Low-Affinity/High-Selectivity Dopamine Transport Inhibition Sufficient to Rescue Cognitive Functions in the Aging Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. hDAT, hSERT and hNET Binding Assay
2.3. Functional GPCRs Agonist and Antagonist Screening
2.4. Kinase Binding Assays
2.5. Determination of Plasma and Brain Levels of S-CE-123
2.6. Neuronal Outgrowth
2.7. Animals and Animal Ethics
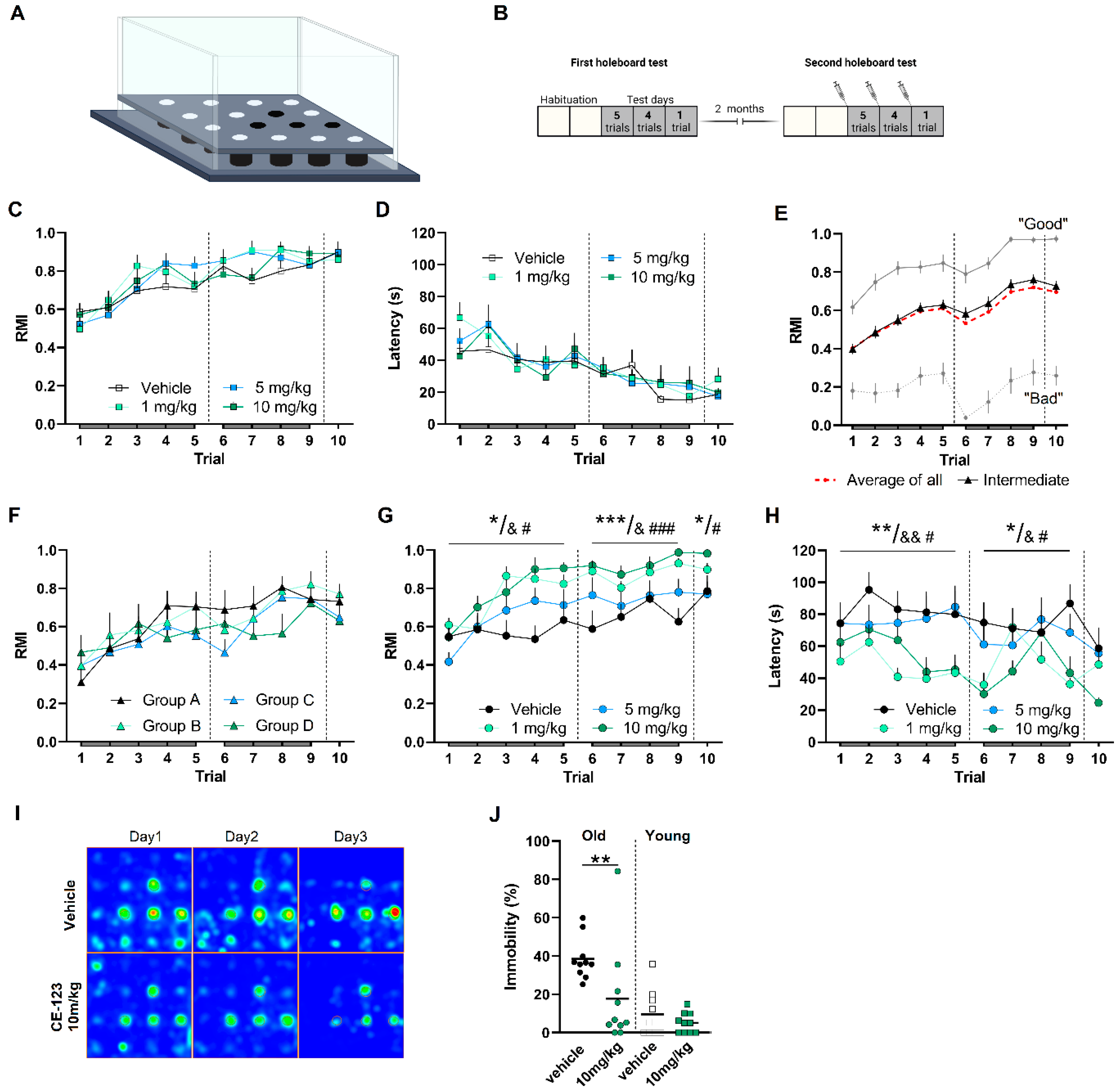
2.8. Hole-Board Spatial Learning and Memory Task
2.9. Morris Water Maze (MWM)
2.10. Open Field (OF)
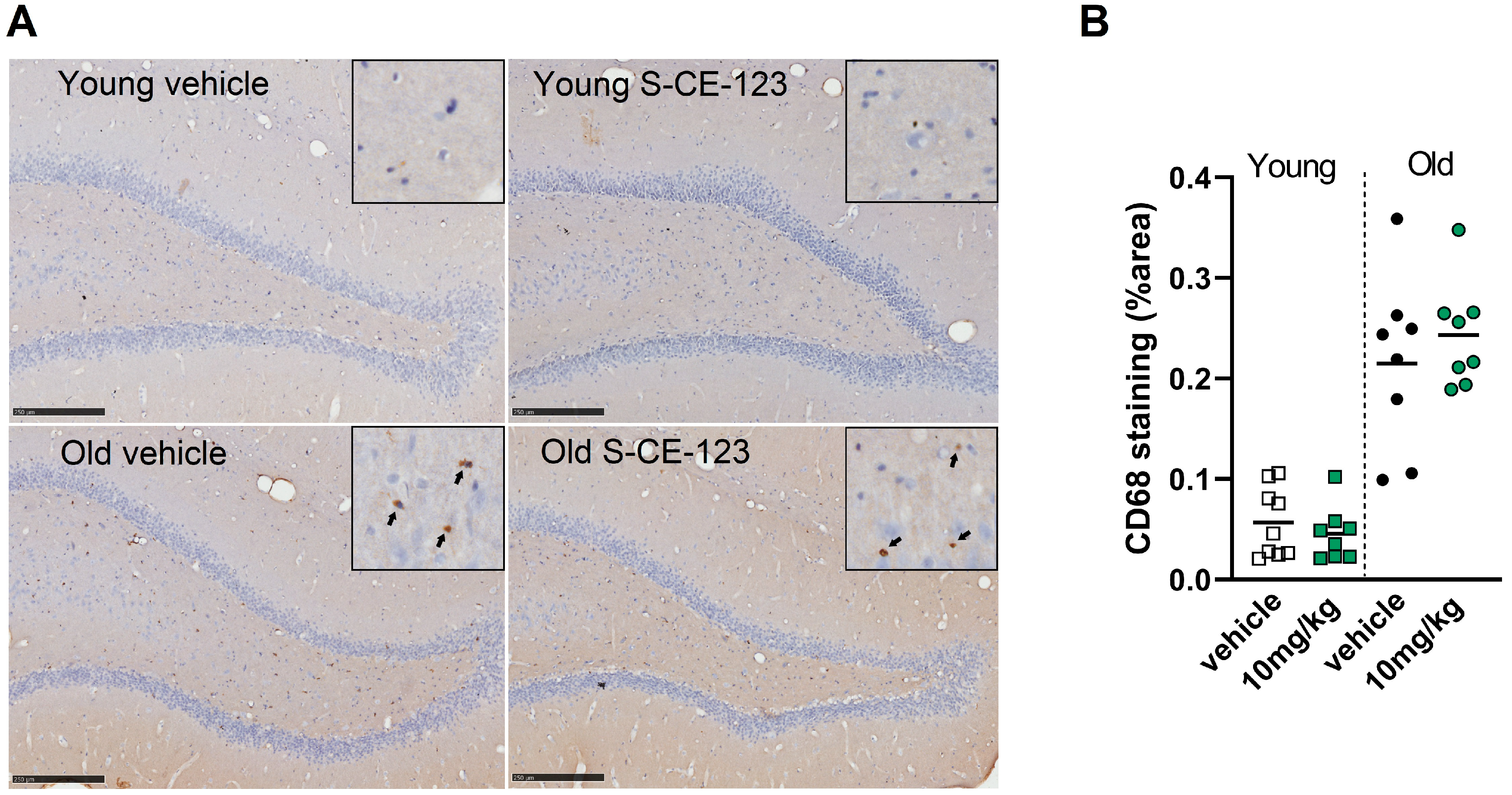
2.11. Immunohistochemistry
2.12. Statistical Analysis
3. Results
3.1. Binding of S-CE-123 to Human Monoamine Transporters
3.2. Analysis of Plasma and Brain Concentrations of S-CE-123
3.3. GPCRs and Kinome Screening
3.4. Effects of S-CE-123 on Neurite Outgrowth
3.5. S-CE-123 Increased Locomotion/Exploration in Familiar, but Not in a Novel Environment
3.5.1. A Hole-Board Test Did Not Show Differences between S-CE-123-Treated and Vehicle-Treated Rats at Young Age
3.5.2. Four Groups of Aged Rats Chosen for the Study Displayed Similar Ability for Spatial Learning and Memory
3.5.3. S-CE-123 Treatment Improves Ability for Spatial Learning and Memory Formation at Advanced Age
3.6. No Treatment Effect in Non-Rewarding Spatial Memory Task
3.7. Acute Treatment with S-CE-123 Has No Effect on Neuroinflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Werlen, E.; Jones, M.W. Modulating the map: Dopaminergic tuning of hippocampal spatial coding and interactions. Prog. Brain Res. 2015, 219, 187–216. [Google Scholar] [CrossRef] [PubMed]
- Singewald, N.; Schmuckermair, C.; Whittle, N.; Holmes, A.; Ressler, K.J. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol. Ther. 2015, 149, 150–190. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.C.; Devilbiss, D.M.; Berridge, C.W. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biol. Psychiatry 2015, 77, 940–950. [Google Scholar] [CrossRef]
- Ashby, F.G.; Valentin, V.V.; von Meer, S.S. Differential effects of dopamine-directed treatments on cognition. Neuropsychiatr. Dis. Treat. 2015, 11, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Linssen, A.M.; Vuurman, E.F.; Sambeth, A.; Riedel, W.J. Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology 2012, 221, 611–619. [Google Scholar] [CrossRef]
- Takeuchi, T.; Duszkiewicz, A.J.; Morris, R.G. The synaptic plasticity and memory hypothesis: Encoding, storage and persistence. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2014, 369, 20130288. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, A.; Rubino, V.; Sambataro, F.; Blasi, G.; Latorre, V.; Fazio, L.; Caforio, G.; Petruzzella, V.; Kolachana, B.; Hariri, A.; et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol. Psychiatry 2006, 60, 1250–1258. [Google Scholar] [CrossRef]
- Foerde, K.; Shohamy, D. The role of the basal ganglia in learning and memory: Insight from Parkinson’s disease. Neurobiol. Learn. Mem. 2011, 96, 624–636. [Google Scholar] [CrossRef]
- Mulder, A.B.; Arts, M.P.; Lopes da Silva, F.H. Short- and long-term plasticity of the hippocampus to nucleus accumbens and prefrontal cortex pathways in the rat, in vivo. Eur. J. Neurosci. 1997, 9, 1603–1611. [Google Scholar] [CrossRef]
- Lopez, J.; Almaguer, W.; Perez, H.; Frey, J.U.; Bergado, J.A. Opposite effects of shell or core stimulation of the nucleus accumbens on long-term potentiation in dentate gyrus of anesthetized rats. Neuroscience 2008, 151, 572–578. [Google Scholar] [CrossRef]
- Sagratella, S.; de Carolis, A.S.; Diana, G.; Domenici, M.R.; Popoli, P. Selective reduction of hippocampal dentate frequency-potentiation in striatally lesioned rats with impaired place learning. Brain Res. 1994, 660, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Peters, R. Ageing and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Dreher, J.C.; Meyer-Lindenberg, A.; Kohn, P.; Berman, K.F. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc. Natl. Acad. Sci. USA 2008, 105, 15106–15111. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Rieckmann, A. Neuromodulation and aging: Implications of aging neuronal gain control on cognition. Curr. Opin. Neurobiol. 2014, 29, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Ramos, K.; Moreno-Castilla, P.; Castro-Cruz, M.; McGaugh, J.L.; Martinez-Coria, H.; LaFerla, F.M.; Bermudez-Rattoni, F. Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn. Mem. 2012, 19, 453–460. [Google Scholar] [CrossRef]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017, 8, 14727. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Fuxe, K.; Borroto-Escuela, D.; Fisone, G.; Agnati, L.F.; Tanganelli, S. Understanding the role of heteroreceptor complexes in the central nervous system. Curr. Protein Pept. Sci. 2014, 15, 647. [Google Scholar] [CrossRef]
- Fuxe, K.; Guidolin, D.; Agnati, L.F.; Borroto-Escuela, D.O. Dopamine heteroreceptor complexes as therapeutic targets in Parkinson’s disease. Expert Opin. Ther. Targets 2015, 19, 377–398. [Google Scholar] [CrossRef]
- Martinez-Pinilla, E.; Rodriguez-Perez, A.I.; Navarro, G.; Aguinaga, D.; Moreno, E.; Lanciego, J.L.; Labandeira-Garcia, J.L.; Franco, R. Dopamine D2 and angiotensin II type 1 receptors form functional heteromers in rat striatum. Biochem. Pharmacol. 2015, 96, 131–142. [Google Scholar] [CrossRef]
- Tsanov, M.; Lyons, D.G.; Barlow, S.; Gonzalez Reyes, R.E.; O’Mara, S.M. The psychostimulant modafinil facilitates water maze performance and augments synaptic potentiation in dentate gyrus. Neuropharmacology 2010, 59, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Jenson, D.; Yang, K.; Acevedo-Rodriguez, A.; Levine, A.; Broussard, J.I.; Tang, J.; Dani, J.A. Dopamine and norepinephrine receptors participate in methylphenidate enhancement of in vivo hippocampal synaptic plasticity. Neuropharmacology 2015, 90, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Swant, J.; Wagner, J.J. Dopamine transporter blockade increases LTP in the CA1 region of the rat hippocampus via activation of the D3 dopamine receptor. Learn. Mem. 2006, 13, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Burgos, H.; Castillo, A.; Flores, O.; Puentes, G.; Morgan, C.; Gatica, A.; Cofre, C.; Hernandez, A.; Laurido, C.; Constandil, L. Effect of modafinil on learning performance and neocortical long-term potentiation in rats. Brain Res. Bull. 2010, 83, 238–244. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.P.; Shen, X.; Tsien, J.Z. Balanced dopamine is critical for pattern completion during associative memory recall. PLoS ONE 2010, 5, e15401. [Google Scholar] [CrossRef]
- Zilles, D.; Meyer, J.; Schneider-Axmann, T.; Ekawardhani, S.; Gruber, E.; Falkai, P.; Gruber, O. Genetic polymorphisms of 5-HTT and DAT but not COMT differentially affect verbal and visuospatial working memory functioning. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Kristofova, M.; Aher, Y.D.; Ilic, M.; Radoman, B.; Kalaba, P.; Dragacevic, V.; Aher, N.Y.; Leban, J.; Korz, V.; Zanon, L.; et al. A daily single dose of a novel modafinil analogue CE-123 improves memory acquisition and memory retrieval. Behav. Brain Res. 2018, 343, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Camats-Perna, J.; Kalaba, P.; Ebner, K.; Sartori, S.B.; Vuyyuru, H.; Aher, N.Y.; Dragacevic, V.; Singewald, N.; Engelmann, M.; Lubec, G. Differential Effects of Novel Dopamine Reuptake Inhibitors on Interference With Long-Term Social Memory in Mice. Front. Behav. Neurosci. 2019, 13, 63. [Google Scholar] [CrossRef]
- Nikiforuk, A.; Kalaba, P.; Ilic, M.; Korz, V.; Dragacevic, V.; Wackerlig, J.; Langer, T.; Hoger, H.; Golebiowska, J.; Popik, P.; et al. A Novel Dopamine Transporter Inhibitor CE-123 Improves Cognitive Flexibility and Maintains Impulsivity in Healthy Male Rats. Front. Behav. Neurosci. 2017, 11, 222. [Google Scholar] [CrossRef]
- Rotolo, R.A.; Dragacevic, V.; Kalaba, P.; Urban, E.; Zehl, M.; Roller, A.; Wackerlig, J.; Langer, T.; Pistis, M.; De Luca, M.A.; et al. The Novel Atypical Dopamine Uptake Inhibitor (S)-CE-123 Partially Reverses the Effort-Related Effects of the Dopamine Depleting Agent Tetrabenazine and Increases Progressive Ratio Responding. Front. Pharmacol. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Gyertyan, I.; Lubec, J.; Ernyey, A.J.; Gerner, C.; Kassai, F.; Kalaba, P.; Kozma, K.; Cobankovic, I.; Brenner, G.; Wackerlig, J.; et al. Cognitive profiling and proteomic analysis of the modafinil analogue S-CE-123 in experienced aged rats. Sci. Rep. 2021, 11, 23962. [Google Scholar] [CrossRef] [PubMed]
- Sagheddu, C.; Pintori, N.; Kalaba, P.; Dragacevic, V.; Piras, G.; Lubec, J.; Simola, N.; De Luca, M.A.; Lubec, G.; Pistis, M. Neurophysiological and Neurochemical Effects of the Putative Cognitive Enhancer (S)-CE-123 on Mesocorticolimbic Dopamine System. Biomolecules 2020, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Lubec, J.; Smidak, R.; Malikovic, J.; Feyissa, D.D.; Korz, V.; Hoger, H.; Lubec, G. Dentate Gyrus Peroxiredoxin 6 Levels Discriminate Aged Unimpaired From Impaired Rats in a Spatial Memory Task. Front. Aging Neurosci. 2019, 11, 198. [Google Scholar] [CrossRef]
- Lee, Y.B.; Nagai, A.; Kim, S.U. Cytokines, chemokines, and cytokine receptors in human microglia. J. Neurosci. Res. 2002, 69, 94–103. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimer’s Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Mohammad, D.; Trepanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef]
- Hoozemans, J.J.; Rozemuller, A.J.; van Haastert, E.S.; Eikelenboom, P.; van Gool, W.A. Neuroinflammation in Alzheimer’s disease wanes with age. J. Neuroinflammation 2011, 8, 171. [Google Scholar] [CrossRef]
- Farso, M.; Menard, C.; Colby-Milley, J.; Quirion, R. Immune marker CD68 correlates with cognitive impairment in normally aged rats. Neurobiol. Aging 2013, 34, 1971–1976. [Google Scholar] [CrossRef]
- Docherty, J.R.; Alsufyani, H.A. Pharmacology of Drugs Used as Stimulants. J. Clin. Pharmacol. 2021, 61 (Suppl. S2), S53–S69. [Google Scholar] [CrossRef]
- Pontieri, F.E.; Tanda, G.; Di Chiara, G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the "shell" as compared with the "core" of the rat nucleus accumbens. Proc. Natl. Acad. Sci. USA 1995, 92, 12304–12308. [Google Scholar] [CrossRef]
- Pontieri, F.E.; Tanda, G.; Orzi, F.; Di Chiara, G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996, 382, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Aragona, B.J.; Cleaveland, N.A.; Stuber, G.D.; Day, J.J.; Carelli, R.M.; Wightman, R.M. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J. Neurosci. 2008, 28, 8821–8831. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, A.; Lecca, D.; Valentini, V.; Bahi, A.; Dreyer, J.L.; Cacciapaglia, F.; Scifo, A.; Piras, G.; Cadoni, C.; Di Chiara, G. Impairment of acquisition of intravenous cocaine self-administration by RNA-interference of dopamine D1-receptors in the nucleus accumbens shell. Neuropharmacology 2015, 89, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.D.; Tran, V.L.; Turchi, J.; Averbeck, B.B. Dopamine modulates novelty seeking behavior during decision making. Behav. Neurosci. 2014, 128, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef]
- Horvitz, J.C. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 2000, 96, 651–656. [Google Scholar] [CrossRef]
- Carey, R.J.; DePalma, G.; Damianopoulos, E. Acute and chronic cocaine behavioral effects in novel versus familiar environments: Open-field familiarity differentiates cocaine locomotor stimulant effects from cocaine emotional behavioral effects. Behav. Brain Res. 2005, 158, 321–330. [Google Scholar] [CrossRef]
- Carey, R.J.; Damianopoulos, E.N. Cocaine conditioning and sensitization: The habituation factor. Pharmacol. Biochem. Behav. 2006, 84, 128–133. [Google Scholar] [CrossRef]
- Pieruccini-Faria, F.; Lord, S.R.; Toson, B.; Kemmler, W.; Schoene, D. Mental Flexibility Influences the Association Between Poor Balance and Falls in Older People—A Secondary Analysis. Front. Aging Neurosci. 2019, 11, 133. [Google Scholar] [CrossRef]
- Rotolo, R.A.; Kalaba, P.; Dragacevic, V.; Presby, R.E.; Neri, J.; Robertson, E.; Yang, J.H.; Correa, M.; Bakulev, V.; Volkova, N.N.; et al. Behavioral and dopamine transporter binding properties of the modafinil analog (S, S)-CE-158: Reversal of the motivational effects of tetrabenazine and enhancement of progressive ratio responding. Psychopharmacology 2020, 237, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, R.A.; Presby, R.E.; Tracy, O.; Asar, S.; Yang, J.H.; Correa, M.; Murray, F.; Salamone, J.D. The novel atypical dopamine transport inhibitor CT-005404 has pro-motivational effects in neurochemical and inflammatory models of effort-based dysfunctions related to psychopathology. Neuropharmacology 2021, 183, 108325. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Geyer, M.A. Action of modafinil--increased motivation via the dopamine transporter inhibition and D1 receptors? Biol. Psychiatry 2010, 67, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Kouhnavardi, S.; Ecevitoglu, A.; Dragacevic, V.; Sanna, F.; Arias-Sandoval, E.; Kalaba, P.; Kirchhofer, M.; Lubec, J.; Niello, M.; Holy, M.; et al. A Novel and Selective Dopamine Transporter Inhibitor, (S)-MK-26, Promotes Hippocampal Synaptic Plasticity and Restores Effort-Related Motivational Dysfunctions. Biomolecules 2022, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Braver, T.S.; Krug, M.K.; Chiew, K.S.; Kool, W.; Westbrook, J.A.; Clement, N.J.; Adcock, R.A.; Barch, D.M.; Botvinick, M.M.; Carver, C.S.; et al. Mechanisms of motivation-cognition interaction: Challenges and opportunities. Cogn. Affect. Behav. Neurosci. 2014, 14, 443–472. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998, 80, 1–27. [Google Scholar] [CrossRef]
- Shohamy, D.; Adcock, R.A. Dopamine and adaptive memory. Trends Cogn. Sci. 2010, 14, 464–472. [Google Scholar] [CrossRef]
- Feenstra, M.G.; Botterblom, M.H. Rapid sampling of extracellular dopamine in the rat prefrontal cortex during food consumption, handling and exposure to novelty. Brain Res. 1996, 742, 17–24. [Google Scholar] [CrossRef]
- Bassareo, V.; Di Chiara, G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur. J. Neurosci. 1999, 11, 4389–4397. [Google Scholar] [CrossRef]
- Spaniol, J.; Schain, C.; Bowen, H.J. Reward-enhanced memory in younger and older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2014, 69, 730–740. [Google Scholar] [CrossRef]
- Schulz, K.; Korz, V. Emotional and cognitive information processing: Relations to behavioral performance and hippocampal long-term potentiation in vivo during a spatial water maze training in rats. Learn. Mem. 2010, 17, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Allard, S.; Gosein, V.; Cuello, A.C.; Ribeiro-da-Silva, A. Changes with aging in the dopaminergic and noradrenergic innervation of rat neocortex. Neurobiol. Aging 2011, 32, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Richter-Levin, G.; Segal, M. Age-related cognitive deficits in rats are associated with a combined loss of cholinergic and serotonergic functions. Ann. N. Y. Acad. Sci. 1993, 695, 254–257. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Jones, S.R.; Fumagalli, F.; Wightman, R.M.; Caron, M.G. Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res. Brain Res. Rev. 1998, 26, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pei, L.; Li, S.; Wang, M.; Liu, F. Extracellular dopamine induces the oxidative toxicity of SH-SY5Y cells. Synapse 2008, 62, 797–803. [Google Scholar] [CrossRef]
- Minzenberg, M.J.; Carter, C.S. Modafinil: A review of neurochemical actions and effects on cognition. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 1477–1502. [Google Scholar] [CrossRef]
- Lillicrap, T.P.; Levi, C.R.; Holliday, E.; Parsons, M.W.; Bivard, A. Short- and Long-Term Efficacy of Modafinil at Improving Quality of Life in Stroke Survivors: A Post Hoc Sub Study of the Modafinil in Debilitating Fatigue After Stroke Trial. Front. Neurol. 2018, 9, 269. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubec, J.; Hussein, A.M.; Kalaba, P.; Feyissa, D.D.; Arias-Sandoval, E.; Cybulska-Klosowicz, A.; Bezu, M.; Stojanovic, T.; Korz, V.; Malikovic, J.; et al. Low-Affinity/High-Selectivity Dopamine Transport Inhibition Sufficient to Rescue Cognitive Functions in the Aging Rat. Biomolecules 2023, 13, 467. https://doi.org/10.3390/biom13030467
Lubec J, Hussein AM, Kalaba P, Feyissa DD, Arias-Sandoval E, Cybulska-Klosowicz A, Bezu M, Stojanovic T, Korz V, Malikovic J, et al. Low-Affinity/High-Selectivity Dopamine Transport Inhibition Sufficient to Rescue Cognitive Functions in the Aging Rat. Biomolecules. 2023; 13(3):467. https://doi.org/10.3390/biom13030467
Chicago/Turabian StyleLubec, Jana, Ahmed M. Hussein, Predrag Kalaba, Daniel Daba Feyissa, Edgar Arias-Sandoval, Anita Cybulska-Klosowicz, Mekite Bezu, Tamara Stojanovic, Volker Korz, Jovana Malikovic, and et al. 2023. "Low-Affinity/High-Selectivity Dopamine Transport Inhibition Sufficient to Rescue Cognitive Functions in the Aging Rat" Biomolecules 13, no. 3: 467. https://doi.org/10.3390/biom13030467
APA StyleLubec, J., Hussein, A. M., Kalaba, P., Feyissa, D. D., Arias-Sandoval, E., Cybulska-Klosowicz, A., Bezu, M., Stojanovic, T., Korz, V., Malikovic, J., Aher, N. Y., Zehl, M., Dragacevic, V., Leban, J. J., Sagheddu, C., Wackerlig, J., Pistis, M., Correa, M., Langer, T., ... Lubec, G. (2023). Low-Affinity/High-Selectivity Dopamine Transport Inhibition Sufficient to Rescue Cognitive Functions in the Aging Rat. Biomolecules, 13(3), 467. https://doi.org/10.3390/biom13030467





