MAPK Is a Mutual Pathway Targeted by Anxiety-Related miRNAs, and E2F5 Is a Putative Target for Anxiolytic miRNAs
Abstract
:1. Introduction
2. Material and Methods
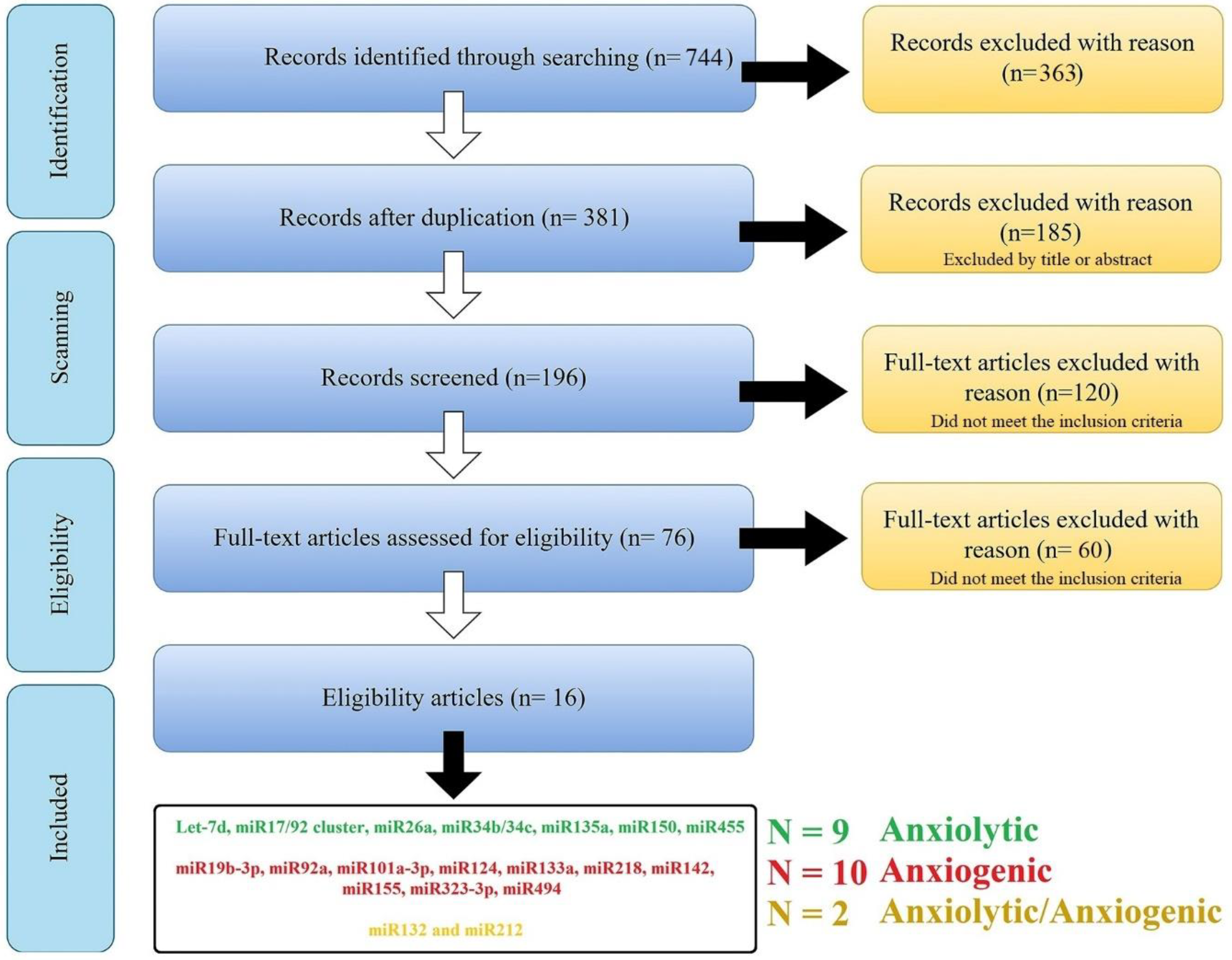
2.1. Searching Methodology
2.2. Target Prediction of Anxiolytic and Anxiogenic ARmiRs
2.3. Anxiolytic and Anxiogenic miRs Pathway Analysis and Selection of Influential miRs
2.4. Common Target Genes among Highlighted ARmiRs and Their Interactions
2.5. Determination of Mutual Target Genes and Their Signaling Pathways
3. Result
3.1. The Experimentally Confirmed Anxiolytic miRs (Reduced Anxiety)
3.1.1. Let-7d
3.1.2. miR-17/92 Cluster
3.1.3. miR-26a
3.1.4. miR-34b and miR-34c
3.1.5. miR-135a
3.1.6. miR-150
3.1.7. miR-455
3.2. The Experimentally Confirmed Anxiogenic miRs (Induced Anxiety)
3.2.1. miR-19b-3p
3.2.2. miR-92a
3.2.3. miR-101a-3p
3.2.4. miR-124a
3.2.5. miR-133a and miR-218
3.2.6. miR-142-5p
3.2.7. miR-155
3.2.8. miR-323-3p
3.2.9. miR-494
3.3. The miRs with Dispute Function (Anxiolytic/Anxiogenic)
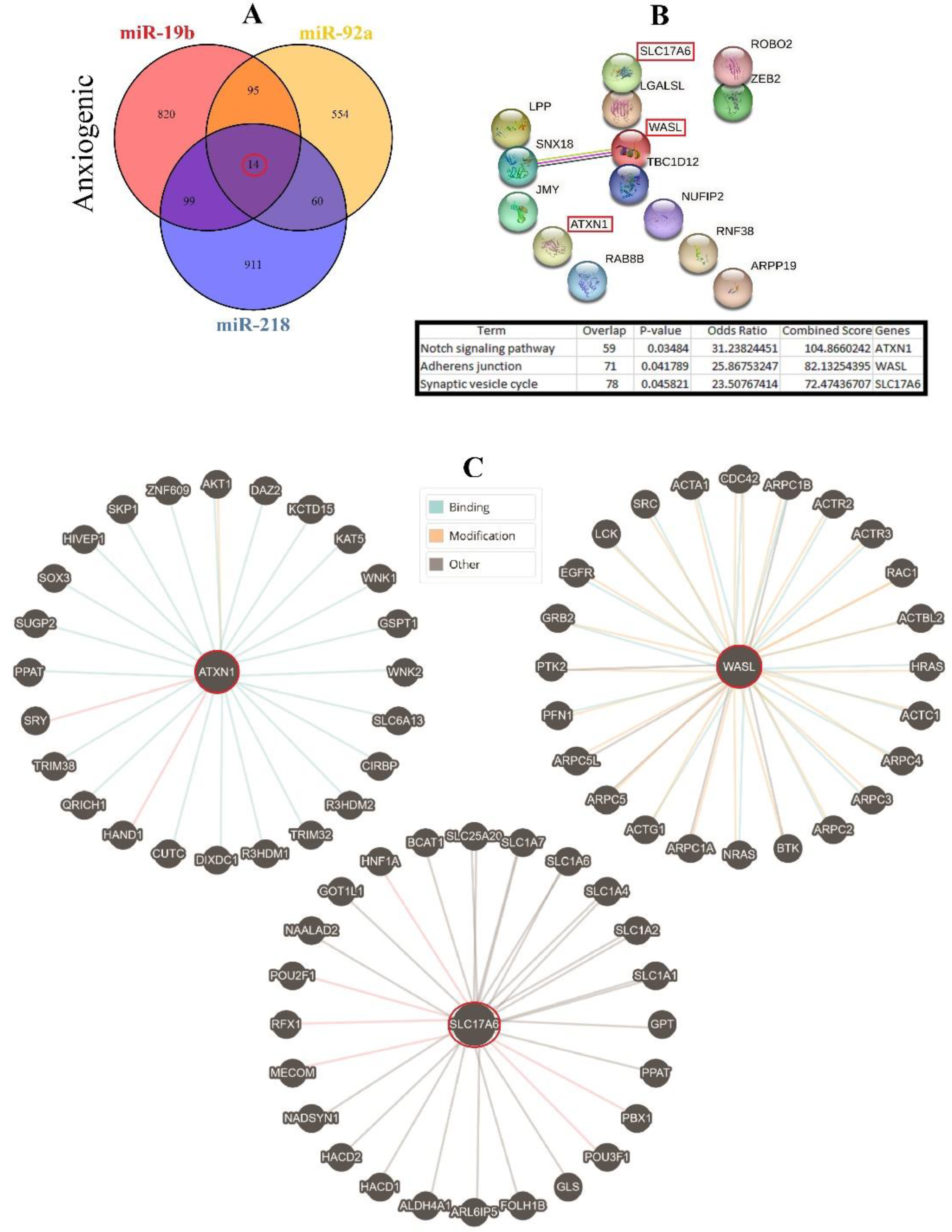
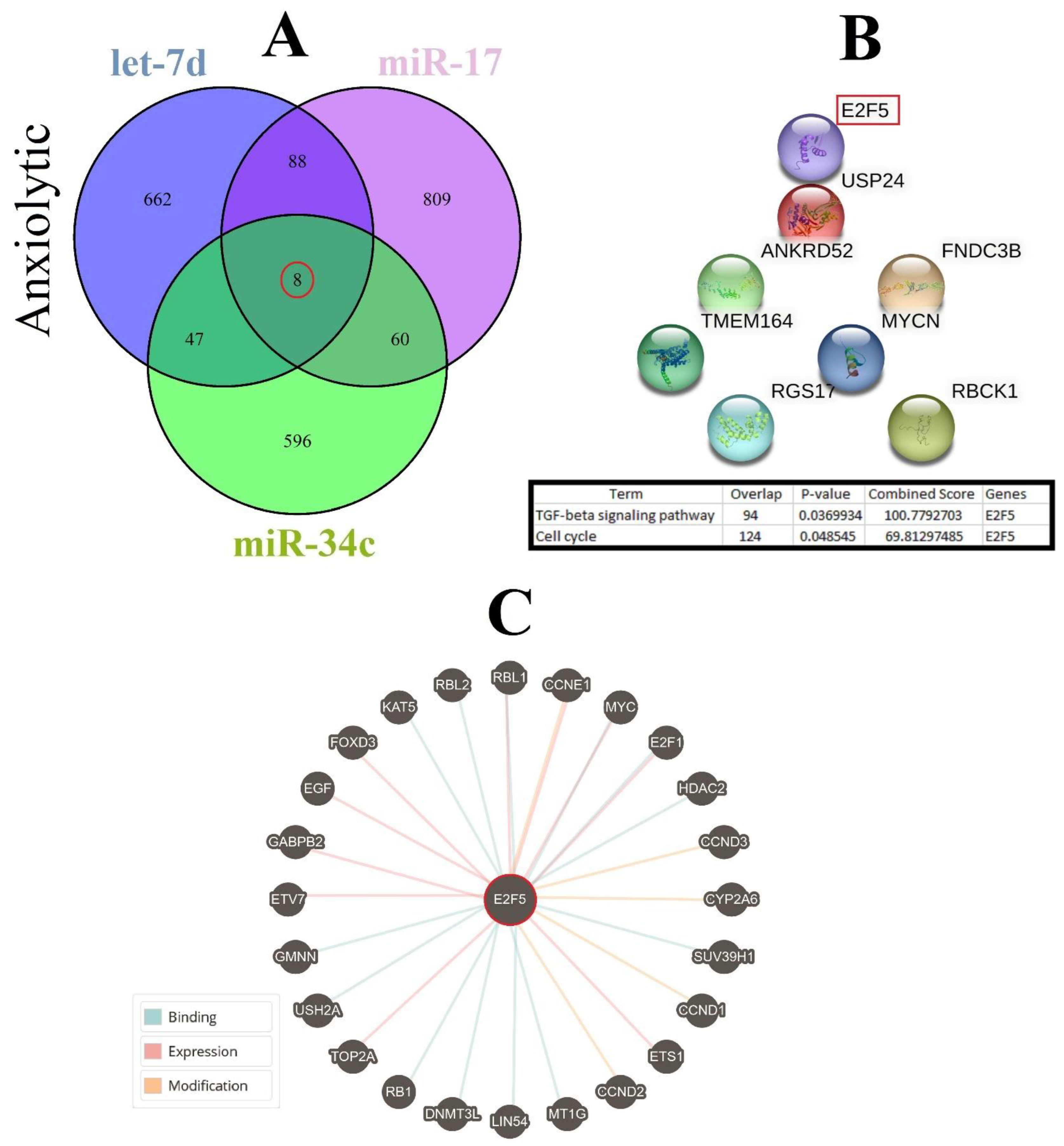
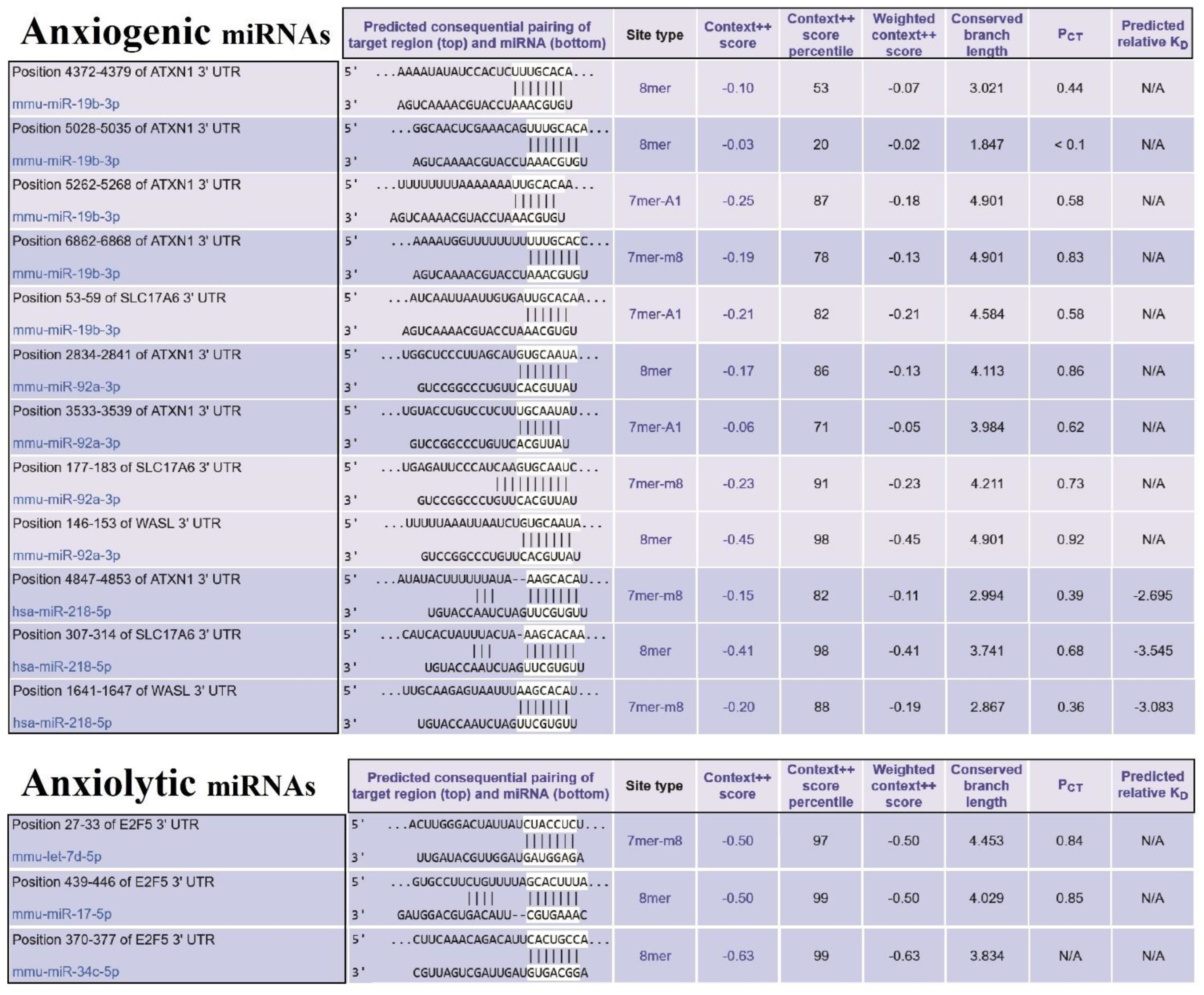
3.4. Construction of Common ARmiRs Gene Targets and Anxiety-Related Genes
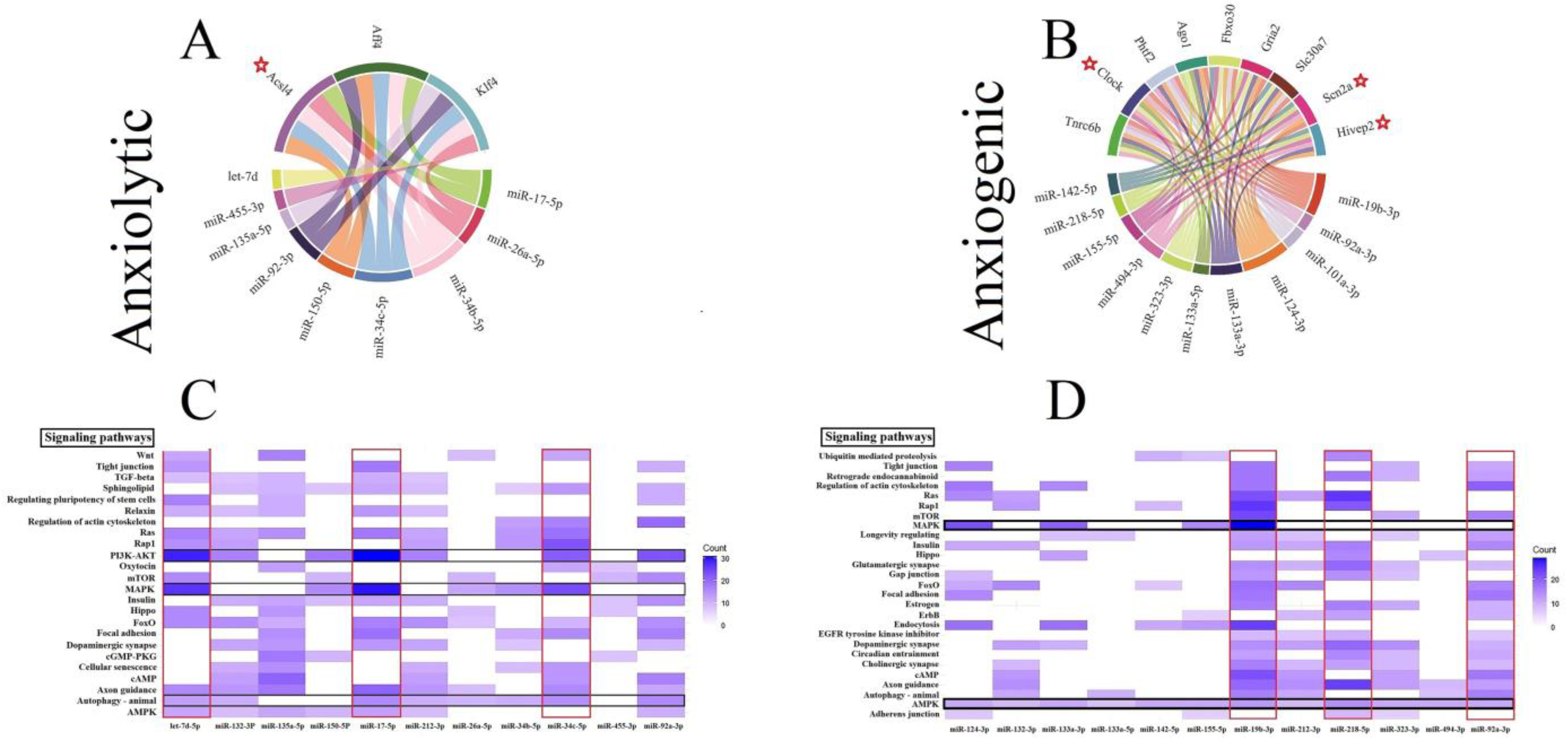
3.5. Construction of Cellular Pathways of ARmiRs Targets
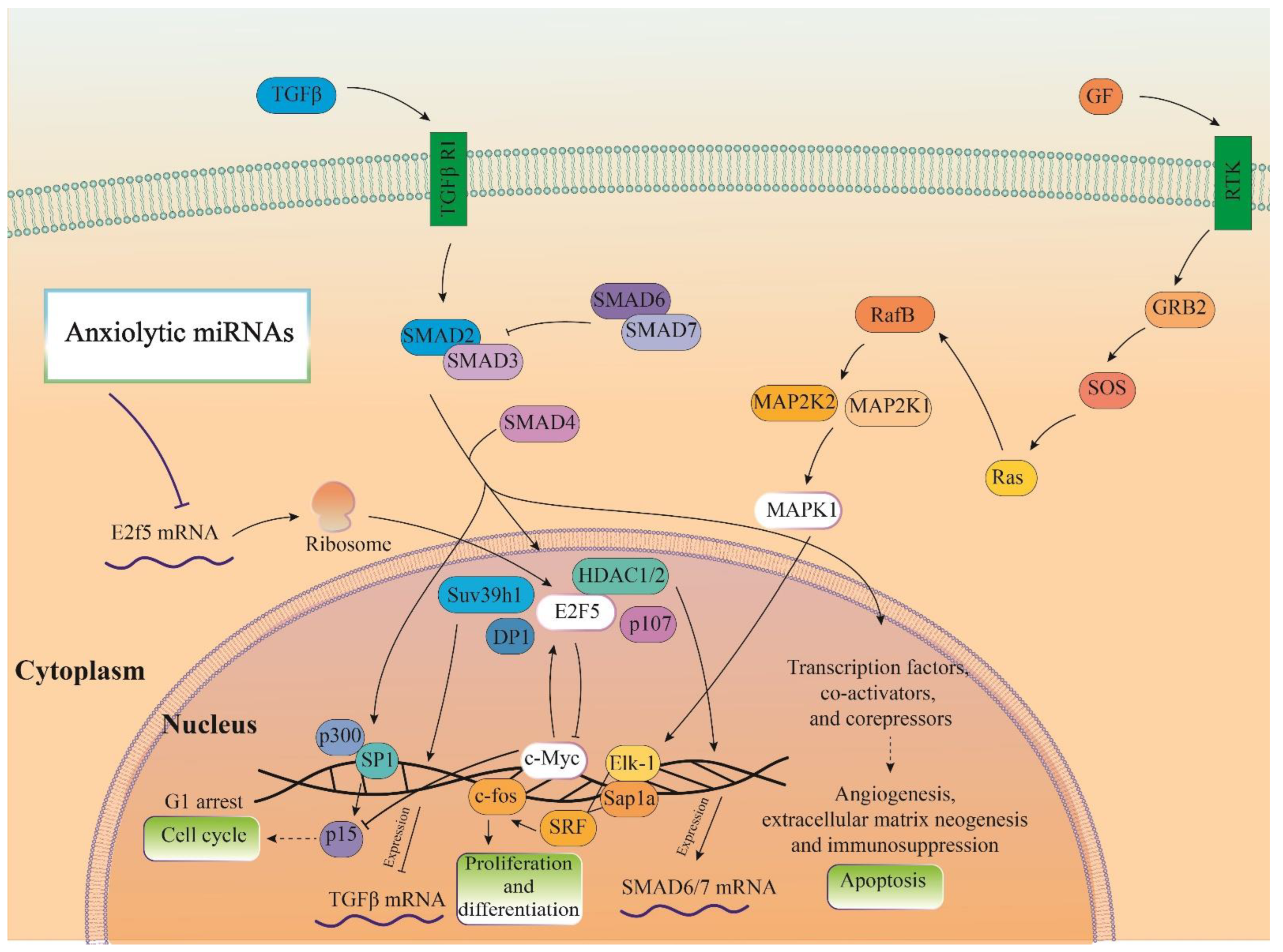
3.6. Cellular Pathways and Common Targets of Anxiogenic miRs
3.7. Cellular Pathways and Common Targets of Anxiolytic miRs
3.8. Distribution of Target Genes Expression Patterns in the Brain and Human Body
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babaev, O.; Chatain, C.P.; Krueger-Burg, D. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacobbe, P.; Flint, A. Diagnosis and Management of Anxiety Disorders. Contin. Lifelong Learn. Neurol. 2018, 24, 893–919. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.P.; Singewald, N. Role of MicroRNAs in Anxiety and Anxiety-Related Disorders. Curr. Top Behav. Neurosci. 2019, 42, 185–219. [Google Scholar] [PubMed]
- Kandola, A.; Vancampfort, D.; Herring, M.; Rebar, A.; Hallgren, M.; Firth, J.; Stubbs, B. Moving to Beat Anxiety: Epidemiology and Therapeutic Issues with Physical Activity for Anxiety. Curr. Psychiatry Rep. 2018, 20, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peedicayil, J. The Potential Role of Epigenetic Drugs in the Treatment of Anxiety Disorders. Neuropsychiatr. Dis. Treat. 2020, 16, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askari, H.; Raeis-Abdollahi, E.; Abazari, M.F.; Akrami, H.; Vakili, S.; Savardashtaki, A.; Tajbakhsh, A.; Sanadgol, N.; Azarnezhad, A.; Rahmati, L.; et al. Recent findings on the role of microRNAs in genetic kidney diseases. Mol. Biol. Rep. 2022, 49, 7039–7056. [Google Scholar] [CrossRef]
- Murphy, C.P.; Singewald, N. Potential of microRNAs as novel targets in the alleviation of pathological fear. Genes Brain Behav. 2018, 17, e12427. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, R.; Schratt, G. miRNA regulation of social and anxiety-related behavior. Cell Mol. Life Sci. 2020, 77, 4347–4364. [Google Scholar] [CrossRef]
- Du, K.; Lu, W.; Sun, Y.; Feng, J.; Wang, J.-H. mRNA and miRNA profiles in the nucleus accumbens are related to fear memory and anxiety induced by physical or psychological stress. J. Psychiatr. Res. 2019, 118, 44–65. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [Green Version]
- Queralt-Rosinach, N.; Piñero, J.; Bravo, À.; Sanz, F.; Furlong, L.I. DisGeNET-RDF: Harnessing the innovative power of the Semantic Web to explore the genetic basis of diseases. Bioinformatics 2016, 32, 2236–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Azari, H.; Karimi, E.; Shekari, M.; Tahmasebi, A.; Nikpoor, A.R.; Negahi, A.A.; Sanadgol, N.; Mousavi, P. Construction of a lncRNA-miRNA-mRNA network to determine the key regulators of the Th1/Th2 imbalance in multiple sclerosis. Epigenomics 2021, 13, 1797–1815. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, 6472. [Google Scholar] [CrossRef]
- Karimi, E.; Azari, H.; Tahmasebi, A.; Nikpoor, A.R.; Negahi, A.A.; Sanadgol, N.; Shekari, M.; Mousavi, P. LncRNA-miRNA network analysis across the Th17 cell line reveals biomarker potency of lncRNA NEAT1 and KCNQ1OT1 in multiple sclerosis. J. Cell Mol. Med. 2022, 26, 2351–2362. [Google Scholar] [CrossRef]
- Wong, J.V.; Franz, M.; Siper, M.C.; Fong, D.; Durupinar, F.; Dallago, C.; Luna, A.; Giorgi, J.; Rodchenkov, I.; Babur, V.; et al. Author-sourced capture of pathway knowledge in computable form using Biofactoid. eLife 2021, 10, 3342. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367, 6482. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Dreyer, J.L. Lentiviral-mediated let-7d microRNA overexpression induced anxiolytic- and anti-depressant-like behaviors and impaired dopamine D3 receptor expression. Eur. Neuropsychopharmacol. 2018, 28, 1394–1404. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Kim, S.-N.; Liu, X.; Zhang, H.; Zhang, C.; Seo, J.-S.; Kim, Y.; Sun, T. miR-17-92 Cluster Regulates Adult Hippocampal Neurogenesis, Anxiety, and Depression. Cell Rep. 2016, 16, 1653–1663. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Chen, J.; Ding, Y.M.; Gui, X.W.; Wu, L.X.; Tian, S.; Wu, W. MicroRNA-26a-2 maintains stress resiliency and antidepressant efficacy by targeting the serotonergic autoreceptor HTR1A. Biochem. Biophys. Res. Commun. 2019, 511, 440–446. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Z.; Tian, J.; Meng, Z.; Ju, M.; Wu, G.; Tian, Z. miR-34b attenuates trauma-induced anxiety-like behavior by targeting CRHR1. Int. J. Mol. Med. 2017, 40, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haramati, S.; Navon, I.; Issler, O.; Ezra-Nevo, G.; Gil, S.; Zwang, R.; Hornstein, E.; Chen, A. microRNA as Repressors of Stress-Induced Anxiety: The Case of Amygdalar miR-34. J. Neurosci. 2011, 31, 14191–14203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andolina, D.; Di Segni, M.; Bisicchia, E.; D’Alessandro, F.; Cestari, V.; Ventura, A.; Concepcion, C.; Puglisi-Allegra, S.; Ventura, R. Effects of lack of microRNA-34 on the neural circuitry underlying the stress response and anxiety. Neuropharmacology 2016, 107, 305–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannironi, C.; Biundo, A.; Rajendran, S.; De Vito, F.; Saba, L.; Caioli, S.; Zona, C.; Ciotti, T.; Caristi, S.; Perlas, E.; et al. miR-135a Regulates Synaptic Transmission and Anxiety-Like Behavior in Amygdala. Mol Neurobiol. 2018, 55, 3301–3315. [Google Scholar] [CrossRef]
- Zhang, W.J.; Cao, W.Y.; Huang, Y.Q.; Cui, Y.H.; Tu, B.X.; Wang, L.F.; Zou, G.J.; Liu, Y.; Hu, Z.L.; Hu, R.; et al. The Role of miR-150 in Stress-Induced Anxiety-Like Behavior in Mice. Neurotox Res. 2019, 35, 160–172. [Google Scholar] [CrossRef]
- Swingler, T.E.; Niu, L.; Pontifex, M.G.; Vauzour, D.; Clark, I.M. The microRNA-455 Null Mouse Has Memory Deficit and Increased Anxiety, Targeting Key Genes Involved in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 554. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Xu, M.; Zhu, J.; Xia, Z. Upregulation of miR-19b-3p exacerbates chronic stress-induced changes in synaptic plasticity and cognition by targeting Drebrin. Neuropharmacology 2022, 207, 108951. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Tan, L.; Wang, Y.; Lu, C.; Chen, R.; Zhang, S.; Gao, Y.; Liu, Y.; Yin, Y.; et al. Correcting miR92a-vGAT-Mediated GABAergic Dysfunctions Rescues Human Tau-Induced Anxiety in Mice. Mol Ther. 2017, 25, 140–152. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.L.; Jackson, N.L.; Ballestas, M.E.; Webb, W.M.; Lubin, F.D.; Clinton, S.M. Amygdalar expression of the microRNA miR-101a and its target Ezh2 contribute to rodent anxiety-like behaviour. Eur. J. Neurosci. 2017, 46, 2241–2252. [Google Scholar] [CrossRef] [Green Version]
- Bahi, A. Hippocampal BDNF overexpression or microR124a silencing reduces anxiety- and autism-like behaviors in rats. Behav. Brain Res. 2017, 326, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, M.; Moser, D.; Koller, A.; Yu, L.; Chen, E.I.; Bennett, D.A.; Canli, T. Integration of postmortem amygdala expression profiling, GWAS, and functional cell culture assays: Neuroticism-associated synaptic vesicle glycoprotein 2A (SV2A) gene is regulated by miR-133a and miR-218. Transl. Psychiatry 2020, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.-L.; Ye, Y.; Nie, P.-Y.; Peng, J.-B.; Fu, C.-H.; Wang, Z.-Y.; Tong, L. Dysregulation of miR-142 results in anxiety-like behaviors following single prolonged stress. Behav. Brain Res. 2019, 365, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Gaudet, A.D.; Gaier, K.R.; Nelson, R.J.; Popovich, P.G. MicroRNA-155 deletion reduces anxiety- and depressive-like behaviors in mice. Psychoneuroendocrinology 2016, 63, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Fiori, L.M.; Kos, A.; Lin, R.; Théroux, J.-F.; Lopez, J.P.; Kühne, C.; Eggert, C.; Holzapfel, M.; Huettl, R.-E.; Mechawar, N.; et al. miR-323a regulates ERBB4 and is involved in depression. Mol. Psychiatry 2021, 26, 4191–4204. [Google Scholar] [CrossRef]
- Teppen, T.L.; Krishnan, H.R.; Zhang, H.; Sakharkar, A.J.; Pandey, S.C. The Potential Role of Amygdaloid MicroRNA-494 in Alcohol-Induced Anxiolysis. Biol. Psychiatry 2016, 80, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Aten, S.; Page, C.E.; Kalidindi, A.; Wheaton, K.; Niraula, A.; Godbout, J.P.; Hoyt, K.R.; Obrietan, K. miR-132/212 is induced by stress and its dysregulation triggers anxiety-related behavior. Neuropharmacology 2019, 144, 256–270. [Google Scholar] [CrossRef]
- Park, J.; Moghaddam, B. Impact of anxiety on prefrontal cortex encoding of cognitive flexibility. Neuroscience 2017, 345, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Feng, S.; Wei, M.; Zhou, W. Role of the anterior agranular insular cortex in the modulation of fear and anxiety. Brain Res. Bull. 2020, 155, 174–183. [Google Scholar] [CrossRef]
- Schiele, M.A.; Domschke, K. Epigenetics at the crossroads between genes, environment and resilience in anxiety disorders. Genes Brain Behav. 2018, 17, e12423. [Google Scholar] [CrossRef] [Green Version]
- Malan-Müller, S.; Hemmings, S.M. The Big Role of Small RNAs in Anxiety and Stress-Related Disorders. Vitam. Horm. 2017, 103, 85–129. [Google Scholar] [PubMed]
- Lin, Q.; Wei, W.; Coelho, C.M.; Li, X.; Baker-Andresen, D.; Dudley, K.; Ratnu, V.S.; Boskovic, Z.; Kobor, M.S.; Sun, Y.E.; et al. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat. Neurosci. 2011, 14, 1115–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, Y.H. MicroRNA-mediated regulation of target genes in several brain regions is correlated to both microRNA-targeting-specific promoter methylation and differential microRNA expression. BioData Min. 2013, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, L.; Klausen, M.; Helboe, L.; Nielsen, F.C.; Werge, T. MicroRNAs show mutually exclusive expression patterns in the brain of adult male rats. PLoS ONE 2009, 4, e7225. [Google Scholar] [CrossRef]
- Cheng, J.; Fan, Y.Q.; Liu, B.H.; Zhou, H.; Wang, J.M.; Chen, Q.X. ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol Rep. 2020, 43, 147–158. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, Z.; Sun, B.; Wang, Y.; Zheng, M.; Ye, L.; Zhang, X. Involvement of cholesterol in hepatitis B virus X protein-induced abnormal lipid metabolism of hepatoma cells via up-regulating miR-205-targeted ACSL4. Biochem. Biophys. Res. Commun. 2014, 445, 651–655. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like factor 4 (KLF4): What we currently know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef]
- Kajiyama, Y.; Iijima, Y.; Chiba, S.; Furuta, M.; Ninomiya, M.; Izumi, A.; Shibata, S.; Kunugi, H. Prednisolone causes anxiety- and depression-like behaviors and altered expression of apoptotic genes in mice hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 159–165. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.S.; Moon, C.; Son, Y. Profiling of gene expression in the brain associated with anxiety-related behaviors in the chronic phase following cranial irradiation. Sci. Rep. 2022, 12, 13162. [Google Scholar] [CrossRef]
- Chen, X.; Wen, J.; Liu, C.; Guo, D. KLF4 downregulates FGF21 to activate inflammatory injury and oxidative stress of LPS-induced ATDC5 cells via SIRT1/NF-κB/p53 signaling. Mol. Med. Rep. 2022, 25, 164. [Google Scholar] [CrossRef]
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedoce, A.D.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Voisin, N.; Schnur, R.E.; Douzgou, S.; Hiatt, S.M.; Rustad, C.F.; Brown, N.J.; Earl, D.L.; Keren, B.; Levchenko, O.; Geuer, S.; et al. Variants in the degron of AFF3 are associated with intellectual disability, mesomelic dysplasia, horseshoe kidney, and epileptic encephalopathy. Am. J. Hum. Genet. 2021, 108, 857–873. [Google Scholar] [CrossRef]
- Granadillo, J.L.; Stegmann, A.P.; Guo, H.; Xia, K.; Angle, B.; Bontempo, K.; Ranells, J.D.; Newkirk, P.; Costin, C.; Viront, J.; et al. Pathogenic variants in TNRC6B cause a genetic disorder characterised by developmental delay/intellectual disability and a spectrum of neurobehavioural phenotypes including autism and ADHD. J. Med. Genet. 2020, 57, 717–724. [Google Scholar] [CrossRef]
- Saran, A.R.; Kalinowska, D.; Oh, S.; Janknecht, R.; DiTacchio, L. JMJD5 links CRY1 function and proteasomal degradation. PLoS Biol. 2018, 16, e2006145. [Google Scholar] [CrossRef] [Green Version]
- De Bundel, D.; Gangarossa, G.; Biever, A.; Bonnefont, X.; Valjent, E. Cognitive dysfunction, elevated anxiety, and reduced cocaine response in circadian clock-deficient cryptochrome knockout mice. Front. Behav. Neurosci. 2013, 7, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkelai, A.; Shohat, S.; Greenbaum, L.; Schechter, T.; Draiman, B.; Chitrit-Raveh, E.; Rienstein, S.; Dagaonkar, N.; Hughes, D.; Aggarwal, V.S.; et al. Expansion of the GRIA2 phenotypic representation: A novel de novo loss of function mutation in a case with childhood onset schizophrenia. J. Hum. Genet. 2021, 66, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, T.; Raveau, M.; Ogiwara, I.; Hattori, S.; Miyamoto, H.; Mazaki, E.; Itohara, S.; Miyakawa, T.; Montal, M.; Yamakawa, K. Scn2a haploinsufficient mice display a spectrum of phenotypes affecting anxiety, sociability, memory flexibility and ampakine CX516 rescues their hyperactivity. Mol. Autism 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Colombo, R.; Schäferhoff, K.; Janiri, L.; Grimmel, M.; Sturm, M.; Grasshoff, U.; Dufke, A.; Haack, T.B.; Kehrer, M. Novel HIVEP2 Variants in Patients with Intellectual Disability. Mol. Syndr. 2019, 10, 195–201. [Google Scholar] [CrossRef]
- Srivastava, S.; Engels, H.; Schanze, I.; Cremer, K.; Wieland, T.; Menzel, M.; Schubach, M.; Biskup, S.; Kreiß, M.; Endele, S.; et al. Loss-of-function variants in HIVEP2 are a cause of intellectual disability. Eur. J. Hum. Genet. 2015, 24, 556–561. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Kowalczyk, E.; Galita, G.; Majsterek, I.; Talarowska, M.; Popławski, T.; Kwiatkowski, P.; Lichota, A.; Sienkiewicz, M. Association of Polymorphic Variants in Argonaute Genes with Depression Risk in a Polish Population. Int. J. Mol. Sci. 2022, 23, 10586. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Pei, P.; Yu, J.; Zhang, Q.; Li, D.; Xie, X.; Wu, J.; Wang, S.; Zhang, T. F-box protein FBXO30 mediates retinoic acid receptor γ ubiquitination and regulates BMP signaling in neural tube defects. Cell Death Dis. 2019, 10, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meister, M.; Tomasovic, A.; Banning, A.; Tikkanen, R. Mitogen-Activated Protein (MAP) Kinase Scaffolding Proteins: A Recount. Int. J. Mol. Sci. 2013, 14, 4854–4884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burotto, M.; Chiou, V.L.; Lee, J.-M.; Kohn, E.C. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef] [Green Version]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 23, 276. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Rao, L.V.M. The Role of microRNAs in Inflammation. Int. J. Mol. Sci. 2022, 23, 15479. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Lee, I.; Briggs, H.A.; He, Y.; Wang, K. Issues and prospects of microRNA-based biomarkers in blood and other body fluids. Molecules 2014, 19, 6080–6105. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. Cananga odorata essential oil reverses the anxiety induced by 1-(3-chlorophenyl) piperazine through regulating the MAPK pathway and serotonin system in mice. J. Ethnopharmacol. 2018, 219, 23–30. [Google Scholar] [CrossRef]
- Mazzolini, J.; Le Clerc, S.; Morisse, G.; Coulonges, C.; Zagury, J.F.; Sieger, D. Wasl is crucial to maintain microglial core activities during glioblastoma initiation stages. Glia 2022, 70, 1027–1051. [Google Scholar] [CrossRef]
- Tong, X.; Gui, H.; Jin, F.; Heck, B.W.; Lin, P.; Ma, J.; Fondell, J.D.; Tsai, C.-C. Ataxin-1 and Brother of ataxin-1 are components of the Notch signalling pathway. EMBO Rep. 2011, 12, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Dimova, D.K.; Dyson, N.J. The E2F transcriptional network: Old acquaintances with new faces. Oncogene 2005, 24, 2810–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Grinchuk, O.V.; Tirado-Magallanes, R.; Ngian, Z.K.; Tay, E.X.Y.; Chuah, Y.H.; Lee, B.W.L.; Feng, J.; Crasta, K.C.; Ong, C.T.; et al. E2F and STAT3 provide transcriptional synergy for histone variant H2AZ activation to sustain glioblastoma chromatin accessibility and tumorigenicity. Cell Death Differ. 2022, 29, 1379–1394. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.-Z.; Wang, Y.-P.; Liu, J.; Hui, X.-B.; Wang, X.-D.; Chen, X.; Liu, D. MicroRNA-129-3p suppresses tumor growth by targeting E2F5 in glioblastoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1044–1050. [Google Scholar]
- Huang, M.; Gong, X. Let-7c Inhibits the Proliferation, Invasion, and Migration of Glioma Cells via Targeting E2F5. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Kwon, M.J.; Lee, E.M.; Kim, K.; Oh, Y.J.; Kim, H.S.; Hwang, D.H.; Kim, B.G. Role of Myc proto-oncogene as a transcriptional hub to regulate the expression of regeneration-associated genes following preconditioning peripheral nerve injury. J. Neurosci. 2021, 41, 446–460. [Google Scholar] [CrossRef]
- Belin, S.; Nawabi, H.; Wang, C.; Tang, S.; Latremoliere, A.; Warren, P.; Schorle, H.; Uncu, C.; Woolf, C.J.; He, Z.; et al. Injury-Induced Decline of Intrinsic Regenerative Ability Revealed by Quantitative Proteomics. Neuron 2015, 86, 1000–1014. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.J.; Ju, X.; Xu, R.J.; Wang, W.H.; Luo, Z.P.; Liu, C.M.; Yang, L.; Li, B.; Chen, J.Q.; Meng, B.; et al. Telomerase Reverse Transcriptase and p53 Regulate Mammalian Peripheral Nervous System and CNS Axon Regeneration Downstream of c-Myc. J. Neurosci. 2019, 39, 9107–9118. [Google Scholar] [CrossRef]


| Name | Analyzed Tissue (Animal) | Proposed Mechanism of Action | Behavioral Test | Ref. | |
|---|---|---|---|---|---|
| Anxiolytic | Let-7d | Hippocampus (C57BL/6, M mice) | Anxiolytic and anti-depressant-like action through targeting of D3R in anxiety | Elevated plus maze, Open field | [19] |
| miR-17 miR-92 | Amygdala, Prefrontal cortex, Hypothalamus (C57BL/6, M mice) | Exhibit anxiolytic and anti-depression-like behavior by regulating Sgk1 | Elevated plus maze, Open field, Restraint stress, forced swim, Tail suspension, Sucrose preference | [20] | |
| miR-26a | Dorsal raphe nucleus (C57BL/6, M mice) | Functions as an antidepressant by targeting HTR1A in serotonergic neurons | Elevated plus maze, Dark-light transfer, Open field, Forced swim | [21] | |
| miR-34b | The paraventricular nucleus (M Wistar Rat) | Targeting CRHR1 and attenuating trauma-induced anxiety by decreasing the hyperactivity of the HPA axis | Elevated plus maze, Open field | [22,23] | |
| miR-34c | Amygdala (C57BL/6, M mice) | Reduces the responsiveness of cells to CRF in CRFR1-expressing neuronal cells | Elevated plus maze, Dark-light transfer, Open field | [24] | |
| miR-135a | Amygdala (C57BL/6J, M mice) | Targets complexin-1 and 2 and modulate presynaptic glutamate neurotransmission | Elevated-plus maze | [24,25] | |
| miR-150 | Hippocampus (C57BL/6, M mice) | Decrease anxiety-like behavior by influencing the synaptic plasticity | Elevated plus maze, Open field | [26] | |
| miR-455 | Hippocampus (C57BL/6J, M mice) | Decrease anxiety and increase recognition memory in Alzheimer | Open field, Novel object recognition | [27] | |
| Anxiogenic | miR-19b-3p | Hippocampus (C57BL/6J, M mice) | Exacerbates CRS-induced cognitive impairment by targeting Drebrin | Elevated plus maze, Open field | [28] |
| miR-92a | Hippocampus (C57BL/6J, M mice) | Targeted vGAT mRNA 3′ UTR, inhibited its translation, and increased anxiety in Tauopathy | Elevated plus maze, Open field, social interaction, Unchanged motor function | [29] | |
| miR-101a-3p | Amygdala (HR/LR, M rats) | Increases anxiety-like behavior at least partially via repression of Ezh2 | Elevated plus maze, Open field | [30] | |
| miR-124a | Dentate gyrus (M/F Wistar rats) | Through its target, BDNF may influence neonatal isolation-induced anxiety-like behaviors | Elevated plus maze, Open field | [31] | |
| miR-133a miR-218 | Amygdala (Postmortem, H) | Targets the 3′UTR of SV2A and increases anxiety-like behavior | Self-reported | [32] | |
| miR-142-5p | Amygdala (M Sprague-Dawley rats) | Targets Npas4 and increases anxiety-like behavior | Elevated plus maze, Open field, Morris water maze | [33] | |
| miR-155 | Hippocampus (C57BL/6J M/F mice) | Increases anxiety-like behavior and inflammatory cytokines (IL-6 and TNF-α) | Elevated plus maze, Open field, Forced swim | [34] | |
| miR-323-3p | Anterior cingulate cortex (M CD-1 mice) Anterior cingulate cortex and lateral habenula (Postmortem, H) | Targets ERBB4 and elevates anxiety-like behaviors | Elevated plus maze, Open field, Tail suspension | [35] | |
| miR-494 | Amygdala (M Sprague-Dawley rats) | Via regulation of chromatin structure in the central nucleus increase anxiety-like behaviors | Elevated plus maze | [36] | |
| UKF | miR-132 miR-212 | Hippocampus, Amygdala (C57BL/6 M/F mice) | Stress-inducing paradigms alter their expression | Elevated plus maze, Open field | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amini, J.; Beyer, C.; Zendedel, A.; Sanadgol, N. MAPK Is a Mutual Pathway Targeted by Anxiety-Related miRNAs, and E2F5 Is a Putative Target for Anxiolytic miRNAs. Biomolecules 2023, 13, 544. https://doi.org/10.3390/biom13030544
Amini J, Beyer C, Zendedel A, Sanadgol N. MAPK Is a Mutual Pathway Targeted by Anxiety-Related miRNAs, and E2F5 Is a Putative Target for Anxiolytic miRNAs. Biomolecules. 2023; 13(3):544. https://doi.org/10.3390/biom13030544
Chicago/Turabian StyleAmini, Javad, Cordian Beyer, Adib Zendedel, and Nima Sanadgol. 2023. "MAPK Is a Mutual Pathway Targeted by Anxiety-Related miRNAs, and E2F5 Is a Putative Target for Anxiolytic miRNAs" Biomolecules 13, no. 3: 544. https://doi.org/10.3390/biom13030544
APA StyleAmini, J., Beyer, C., Zendedel, A., & Sanadgol, N. (2023). MAPK Is a Mutual Pathway Targeted by Anxiety-Related miRNAs, and E2F5 Is a Putative Target for Anxiolytic miRNAs. Biomolecules, 13(3), 544. https://doi.org/10.3390/biom13030544








