The Impact of Flavonoid-Loaded Nanoparticles in the UV Protection and Safety Profile of Topical Sunscreens
Abstract
1. Introduction
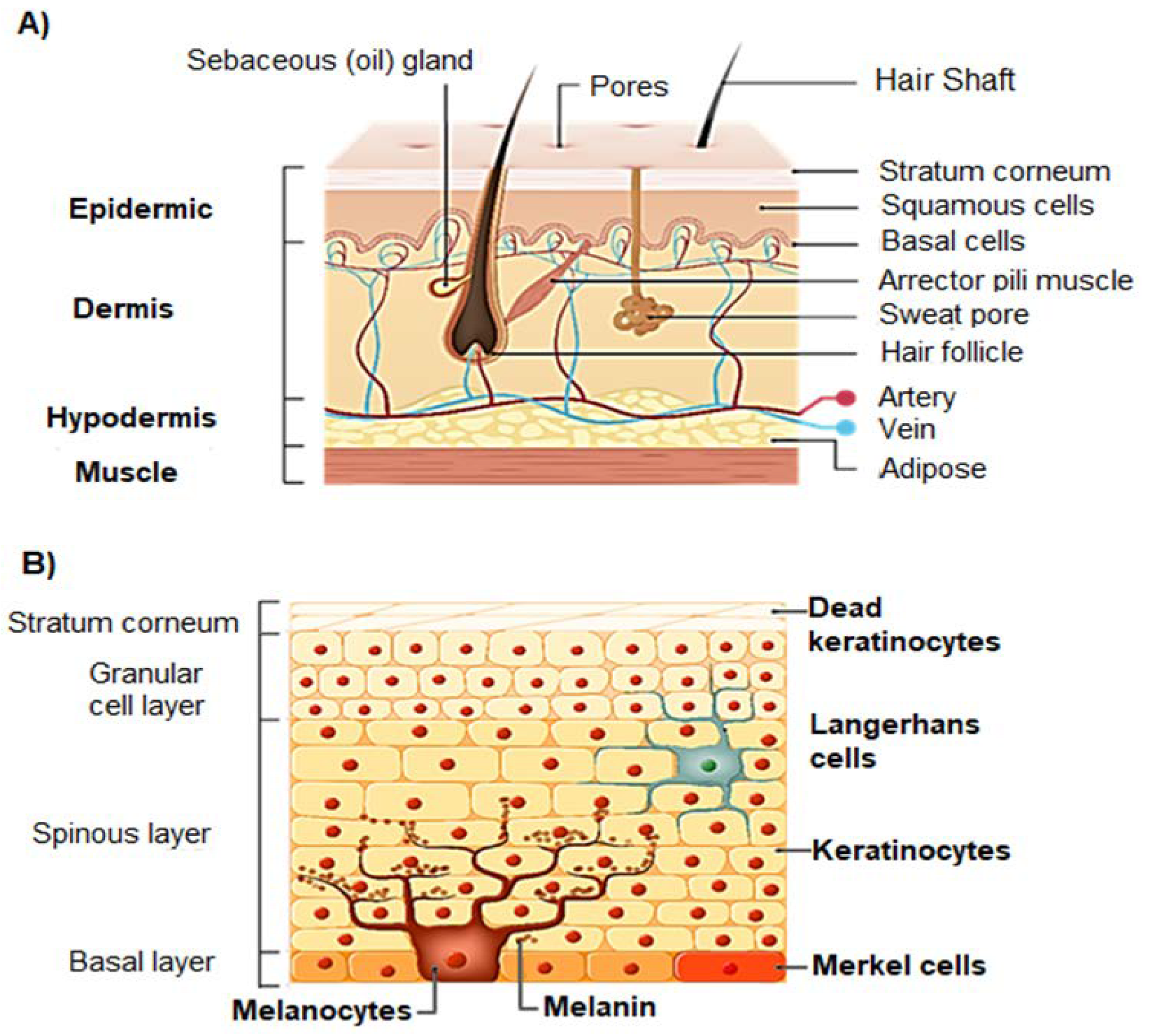
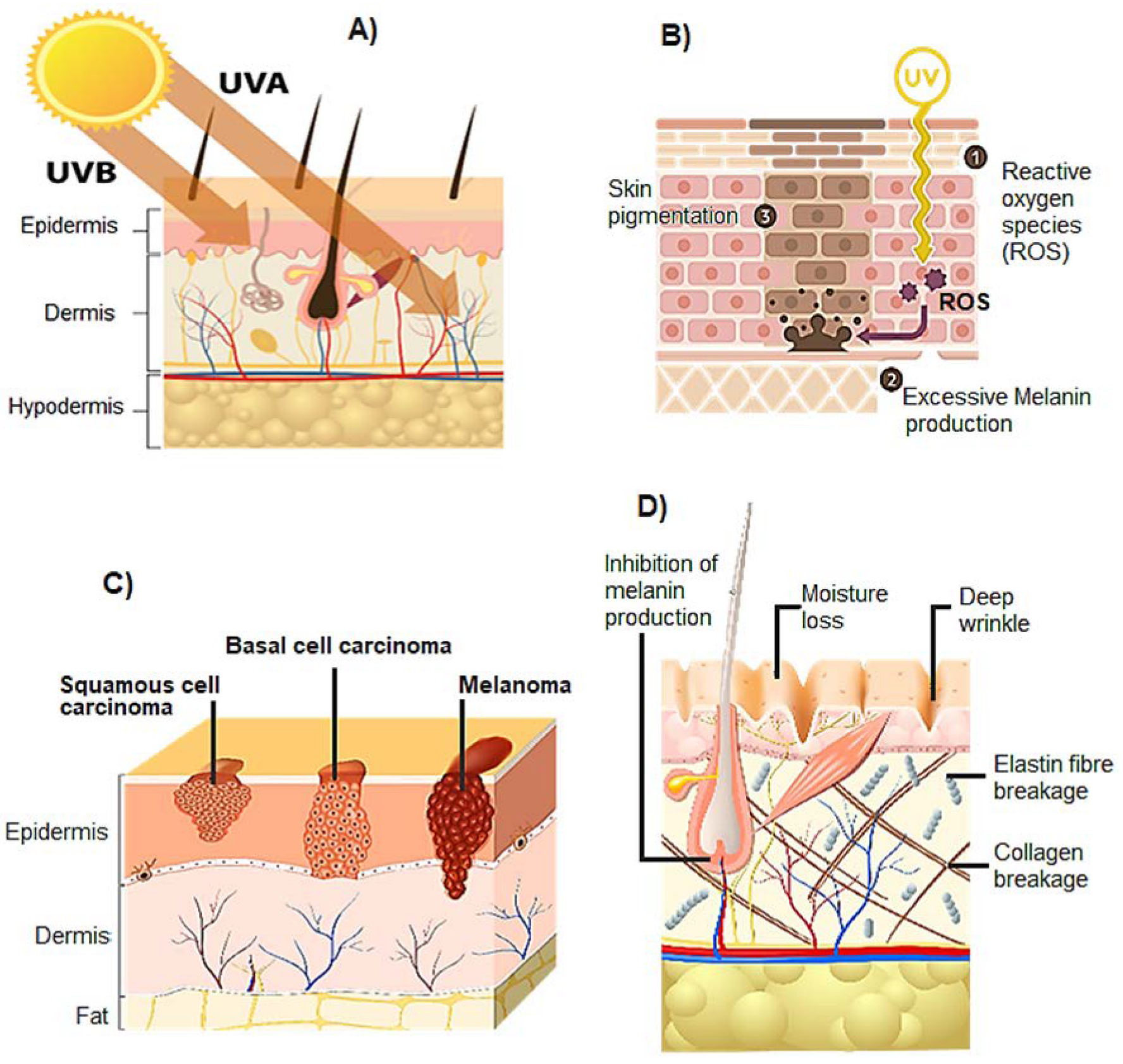
2. Structure and Effect of UV Radiation on Skin
3. Sunscreens and UV Protection
3.1. Sunscreens
3.2. Conventional UV Filters
3.2.1. Organic Filters
3.2.2. Inorganic Filters
3.3. Regulatory Considerations on Sunscreens
4. The Next Generation of Sunscreens: Phytoactive UVA Filters
4.1. Quercetin and Rutin
4.2. Silymarin
4.3. Pomegranate
4.4. Lignin
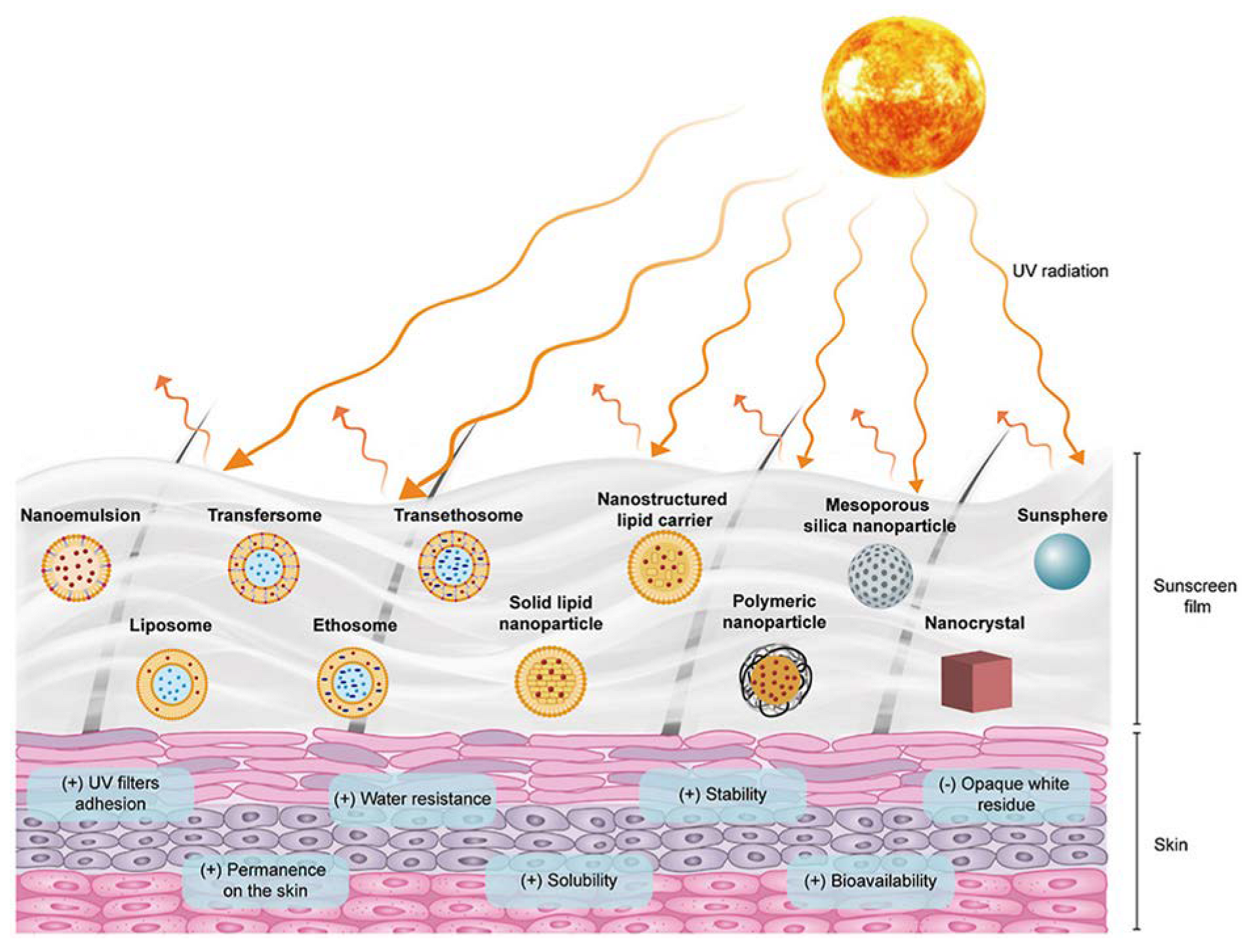
5. Nanocarriers to Improve UVA Photoprotection
5.1. Liposomes
5.2. Solid Lipid Nanoparticles
5.3. Polymeric Nanoparticles
5.4. Gold Nanoparticles
6. Phytoactive Nanoformulations with UVA Photoprotection Activity
6.1. Quercetin-Loaded Nanoparticles
6.2. Rutin-Loaded Nanoparticles
6.3. Silymarin-Loaded Nanoparticles
6.4. Pomegranate-Loaded Nanoparticles
6.5. Lignin-Loaded Nanoparticles
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
References
- Calzavara-Pinton, P.G.; Ortel, B. Pigmentation after solar radiation. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; The Royal Society of Chemistry: Cambridge, UK, 2007; Volume 10, pp. 65–97. [Google Scholar]
- Kovacs, E.G.; Di Pietro, L.A.Y. Fibrogenic cytokines and connective tissue production. FASEB J. 1994, 8, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Matta, M.K.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.; Florian, J.; Oh, L.; Bashaw, E.; Zineh, I.; Sanabria, C.; et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients. JAMA 2019, 321, 2082–2091. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Preethi, S.; Patil, A.B.; Jain, V. A comparison of Natural and Synthetic Sunscreen Agents: A Review. Int. J. Pharm. Res. 2021, 13, 3494–3505. [Google Scholar]
- León, Z.; Chisvert, A.; Tarazona, I.; Salvador, A. Solid-phase extraction liquid chromatography–tandem mass spectrometry analytical method for the determination of 2-hydroxy-4-methoxybenzophenone and its metabolites in both human urine and semen. Anal. Bioanal. Chem. 2010, 398, 831–843. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; del Mar Olmo-Campos, M.; Valeta-Juan, G.; Pleguezuelos-Hernández, V.; Barceló, D.; Díaz-Cruz, M.S. Determination of UV filters in human breast milk using turbulent flow chromatography and babies’ daily intake estimation. Environ. Res. 2018, 161, 532–539. [Google Scholar] [CrossRef]
- Merhi, S.; Salameh, P.; Kaplan, P.; Banerjee, S.; Lajnef, M.; Dumont, E.; Ezzedine, K. An Ecological Study Indicates the Importance of Ultraviolet A Protection in Sunscreens. Acta Derm. Venereol. 2021, 101, adv00480. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Imai, N.; Mashima, R.; Konaka, R.; Inoue, M.; Dunlap, W.C. Singlet Oxygen from Irradiated Titanium Dioxide and Zinc Oxide. Methods Enzymol. 2000, 319, 29–37. [Google Scholar]
- Shindo, Y.; Witt, E.; Packer, L. Antioxidant Defense Mechanisms in Murine Epidermis and Dermis and Their Responses to Ultraviolet Light. J. Investig. Dermatol. 1993, 100, 260–265. [Google Scholar] [CrossRef]
- Fivenson, D.; Sabzevari, N.; Qiblawi, S.; Blitz, J.; Norton, B.B.; Norton, S.A. Sunscreens: UV filters to protect us: Part 2-Increasing awareness of UV filters and their potential toxicities to us and our environment. Int. J. Women’s Dermatol. 2021, 7, 45–69. [Google Scholar] [CrossRef]
- Sabzevari, N.; Qiblawi, S.; Norton, S.A.; Fivenson, D. Sunscreens: UV filters to protect us: Part 1: Changing regulations and choices for optimal sun protection. Int. J. Women’s Dermatol. 2021, 7, 28–44. [Google Scholar] [CrossRef] [PubMed]
- De Cooman, L.; Everaert, E.; De Keukeleire, D. Quantitative analysis of hop acids, essential oils and flavonoids as a clue to the identification of hop varieties. Phytochem. Anal. 1998, 9, 145–150. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.L.S.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Agarwal, S.B.; Godse, K.; Patil, S.; Nadkarni, N. Knowledge and attitude of general population toward effects of sun exposure and use of sunscreens. Indian J. Dermatol. 2018, 63, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Quelhas, S.; Silva, A.M.; Souto, E.B. Nanoemulsions for delivery of flavonoids: Formulation and in vitro release of rutin as model drug. Pharm. Dev. Technol. 2014, 19, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Saupe, A.; Müller, R.H.; Souto, E.B. Nanostructured lipid carriers as novel carrier for sunscreen formulations. Int. J. Cosmet. Sci. 2007, 29, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.; Yoshida, C.; Leonardi, G.; Cano, A.; Sanchez-Lopez, E.; Zielinska, A.; Viseras, C.; Severino, P.; Silva, C.; Barbosa, R. Lipid-Polymeric Films: Composition, Production and Applications in Wound Healing and Skin Repair. Pharmaceutics 2021, 13, 1199. [Google Scholar] [CrossRef]
- Batistela, M.A.; Chorilli, M.; Leonardi, G.R. Approach to the process knowledge of skin aging among different ethnics. Rev. Bras. Farm. 2007, 88, 59–62. [Google Scholar]
- Das Kurmi, B.; Tekchandani, P.; Paliwal, R.; Rai Paliwal, S. Transdermal Drug Delivery: Opportunities and Challenges for Controlled Delivery of Therapeutic Agents Using Nanocarriers. Curr. Drug Metab. 2017, 18, 481–495. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Cricker, A. The epidemiology of solar radiation and skin cancer. In Sun Protection in Man; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 131–153. [Google Scholar]
- de Gruijl, F.R.; van Kranen, H.J. UV radiation, mutation and oncogenic pathways in skin cancer. In Sun Protection in Man; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 287–302. [Google Scholar]
- Wlaschek, M.; Tantcheva-Poór, I.; Brenneisen, P.; Kuhr, L.; Razi-Wolf, Z.; Hellweg, C.; Schneider, L.A.; Meewes, C.; Scharffetter-Kochanek, K. The negative effects of solar and artificial radiation: Photoaging of the skin, its clinical appearance and underlying mechanisms. In Sun Protection in Man; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 115–130. [Google Scholar]
- Wlaschek, M.; Schneider, L.A.; Kohn, M.; Nüßeler, E.; Treiber, N.; Scharffetter-Kochanek, K. Aging after Solar Radiation. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; The Royal Society of Chemistry: Cambridge, UK, 2007; pp. 191–210. [Google Scholar]
- Urbach, F. The negative effects of solar radiation: A clinical overview. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 39–67. [Google Scholar]
- Chung, J.O. The effects on sunlight on the skin of Asians. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 69–90. [Google Scholar]
- Young, A.R.; Sheehan, J.R. Damage from Acute vs Chronic Solar Exposure. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; The Royal Society of Chemistry: Cambridge, UK, 2007; Volume 10, pp. 3–23. [Google Scholar]
- Foster, K.W.; Katiyar, S.K.; Yusuf, N.; Elmets, C.A. Inflammation after solar radiation. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; The Royal Society of Chemistry: Cambridge, UK, 2007; pp. 25–63. [Google Scholar]
- Heenen, M.; Giacomoni, P.U.; Golstein, P. Erythema, a link between UV-induced DNA damage, cell death and clinical effects. In Sun Protection in Man; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 277–285. [Google Scholar]
- Norval, M. Effects of solar radiation on the human immune system. In Sun Protection in Man; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 91–113. [Google Scholar] [CrossRef]
- Halliday, G.M.; Rana, S. The effects of solar radiation on the immune response in humans. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; The Royal Society of Chemistry: Cambridge, UK, 2007; pp. 127–163. [Google Scholar]
- Ravanat, J.L.; Douki, T.; Cadet, J. UV damage to Nucleic Acid components. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 207–230. [Google Scholar]
- Douki, T. UV-induced DNA damage. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; The Royal Society of Chemistry: Cambridge, UK, 2007; pp. 227–269. [Google Scholar]
- Davies, M.J.; Truscott, R.J.W. Photo-oxidation of Proteins and its Consequences. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 251–275. [Google Scholar]
- Girotti, A.W. Lipid photooxidative damage in biological membranes: Reaction mechanisms, cytotoxic consequences, and defense strategies. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 231–250. [Google Scholar]
- Girotti, A.W.; Giacomoni, P.U. Lipid and Protein damage provoked by Ultraviolet Radiation: Mechanisms of indirect photooxidative damage. In Biophysical and Physiological Effects of Solar Radiation on Human Skin; Giacomoni, P.U., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2007; pp. 271–291. [Google Scholar]
- Audic, A.; Giacomoni, P.U. Dna nicking by ultraviolet radiation is enhanced in the presence of iron and of oxygen. Photochem. Photobiol. 1993, 57, 508–512. [Google Scholar] [CrossRef]
- Ley, R.D. Photoreactivation of UV-induced pyrimidine dimers and erythema in the marsupial Monodelphis domestica. Proc. Natl. Acad. Sci. USA 1985, 82, 2409–2411. [Google Scholar] [CrossRef] [PubMed]
- Stoien, J.D.; Wang, R.J. Effect of Near-Ultraviolet and Visible Light on Mammalian Cells in Culture II. Formation of Toxic Photoproducts in Tissue Culture Medium by Blacklight. Proc. Natl. Acad. Sci. USA 1974, 71, 3961–3965. [Google Scholar] [CrossRef] [PubMed]
- Baier, J.; Maisch, T.; Maier, M.; Engel, E.; Landthaler, M.; Bäumler, W. Singlet Oxygen Generation by UVA Light Exposure of Endogenous Photosensitizers. Biophys. J. 2006, 91, 1452–1459. [Google Scholar] [CrossRef]
- Balny, C.; Douzou, P. Production of superoxide ions by photosensitization of dyes. Biochem. Biophys. Res. Commun. 1974, 56, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Malorni, W.; Donelli, G.; Straface, E.; Santini, M.T.; Paradisi, S.; Giacomoni, P.U. Both UVA and UVB induce cytoskeletal dependent surface blebbing in epidermoid cells. J. Photochem. Photobiol. B Biol. 1994, 26, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Malorni, W.; Iosi, F.; Mirabelli, F.; Bellomo, G. Cytoskeleton as a target in menadione-induced oxidative stress in cultured mammalian cells: Alterations underlying surface bleb formation. Chem. Interact. 1991, 80, 217–236. [Google Scholar] [CrossRef]
- Giacomoni, P.U.; Nadaud, J.F.; Straface, E.; Donelli, G.; Heenen, M.; Malorni, W. Morphological alterations and cell blebbing in UV-irradiated human epidermis. Arch. Dermatol. Res. 1998, 290, 163–166. [Google Scholar] [CrossRef]
- Straface, E.; Falchi, M.; Giacomoni, P.U.; Malorni, W. Cytotoxicity and morphological endpoints of exposure to UV: Cultured cells as a model system. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 321–336. [Google Scholar]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Svobodová, A.R.; Gabrielová, E.; Ulrichová, J.; Zálešák, B.; Biedermann, D.; Vostálová, J. A pilot study of the UVA-photoprotective potential of dehydrosilybin, isosilybin, silychristin, and silydianin on human dermal fibroblasts. Arch. Dermatol. Res. 2019, 311, 477–490. [Google Scholar] [CrossRef]
- Montenegro, L.; Puglisi, G. Evaluation of sunscreen safety by in vitro skin permeation studies: Effects of vehicle composition. Die Pharm. Int. J. Pharm. Sci. 2013, 68, 34–40. [Google Scholar]
- Cefali, L.C.; Ataide, J.A.; Fernandes, A.R.; Sousa, I.M.D.O.; Gonçalves, F.C.D.S.; Eberlin, S.; Dávila, J.L.; Jozala, A.F.; Chaud, M.V.; Sanchez-Lopez, E.; et al. Flavonoid-Enriched Plant-Extract-Loaded Emulsion: A Novel Phytocosmetic Sunscreen Formulation with Antioxidant Properties. Antioxidants 2019, 8, 443. [Google Scholar] [CrossRef]
- Widsten, P.; Tamminen, T.; Liitiä, T. Natural Sunscreens Based on Nanoparticles of Modified Kraft Lignin (CatLignin). ACS Omega 2020, 5, 13438–13446. [Google Scholar] [CrossRef]
- Herzinger, T. Sun protection factor 50+: Pro and contra. Hautarzt 2017, 68, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.H.; Nesbitt, L.T. Sun Exposure Behavior and Protection: Recommendations for Travelers. J. Travel Med. 2013, 20, 108–118. [Google Scholar] [CrossRef]
- Lu, C.J.; Benner, R.; Fichot, C.G.; Fukuda, H.; Yamashita, Y.; Ogawa, H. Sources and transformations of dissolved lignin phenols and chromophoric dissolved organic matter in Otsuchi Bay, Japan. Front. Mar. Sci. 2016, 3, 85. [Google Scholar] [CrossRef]
- Young, A.R.; Sheehan, J.R. UV-induced pigmentation in human skin. In Sun Protection in Man; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 357–375. [Google Scholar]
- van der Pols, J.C.; Williams, G.M.; Pandeya, N.; Logan, V.; Green, A.C. Prolonged Prevention of Squamous Cell Carcinoma of the Skin by Regular Sunscreen Use. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2546–2548. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Arianto, A.; Cella, G.; Bangun, H. Preparation and Evaluation of Sunscreen Nanoemulsions with Synergistic Efficacy on SPF by Combination of Soybean Oil, Avobenzone, and Octyl Methoxycinnamate. Open Access Maced. J. Med. Sci. 2019, 7, 2751–2756. [Google Scholar] [CrossRef]
- Gause, S.; Chauhan, A. UV-blocking potential of oils and juices. Int. J. Cosmet. Sci. 2016, 38, 354–363. [Google Scholar] [CrossRef]
- Han, Y. Rutin has therapeutic effect on septic arthritis caused by Candida albicans. Int. Immunopharmacol. 2009, 9, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Bonina, F.; Lanza, M.; Montenegro, L.; Puglisi, C.; Tomaino, A.; Trombetta, D.; Castelli, F.; Saija, A. Flavonoids as potential protective agents against photo-oxidative skin damage. Int. J. Pharm. 1996, 145, 87–94. [Google Scholar] [CrossRef]
- Kim, H.K.; Namgoong, S.Y.; Kim, H.P. Antiinflammatory activity of flavonoids: Mouse ear edema inhibition. Arch. Pharmacal Res. 1993, 16, 18–24. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B Biointerfaces 2014, 123, 566–574. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Koop, D.R. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem. Pharmacol. 1999, 57, 941–949. [Google Scholar] [CrossRef]
- Mauludin, R.; Müller, R.H.; Keck, C.M. Kinetic solubility and dissolution velocity of rutin nanocrystals. Eur. J. Pharm. Sci. 2009, 36, 502–510. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Jiménez-Aliaga, K.; Bermejo-Bescós, P.; Benedí, J.; Martín-Aragón, S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011, 89, 939–945. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Chat, O.A.; Najar, M.H.; Mir, M.A.; Rather, G.M.; Dar, A.A. Effects of surfactant micelles on solubilization and DPPH radical scavenging activity of Rutin. J. Colloid Interface Sci. 2011, 355, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Mauludin, R.; Müller, R.H.; Keck, C.M. Development of an oral rutin nanocrystal formulation. Int. J. Pharm. 2009, 370, 202–209. [Google Scholar] [CrossRef] [PubMed]
- IV CPT. Method for the In Vitro Determination of UVA Protection Provided by Sunscreen Products. COLIPA, Belgium, 2006.
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 591, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pintoa, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF enhancement provided by rutin in a multifunctional sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agarwal, R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur. J. Cancer 2005, 41, 1969–1979. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Vaid, M. Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn). Int. J. Oncol. 2010, 36, 1053–1060. [Google Scholar] [CrossRef]
- Rajnochová Svobodová, A.; Gabrielová, E.; Michaelides, L.; Kosina, P.; Ryšavá, A.; Ulrichová, J.; Zálešák, B.; Vostálová, J. UVAphotoprotective potential of silymarin and silybin. Arch. Dermatol. Res. 2018, 310, 413–424. [Google Scholar] [CrossRef]
- Vostálová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Rajnochova Svobodová, A. Skin Protective Activity of Silymarin and its Flavonolignans. Molecules 2019, 24, 1022. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Lopedota, A.; Fini, P.; Laurenzana, A.; Fibbi, G.; Fanelli, F.; Petrella, A.; Laquintana, V.; Denora, N.; et al. One pot environmental friendly synthesis of gold nanoparticles using Punica Granatum Juice: A novel antioxidant agent for future dermatological and cosmetic applications. J. Colloid Interface Sci. 2018, 521, 50–61. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, Y.; Wang, J.; Qiu, X.; Zeng, H. Bioinspired Lignin-Polydopamine Nanocapsules with Strong Bioadhesion for Long-Acting and High-Performance Natural Sunscreens. Biomacromolecules 2020, 21, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Estimating of Structural Composition of Wood and Non-Wood Biomass Samples. Energy Sources 2005, 27, 761–767. [Google Scholar] [CrossRef]
- Opsahl, S.; Benner, R. Photochemical reactivity of dissolved lignin in river and ocean waters. Limnol. Oceanogr. 1998, 43, 1297–1304. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef]
- Argyropoulos, D.S. Quantitative Phosphorus-31 NMR Analysis of Lignins, a New Tool for the Lignin Chemist. J. Wood Chem. Technol. 1994, 14, 45–63. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Venditti, R.; Jur, J.; Gorga, R.E.; Pawlak, J.J. Cellulose-Lignin Biodegradable and Flexible UV Protection Film. ACS Sustain. Chem. Eng. 2017, 5, 625–631. [Google Scholar] [CrossRef]
- Lou, H.; Lai, H.; Wang, M.; Pang, Y.; Yang, D.; Qiu, X.; Wang, B.; Zhang, H. Preparation of Lignin-Based Superplasticizer by Graft Sulfonation and Investigation of the Dispersive Performance and Mechanism in a Cementitious System. Ind. Eng. Chem. Res. 2013, 52, 16101–16109. [Google Scholar] [CrossRef]
- Barsberg, S.; Elder, T.; Felby, C. Lignin−Quinone Interactions: Implications for Optical Properties of Lignin. Chem. Mater. 2003, 15, 649–655. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Li, S.-X.; Li, M.-F.; Bian, J.; Wu, X.-F.; Peng, F.; Ma, M.-G. Preparation of organic acid lignin submicrometer particle as a natural broad-spectrum photo-protection agent. Int. J. Biol. Macromol. 2019, 132, 836–843. [Google Scholar] [CrossRef]
- Yu, J.; Li, L.; Qian, Y.; Lou, H.; Yang, D.; Qiu, X. Facile and Green Preparation of High UV-Blocking Lignin/Titanium Dioxide Nanocomposites for Developing Natural Sunscreens. Ind. Eng. Chem. Res. 2018, 57, 15740–15748. [Google Scholar] [CrossRef]
- Jovanović, B.; Guzmán, H.M. Effects of titanium dioxide (TiO2) nanoparticles on caribbean reef-building coral (Montastraea faveolata). Environ. Toxicol. Chem. 2014, 33, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Narla, S.; Lim, H.W. Sunscreen: FDA regulation, and environmental and health impact. Photochem. Photobiol. Sci. 2020, 19, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Bennett, S.; Keller, A.A.; Pease, S.; Lenihan, H.S. TiO2 Nanoparticles Are Phototoxic to Marine Phytoplankton. PLoS ONE 2012, 7, e30321. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Tucci, P. Titanium dioxide nanoparticles: A risk for human health? Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Dufour, E.K. Nano-sized cosmetic formulations or solid nanoparticles in sunscreens: A risk to human health? Arch Toxicol. 2012, 86, 1063–1075. [Google Scholar] [CrossRef]
- Hansen, S.F.; Baun, A. European Regulation Affecting Nanomaterials—Review of Limitations and Future Recommendations. Dose-Response 2012, 10, 364–383. [Google Scholar] [CrossRef]
- Europa ECoPH. What Are Potential Harmful Effects of Nanoparticles? Europa ECoPH, 2007.
- Ze, Y.; Hu, R.; Wang, X.; Sang, X.; Ze, X.; Li, B.; Su, J.; Wang, Y.; Guan, N.; Zhao, X.; et al. Neurotoxicity and gene-expressed profile in brain-injured mice caused by exposure to titanium dioxide nanoparticles. J. Biomed. Mater. Res. Part A 2014, 102, 470–478. [Google Scholar] [CrossRef]
- El-Boury, S.; Couteau, C.; Boulande, L.; Paparis, E.; Coiffard, L.J.M. Effect of the combination of organic and inorganic filters on the sun protection factor (SPF) determined by in vitro method. Int. J. Pharm. 2007, 340, 1–5. [Google Scholar] [CrossRef]
- Couteau, C.; Chammas, R.; Boury, S.A.-E.; Choquenet, B.; Paparis, E.; Coiffard, L.J. Combination of UVA-filters and UVB-filters or inorganic UV filters—Influence on the sun protection factor (SPF) and the PF-UVA determined by in vitro method. J. Dermatol. Sci. 2008, 50, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.; Mendes, F.; Filipe, P.; Reis, S.; Fonte, P. Nanocarrier-Mediated Topical Insulin Delivery for Wound Healing. Materials 2021, 14, 4257. [Google Scholar] [CrossRef] [PubMed]
- Honeywell-Nguyen, P.L.; Bouwstra, J.A. Vesicles as a tool for transdermal and dermal delivery. Drug Discov. Today: Technol. 2005, 2, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Otberg, N.; Thiede, G.; Richter, H.; Sterry, W.; Panzner, S.; Lademann, J. Innovative Liposomes as a Transfollicular Drug Delivery System: Penetration into Porcine Hair Follicles. J. Investig. Dermatol. 2006, 126, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Song, C.-K.; Nagayya-Sriraman, S.; Baskaran, R.; Yong, C.-S.; Choi, H.-G.; Kim, D.-D.; Woo, J.S.; Yoo, B.-K. Physicochemical characterization and skin permeation of liposome formulations containing clindamycin phosphate. Arch. Pharmacal Res. 2009, 32, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Carrer, D.C.; Vermehren, C.; Bagatolli, L.A. Pig skin structure and transdermal delivery of liposomes: A two photon microscopy study. J. Control Release 2008, 132, 12–20. [Google Scholar] [CrossRef]
- Jose, J.; Charyulu, R.N. Prolonged drug delivery system of an antifungal drug by association with polyamidoamine dendrimers. Int. J. Pharm. Investig. 2016, 6, 123–127. [Google Scholar] [CrossRef]
- Marquele-Oliveira, F.; Santana, D.C.D.A.; Taveira, S.F.; Vermeulen, D.M.; de Oliveira, A.R.M.; da Silva, R.S.; Lopez, R.F.V. Development of nitrosyl ruthenium complex-loaded lipid carriers for topical administration: Improvement in skin stability and in nitric oxide release by visible light irradiation. J. Pharm. Biomed. Anal. 2010, 53, 843–851. [Google Scholar] [CrossRef]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef]
- Sgorla, D.; Bunhak, J.; Cavalcanti, O.A.; Fonte, P.; Sarmento, B. Exploitation of lipid-polymeric matrices at nanoscale for drug delivery applications. Expert Opin. Drug Deliv. 2016, 13, 1301–1309. [Google Scholar] [CrossRef]
- Wissing, S.; Müller, R. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Panos, I.; Acosta, N.; Heras, A. New drug delivery systems based on chitosan. Curr. Drug Discov. Technol. 2008, 5, 333–341. [Google Scholar] [CrossRef]
- Alvarez-Roman, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control Release 2004, 99, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Khuda-Bukhsh, A.; Das, S. PLGA-loaded nanomedicines in melanoma treatment: Future prospect for efficient drug delivery. Indian J. Med. Res. 2016, 144, 181–193. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, P.C. The safety of nanoparticles in sunscreens: An update for general practice. Aust. Fam. Physician 2016, 45, 397–399. [Google Scholar]
- Santos, A.C.; Marto, J.; Cha-Cha, R.; Martins, A.M. Nanotechnology-based sunscreens—A review. Mater. Today Chem. 2022, 23, 100709. [Google Scholar] [CrossRef]
- Liu, D.; Hu, H.; Lin, Z.; Chen, D.; Zhu, Y.; Hou, S.; Shi, X. Quercetin deformable liposome: Preparation and efficacy against ultraviolet B induced skin damages in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2013, 127, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Keck, C.M. Second generation of drug nanocrystals for delivery of poorly soluble drugs: SmartCrystal technology. Eur. J. Pharm. Sci. 2008, 34, S20–S21. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Dorandeu, C.; Müller, R.H.; Bégu, S. Dermal quercetin smartCrystals(R): Formulation development, antioxidant activity and cellular safety. Eur. J. Pharm. Biopharm. 2016, 102, 51–63. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. A Novel Phase Inversion-Based Process for the Preparation of Lipid Nanocarriers. Pharm. Res. 2002, 19, 875–880. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Shamseddin, A.; Aubert-Pouëssel, A.; Devoisselle, J.; Bégu, S. Dermal quercetin lipid nanocapsules: Influence of the formulation on antioxidant activity and cellular protection against hydrogen peroxide. Int. J. Pharm. 2017, 518, 167–176. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.-M.; Müller, R.; Bégu, S. Liposomes, lipid nanocapsules and smartCrystals®: A comparative study for an effective quercetin delivery to the skin. Int. J. Pharm. 2018, 542, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Franceschinis, E.; Bertelli, D.; Iannuccelli, V. Comparative Evaluation of the Effect of Permeation Enhancers, Lipid Nanoparticles and Colloidal Silica on in vivo Human Skin Penetration of Quercetin. Ski. Pharmacol. Physiol. 2013, 26, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Hoeller, S.; Sperger, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int. J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Eberlin, S.; da Silva Gonçalves, F.C.; Fernandes, A.R.; Marto, J.; Ribeiro, H.M.; Foglio, M.A.; Mazzola, P.G.; Souto, E.B. In vitro SPF and Photostability Assays of Emulsion Containing Nanoparticles with Vegetable Extracts Rich in Flavonoids. AAPS PharmSciTech 2018, 20, 9. [Google Scholar] [CrossRef]
- de Oliveira, C.A.; Peres, D.D.; Graziola, F.; Chacra, N.A.B.; de Araújo, G.L.B.; Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M.; et al. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Netto, G.; Jose, J. Development, characterization, and evaluation of sunscreen cream containing solid lipid nanoparticles of silymarin. J. Cosmet. Dermatol. 2018, 17, 1073–1083. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Lee, S.C.; Tran, T.M.T.; Choi, J.W.; Won, K. Lignin for white natural sunscreens. Int. J. Biol. Macromol. 2019, 122, 549–554. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D.; Wang, H.-M.; Yuan, T.-Q.; Sun, R.-C. Green and Facile Preparation of Regular Lignin Nanoparticles with High Yield and Their Natural Broad-Spectrum Sunscreens. ACS Sustain. Chem. Eng. 2019, 7, 2658–2666. [Google Scholar] [CrossRef]
- Lee, S.C.; Yoo, E.; Lee, S.H.; Won, K. Preparation and Application of Light-Colored Lignin Nanoparticles for Broad-Spectrum Sunscreens. Polymers 2020, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hernández, J.M.; Escalante, A.; Murillo-Vázquez, R.N.; Delgado, E.; González, F.J.; Toríz, G. Use of Agave tequilana-lignin and zinc oxide nanoparticles for skin photoprotection. J. Photochem. Photobiol. B Biol. 2016, 163, 156–161. [Google Scholar] [CrossRef]
- Karisma, V.W.; Wu, W.; Lei, M.; Liu, H.; Nisar, M.F.; Lloyd, M.D.; Pourzand, C.; Zhong, J.L. UVA-Triggered Drug Release and Photo-Protection of Skin. Front. Cell Dev. Biol. 2021, 9, 598717. [Google Scholar] [CrossRef] [PubMed]


| UV Filter | Type | Spectrum Activity | Maximum % in Sunscreens | Approvals and Possible Complications |
|---|---|---|---|---|
| Avobenzone | Organic or chemical | UVA | 3% U.S. 5% EU, AUS 10% JP | Non-GRASE III Photodegradation Photosensitization |
| Octinoxate | Organic or chemical | UVA UVB | 7.5% U.S. 10% EU, AUS 20% JP | Non-GRASE III Photodegradation Endocrine-disruption potential Skin absorption Breast milk detection |
| Octocrylene | Organic or chemical | UVA UVB | 10%—worldwide | Non-GRASE III Photosensitization Skin absorption Breast milk detection |
| Oxybenzone | Organic or chemical | UVA UVB | 6% U.S. 10% EU, AUS 5% JP | Non-GRASE III Possible photocarcinogen Skin absorption Breast milk detection Endocrine-disruption potential |
| Ecamsule | Organic or chemical | UVA | 3% U.S. 10% EU, AUS, JP | No GRASE rating |
| PABA | Organic or chemical | UVB | Non-GRASE II Banned in Europe Allergen, contact dermatitis Possible photocarcinogen | |
| Trolamine salicylate | Organic or chemical | UVB | 12% U.S., CA, AUS 2.5% EU | Non-GRASE II Skin absorption Salicylism risk |
| Titanium dioxide | Inorganic or physical | UVA UVB | 25% U.S., EU, JP No limit—AUS | GRASE I |
| Zinc oxide | Inorganic or physical | UVA UVB | 25% U.S., EU, JP No limit—AUS | GRASE I |
| Flavonoid | Pharmaceutical Formulation | Physicochemical Stability | UV Filters | UV Protection | PFUVA | UVA:UVB Ratio | SPF | Antioxidant Activity | Photostability | Skin Permeation | Skin Adverse Reactions | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rutin | O/W Emulsion | - | - | Present | 2.4 | 0.78 | 2.94 | Present | Present | Stratum corneum and deeper epidermis | None | [49] |
| O/W Emulsion | - | Avo benzone and octyl dimethyl PABA | Present | - | - | 70% increase compared with free rutin | 40% increase compared with free rutin | - | - | None | [74] | |
| Nano Emulsion | 127 nm 3.49 mV | - | - | - | - | - | - | - | - | - | [16] | |
| Chitosan/tripolyphosphate (TPP) NP loaded with flavonoids-enriched vegetable extracts | Encapsulation efficiency = 75.89% | - | Present | 2.0 | 0.69 | - | - | Present | - | - | [126] | |
| Rutin-loaded gelatin NP | Encapsulation efficiency = 51.8% | Ethyl hexyl dimethyl PABA, ethyl hexyl methoxycinnamate, methoxydibenzoyl methane | Present | - | 0.78 | - | 74% increase compared with free rutin | Present | NP adherence onto skin surface, forming a protective film | None | [127] | |
| Quercetin | Liposomes Lipid nano capsules Smart Crystals® | 179 nm 26 nm 203 nm | - | Present | - | - | - | More pronounced effect with lipid nano capsule and liposome | - | Lower skin penetration with Smart Crystals® | None | [123] |
| Silymarin | Solid lipid NP | - | - | Present | - | - | 13.80—in vitro 14.10—in vivo | - | Present | - | - | [128] |
| Pomegranate | Pomegranate-juice-coated gold NP | 100 nm Stable under extreme conditions of pH value and temperature | - | Present | - | - | 3–18 | Present | Present | Absent at 1.80 × 10−12–3.60 × 10−12 M [AuNP] | [79] | |
| Lignin | Cellulolytic enzyme lignin NP | Spherical shape | Octo crylene, ethyl hexyl salicylate, butyl methoxydiben zoylmethane, ethyl hexyl triazone | Present | Five-fold in crease relative to sun screen wi thout lignin nanoparticles | - | Five-fold increase allowed by the loaded NP | - | - | - | - | [133] |
| Lignin-poly dopamine nano capsule | Spherical shape | Avo benzone, ethyl methoxycinna mate | Present | - | - | 195.33 | Present | Present | Stratum corneum | None | [81] | |
| Organic acid lignin nanoparticle | Spherical shape Size < 100 nm Stable in size after 180 days | - | Present | - | 0.69–0.72 | 2.80–3.53-fold increase | Present | - | Stratum corneum | - | [90] | |
| Lignin nanoparticle (lignin obtained from A. tequilana Weber bagasse by soda and organosolv pulping) | - | Zinc oxide NP | Present | - | 0.7–0.95 | 1.5-fold increase | - | Present | - | - | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, M.; Rehman, M.; Soares, R.; Fonte, P. The Impact of Flavonoid-Loaded Nanoparticles in the UV Protection and Safety Profile of Topical Sunscreens. Biomolecules 2023, 13, 493. https://doi.org/10.3390/biom13030493
Fonseca M, Rehman M, Soares R, Fonte P. The Impact of Flavonoid-Loaded Nanoparticles in the UV Protection and Safety Profile of Topical Sunscreens. Biomolecules. 2023; 13(3):493. https://doi.org/10.3390/biom13030493
Chicago/Turabian StyleFonseca, Magda, Mubashar Rehman, Raquel Soares, and Pedro Fonte. 2023. "The Impact of Flavonoid-Loaded Nanoparticles in the UV Protection and Safety Profile of Topical Sunscreens" Biomolecules 13, no. 3: 493. https://doi.org/10.3390/biom13030493
APA StyleFonseca, M., Rehman, M., Soares, R., & Fonte, P. (2023). The Impact of Flavonoid-Loaded Nanoparticles in the UV Protection and Safety Profile of Topical Sunscreens. Biomolecules, 13(3), 493. https://doi.org/10.3390/biom13030493









