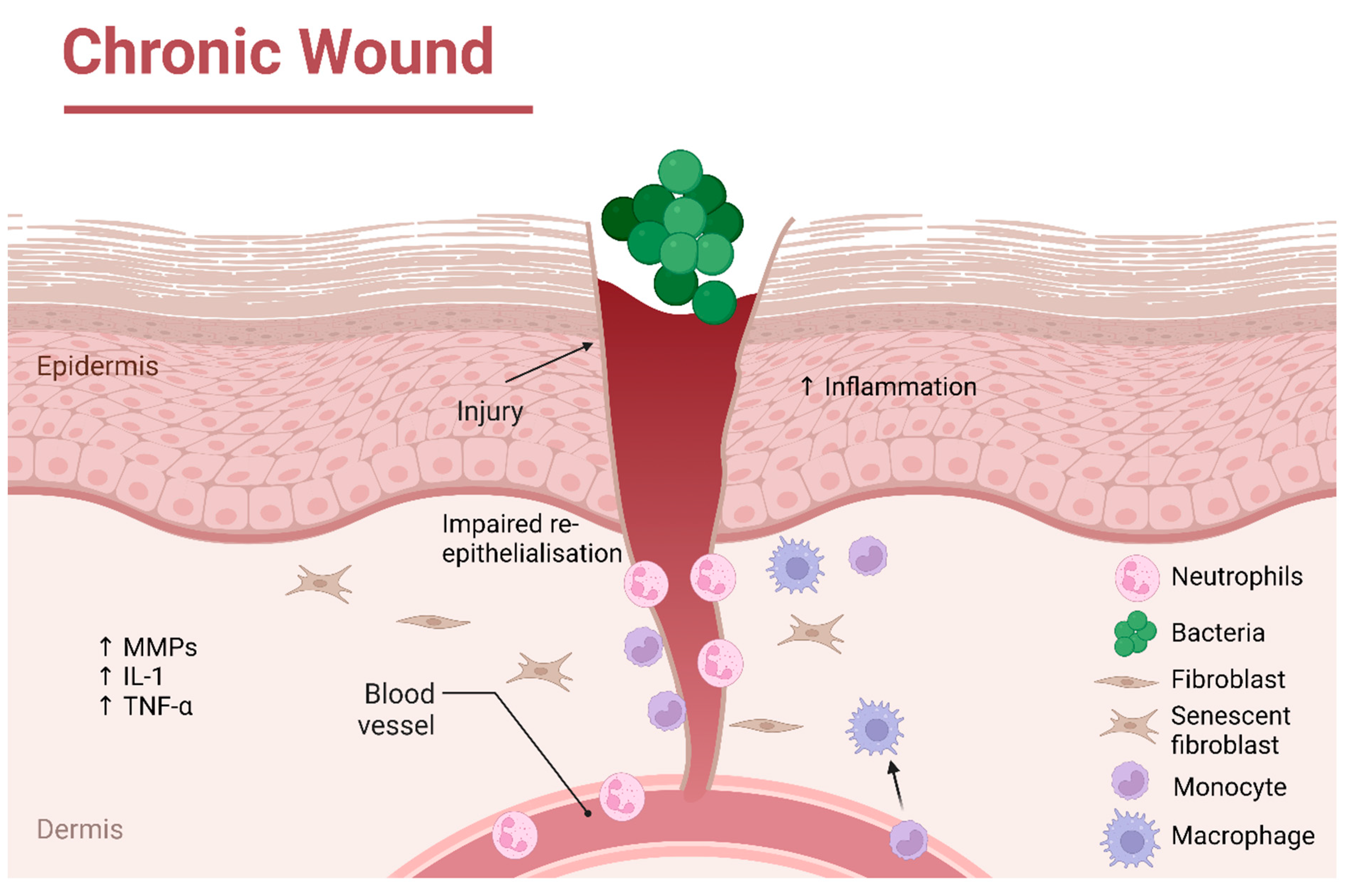
1. Introduction
2. Clinical Trials within Last 20 Years
3. Diabetic Foot Ulcers
4. Pressure Ulcers
5. Venous Leg Ulcers
6. Bioactive Small Molecules from Natural Sources with Promising Wound-Healing Potential
6.1. Manuka Honey
6.2. Aloe vera
6.3. Tree Latex, Plant Exudates and Bark Extracts
6.4. Epoxy-Tiglianes
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Posnett, J.; Franks, P.J. The burden of chronic wounds in the UK. Nurs. Times 2008, 104, 44–45. [Google Scholar]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015, 5, e009283. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that different wound types impose on the UK’s National Health Service. Int. Wound J. 2016, 14, 322–330. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.L.; Mansbridge, J.N.; Carter, W.G.; Fujita, M.; Olerud, J.E. Keratinocyte migration, proliferation and differentiation in chronic ulcers from patients with diabetes and normal wounds. J. Histochem. Cytochem. 2008, 56, 687–696. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 1: Normal and Chronic Wounds: Biology, Causes, and Approaches to Care. Adv. Skin. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Diegelmann, R.F. Excessive neutrophils characterize chronic pressure ulcers. Wound Repair Regen. 2003, 11, 490–495. [Google Scholar] [CrossRef]
- Wilgus, T.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef]
- Rennekampff, H.O.; Hansbrough, J.F.; Kiessig, V.; Dore, C.; Sticherling, M.; Schroder, J.M. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J. Surg. Res. 2000, 93, 41–54. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Stojadinovic, O.; Brem, H.; Vouthounis, C.; Lee, B.; Fallon, J.; Stallcup, M.; Merchant, A.; Galiano, R.D.; Tomic-Canic, M. Molecular pathogenesis of chronic wounds: The role of β-catenin and c-myc in the inhibition of epithelialization and wound healing. Am. J. Pathol. 2005, 167, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Rep. Reg. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Khullar, L.; Harjai, K.; Chhibber, S. Therapeutic and pro-healing potential of advanced wound dressings loaded with bioactive agents. Future Microbiol. Epub ahead of print. 2022. [Google Scholar] [CrossRef]
- Sethuram, L.; Thomas, J. Therapeutic applications of electrospun nanofibers impregnated with various biological macromolecules for effective wound healing strategy—A review. Biomed Pharmacother. 2023, 157, 113996. [Google Scholar] [CrossRef]
- Papanas, N.; Maltezos, E. Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Saf. 2010, 33, 455–461. [Google Scholar] [CrossRef]
- Blume, P.; Bowlby, M.; Schmidt, B.M.; Donegan, R. Safety and efficacy of Becaplermin gel in the treatment of diabetic foot ulcers. Chronic Wound Care Manag. Res. 2014, 2014, 11–14. [Google Scholar]
- Islam, A.T.M.R.; Hasan, M.; Islam, T.; Rahman, A.; Mitra, S.; Das, S.K. Ethnobotany of Medicinal Plants Used by Rakhine Indigenous Communities in Patuakhali and Barguna District of Southern Bangladesh. J. Evid. Based Integr. Med. 2020, 25, 2515690X20971586. [Google Scholar] [CrossRef]
- Fana, S.E.; Ahmadpour, F.; Rasouli, H.R.; Tehrani, S.S.; Maniati, M. The effects of natural compounds on wound healing in Iranian traditional medicine: A comprehensive review, Complement. Ther. Clin. Pract. 2021, 42, 101275. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, A.; Falahatzadeh, M.; Raoofi, E.; Zarshenas, M.M. An Evidence-Based Review on Wound Healing Herbal Remedies From Reports of Traditional Persian Medicine. J. Evid. Based Complement. Altern. Med. 2017, 22, 334–343. [Google Scholar] [CrossRef]
- Dorai, A.A. Wound care with traditional, complementary and alternative medicine. Indian J. Plast. Surg. 2012, 45, 418–424. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Ma, B.R.; Wehrli, L.N.; Maverakis, E. Phytochemicals and Naturally Derived Substances for Wound Healing. Adv. Wound Care 2012, 1, 213–217. [Google Scholar] [CrossRef]
- Budovsky, A.; Yarmolinsky, L.; Ben-Shabat, S. Effect of medicinal plants on wound healing. Wound Rep. Reg. 2015, 23, 171–183. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=%22ulcer%22+%2B+%22plant%22&filter=pubt.clinicaltrial&filter=years.2002-2022&sort=date (accessed on 14 February 2023).
- Available online: https://www.fda.gov/patients/drug-development-process/step-3-clinical-research (accessed on 14 February 2023).
- Tonaco, L.A.B.; Gomes, F.L.; Velasquez-Melendez, G.; Lopes, M.T.P.; Salas, C.E. The Proteolytic Fraction from Latex of Vasconcellea cundinamarcensis (P1G10) Enhances Wound Healing of Diabetic Foot Ulcers: A Double-Blind Randomized Pilot Study. Adv Ther. 2018, 35, 494–502. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT03700580 (accessed on 14 February 2023).
- Gomes, F.S.L.; Spínola, C.V.; Ribeiro, H.A.; Lopes, M.T.P.; Cassali, G.D.; Salas, C.E. Wound-healing activity of a proteolytic fraction from Carica candamarcensis on experimentally induced burn. Burns 2010, 36, 277–283. [Google Scholar] [CrossRef]
- Lemos, F.O.; Ferreira, L.A.M.; Cardoso, V.N.; Cassali, G.D.; Salas, C.E.; Lopes, M.T.P. Skin-healing activity and toxicological evaluation of a proteinase fraction from Carica candamarcensis. Eur. J. Dermatol. 2011, 21, 722–730. [Google Scholar] [CrossRef]
- Freitas, K.M.; Barcelos, L.S.; Caliari, M.V.; Salas, C.E.; Lopes, M.T.P. Healing activity of proteolytic fraction (P1G10) from Vasconcellea cundinamarcensis in a cutaneous wound excision model. Biomed. Pharmacother. 2017, 96, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Errichi, B.M.; Ledda, A.; Di Renzo, A.; Stuard, S.; Dugall, M.; Pellegrini, L.; Gizzi, G.; Rohdewald, P.; et al. Diabetic Ulcers: Microcirculatory Improvement and Faster Healing With Pycnogenol. Clin. Appl. Thromb. Hemost. 2006, 12, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, H.-J.; Rohdewalk, P. French Maritime Pine Bark Extract Pycnogenol Dose Dependently Lowers Glucose in Type 2 Diabetic Patients. Diabetes Care 2004, 27, 839. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Errichi, B.M.; Ledda, A.; Di Renzo, A.; Stuard, S.; Dugall, M.; Pellegrini, L.; Rohdewald, P.; Ippolito, E.; et al. Venous Ulcers: Microcirculatory Improvement and Faster Healing with Local Use of Pycnogenol®. Angiology 2005, 56, 699–705. [Google Scholar] [CrossRef]
- Blazsó, G.; Gábor, M.; Schönlau, F.; Rohdewald, P. Pycnogenol® Accelerates Wound Healing and Reduces Scar Formation. Phytother. Res. 2004, 18, 579–581. [Google Scholar] [CrossRef]
- Dogan, E.; Yanmaz, L.; Gedikli, S.; Ersoz, U.; Okumus, Z. The Effect of Pycnogenol on Wound Healing in Diabetic Rats. Ostomy Wound Manag. 2017, 63, 41–47. [Google Scholar]
- Buzzi, M.; de Freitas, F.; Winter, M. A Prospective, Descriptive Study to Assess the Clinical Benefits of Using Calendula officinalis Hydroglycolic Extract for the Topical Treatment of Diabetic Foot Ulcers. Ostomy Wound Manag. 2016, 62, 8–24. [Google Scholar]
- Jan, N.; Andrabi, K.I.; John, R. Calendula officinalis—An Important Medicinal Plant with Potential Biological Properties. Proc. Indian Natn. Sci. Acad. 2017, 83, 769–787. [Google Scholar]
- Hamburger, M.; Adler, S.; Baumann, D.; Forg, A.; Weinreich, B. Preparative purification of the major anti-inflammatory triterpenoid esters from Marigold (Calendula officinalis). Fitoterapia 2003, 74, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Rep. Reg. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Duran, V.; Matic, M.; Jovanovć, M.; Mimica, N.; Gajinov, Z.; Poljacki, M.; Boza, P. Results of the clinical examination of an ointment with marigold (Calendula officinalis) extract in the treatment of venous leg ulcers. Int. J. Tissue React. 2005, 27, 101–106. [Google Scholar]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Kesavan, R.; Kavitha, K.V.; Kumpatla, S. A pilot study on the effects of a polyherbal formulation cream on diabetic foot ulcers. Indian J. Med. Res. 2011, 134, 168–173. [Google Scholar] [PubMed]
- Najafian, Y.; Khorasani, Z.M.; Najafi, M.N.; Hamedi, S.S.; Mahjour, M.; Feyzabadi, Z. Efficacy of Aloe vera/Plantago major Gel in Diabetic Foot Ulcer: A Randomized Double-Blind Clinical Trial. Curr. Drug Discov. Technol. 2019, 16, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ashkani-Esfahani, S.; Khoshneviszadeh, M.; Noorafshan, A.; Miri, R.; Rafiee, S.; Hemyari, K.; Kardeh, S.; Hosseinabadi, O.K.; Fani, D.; Faridi, E. The Healing Effect of Plantago Major and Aloe Vera. Mixture in Excisional Full Thickness Skin Wounds: Stereological Study. World J. Plast. Surg. 2019, 8, 51–57. [Google Scholar] [CrossRef]
- Zubair, M.; Nybom, H.; Lindholm, C.; Brandner, J.M.; Rumpunen, K. Promotion of wound healing by Plantago major L. leaf extracts—Ex-vivo experiments confirm experiences from traditional medicine. Nat. Prod. Res. 2016, 30, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Zamilpa, A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis in patients with diabetic foot ulcer: A randomized, controlled pilot study. Planta Med. 2015, 81, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Zamilpa-Álvarez, A.; Jiménez-Ferrer, E.; Tortoriello, J. Exploratory Study on the Effectiveness of a Standardized Extract from Ageratina pichinchensis in Patients with Chronic Venous Leg Ulcers. Planta Med. 2012, 78, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Zamilpa, A.; Díaz-García, E.R.; Tortoriello, J. Pharmacological effect of Ageratina pichinchensis on wound healing in diabetic rats and genotoxicity evaluation. J. Ethnopharmacol. 2014, 156, 222–227. [Google Scholar] [CrossRef]
- Stepán, J.; Ehrlichova, J.; Hladikova, M. Therapy results and safety of use of Symphytum Herba extract cream in the treatment of decubitus. J. Gerontol. Geriatr. 2014, 47, 228–235. [Google Scholar]
- Frost, R.; MacPherson, H.; O’Meara, S. A critical scoping review of external uses of comfrey (Symphytum spp.). Complement. Ther. Med. 2013, 21, 724–745. [Google Scholar] [CrossRef]
- Sipponen, A.; Jokinen, J.J.; Sipponen, P.; Papp, A.; Sarna, S.; Lohi, J. Beneficial effect of resin salve in treatment of severe pressure ulcers: A prospective, randomized and controlled multicentre trial. Br. J. Dermatol. 2008, 158, 1055–1062. [Google Scholar] [CrossRef]
- Sipponen, A.; Kuokkanen, O.; Tiihonen, R.; Kauppinen, H.; Jokinen, J.J. Natural coniferous resin salve used to treat complicated surgical wounds: Pilot clinical trial on healing and costs. Int. J. Dermatol. 2012, 51, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, A.; Peltola, R.; Jokinen, J.J.; Laitinen, K.; Lohi, J.; Rautio, M.; Männistö, M.; Sipponen, P.; Lounatmaa, K. Effects of Norway Spruce (Picea abies) Resin on Cell Wall and Cell Membrane of Staphylococcus aureus. Ultrastruct. Pathol. 2009, 33, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.J.; Sipponen, A. Refined Spruce Resin to Treat Chronic Wounds: Rebirth of an Old Folkloristic Therapy. Adv. Wound Care 2016, 5, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Rautio, M.; Sipponen, A.; Peltola, R.; Lohi, J.; Jokinen, J.J.; Papp, A.; Carlson, P.; Sipponen, P. Antibacterial effects of home-made resin salve from Norway spruce (Picea abies). APMIS 2007, 115, 335–340. [Google Scholar] [CrossRef]
- Romanelli, M.; Macchia, M.; Panduri, S.; Paggi, B.; Saponati, G.; Dini, V. Clinical evaluation of the efficacy and safety of a medical device in various forms containing Triticum vulgare for the treatment of venous leg ulcers—A randomized pilot study. Drug Des. Devel. Ther. 2015, 9, 2787–2792. [Google Scholar] [CrossRef]
- Funel, N.; Dini, V.; Janowska, A.; Loggini, B.; Minale, M.; Grieco, F.; Riccio, S.; Romanelli, M. Triticum vulgare Extract Modulates Protein-Kinase B and Matrix Metalloproteinases 9 Protein Expression in BV-2 Cells: Bioactivity on Inflammatory Pathway Associated with Molecular Mechanism Wound Healing. Mediat. Inflamm. 2020, 27, 2851949. [Google Scholar] [CrossRef]
- D’Agostino, A.; Pirozzi, A.V.A.; Finamore, R.; Grieco, F.; Minale, M.; Schiraldi, C. Molecular Mechanisms at the Basis of Pharmaceutical Grade Triticum vulgare Extract Efficacy in Prompting Keratinocytes Healing. Molecules 2020, 25, 431. [Google Scholar] [CrossRef]
- Martini, P.; Mazzatenta, C.; Saponati, G. Efficacy and Tolerability of Fitostimoline in Two Different. Forms (Soaked Gauzes and Cream) and Citrizan Gel in the Topical Treatment of Second-Degree Superficial Cutaneous Burns. Dermatol. Res. Pract. 2011, 2011, 978291. [Google Scholar] [CrossRef]
- Di Giulio, P.; Saiani, L.; Laquintana, D.; Palese, A.; Perli, S.; Andreatta, M.; Rosa, F.; Chini, P.; Soraperra, F.; Ventura, I.; et al. Studio clinico randomizzato controllato in doppio cieco sull’efficacia dei trattamenti delle lesioni da decubito [A double blind randomised clinical trial to assess the efficacy of the treatments of the superficial pressure sores]. Assist. Inferm. E Ric. 2004, 23, 201–208. Erratum in Assist Inferm Ric. 2005, 24, 51.(In Italian) [Google Scholar]
- Rivera-Arce, E.; Chávez-Soto, M.A.; Herrera-Arellano, A.; Arzate, S.; Agüero, J.; Feria-Romero, I.A.; Cruz-Guzmán, A.; Lozoya, X. Therapeutic effectiveness of a Mimosa tenuiflora cortex extract in venous leg ulceration treatment. J. Ethnopharmacol. 2007, 109, 523–528. [Google Scholar] [CrossRef]
- Lammoglia-Ordiales, L.; Vega-Memije, M.E.; Herrera-Arellano, A.; Rivera-Arce, E.; Agüero, J.; Vargas-Martinez, F.; Contreras-Ruiz, J. A randomised comparative trial on the use of a hydrogel with tepescohuite extract (Mimosa tenuiflora cortex extract-2G) in the treatment of venous leg ulcers. Int. Wound J. 2012, 9, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.L.; Evangelista, A.J.J. Mimosa tenuiflora’s antimicrobial activity on bacteria and fungi from medical importance: An integrative review. Arch. Microbiol. 2021, 203, 3399–3406. [Google Scholar] [CrossRef] [PubMed]
- Zippel, J.; Deters, A.; Hensel, A. Arabinogalactans from Mimosa tenuiflora (Willd.) Poiret bark as active principles for wound-healing properties: Specific enhancement of dermal fibroblast activity and minor influence on HaCaT keratinocytes. J. Ethnopharmacol. 2009, 124, 391–396. [Google Scholar] [CrossRef]
- Binić, I.; Janković, A.; Janković, D.; Janković, I.; Vručinić, Z. Evaluation of Healing and Antimicrobiological Effects of Herbal Therapy on Venous Leg Ulcer: Pilot Study. Phytother. Res. 2010, 24, 277–282. [Google Scholar] [PubMed]
- Leach, M.J.; Pincombe, J.; Foster, G. Using horsechestnut seed extract in the treatment of venous leg ulcers: A cost-benefit analysis. Ostomy Wound Manag. 2006, 52, 68–70, 72–74, 76–78. [Google Scholar]
- Leach, M.J.; Pincombe, J.; Foster, G. Clinical efficacy of horsechestnut seed extract in the treatment of venous ulceration. J. Wound Care 2006, 15, 159–167. [Google Scholar] [CrossRef]
- Aksoy, H.; Çevik, Ö.; Şen, A.; Göğer, F.; Şekerler, T.; Şener, A. Effect of Horse-chestnut seed extract on matrix metalloproteinase-1 and -9 during diabetic wound healing. J. Food Biochem. 2019, 43, e12758. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Min, D.; Bolton, T.; Nubé, V.; Twigg, S.M.; Yue, D.K.; McLennan, S.V. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009, 32, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Kundaković, T.; Milenković, M.; Zlatković, S.; Nikolić, V.; Nikolić, G.; Binić, I. Treatment of venous ulcers with the herbal-based ointment Herbadermal®: A prospective non-randomized pilot study. Forsch. Komplementmed. 2012, 19, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.; Merfort, I.; Schempp, C.M. Botanicals in Dermatology: An Evidence-Based Review. Am. J. Clin. Dermatol. 2010, 11, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Izadi, M.; Sayyadi, N.; Rezaee, R.; Jonaidi-Jafari, N.; Beiraghdar, F.; Zamani, A.; Sahebkar, A. Comparative trial of Aloe vera/olive oil combination cream versus phenytoin cream in the treatment of chronic wounds. J. Wound Care 2015, 24, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Madani, S.A.; Abediankenari, S. The Review on Properties of Aloe Vera in Healing of Cutaneous Wounds. Biomed. Res. Int. 2015, 2015, 714216. [Google Scholar] [CrossRef]
- Hasamnis, A.A.; Mohanty, B.K.; Muralikrishna, P.S. Evaluation of Wound Healing Effect of Topical Phenytoin on Excisional Wound in Albino Rats. J. Young Pharm. 2010, 2, 59–62. [Google Scholar] [CrossRef]
- Talas, G.; Brown, R.A.; McGrouther, D.A. Role of Phenytoin in Wound Healing—A Wound. Pharmacol. Perspect. Biochem. Pharmacol. 1999, 57, 1085–1094. [Google Scholar]
- Panahi, Y.; Rastgar, N.; Zamani, A.; Sahebkar, A. Comparing the Therapeutic Effects of Aloe vera and Olive Oil Combination Cream versus Topical Betamethasone for Atopic Dermatitis: A Randomized Double-blind Clinical Trial. J. Pharmacopunct. 2020, 23, 173–178. [Google Scholar] [CrossRef]
- Hernandez, R. The use of systemic antibiotics in the treatment of chronic wounds. Dermatol. Ther. 2006, 19, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Food & Drug Administration (FDA). 2007. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf5/K053095.pdf (accessed on 9 July 2021).
- Breasted, J.H. The Edwin Smith Papyrus: Published in Facsimile and Hieroglyphic Transliteration; University of Chicago Press: Chicago, IL, USA, 1930. [Google Scholar]
- Sipos, P.; Gyõry, H.; Hagymási, K.; Ondrejka, P.; Blázovics, A. Special wound healing methods used in ancient egypt and the mythological background. World J. Surg. 2004, 28, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2015, 3, CD005083. [Google Scholar] [CrossRef]
- Molan, P.C. Potential of Honey in the Treatment of Wounds and Burns. Am. J. Clin. Dermatol. 2001, 2, 13–19. [Google Scholar] [CrossRef]
- Lee, D.S.; Sinno, S.; Khachemoune, A. Honey and Wound Healing: An Overview. Am. J. Clin. Dermatol. 2011, 12, 181–190. [Google Scholar] [CrossRef]
- Anastasiou, I.A.; Eleftheriadou, I.; Tentolouris, A.; Samakidou, G.; Papanas, N.; Tentolouris, N. Therapeutic Properties of Honey for the Management of Wounds; Is There a Role in the Armamentarium of Diabetic Foot Ulcer Treatment? Results From In vitro and In vivo Studies. Int. J. Low Extrem. Wounds 2021, 15347346211026819. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Cokcetin, N.N.; Pappalardo, M.; Campbell, L.T.; Brooks, P.; Carter, D.A.; Blair, S.E.; Harry, E.J. The Antibacterial Activity of Australian Leptospermum Honey Correlates with Methylglyoxal Levels. PLoS ONE 2016, 11, e0167780. [Google Scholar] [CrossRef]
- Atrott, J.; Henle, T. Methylglyoxal in Manuka Honey—Correlation with Antibacterial Properties. Czech J. Food Sci. 2009, 27, S163–S165. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Burton, N.; Cooper, R. Manuka honey inhibits cell division in methicillin-resistant. Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 2536–2542. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.E.; Cooper, R. Synergy between oxacillin and manuka honey sensitizes methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 2012, 67, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Jenkins, R. Are there feasible prospects for manuka honey as an alternative to conventional antimicrobials? Expert Rev. Anti Infect. Ther. 2012, 10, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; Jenkins, L.; Henriques, A.F.M.; Duggan, R.S.; Burton, N.F. Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Robson, V.; Dodd, S.; Thomas, S. Standardized antibacterial honey (Medihoney™) with standard therapy in wound care: Randomized clinical trial. J. Adv. Nurs. 2009, 65, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Biglari, B.; vd Linden, P.H.; Simon, A.; Aytac, S.; Gerner, H.J.; Moghaddam, A. Use of Medihoney as a non-surgical therapy for chronic pressure ulcers in patients with spinal cord injury. Spinal. Cord. 2012, 50, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, J.; Müller, P.; Turnbull, L.; Burke, C.M.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Antibiotic-specific differences in the response of Staphylococcus aureus to treatment with antimicrobials combined with Manuka honey. Front. Microbiol. 2015, 5, 779. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T. Use of manuka honey for autolytic debridement in necrotic and sloughy wounds. JCN 2018, 32, 38–43. [Google Scholar]
- Simon, A.; Traynor, K.; Santos, K.; Blaser, G.; Bode, U.; Molan, P. Medical Honey for Wound Care—Still the ‘Latest Resort’? Evid. Based Complement. Altern. Med. 2009, 6, 165–173. [Google Scholar] [CrossRef]
- Ingle, R.; Levin, J.; Polinder, K. Wound healing with honey—A randomised controlled trial. S. Afr. Med. J. 2006, 96, 831–835. [Google Scholar] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/results?term=manuka+honey (accessed on 14 February 2023).
- Tsang, K.-K.; Kwong, E.W.-Y.; To, T.S.-S.; Chung, J.W.-Y.; Wong, T.K.-S. A Pilot Randomized, Controlled Study of Nanocrystalline Silver, Manuka Honey, and Conventional Dressing in Healing Diabetic Foot Ulcer. Evid. Based Complement. Alternat. Med. 2017, 2017, 5294890. [Google Scholar] [CrossRef]
- Sankar, J.; Lalitha, A.V.; Rameshkumar, R.; Mahadevan, S.; Kabra, S.K.; Lodha, R. Use of Honey Versus Standard Care for Hospital-Acquired Pressure Injury in Critically Ill Children: A Multicenter Randomized Controlled Trial. Pediatr. Crit. Care Med. 2021, 22, e349–e362. [Google Scholar] [CrossRef]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal Activity of Different Honeys against Pathogenic Bacteria. Arch. Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. A Comparison of Wound Healing following Treatment with Lavandula x allardii Honey or Essential Oil. Phytother. Res. 2006, 20, 755–757. [Google Scholar] [CrossRef]
- Salehi, B.; Albayrak, S.; Antolak, H.; Kręgiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Fokou, P.V.T.; Yousef, Z.; Zakaria, Z.A.; et al. Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy. Int. J. Mol. Sci. 2018, 19, 2843. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cui, L.; Li, J.; Guan, S.; Zhang, K.; Li, J. Aloe vera: A Medicinal Plant Used in Skin Wound Healing. Tissue Eng. Part B Rev. 2021, 27, 455–474. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP technical report on the toxicology and carcinogenesis studies of anthraquinone (CAS No. 84-65-1) in F344/N rats and B6C3F1 mice (Feed Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 2005, 494, 1–358. [Google Scholar]
- Boudreau, M.D.; Mellick, P.; Olson, G.; Felton, R.; Thorn, B.; Beland, F. Clear Evidence of Carcinogenic Activity by a Whole Leaf Extract of Aloe barbadenis Miller (Aloe vera) in F344/N Rats. Toxicol. Sci. 2013, 131, 26–39. [Google Scholar] [CrossRef]
- Fox, L.T.; Mazumder, A.; Dwivedi, A.; Gerber, M.; du Plessis, J.; Hamman, J.H. In vitro wound healing and cytotoxic activity of the gel and whole-leaf materials from selected aloe species. J. Ethnopharmacol. 2017, 200, 1–7. [Google Scholar] [CrossRef]
- Shafaie, S.; Andalib, S.; Shafaei, H.; Montaseri, A.; Tavakolizadeh, M. Differential Biological Behavior of Fibroblasts and Endothelial Cells under Aloe vera Gel Culturing. Int. J. Mol. Cell. Med. 2020, 9. [Google Scholar] [CrossRef]
- Koivisto, L.; Heino, J.; Häkkinen, L.; Larjava, H. Integrins in Wound Healing. Adv. Wound Care 2014, 3, 762–783. [Google Scholar] [CrossRef]
- Khorasani, G.; Hosseinimehr, S.J.; Azadbakht, M.; Zamani, A.; Mahdavi, M.R. Aloe Versus Silver Sulfadiazine Creams for Second-Degree Burns: A Randomized Controlled Study. Surg. Today 2009, 39, 587–591. [Google Scholar] [CrossRef]
- Maenthaisong, R.; Chaiyakunapruk, N.; Niruntraporn, S.; Kongkaew, C. The efficacy of aloe vera used for burn wound healing: A systematic review. Burns 2007, 33, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.S.; Rahmati, J.; Ehsani, A.H.; Takzare, A.; Partoazar, A.; Takzaree, N. Efficacy of a Natural Topical Skin Ointment for Managing Split-Thickness Skin Graft Donor Sites: A Pilot Double-blind Randomized Controlled Trial. Adv. Skin. Wound Care 2020, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Meza-Valle, K.Z.; Saucedo-Acuña, R.A.; Tovar-Carrillo, K.L.; Cuevas-González, J.C.; Zaragoza-Contreras, E.A.; Melgoza-Lozano, J. Characterization and Topical Study of Aloe Vera Hydrogel on Wound-Healing Process. Polymers 2021, 13, 3958. [Google Scholar] [CrossRef]
- Teplicki, E.; Ma, Q.; Castillo, D.E.; Zarei, M.; Hustad, A.P.; Chen, J.; Li, J. The Effects of Aloe vera on Wound Healing in Cell Proliferation, Migration, and Viability. Wounds 2018, 30, 263–268. [Google Scholar] [PubMed]
- Olczyk, P.; Mencner, A.; Komosinska-Vassev, K. The Role of the Extracellular Matrix Components in Cutaneous Wound Healing. Biomed. Res. Int. 2014, 747584. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.D.P.; Mota, M.R.; Brizeno, L.A.; Nogueira, F.C.; Ferreira, E.G.; Pereira, M.G.; Assreuy, A.M. Modulator effect of a polysaccharide-rich extract from Caesalpinia ferrea stem barks in rat cutaneous wound healing: Role of TNF-α, IL-1β, NO, TGF-β. J. Ethnopharmacol. 2016, 187, 213–223. [Google Scholar] [CrossRef] [PubMed]
- de Paulo Pereira, L.; da Silva, R.O.; Bringel, P.H.D.S.F.; da Silva, K.E.S.; Assreuy, A.M.S.; Pereira, M.G. Polysaccharide fractions of Caesalpinia ferrea pods: Potential anti-inflammatory usage. J. Ethnopharmacol. 2012, 139, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.L.; Dally, J.; Lundy, F.T.; Langat, M.; Kiapranis, R.; Tsolaki, A.G.; Moseley, R.; Prescott, T.A.K. Lepiniopsis ternatensis sap stimulates fibroblast proliferation and down regulates macrophage TNF-α secretion. Fitoterapia 2020, 141, 104478. [Google Scholar] [CrossRef]
- Moses, R.L.; Fang, R.; Dally, J.; Briggs, M.; Lundy, F.T.; Kiapranis, R.; Moseley, R.; Prescott, T.A.K. Evaluation of Cypholophus macrocephalus sap as a treatment for infected cutaneous ulcers in Papua New Guinea. Fitoterapia 2020, 143, 104554. [Google Scholar] [CrossRef]
- Deli, J.; González-Beiras, C.; Guldan, G.S.; Moses, R.L.; Dally, J.; Moseley, R.; Lundy, F.T.; Corbacho-Monne, M.; Walker, S.L.; Cazorla, M.U.; et al. Clinical evaluation of Ficus septica exudate, a traditional medicine used in Papua New Guinea for treating small infected cutaneous ulcers. Phytomedicine 2022, 99, 154026. [Google Scholar] [CrossRef]
- Wall, I.B.; Moseley, R.; Baird, D.M.; Kipling, D.; Giles, P.; Laffafian, I.; Price, P.E.; Thomas, D.W.; Stephens, P. Fibroblast Dysfunction Is a Key Factor in the Non-Healing of Chronic Venous Leg Ulcers. J. Invest. Dermatol. 2008, 128, 2526–2540. [Google Scholar] [CrossRef]
- Moseley, R.; Stewart, J.E.; Stephens, P.; Waddington, R.J.; Thomas, D.W. Extracellular matrix metabolites as potential biomarkers of disease activity in wound fluid: Lessons learned from other inflammatory diseases? Br. J. Dermatol. 2004, 150, 401–413. [Google Scholar] [CrossRef]
- Abdennabi, R.; Bardaa, S.; Mehdi, M.; Rateb, M.E.; Raab, A.; Alenezi, F.N.; Sahnoun, Z.; Gharsallah, N.; Belbahri, L. Phoenix dactylifera L. sap enhances wound healing in Wistar rats: Phytochemical and histological assessment. Int. J. Biol. Macromol. 2016, 88, 443–450. [Google Scholar] [CrossRef]
- Taleb, H.; Maddocks, S.E.; Morris, R.K.; Kanekanian, A.D. Chemical characterisation and the anti-inflammatory, anti-angiogenic and antibacterial properties of date fruit (Phoenix dactylifera L.). J. Ethnopharmacol. 2016, 194, 457–468. [Google Scholar] [CrossRef]
- El-Far, A.H.; Oyinloye, B.E.; Sepehrimanesh, M.; Gab Allah, M.A.; Abu-Reidah, I.; Shaheen, H.M.; Razeghian-Jahromi, I.; El-Wahab, A.; Alsenosy, A.; Noreldin, A.E.; et al. Date Palm (Phoenix dactylifera): Novel Findings and Future Directions for Food and Drug Discovery. Curr. Drug Discov. Technol. 2019, 16, 2–10. [Google Scholar] [CrossRef]
- Kriaa, W.; Fetoui, H.; Makni, M.; Zeghal, N.; Drira, N.-E. Phenolic Contents and Antioxidant Activities of Date Palm (Phoenix Dactylifera L.) Leaves. Int. J. Food Prop. 2012, 15, 1220–1232. [Google Scholar] [CrossRef]
- Available online: https://qbiotics.com/product-pipeline/ecologic (accessed on 14 February 2023).
- Grant, E.L.; Wallace, H.M.; Trueman, S.J.; Reddell, P.W.; Ogbourne, S.M. Floral and reproductive biology of the medicinally significant rainforest tree, Fontainea picrosperma (Euphorbiaceae). Ind. Crops Prod. 2017, 108, 416–422. [Google Scholar] [CrossRef]
- De Ridder, T.R.; Campbell, J.E.; Burke-Schwarz, C.; Clegg, D.; Elliot, E.L.; Geller, S.; Kozak, W.; Pittenger, S.T.; Pruitt, J.B.; Riehl, J.; et al. Randomized controlled clinical study evaluating the efficacy and safety of intratumoral treatment of canine mast cell tumors with tigilanol tiglate (EBC-46). J. Vet. Intern. Med. 2020, 35, 415–429. [Google Scholar] [CrossRef]
- Jones, P.D.; Campbell, J.E.; Brown, G.; Johannes, C.M.; Reddell, P. Recurrence-free interval 12 months after local treatment of mast cell tumors in dogs using intratumoral injection of tigilanol tiglate. J. Vet. Intern. Med. 2021, 35, 451–455. [Google Scholar] [CrossRef]
- Reddell, P.; De Ridder, T.R.; Morton, J.M.; Jones, P.D.; Campbell, J.E.; Brown, G.; Johannes, C.M.; Schmidt, P.F.; Gordon, V. Wound formation, wound size, and progression of wound healing after intratumoral treatment of mast cell tumors in dogs with tigilanol tiglate. J. Vet. Intern. Med. 2021, 35, 430–441. [Google Scholar] [CrossRef]
- Boyle, G.M.; D’Souza, M.M.; Pierce, C.J.; Adams, R.A.; Cantor, A.S.; Johns, J.P.; Maslovskaya, L.; Gordon, V.A.; Reddell, P.W.; Parsons, P.G. Intra-lesional injection of the novel PKC activator EBC-46 rapidly ablates tumors in mouse models. PLoS ONE 2014, 9, e108887. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.L.; Boyle, G.M.; Howard-Jones, R.A.; Errington, R.J.; Johns, J.P.; Gordon, V.; Reddell, P.; Steadman, R.; Moseley, R. Novel epoxy-tiglianes-stimulate skin keratinocyte wound healing responses and re-epithelialization via protein kinase C activation. Biochem. Pharmacol. 2020, 178, 114048. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Ren, J.-H.; Xia, K.; Wang, S.-H.; Yin, T.-F.; Xie, D.-H.; Li, L.-H. Effect of mitomycin on normal dermal fibroblast and HaCat cell: An in vitro study. J. Zhejiang Univ-Sci. B Biomed. Biotechnol. 2012, 13, 997–1005. [Google Scholar] [CrossRef] [PubMed]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moses, R.L.; Prescott, T.A.K.; Mas-Claret, E.; Steadman, R.; Moseley, R.; Sloan, A.J. Evidence for Natural Products as Alternative Wound-Healing Therapies. Biomolecules 2023, 13, 444. https://doi.org/10.3390/biom13030444
Moses RL, Prescott TAK, Mas-Claret E, Steadman R, Moseley R, Sloan AJ. Evidence for Natural Products as Alternative Wound-Healing Therapies. Biomolecules. 2023; 13(3):444. https://doi.org/10.3390/biom13030444
Chicago/Turabian StyleMoses, Rachael L., Thomas A. K. Prescott, Eduard Mas-Claret, Robert Steadman, Ryan Moseley, and Alastair J. Sloan. 2023. "Evidence for Natural Products as Alternative Wound-Healing Therapies" Biomolecules 13, no. 3: 444. https://doi.org/10.3390/biom13030444
APA StyleMoses, R. L., Prescott, T. A. K., Mas-Claret, E., Steadman, R., Moseley, R., & Sloan, A. J. (2023). Evidence for Natural Products as Alternative Wound-Healing Therapies. Biomolecules, 13(3), 444. https://doi.org/10.3390/biom13030444








