1. Introduction
Uveal melanoma (UM) is a rare malignant tumor of the eye with an incidence of 5.1 cases per million per year [
1], yet it is the most common intraocular malignancy in adults and accounts for about 5% of all melanomas [
2]. About half of the patients are cured by local treatment [
3], while the others will eventually metastasize, especially to the liver [
4]. Even with a breakthrough in the treatment of cutaneous melanoma (CM) with checkpoint inhibitors [
5] and targeted treatment [
6], prognosis of advanced UM remains poor [
7], with little response seen in conventional immunotherapies so far [
8]. Recently, tebentafusp was approved for treatment, a bispecific protein that prolongs patient survival significantly [
9]. Even patients with radiologically progressing tumors may benefit.
S100 is a family of calcium-binding proteins that are primarily found in nerve cells and glial cells. However, some S100 proteins have been found to be elevated in the blood of patients with certain types of cancer and may serve as serum tumor markers [
10]. For example, S100B is a protein that is normally found in the brain, but it can also be produced by certain types of cancer, such as melanoma and glioma [
11]. Other cancers, such as breast cancer [
12], colorectal cancer [
13], and lung cancer [
14], may be associated with elevated levels of other members of the S100 protein family, such as S100A4. Also, their role in inflammatory diseases is increasingly reported [
15,
16]. The use of S100B, in this manuscript abbreviated as S100, is common as a serum tumor marker for cutaneous melanoma and has been established for a long time [
17]. Its expression on melanoma cells dates back to a first publication in 1980 [
18]. S100 measurements are now universally recommended in national and international guidelines on cutaneous melanoma [
19] and belong to commonly available and routinely performed laboratory tests in the follow-up of melanoma patients. While the use of S100 in cutaneous melanoma is well established, there is scarce and inconclusive evidence on the performance of S100 protein as a serum tumor marker in advanced uveal melanoma: while some studies suggest a link with tumor burden [
20,
21,
22,
23], Strobel et al. question its value and sensitivity [
24]. Of note, none of these studies included more than 32 patients in a metastasized stage. With S100 measurements routinely performed at many institutions, there is currently a lack of evidence on its performance in predicting progression of UM patients. Even though novel biomarkers may be more promising in the future [
25,
26,
27,
28,
29], the value of S100 as a serum tumor marker in UM is still a relevant issue of daily patient care due to its widespread availability.
Similarly to the available literature on serum S100, few studies have reported on the expression of S100 in uveal melanoma. Available studies suggest an expression of S100 in about 66% of metastases [
30], with a strong expression in less than 50% [
31].
The aims of our analysis were to assess the frequency of S100 protein elevation in patients with advanced UM, to find a connection with the clinical course of the disease, and to correlate serum S100 elevation with S100 expression of metastases.
2. Materials and Methods
2.1. Patients
This was a monocenter, retrospective study performed at the Department of Dermatology and National Center for Tumor Diseases at the Heidelberg University Hospital. We systematically included all patients treated for stage IV UM at our institution with digital records available from 2010 to November 2022. Treatment was defined as any systemic anticancer treatment, liver-directed local treatment, or supportive treatment. Patients were excluded if they were seen only for a second opinion and treated elsewhere. There were no further exclusions made from the cohort. Missing values are reported in the results section wherever applicable.
2.2. Data Collection
All patients were treated in routine clinical practice and not for the purpose of this study. Clinical records were screened retrospectively and data merged centrally. All available values of S100 and LDH were documented. Treatment lines and dates of progression, as well as data on survival were documented. We also reviewed available histopathological staining of metastases for their expression of S100 protein, gp100 (HMB-45) [
32] and melan A [
33]. Where available, expression of S100 was evaluated by H-score [
34]: A strong expression was defined as H-scores of 2 and 3, a weak expression was defined as an H-score of 1, and no expression was defined as an H-score of 0. External histopathological reports were included only if the description on S100 staining gave clear evidence on the respective grading by H-scores (e.g., strong expression, weak expression).
2.3. Statistical Analyses
Statistical analysis was performed using IBM SPSS Statistics, version 27.
Time points of available imaging (CT and MRT scans as clinically indicated, routinely performed every three months), available S100 serum levels and available LDH levels were collected. Radiographic response to treatment, including the criteria for progressive disease, was defined by RECIST criteria version 1.1 [
35]. A connection of S100 levels with tumor progression was assumed with rising levels concurrently with rising LDH serum levels and radiologically confirmed progression. A connection of S100 levels with the disease was ruled out if radiographic progression and pathological elevation of LDH levels were observed with no increase in S100 levels.
Overall survival (OS) was calculated from first diagnosis of stage IV disease until death from any cause. In patients alive at time of final data analysis as well as in patients lost to follow-up, the date of last contact was used for censored calculation. Survival was estimated by the Kaplan–Meier method. Univariate comparisons of Kaplan–Meier estimators were performed using the log-rank test. Two-sided Fisher’s exact and chi-squared tests were used for comparisons among groups with categorical variables. Multivariate analyses were performed by Cox regression. p values were considered significant at p < 0.05.
2.4. Ethical Approval
Retrospective analyses of clinical data were approved by the institutional review board of the Medical Faculty of the University Hospital Heidelberg (S-454/2015). The ethics committee had agreed to the retrospective analysis of routinely collected clinical data without prior informed consent of patients.
3. Results
3.1. Patient Characteristics
A total of 101 patients were included, 56 female (55.4%) and 45 male (44.6%). Median age at diagnosis of stage IV disease was 63 years (range 17–89). Patients had a median OS of 17.3 months (range 1.4–100.5 months), and 27 were alive at the time of final follow-up in November 2022. Most patients (96, 95%) suffered from hepatic metastases. Patients received a median of two systemic lines of treatment (range 0–6), but only 10 patients (9.9%) responded to any kind of systemic treatment. 10 patients (9.9%) were in an ongoing remission after excision, local or systemic treatment of metastases. A summary of patient characteristics is shown in
Table 1.
3.2. Serum S100 Elevation
Over the course of the disease, a median of three S100 serum levels were assessed per patient (range 0–19). Any S100 level measurement was available in 98 patients (97%). Based on treatment duration and survival, the number of assessed values differed between patients: 25 (24.8%) had a single available value, 42 (41.6%) had two to four available values, 21 (20.8%) had five to seven available values, and 10 (9.9%) had eight or more available values (one patient had 8, three patients had 9, one patient had 10, one patient had 11, two patients had 13, one patient had 15, and one patient had 19 available values). Any S100 elevation was observed in 53 patients (54.1%); in median, the highest measured S100 serum level was 2.78 xULN (upper limit of normal) (range 1.04–219.86) in these patients. In sum, 58 patients (57.4%) had available S100 levels at the first diagnosis of stage IV disease. Of these, 12 (20.7%) had an elevated S100 level and 46 (79.3%) had normal values at first diagnosis of stage IV. Of the patients with an S100 elevation throughout the course of disease, 12 of 31 (38.7%, 23 missing values at first diagnosis) had an elevation already, while 19 of 31 (61.3%) had normal values at first diagnosis of stage IV.
3.3. Connection of Serum S100 with Tumor Progression
To assess the connection of S100 levels with the clinical course of the uveal melanoma progression, time points of radiologically confirmed progression with available S100 and LDH serum levels were collected. Increasing serum S100 (or pathologically elevated S100 levels in patients with only one value available) during progressive disease and concurrent increase of LDH levels were detected in 45 of 83 patients (54.2%) with available data at time of progression. In 38 of 83 patients (45.8%), no increase in S100 levels was observed during radiographically confirmed progression and rising pathological LDH levels, and thus there was no connection between S100 serum levels with the clinical course of the uveal melanoma. In 18 patients, there was no relative time point available for evaluation. This was due to lack of baseline values or lack of disease progression.
There was no statistically significant difference between the groups of patients with or without S100 connection to disease status regarding age (p = 0.590), time between treatment of the primary to stage IV disease (p = 0.096) and OS since first diagnosis of stage IV (p = 0.194).
A summary on S100 serum levels and the connection of serum S100 with tumor progression is given in
Table 2.
3.4. Expression of S100 in Uveal Melanoma Metastasis and Correlation of Expression with Serum S100 Levels
A total of 57 in-house histopathological stains and 16 external histopathological reports of metastases were available (73/101, 72.3% of patients), of which 44 (60.3%) samples were collected to verify the first diagnosis of stage IV disease and 29 (39.7%) were taken at a later stage. Overall, 41 metastases were liver biopsies (56.2%), 23 were subcutaneous metastases (31.5%), three were bone metastases (4.1%), while other localizations (6 samples, 5.9%) included lung metastases (two), metastasis of the small intestine, malignant pleural effusion, brain metastasis, and peritoneal metastasis.
Of 56 available S100 stains, 26 showed a strong expression (as defined by H-score of 2–3) and 19 a weak expression (46.4% and 33.9%, respectively) (
Figure 1). No expression was observed in 11 samples (19.6%). Melan A was expressed in 50 of 51 tumors (98%), Hmb45 in 57 of 60 tumors (95%).
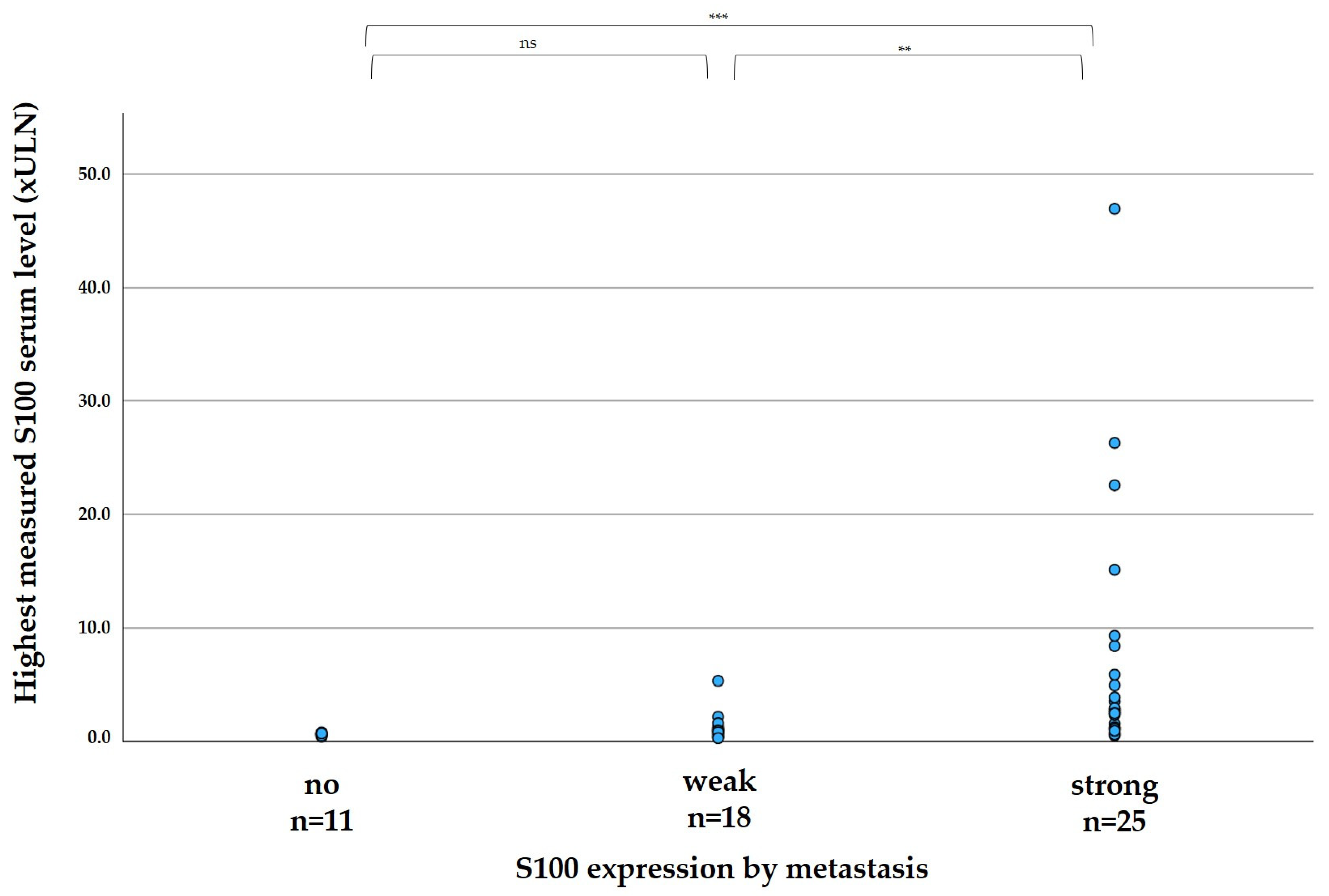
The elevation of S100 in patient serum correlated significantly with the expression of S100 in the tumor, as shown in
Figure 2. In median, the highest measured serum S100 per patient was 0.60 xULN in patients with no expression of S100 in metastases (range 0.40–0.78 xULN), 0.91 in patients with weak S100 expression of metastases (range 0.28–5.31 xULN), and 2.77 xULN in patients with strong expression of metastases (range 0.51–46.96 xULN).
In sum, 44 patients had both available tumor tissue and sufficient data to assess the connection between the course of serum S100 levels and disease progression.
Table 3 demonstrates whether S100 levels increased with disease progression based on S100 expression: If S100 were expressed by metastases, serum S100 levels rose in all 21 patients during the course of progressive disease. On the other hand, if S100 were not expressed by metastases, S100 serum levels stayed normal even during LDH elevation and radiologically confirmed progression. In patients with weak expression of S100, only 3 of 14 patients developed increasing S100 serum levels throughout the course of the disease (
p < 0.001).
4. Discussion
S100 protein has been used in UM as a serum tumor marker due to its similarity to CM by many physicians, with currently inconclusive studies on its actual value to predict progression of UM. In clinical routine, it may be used in the setting of early detection in the absence of metastases or to monitor the course of disease in patients already suffering from advanced disease. False-negative or false-positive results may lead to wrong treatment decisions. Thus, the aim of our study was to determine whether serum S100 is associated with the course of disease in UM patients, and to potentially link these serum tumor levels to the expression of S100 in the tumor.
In our cohort, only 54% of advanced UM patients ever showed elevated S100 serum levels; conversely, almost half of the patients never showed an increase. The same ratio was found in our analysis to assess the connection between rising S100 serum levels and progression of UM, as defined by rising LDH levels and radiographically confirmed progression. Overall, our data show that S100 protein was a functional tumor marker only for about half of our patients with advanced UM.
Even in the patients with rising S100 levels throughout the course of disease, only 39% already showed increased S100 values at the time of first clinical detection of metastases. Among our entire cohort, only 21% had elevated serum S100 at the first diagnosis of metastases. This shows a major limitation of using serum S100 in tumor aftercare after primary resection, as S100 levels may not be elevated at all or only late in the course of disease. In addition, false-positive results are common [
36]. S100 serum levels are thus not suitable for reliable early detection of metastases, an utterly important function of any tumor marker [
37]. In this situation, with no metastases present, a confounder may lie in a difference in S100 expression between the primary stage and metastasis, which has been described previously [
30].
However, we show that its value in monitoring advanced disease can be predicted by immunohistochemistry of metastases. Whenever there was a strong expression of S100 protein by metastases, its serum levels rose invariably during the course of disease. On the other hand, none of the patients without expression of S100 showed an increase during the course of disease, and thus there is no rationale in measuring these serum levels. For patients with weak expression of S100, an increase was observed in a minority of patients, potentially indicating a high necessary tumor load to lead to an increase in serum levels. Thus, though S100 protein is a functional serum tumor marker for UM expressing S100, it is unusable as a serum tumor marker for UM not expressing S100, and has limited value in UM with weak expression of S100. As a limitation to this finding, the use of this correlation is only possible in patients with already diagnosed stage IV disease, and not during follow-up after primary treatment only.
In daily practice, our data may help the clinician to better put available values into perspective. Especially a false sense of security should be avoided with normal S100 levels, as there may never be an increase during the course of the disease. On the other hand, S100 serum levels may be valuable information in monitoring disease progression in tumors with an expression of S100.
We are aware of many limitations of our study, which mainly include its retrospective nature, limited patient numbers, and nonstandardized monitoring of data during clinical routine.
5. Conclusions
Serum S100 protein levels are linked to disease progression in about half of advanced UM patients; however, S100 performed poorly in early detection of metastases in our collective. Its value is closely linked to expression of S100 protein by metastases, and thus it can be predicted by immunohistochemistry.
Author Contributions
Conceptualization, M.S. and J.C.H.; methodology, M.S. and J.C.H.; validation, M.S., A.H.E. and J.C.H.; formal analysis, M.S. and J.C.H.; investigation, M.S. and J.C.H.; resources, M.S. and J.C.H.; data curation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, A.H.E. and J.C.H.; visualization, M.S. and J.C.H.; supervision, A.H.E. and J.C.H.; project administration, M.S. and J.C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of University of Heidelberg (S-454/2015).
Informed Consent Statement
Patient consent was waived as allowed by the institutional review board, which had agreed to the retrospective analysis of routinely collected clinical data without prior informed consent of patients.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
M.S. received honoraria and travel grants from AbbVie, Bristol Myers Squibb (BMS), Merck, Merck Sharp & Dohme (MSD), Novartis, and Pfizer. A.H.E.: advisory honoraria from Biotest AG, MSD Oncology, Galderma, Janssen-Cilag, and AbbVie, as well as speaker’s honoraria from Roche Pharma. J.C.H.: honoraria for talks from BMS, MSD, Roche, Novartis; advisory board member for MSD and Pierre Fabre; scientific grant support from BMS; travel grants from BMS and Pierre Fabre.
References
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [Green Version]
- Damato, B. Developments in the management of uveal melanoma. Clin. Exp. Ophthalmol. 2004, 32, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef]
- Khoja, L.; Atenafu, E.G.; Suciu, S.; Leyvraz, S.; Sato, T.; Marshall, E.; Keilholz, U.; Zimmer, L.; Patel, S.P.; Piperno-Neumann, S.; et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: An international rare cancers initiative (IRCI) ocular melanoma study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Schank, T.E.; Hassel, J.C. Immunotherapies for the Treatment of Uveal Melanoma-History and Future. Cancers 2019, 11, 1048. [Google Scholar] [CrossRef] [Green Version]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Bresnick, A.R.; Weber, D.J.; Zimmer, D.B. S100 proteins in cancer. Nat. Rev. Cancer 2015, 15, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Mao, X.; Ye, L.; Cheng, H.; Dai, X. The Role of the S100 Protein Family in Glioma. J. Cancer 2022, 13, 3022–3030. [Google Scholar] [CrossRef]
- Li, F.; Men, X.; Zhang, W. S100 protein in breast tumor. Indian J. Cancer 2014, 51 (Suppl. S3), e67–e71. [Google Scholar] [CrossRef]
- Moravkova, P.; Kohoutova, D.; Rejchrt, S.; Cyrany, J.; Bures, J. Role of S100 Proteins in Colorectal Carcinogenesis. Gastroenterol. Res. Pract. 2016, 2016, 2632703. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Du, G.; Wang, D. The S100 protein family in lung cancer. Clin. Chim. Acta 2021, 520, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Sreejit, G.; Flynn, M.C.; Patil, M.; Krishnamurthy, P.; Murphy, A.J.; Nagareddy, P.R. S100 family proteins in inflammation and beyond. Adv. Clin. Chem. 2020, 98, 173–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Li, X.F.; Wu, S.; Niu, X.N.; Yin, S.Q.; Huang, C.; Li, J. Role of the S100 protein family in rheumatoid arthritis. Arthritis Res. 2022, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Harpio, R.; Einarsson, R. S100 proteins as cancer biomarkers with focus on S100B in malignant melanoma. Clin. Biochem. 2004, 37, 512–518. [Google Scholar] [CrossRef]
- Gaynor, R.; Irie, R.; Morton, D.; Herschman, H.R. S100 protein is present in cultured human malignant melanomas. Nature 1980, 286, 400–401. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics—Update 2019. Eur. J. Cancer 2020, 126, 141–158. [Google Scholar] [CrossRef] [Green Version]
- Barak, V.; Frenkel, S.; Kalickman, I.; Maniotis, A.J.; Folberg, R.; Pe’er, J. Serum markers to detect metastatic uveal melanoma. Anticancer Res. 2007, 27, 1897–1900. [Google Scholar]
- Barak, V.; Kaiserman, I.; Frenkel, S.; Hendler, K.; Kalickman, I.; Pe’er, J. The dynamics of serum tumor markers in predicting metastatic uveal melanoma (part 1). Anticancer Res. 2011, 31, 345–349. [Google Scholar] [PubMed]
- Hendler, K.; Pe’er, J.; Kaiserman, I.; Baruch, R.; Kalickman, I.; Barak, V.; Frenkel, S. Trends in liver function tests: A comparison with serum tumor markers in metastatic uveal melanoma (part 2). Anticancer Res. 2011, 31, 351–357. [Google Scholar] [PubMed]
- Missotten, G.S.; Korse, C.M.; van Dehn, C.; Linders, T.C.; Keunen, J.E.; Jager, M.J.; Bonfrer, J.M. S-100B protein and melanoma inhibitory activity protein in uveal melanoma screening. A comparison with liver function tests. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2007, 28, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.; Bode, B.; Dummer, R.; Veit-Haibach, P.; Fischer, D.R.; Imhof, L.; Goldinger, S.; Steinert, H.C.; von Schulthess, G.K. Limited value of 18F-FDG PET/CT and S-100B tumour marker in the detection of liver metastases from uveal melanoma compared to liver metastases from cutaneous melanoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1774–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, K.N.; Jager, M.J.; de Klein, A.; Kiliç, E. Uveal melanoma: Towards a molecular understanding. Prog. Retin Eye Res. 2020, 75, 100800. [Google Scholar] [CrossRef]
- Djulbegovic, M.B.; Taylor, D.J.; Uversky, V.N.; Galor, A.; Shields, C.L.; Karp, C.L. Intrinsic Disorder in BAP1 and Its Association with Uveal Melanoma. Genes 2022, 13, 1703. [Google Scholar] [CrossRef]
- Nareyeck, G.; Zeschnigk, M.; Prescher, G.; Lohmann, D.R.; Anastassiou, G. Establishment and characterization of two uveal melanoma cell lines derived from tumors with loss of one chromosome 3. Exp. Eye Res. 2006, 83, 858–864. [Google Scholar] [CrossRef]
- Wierenga, A.P.A.; Brouwer, N.J.; Gelmi, M.C.; Verdijk, R.M.; Stern, M.H.; Bas, Z.; Malkani, K.; van Duinen, S.G.; Ganguly, A.; Kroes, W.G.M.; et al. Chromosome 3 and 8q Aberrations in Uveal Melanoma Show Greater Impact on Survival in Patients with Light Iris versus Dark Iris Color. Ophthalmology 2022, 129, 421–430. [Google Scholar] [CrossRef]
- Yang, C.; Wang, R.; Hardy, P. Potential of miRNA-Based Nanotherapeutics for Uveal Melanoma. Cancers 2021, 13, 5192. [Google Scholar] [CrossRef]
- Luyten, G.P.; Mooy, C.M.; Post, J.; Jensen, O.A.; Luider, T.M.; de Jong, P.T. Metastatic uveal melanoma. A morphologic and immunohistochemical analysis. Cancer 1996, 78, 1967–1971. [Google Scholar] [CrossRef]
- Burnier, M.N., Jr.; McLean, I.W.; Gamel, J.W. Immunohistochemical evaluation of uveal melanocytic tumors. Expression of HMB-45, S-100 protein, and neuron-specific enolase. Cancer 1991, 68, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Steuhl, K.P.; Rohrbach, J.M.; Knorr, M.; Thiel, H.J. Significance, specificity, and ultrastructural localization of HMB-45 antigen in pigmented ocular tumors. Ophthalmology 1993, 100, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.F.; Odashiro, A.N.; Saraiva, V.S.; Logan, P.; Antecka, E.; Burnier, M.N., Jr. Immunohistochemical expression of melan-A and tyrosinase in uveal melanoma. J. Carcinog. 2007, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Investig. J. Tech. Methods Pathol. 2018, 98, 844–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Gebhardt, C.; Lichtenberger, R.; Utikal, J. Biomarker value and pitfalls of serum S100B in the follow-up of high-risk melanoma patients. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2016, 14, 158–164. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early detection of cancer: Past, present, and future. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 57–65. [Google Scholar] [CrossRef] [Green Version]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).