Normal β-Cell Glut2 Expression Is not Required for Regulating Glucose-Stimulated Insulin Secretion and Systemic Glucose Homeostasis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Generation of Mice
2.2. Islet Isolation Procedure
2.3. Oral Glucose Tolerance Test
2.4. Glucose Stimulated Insulin Secretion
2.5. RT-qPCR
2.6. Immunohistochemistry
2.7. Statistical Analyses
3. Results
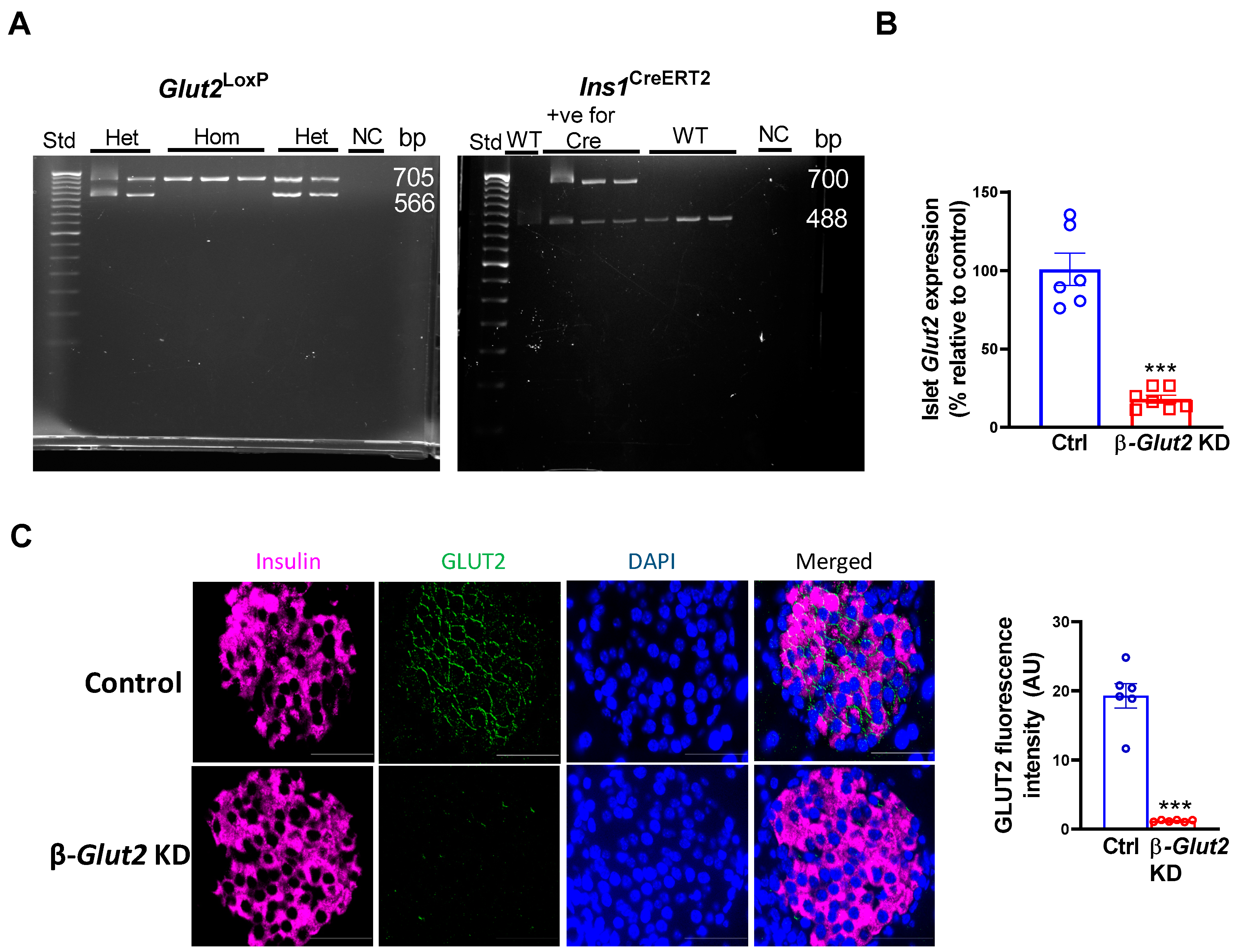
3.1. Generation and Validation of β-cell Glut2 Knockdown Mice
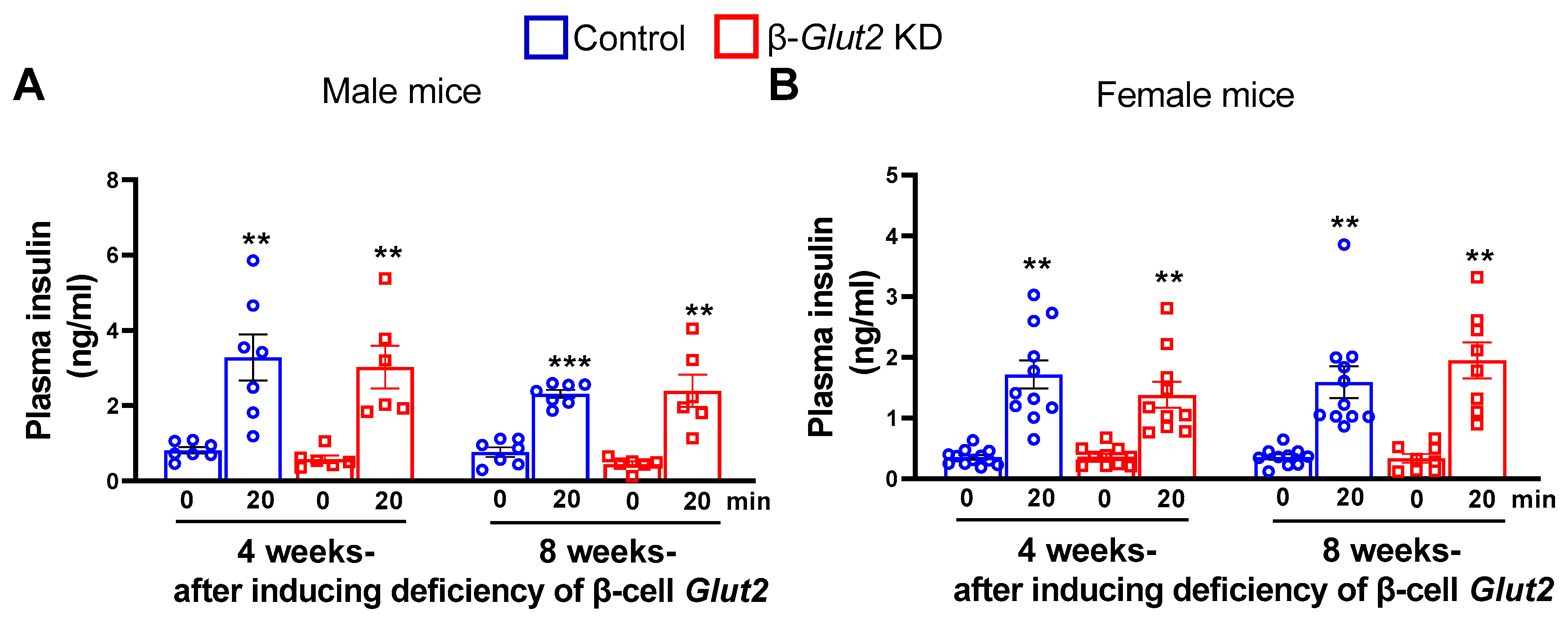
3.2. β-Cell Glut2 Knockdown Mice Have Normal Glucose-Stimulated Insulin Secretion
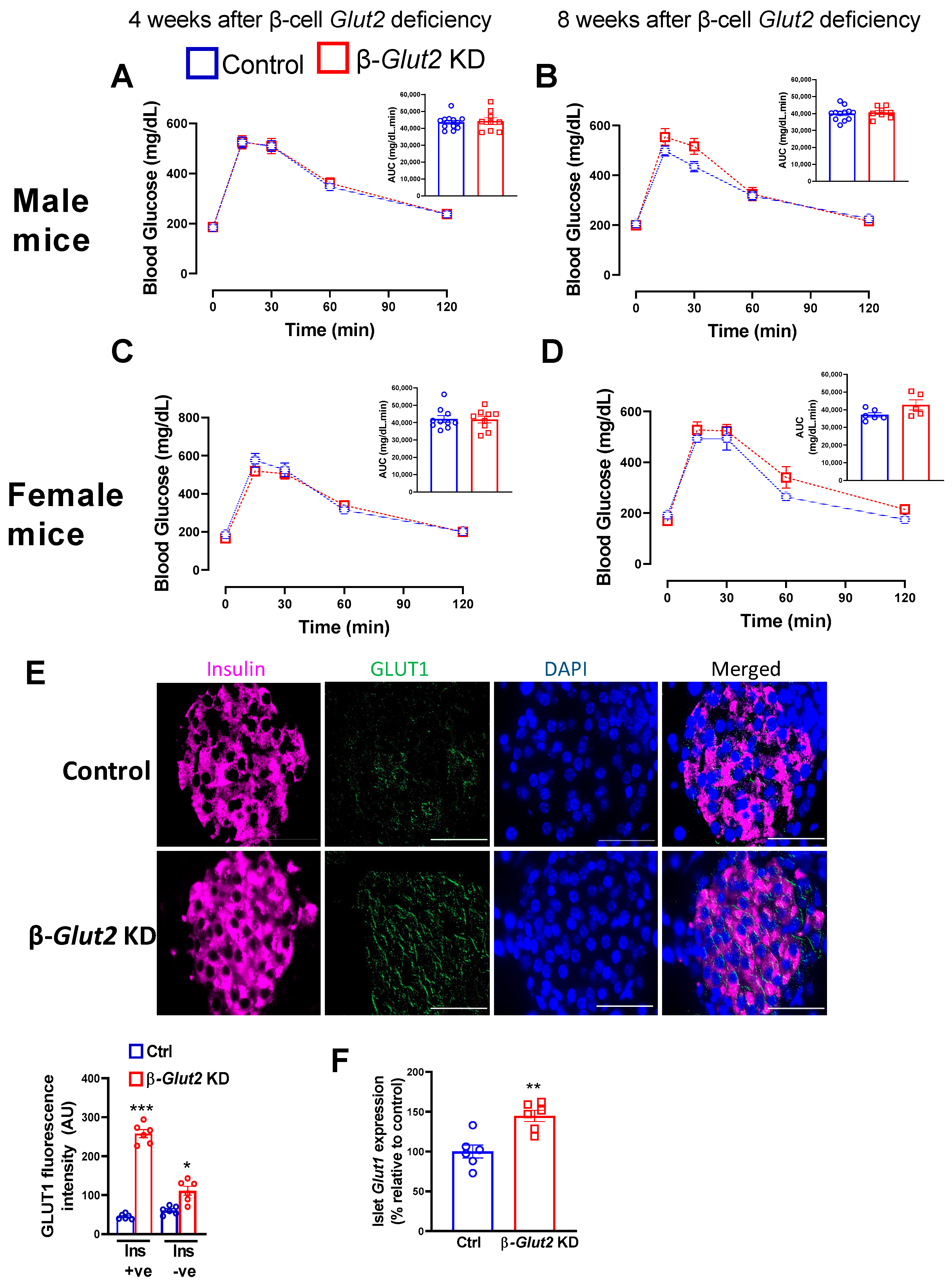
3.3. β-Cell Glut2 Knockdown Mice Have Normal Glucose Tolerance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santer, R.; Schneppenheim, R.; Dombrowski, A.; Götze, H.; Steinmann, B.; Schaub, J. Mutations in GLUT2, the gene for the liver-type glucose transporter, in patients with Fanconi-Bickel syndrome. Nat. Genet. 1997, 17, 324–326. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Aranias, T.; Viel, T.; Chateau, D.; Le Gall, M.; Waligora-Dupriet, A.-J.; Melchior, C.; Rouxel, O.; Kapel, N.; Gourcerol, G.; et al. Intestinal invalidation of the glucose transporter GLUT2 delays tissue distribution of glucose and reveals an unexpected role in gut homeostasis. Mol. Metab. 2017, 6, 61–72. [Google Scholar] [CrossRef]
- Seyer, P.; Vallois, D.; Poitry-Yamate, C.; Schütz, F.; Metref, S.; Tarussio, D.; Maechler, P.; Staels, B.; Lanz, B.; Grueter, R.; et al. Hepatic glucose sensing is required to preserve β cell glucose competence. J. Clin. Investig. 2013, 123, 1662–1676. [Google Scholar] [CrossRef] [PubMed]
- de Souza Cordeiro, L.M.; Bainbridge, L.; Devisetty, N.; McDougal, D.H.; Peters, D.J.M.; Chhabra, K.H. Loss of function of renal Glut2 reverses hyperglycaemia and normalises body weight in mouse models of diabetes and obesity. Diabetologia 2022, 65, 1032–1047. [Google Scholar] [CrossRef]
- de Souza Cordeiro, L.M.; Elsheikh, A.; Devisetty, N.; Morgan, D.A.; Ebert, S.N.; Rahmouni, K.; Chhabra, K.H. Hypothalamic MC4R regulates glucose homeostasis through adrenaline-mediated control of glucose reabsorption via renal GLUT2 in mice. Diabetologia 2021, 64, 181–194. [Google Scholar] [CrossRef]
- Chhabra, K.H.; Adams, J.M.; Fagel, B.; Lam, D.D.; Qi, N.; Rubinstein, M.; Low, M.J. Hypothalamic POMC Deficiency Improves Glucose Tolerance Despite Insulin Resistance by Increasing Glycosuria. Diabetes 2016, 65, 660–672. [Google Scholar] [CrossRef]
- Guillam, M.-T.; Hümmler, E.; Schaerer, E.; Wu, J.Y.; Birnbaum, M.J.; Beermann, F.; Schmidt, A.; Dériaz, N.; Thorens, B. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat. Genet. 1997, 17, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Nambam, B.; Weinstein, D.A.; Shoemaker, L.R. Late Diagnosis of Fanconi-Bickel Syndrome: Challenges With the Diagnosis and Literature Review. J. Inborn Errors Metab. Screen. 2016, 4, 2326409816679430. [Google Scholar] [CrossRef]
- Sansbury, F.H.; Flanagan, S.E.; Houghton, J.A.L.; Shuixian Shen, F.L.; Al-Senani, A.M.S.; Habeb, A.M.; Abdullah, M.; Kariminejad, A.; Ellard, S.; Hattersley, A.T. SLC2A2 mutations can cause neonatal diabetes, suggesting GLUT2 may have a role in human insulin secretion. Diabetologia 2012, 55, 2381–2385. [Google Scholar] [CrossRef]
- Setoodeh, A.; Rabbani, A. Transient neonatal diabetes as a presentation of Fanconi-Bickel Syndrome. Acta Med. Iran. 2012, 50, 836–838. [Google Scholar]
- De Vos, A.; Heimberg, H.; Quartier, E.; Huypens, P.; Bouwens, L.; Pipeleers, D.; Schuit, F. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J. Clin. Investig. 1995, 96, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef]
- Thorens, B.; Tarussio, D.; Maestro, M.A.; Rovira, M.; Heikkilä, E.; Ferrer, J. Ins1(Cre) knock-in mice for beta cell-specific gene recombination. Diabetologia 2015, 58, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, K.H.; Xia, H.; Pedersen, K.B.; Speth, R.C.; Lazartigues, E. Pancreatic angiotensin-converting enzyme 2 improves glycemia in angiotensin II-infused mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E874–E884. [Google Scholar] [CrossRef]
- Zmuda, E.J.; Powell, C.A.; Hai, T. A method for murine islet isolation and subcapsular kidney transplantation. J. Vis. Exp. 2011, e2096. [Google Scholar] [CrossRef]
- Thorens, B.; Guillam, M.-T.; Beermann, F.; Burcelin, R.; Jaquet, M. Transgenic Reexpression of GLUT1 or GLUT2 in Pancreatic β Cells Rescues GLUT2-null Mice from Early Death and Restores Normal Glucose-stimulated Insulin Secretion. J. Biol. Chem. 2000, 275, 23751–23758. [Google Scholar] [CrossRef]
- Liang, Y.; Cushman, S.M.; Whitesell, R.R.; Matschinsky, F.M. GLUT1 is adequate for glucose uptake in GLUT2-deficient insulin-releasing beta-cells. Horm. Metab. Res. 1997, 29, 255–260. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology; Elsevier, Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Guillam, M.T.; Dupraz, P.; Thorens, B. Glucose uptake, utilization, and signaling in GLUT2-null islets. Diabetes 2000, 49, 1485–1491. [Google Scholar] [CrossRef]
- Pang, K.; Mukonoweshuro, C.; Wong, G.G. Beta cells arise from glucose transporter type 2 (Glut2)-expressing epithelial cells of the developing rat pancreas. Proc. Natl. Acad. Sci. USA 1994, 91, 9559–9563. [Google Scholar] [CrossRef]
- Pena, L.; Charrow, J. Fanconi-Bickel syndrome: Report of life history and successful pregnancy in an affected patient. Am. J. Med. Genet. A 2011, 155, 415–417. [Google Scholar] [CrossRef] [PubMed]
- von Schnakenburg, C.; Santer, R. Fanconi–Bickel syndrome and fertility. Am. J. Med. Genet. Part. A 2011, 155, 2607. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, P.; Sinha, A.; Jain, V.; Houghton, J.; Hari, P.; Bagga, A. Fanconi syndrome and neonatal diabetes: Phenotypic heterogeneity in patients with GLUT2 defects. CEN Case Rep. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Tal, M.; Wu, Y.J.; Leiser, M.; Surana, M.; Lodish, H.; Fleischer, N.; Weir, G.; Efrat, S. [Val12] HRAS downregulates GLUT2 in beta cells of transgenic mice without affecting glucose homeostasis. Proc. Natl. Acad. Sci. USA 1992, 89, 5744–5748. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.; Augustin, R.; Gatineau, E.; Mayoux, E.; Bensellam, M.; Antoine, N.; Khattab, F.; Lai, B.-K.; Brusa, D.; Stierstorfer, B.; et al. SGLT2 is not expressed in pancreatic α- and β-cells, and its inhibition does not directly affect glucagon and insulin secretion in rodents and humans. Mol. Metab. 2020, 42, 101071. [Google Scholar] [CrossRef]
- Suga, T.; Kikuchi, O.; Kobayashi, M.; Matsui, S.; Yokota-Hashimoto, H.; Wada, E.; Kohno, D.; Sasaki, T.; Takeuchi, K.; Kakizaki, S.; et al. SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels. Mol. Metab. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bathina, S.; Faniyan, T.S.; Bainbridge, L.; Davis, A.; Chhabra, K.H. Normal β-Cell Glut2 Expression Is not Required for Regulating Glucose-Stimulated Insulin Secretion and Systemic Glucose Homeostasis in Mice. Biomolecules 2023, 13, 540. https://doi.org/10.3390/biom13030540
Bathina S, Faniyan TS, Bainbridge L, Davis A, Chhabra KH. Normal β-Cell Glut2 Expression Is not Required for Regulating Glucose-Stimulated Insulin Secretion and Systemic Glucose Homeostasis in Mice. Biomolecules. 2023; 13(3):540. https://doi.org/10.3390/biom13030540
Chicago/Turabian StyleBathina, Siresha, Tumininu S. Faniyan, Lauren Bainbridge, Autumn Davis, and Kavaljit H. Chhabra. 2023. "Normal β-Cell Glut2 Expression Is not Required for Regulating Glucose-Stimulated Insulin Secretion and Systemic Glucose Homeostasis in Mice" Biomolecules 13, no. 3: 540. https://doi.org/10.3390/biom13030540
APA StyleBathina, S., Faniyan, T. S., Bainbridge, L., Davis, A., & Chhabra, K. H. (2023). Normal β-Cell Glut2 Expression Is not Required for Regulating Glucose-Stimulated Insulin Secretion and Systemic Glucose Homeostasis in Mice. Biomolecules, 13(3), 540. https://doi.org/10.3390/biom13030540




