High-Fat Diet Related Lung Fibrosis-Epigenetic Regulation Matters
Abstract
:1. Introduction
2. Epithelial Cell Injury and Abnormal Activation
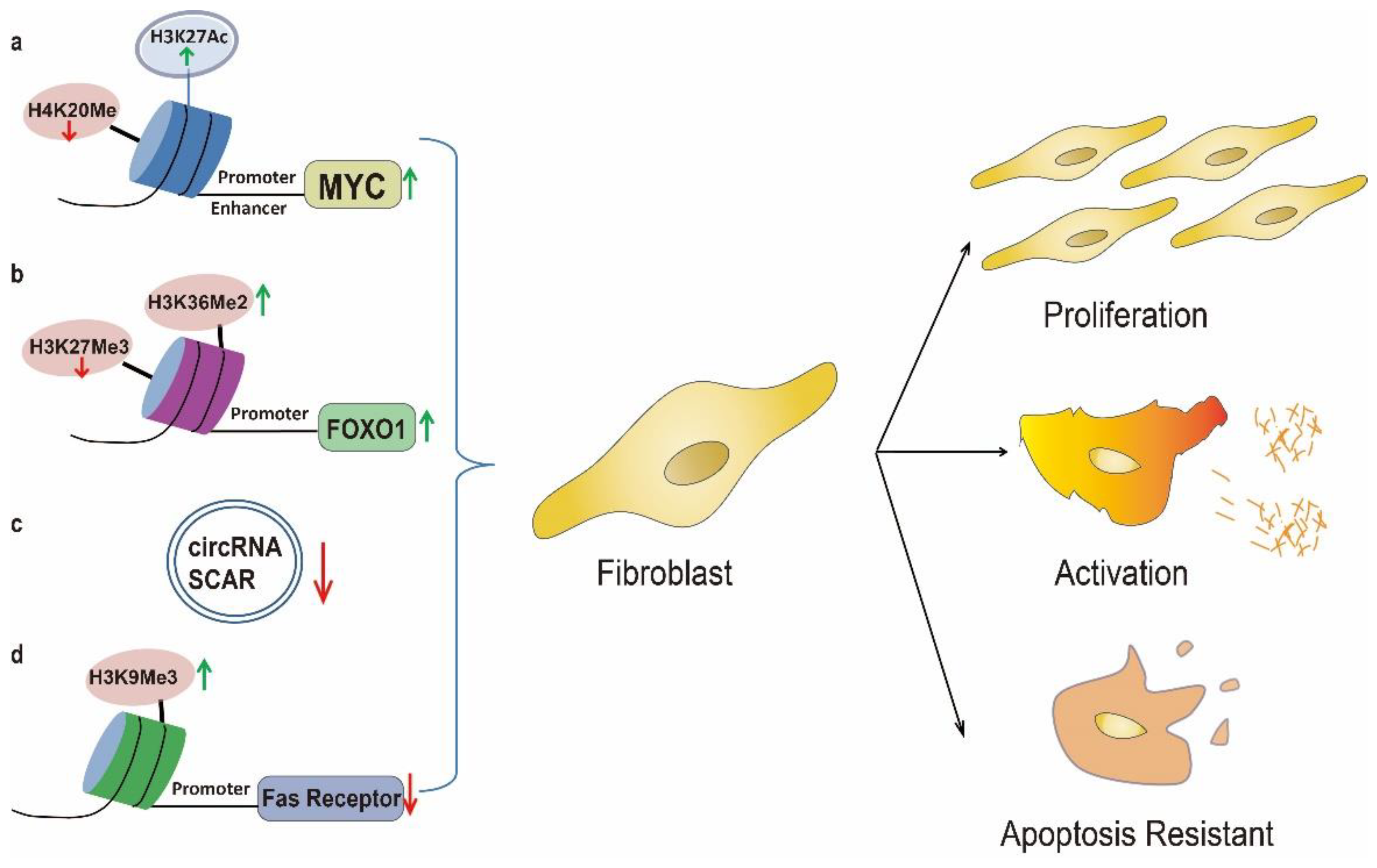
3. Uncontrolled Fibroblast Activation
4. Chronic Inflammation
5. Clinical Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-methylcytosine | (5mC) |
| Alveolar epithelial cells | (AECs) |
| Bromodomain protein subfamily 4 | (BRD4) |
| Bronchoalveolar lavage fluid | (BALF) |
| Damage-associated transient progenitor | (DATP) |
| Dedicator of cytokinesis 2 | (DOCK2) |
| DNA damage response | (DDR) |
| DNA methyltransferases | (DNMTs) |
| Endoplasmic reticulum | (ER) |
| Epithelial mesenchymal transition | (EMT) |
| Extra cellular matrix | (ECM) |
| Fat mass | (FM) |
| Fat-free mass | (FFM) |
| High-fat diet | (HFD) |
| Histone H3 dimethylation at lysine 36 | (H3K36Me2) |
| Histone H3 trimethylation at lysine 27 | (H3K27Me3) |
| Histone H3 trimethylation at lysine 9 | (H3K9Me3) |
| Histone H4 acetylation at lysine 12 | (H4K12Ac) |
| Histone H4 acetylation at lysine 16 | (H4K16Ac) |
| Idiopathic pulmonary fibrosis | (IPF) |
| Interleukin6 | (IL-6) |
| N6-methyladenosine | (m6A) |
| Palmitic acid | (PA) |
| Polyunsaturated fatty acids | (PUFAs) |
| Pulmonary fibrosis | (PF) |
| Saturated fatty acids | (SFAs) |
| Steatohepatitis-associated circRNA ATP5B Regulator | (SCAR) |
| Toll like receptor 4 | (TLR4) |
| Transforming growth factor-beta | (TGF-β) |
| Tumor Necrosis Factor α | (TNFα) |
| Type 1 alveolar epithelial cells | (AEC1s) |
| Type 2 alveolar epithelial cells | (AEC2s) |
References
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. 2022, 17, 515–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, J. Mechanical forces: The missing link between idiopathic pulmonary fibrosis and lung cancer. Eur. J. Cell Biol. 2022, 101, 151234. [Google Scholar] [CrossRef]
- Wijsenbeek, M.; Suzuki, A.; Maher, T.M. Interstitial lung diseases. Lancet 2022, 400, 769–786. [Google Scholar] [CrossRef]
- Zhao, X.; Kwan, J.Y.Y.; Yip, K.; Liu, P.P.; Liu, F.F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 2020, 19, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Calyeca, J.; Rojas, M.; Mora, A.L. Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol. 2020, 33, 101509. [Google Scholar] [CrossRef]
- Yang, X.H.; Wang, F.F.; Chi, X.S.; Wang, X.M.; Cong, J.P.; Hu, Y.; Zhang, Y.Z. Disturbance of serum lipid metabolites and potential biomarkers in the Bleomycin model of pulmonary fibrosis in young mice. BMC Pulm. Med. 2022, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hao, C.; Li, Y.; Guo, Y.; Si, H.; He, J.; Deng, M.; Niu, Z.; Wang, C.; Xu, X.; et al. Lysophosphatidylcholine acyltransferase 1 alleviates silica-induced pulmonary fibrosis by modulating lipid metabolism. Biomed. Pharmacother. 2022, 155, 113638. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.; Tan, D.B.A.; Clynick, B.; Bong, S.H.; Rawlinson, C.; Gummer, J.; Corte, T.J.; Glaspole, I.; Moodley, Y.P.; Trengove, R. Untargeted metabolomics of human plasma reveal lipid markers unique to chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Proteomics. Clin. Appl. 2021, 15, e2000039. [Google Scholar] [CrossRef]
- Malik, V.S.; Willet, W.C.; Hu, F.B. Nearly a decade on—Trends, risk factors and policy implications in global obesity. Nat. Reviews. Endocrinol. 2020, 16, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Bendor, C.D.; Bardugo, A.; Pinhas-Hamiel, O.; Afek, A.; Twig, G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc. Diabetol. 2020, 19, 79. [Google Scholar] [CrossRef]
- Shin, A.C.; MohanKumar, S.M.; Sirivelu, M.P.; Claycombe, K.J.; Haywood, J.R.; Fink, G.D.; MohanKumar, P.S. Chronic exposure to a high-fat diet affects stress axis function differentially in diet-induced obese and diet-resistant rats. Int. J. Obes. 2010, 34, 1218–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, U.; Suratt, B.T.; Bates, J.H.T.; Dixon, A.E. Beyond BMI: Obesity and Lung Disease. Chest 2018, 153, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Yokoyama, T.; Chida, K.; Azuma, A.; Suda, T.; Kudoh, S.; Sakamoto, N.; Okamoto, K.; Kobashi, G.; et al. Dietary fat and meat intake and idiopathic pulmonary fibrosis: A case-control study in Japan. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Against Tuberc. Lung Dis. 2006, 10, 333–339. [Google Scholar]
- Guo, X.; Sunil, C.; Qian, G. Obesity and the Development of Lung Fibrosis. Front. Pharmacol. 2021, 12, 812166. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.N.; Bajwa, E.K.; Thompson, B.T.; Christiani, D.C. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax 2010, 65, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.R.; Kim, J.S.; Allison, M.; Giles, J.T.; Hoffman, E.A.; Ding, J.; Barr, R.G.; Podolanczuk, A. Adiposity and Interstitial Lung Abnormalities in Community-Dwelling Adults: The MESA Cohort Study. Chest 2021, 160, 582–594. [Google Scholar] [CrossRef]
- Hegab, A.E.; Ozaki, M.; Meligy, F.Y.; Kagawa, S.; Ishii, M.; Betsuyaku, T. High fat diet activates adult mouse lung stem cells and accelerates several aging-induced effects. Stem Cell Res. 2018, 33, 25–35. [Google Scholar] [CrossRef]
- Han, H.; Chung, S.I.; Park, H.J.; Oh, E.Y.; Kim, S.R.; Park, K.H.; Lee, J.H.; Park, J.W. Obesity-induced Vitamin D Deficiency Contributes to Lung Fibrosis and Airway Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2021, 64, 357–367. [Google Scholar] [CrossRef]
- Thompson, J.A.; Johnston, R.A.; Price, R.E.; Hubbs, A.F.; Kashon, M.L.; McKinney, W.; Fedan, J.S. High-fat Western diet consumption exacerbates silica-induced pulmonary inflammation and fibrosis. Toxicol. Rep. 2022, 9, 1045–1053. [Google Scholar] [CrossRef]
- Hegab, A.E.; Ozaki, M.; Kagawa, S.; Fukunaga, K. Effect of High Fat Diet on the Severity and Repair of Lung Fibrosis in Mice. Stem Cells Dev. 2021, 30, 908–921. [Google Scholar] [CrossRef]
- Chu, S.G.; Villalba, J.A.; Liang, X.; Xiong, K.; Tsoyi, K.; Ith, B.; Ayaub, E.A.; Tatituri, R.V.; Byers, D.E.; Hsu, F.F.; et al. Palmitic Acid-Rich High-Fat Diet Exacerbates Experimental Pulmonary Fibrosis by Modulating Endoplasmic Reticulum Stress. Am. J. Respir. Cell Mol. Biol. 2019, 61, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Oh, E.Y.; Han, H.; Yang, M.; Park, H.J.; Park, K.H.; Lee, J.H.; Park, J.W. Insulin resistance mediates high-fat diet-induced pulmonary fibrosis and airway hyperresponsiveness through the TGF-beta1 pathway. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Z.; Huang, Y.; Liu, D.; Chen, X.; Wang, D.; Huang, D.; Zhao, L.; Xiao, X. Obesity induced by neonatal overfeeding worsens airway hyperresponsiveness and inflammation. PLoS ONE 2012, 7, e47013. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.N.; Greenberg, Y.; Hosseinkhani, M.R.; Long, E.K.; Bahaie, N.S.; Rao, A.; Ha, S.G.; Rao, S.P.; Bernlohr, D.A.; Sriramarao, P. High-fat diet promotes lung fibrosis and attenuates airway eosinophilia after exposure to cockroach allergen in mice. Exp. Lung Res. 2013, 39, 365–378. [Google Scholar] [CrossRef] [Green Version]
- Vedova, M.C.D.; Soler Garcia, F.M.; Munoz, M.D.; Fornes, M.W.; Gomez Mejiba, S.E.; Gomez, N.N.; Ramirez, D.C. Diet-Induced Pulmonary Inflammation and Incipient Fibrosis in Mice: A Possible Role of Neutrophilic Inflammation. Inflammation 2019, 42, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, H.; Matsui, H.; Ueno, M.; Maeno, T.; Iso, T.; Syamsunarno, M.R.; Anjo, S.; Matsuzaka, T.; Shimano, H.; Yokoyama, T.; et al. Deranged fatty acid composition causes pulmonary fibrosis in Elovl6-deficient mice. Nat. Commun. 2013, 4, 2563. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.D.; Yin, L.; Archer, S.; Lu, C.; Zhao, G.; Yao, Y.; Wu, L.; Hsin, M.; Waddell, T.K.; Keshavjee, S.; et al. Metabolic heterogeneity of idiopathic pulmonary fibrosis: A metabolomic study. BMJ Open Respir. Res. 2017, 4, e000183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Huang, J.; Li, J.S.; Chen, H.; Huang, K.; Zheng, L. Accumulation of endoplasmic reticulum stress and lipogenesis in the liver through generational effects of high fat diets. J. Hepatol. 2012, 56, 900–907. [Google Scholar] [CrossRef]
- Gonzalez-Becerra, K.; Ramos-Lopez, O.; Barron-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez-Lopez, E.; Martinez, J.A. Fatty acids, epigenetic mechanisms and chronic diseases: A systematic review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef] [Green Version]
- Hogg, S.J.; Beavis, P.A.; Dawson, M.A.; Johnstone, R.W. Targeting the epigenetic regulation of antitumour immunity. Nat. Reviews. Drug Discov. 2020, 19, 776–800. [Google Scholar] [CrossRef]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Qi, X.; Liu, L.; Ma, S.; Liu, J.; Wu, J. Epigenetic Regulation of m6A Modifications in Human Cancer. Mol. Therapy. Nucleic Acids 2020, 19, 405–412. [Google Scholar] [CrossRef]
- Ohkouchi, S.; Kanehira, M.; Saigusa, D.; Ono, M.; Tazawa, R.; Terunuma, H.; Hirano, T.; Numakura, T.; Notsuda, H.; Inoue, C.; et al. Metabolic and Epigenetic Regulation of SMAD7 by STC1 Ameliorates Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 67, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, M.; Jakhete, S.M.; Manekar, A.G.; Sasikumar, S. Specific epigenetic regulators serve as potential therapeutic targets in idiopathic pulmonary fibrosis. Heliyon 2022, 8, e09773. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, R.; Shi, W.; Chen, X.; Yi, J.; Yang, X.; Jin, S. Epigenetic regulation in radiation-induced pulmonary fibrosis. Int. J. Radiat. Biol. 2022, 99, 384–395. [Google Scholar] [CrossRef]
- Yang, D.; Xu, P.; Su, H.; Zhong, W.; Xu, J.; Su, Z.; Liu, X. The histone methyltransferase DOT1L is a new epigenetic regulator of pulmonary fibrosis. Cell Death Dis. 2022, 13, 60. [Google Scholar] [CrossRef]
- Hanmandlu, A.; Zhu, L.; Mertens, T.C.J.; Collum, S.; Bi, W.; Xiong, F.; Wang, R.; Amirthalingam, R.T.; Ren, D.; Han, L.; et al. Transcriptomic and Epigenetic Profiling of Fibroblasts in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 66, 53–63. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Z.; Fan, H.; Hao, C.; Yao, W. Epigenetic Changes and Functions in Pneumoconiosis. Oxidative Med. Cell. Longev. 2022, 2022, 2523066. [Google Scholar] [CrossRef]
- Burgoyne, R.A.; Fisher, A.J.; Borthwick, L.A. The Role of Epithelial Damage in the Pulmonary Immune Response. Cells 2021, 10, 2763. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tata, A.; Konkimalla, A.; Katsura, H.; Lee, R.F.; Ou, J.; Banovich, N.E.; Kropski, J.A.; Tata, P.R. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat. Cell Biol. 2020, 22, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Kathiriya, J.J.; Wang, C.; Zhou, M.; Brumwell, A.; Cassandras, M.; Le Saux, C.J.; Cohen, M.; Alysandratos, K.D.; Wang, B.; Wolters, P.; et al. Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5(+) basal cells. Nat. Cell Biol. 2022, 24, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Katzen, J.; Beers, M.F. Contributions of alveolar epithelial cell quality control to pulmonary fibrosis. J. Clin. Investig. 2020, 130, 5088–5099. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Chung, K.W. Targeting fatty acid metabolism for fibrotic disorders. Arch. Pharmacal Res. 2021, 44, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Buren, J.; Ericsson, M.; Damasceno, N.R.T.; Sjodin, A. A Ketogenic Low-Carbohydrate High-Fat Diet Increases LDL Cholesterol in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2021, 13, 814. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, S.; Zhu, T.; Zhang, Y.; Lian, X. Atherogenic high cholesterol/high fat diet induces TLRs-associated pulmonary inflammation in C57BL/6J mice. Inflamm. Res. 2017, 66, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, L.; Wei, Y.; Gu, J.; Liang, J.; Li, S.; Cui, Y.; Liu, R.; Huang, H.; Yang, C.; et al. Cabozantinib ameliorates lipopolysaccharide-induced lung inflammation and bleomycin--induced early pulmonary fibrosis in mice. Int. Immunopharmacol. 2021, 101, 108327. [Google Scholar] [CrossRef]
- Dowson, C.; O’Reilly, S. DNA methylation in fibrosis. Eur. J. Cell Biol. 2016, 95, 323–330. [Google Scholar] [CrossRef]
- Remely, M.; Aumueller, E.; Jahn, D.; Hippe, B.; Brath, H.; Haslberger, A.G. Microbiota and epigenetic regulation of inflammatory mediators in type 2 diabetes and obesity. Benef. Microbes 2014, 5, 33–43. [Google Scholar] [CrossRef]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M.; et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef]
- Auyeung, V.C.; Downey, M.S.; Thamsen, M.; Wenger, T.A.; Backes, B.J.; Sheppard, D.; Papa, F.R. IRE1alpha drives lung epithelial progenitor dysfunction to establish a niche for pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L564–L580. [Google Scholar] [CrossRef]
- Schuliga, M.; Kanwal, A.; Read, J.; Blokland, K.E.C.; Burgess, J.K.; Prele, C.M.; Mutsaers, S.E.; Grainge, C.; Thomson, C.; James, A.; et al. A cGAS-dependent response links DNA damage and senescence in alveolar epithelial cells: A potential drug target in IPF. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L859–L871. [Google Scholar] [CrossRef]
- Millan-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone post-translational modifications—Cause and consequence of genome function. Nat. Reviews. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Shvedunova, M.; Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Reviews. Mol. Cell Biol. 2022, 23, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tian, M.; Shliaha, P.V.; Zhang, J.; Jiang, S.; Nan, B.; Alam, M.N.; Jensen, O.N.; Shen, H.; Huang, Q. Real-world particulate matters induce lung toxicity in rats fed with a high-fat diet: Evidence of histone modifications. J. Hazard. Mater. 2021, 416, 126182. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wen, L.; Shi, Q.F.; Gao, F.; Huang, B.; Meng, J.; Hu, C.P.; Wang, C.M. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-kappaB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 2020, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Liu, K.Y.; Jeng, W.Y.; Chiang, C.M.; Chai, C.Y.; Chiou, S.S.; Huang, M.S.; Yokoyama, K.K.; Wang, S.N.; Huang, S.K.; et al. PCAF-mediated acetylation of ISX recruits BRD4 to promote epithelial-mesenchymal transition. EMBO Rep. 2020, 21, e48795. [Google Scholar] [CrossRef]
- Uthaya Kumar, D.B.; Motakis, E.; Yurieva, M.; Kohar, V.; Martinek, J.; Wu, T.C.; Khoury, J.; Grassmann, J.; Lu, M.; Palucka, K.; et al. Bronchial epithelium epithelial-mesenchymal plasticity forms aberrant basaloid-like cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L822–L841. [Google Scholar] [CrossRef]
- Tsukui, T.; Sun, K.H.; Wetter, J.B.; Wilson-Kanamori, J.R.; Hazelwood, L.A.; Henderson, N.C.; Adams, T.S.; Schupp, J.C.; Poli, S.D.; Rosas, I.O.; et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 2020, 11, 1920. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Bajinka, O.; Wang, L.; Wu, G.; Tan, Y. High-fat diet promotes epithelial-mesenchymal transition through enlarged growth of opportunistic pathogens and the intervention of saturated hydrogen. Am. J. Transl. Res. 2021, 13, 6016–6030. [Google Scholar]
- Yi, S.J.; Lee, H.; Lee, J.; Lee, K.; Kim, J.; Kim, Y.; Park, J.I.; Kim, K. Bone Remodeling: Histone Modifications as Fate Determinants of Bone Cell Differentiation. Int. J. Mol. Sci. 2019, 20, 3147. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, H.; Yi, S.J.; Kim, K. Gene regulation by histone-modifying enzymes under hypoxic conditions: A focus on histone methylation and acetylation. Exp. Mol. Med. 2022, 54, 878–889. [Google Scholar] [CrossRef]
- Wan, Q.L.; Meng, X.; Wang, C.; Dai, W.; Luo, Z.; Yin, Z.; Ju, Z.; Fu, X.; Yang, J.; Ye, Q.; et al. Histone H3K4me3 modification is a transgenerational epigenetic signal for lipid metabolism in Caenorhabditis elegans. Nat. Commun. 2022, 13, 768. [Google Scholar] [CrossRef]
- Hu, L.; Yu, Y.; Huang, H.; Fan, H.; Hu, L.; Yin, C.; Li, K.; Fulton, D.J.; Chen, F. Epigenetic Regulation of Interleukin 6 by Histone Acetylation in Macrophages and Its Role in Paraquat-Induced Pulmonary Fibrosis. Front. Immunol. 2016, 7, 696. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.L.; Wu, D.D.; Yao, Y.; Yu, F.Y.; Yang, L.; Tan, H.W.; Hylkema, M.N.; Rots, M.G.; Xu, Y.M.; Lau, A.T.Y. Epiproteome profiling of cadmium-transformed human bronchial epithelial cells by quantitative histone post-translational modification-enzyme-linked immunosorbent assay. J. Appl. Toxicol. JAT 2018, 38, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.H.G.; Paliogiannis, P.; Nasrallah, G.K.; Giordo, R.; Eid, A.H.; Fois, A.G.; Zinellu, A.; Mangoni, A.A.; Pintus, G. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cell. Mol. Life Sci. CMLS 2021, 78, 2031–2057. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, Y.; Huang, H.; Hu, Y.; Fu, S.; Wang, Z.; Shi, M.; Zhao, X.; Yuan, J.; Li, J.; et al. Progressive Pulmonary Fibrosis Is Caused by Elevated Mechanical Tension on Alveolar Stem Cells. Cell 2020, 180, 107–121.e17. [Google Scholar] [CrossRef]
- Izquierdo, V.; Palomera-Avalos, V.; Pallas, M.; Grinan-Ferre, C. Resveratrol Supplementation Attenuates Cognitive and Molecular Alterations under Maternal High-Fat Diet Intake: Epigenetic Inheritance over Generations. Int. J. Mol. Sci. 2021, 22, 1453. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Dong, H.; Sun, B.; Hu, Y.; Yang, Y.; Jia, Y.; Jia, L.; Zhong, X.; Zhao, R. METTL3/METTL14 Transactivation and m6A-Dependent TGF-beta1 Translation in Activated Kupffer Cells. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 839–856. [Google Scholar] [CrossRef]
- Pandey, A.; Goru, S.K.; Kadakol, A.; Malek, V.; Sharma, N.; Gaikwad, A.B. H2AK119 monoubiquitination regulates Angiotensin II receptor mediated macrophage infiltration and renal fibrosis in type 2 diabetic rats. Biochimie 2016, 131, 68–76. [Google Scholar] [CrossRef]
- Siddeek, B.; Mauduit, C.; Chehade, H.; Blin, G.; Liand, M.; Chindamo, M.; Benahmed, M.; Simeoni, U. Long-term impact of maternal high-fat diet on offspring cardiac health: Role of micro-RNA biogenesis. Cell Death Discov. 2019, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Thannickal, V.J.; Horowitz, J.C. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc. Am. Thorac. Soc. 2006, 3, 350–356. [Google Scholar] [CrossRef]
- Stancil, I.T.; Michalski, J.E.; Davis-Hall, D.; Chu, H.W.; Park, J.A.; Magin, C.M.; Yang, I.V.; Smith, B.J.; Dobrinskikh, E.; Schwartz, D.A. Pulmonary fibrosis distal airway epithelia are dynamically and structurally dysfunctional. Nat. Commun. 2021, 12, 4566. [Google Scholar] [CrossRef] [PubMed]
- Carraro, G.; Mulay, A.; Yao, C.; Mizuno, T.; Konda, B.; Petrov, M.; Lafkas, D.; Arron, J.R.; Hogaboam, C.M.; Chen, P.; et al. Single-Cell Reconstruction of Human Basal Cell Diversity in Normal and Idiopathic Pulmonary Fibrosis Lungs. Am. J. Respir. Crit. Care Med. 2020, 202, 1540–1550. [Google Scholar] [CrossRef]
- Jones, S.F.; Infante, J.R. Molecular Pathways: Fatty Acid Synthase. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 5434–5438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plataki, M.; Fan, L.; Sanchez, E.; Huang, Z.; Torres, L.K.; Imamura, M.; Zhu, Y.; Cohen, D.E.; Cloonan, S.M.; Choi, A.M. Fatty acid synthase downregulation contributes to acute lung injury in murine diet-induced obesity. JCI Insight 2019, 5, e127823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality control of the mitochondrion. Dev. Cell 2021, 56, 881–905. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, D.; Tian, Y.; Xie, Z.; Zou, M.H. BRD4 inhibition by JQ1 prevents high-fat diet-induced diabetic cardiomyopathy by activating PINK1/Parkin-mediated mitophagy in vivo. J. Mol. Cell. Cardiol. 2020, 149, 1–14. [Google Scholar] [CrossRef]
- Knoell, J.; Chillappagari, S.; Knudsen, L.; Korfei, M.; Dartsch, R.; Jonigk, D.; Kuehnel, M.P.; Hoetzenecker, K.; Guenther, A.; Mahavadi, P. PACS2-TRPV1 axis is required for ER-mitochondrial tethering during ER stress and lung fibrosis. Cell. Mol. Life Sci. CMLS 2022, 79, 151. [Google Scholar] [CrossRef]
- Dobrinskikh, E.; Hennessy, C.E.; Kurche, J.S.; Kim, E.; Estrella, A.M.; Cardwell, J.; Yang, I.V.; Schwartz, D.A. Epithelial ER Stress Enhances the Risk of Muc5b Associated Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2023, 68, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, T.L.; Sutherland, J.P.; Wolfe, P.; Allian-Sauer, M.; Capell, W.H.; Talley, N.D.; Wyatt, H.R.; Foster, G.D.; Hill, J.O.; Eckel, R.H. Lack of suppression of circulating free fatty acids and hypercholesterolemia during weight loss on a high-fat, low-carbohydrate diet. Am. J. Clin. Nutr. 2010, 91, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Staab-Weijnitz, C.A. Fighting the Fiber: Targeting Collagen in Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 66, 363–381. [Google Scholar] [CrossRef]
- Kaufman, J.; Graf, B.A.; Leung, E.C.; Pollock, S.J.; Koumas, L.; Reddy, S.Y.; Blieden, T.M.; Smith, T.J.; Phipps, R.P. Fibroblasts as sentinel cells: Role of the CDcd40-CDcd40 ligand system in fibroblast activation and lung inflammation and fibrosis. Chest 2001, 120, 53S–55S. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Adeyanju, O.; Sunil, C.; Huang, S.K.; Chen, S.Y.; Tucker, T.A.; Idell, S.; Guo, X. Dedicator of Cytokinesis 2 (DOCK2) Deficiency Attenuates Lung Injury Associated with Chronic High-Fat and High-Fructose Diet-Induced Obesity. Am. J. Pathol. 2022, 192, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Park, J.; Gupta, O.T.; Holland, W.L.; Auerbach, P.; Zhang, N.; Goncalves Marangoni, R.; Nicoloro, S.M.; Czech, M.P.; Varga, J.; et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 2014, 5, 3485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, T.; Sugiura, H.; Koarai, A.; Ichikawa, T.; Minakata, Y.; Matsunaga, K.; Nakanishi, M.; Hirano, T.; Akamatsu, K.; Yanagisawa, S.; et al. Increase of 27-hydroxycholesterol in the airways of patients with COPD: Possible role of 27-hydroxycholesterol in tissue fibrosis. Chest 2012, 142, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Sehlmeyer, K.; Ruwisch, J.; Roldan, N.; Lopez-Rodriguez, E. Alveolar Dynamics and Beyond—The Importance of Surfactant Protein C and Cholesterol in Lung Homeostasis and Fibrosis. Front. Physiol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Levine, S.J. Emerging Roles of Apolipoprotein E and Apolipoprotein A-I in the Pathogenesis and Treatment of Lung Disease. Am. J. Respir. Cell Mol. Biol. 2016, 55, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildirim, M.; Kayalar, O.; Atahan, E.; Oztay, F. Atorvastatin attenuates pulmonary fibrosis in mice and human lung fibroblasts, by the regulation of myofibroblast differentiation and apoptosis. J. Biochem. Mol. Toxicol. 2022, 36, e23074. [Google Scholar] [CrossRef]
- Gu, X.; Han, Y.Y.; Yang, C.Y.; Ji, H.M.; Lan, Y.J.; Bi, Y.Q.; Zheng, C.; Qu, J.; Cheng, M.H.; Gao, J. Activated AMPK by metformin protects against fibroblast proliferation during pulmonary fibrosis by suppressing FOXM1. Pharmacol. Res. 2021, 173, 105844. [Google Scholar] [CrossRef]
- Rashkovan, M.; Albero, R.; Gianni, F.; Perez-Duran, P.; Miller, H.I.; Mackey, A.L.; Paietta, E.M.; Tallman, M.S.; Rowe, J.M.; Litzow, M.R.; et al. Intracellular Cholesterol Pools Regulate Oncogenic Signaling and Epigenetic Circuitries in Early T-cell Precursor Acute Lymphoblastic Leukemia. Cancer Discov. 2022, 12, 856–871. [Google Scholar] [CrossRef]
- Labbe, D.P.; Zadra, G.; Yang, M.; Reyes, J.M.; Lin, C.Y.; Cacciatore, S.; Ebot, E.M.; Creech, A.L.; Giunchi, F.; Fiorentino, M.; et al. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat. Commun. 2019, 10, 4358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, H.; Tang, Y.; Mao, Y.; Zhou, X.; Xu, T.; Liu, W.; Su, X. C-MYC induces idiopathic pulmonary fibrosis via modulation of miR-9-5p-mediated TBPL1. Cell. Signal. 2022, 93, 110274. [Google Scholar] [CrossRef] [PubMed]
- Buras, E.D.; Converso-Baran, K.; Davis, C.S.; Akama, T.; Hikage, F.; Michele, D.E.; Brooks, S.V.; Chun, T.H. Fibro-Adipogenic Remodeling of the Diaphragm in Obesity-Associated Respiratory Dysfunction. Diabetes 2019, 68, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, R.; Meier, U.; Markart, P.; Grimminger, F.; Velcovsky, H.G.; Morr, H.; Seeger, W.; Gunther, A. Altered fatty acid composition of lung surfactant phospholipids in interstitial lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L1079–L1085. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pamulapati, H.; Tikoo, K. Fatty acid induced metabolic memory involves alterations in renal histone H3K36me2 and H3K27me3. Mol. Cell. Endocrinol. 2016, 422, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Jin, X.Q.; Yu, W.Y.; Dong, Y.Y.; Ying, H.Z.; Yu, C.H. 1beta-Hydroxyalantolactone from Inulae Flos alleviated the progression of pulmonary fibrosis via inhibiting JNK/FOXO1/NF-kappaB pathway. Int. Immunopharmacol. 2021, 101, 108339. [Google Scholar] [CrossRef] [PubMed]
- Ferst, J.G.; Glanzner, W.G.; Gutierrez, K.; de Macedo, M.P.; Ferreira, R.; Gasperin, B.G.; Duggavathi, R.; Goncalves, P.B.; Bordignon, V. Supplementation of oleic acid, stearic acid, palmitic acid and beta-hydroxybutyrate increase H3K9me3 in endometrial epithelial cells of cattle cultured in vitro. Anim. Reprod. Sci. 2021, 233, 106851. [Google Scholar] [CrossRef]
- Huang, S.K.; Scruggs, A.M.; Donaghy, J.; Horowitz, J.C.; Zaslona, Z.; Przybranowski, S.; White, E.S.; Peters-Golden, M. Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell Death Dis. 2013, 4, e621. [Google Scholar] [CrossRef] [Green Version]
- Pessoa Rodrigues, C.; Chatterjee, A.; Wiese, M.; Stehle, T.; Szymanski, W.; Shvedunova, M.; Akhtar, A. Histone H4 lysine 16 acetylation controls central carbon metabolism and diet-induced obesity in mice. Nat. Commun. 2021, 12, 6212. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Zhou, J.Q.; Krick, S.; Barnes, J.W.; Thannickal, V.J.; Sanders, Y.Y. Modulation of H4K16Ac levels reduces pro-fibrotic gene expression and mitigates lung fibrosis in aged mice. Theranostics 2022, 12, 530–541. [Google Scholar] [CrossRef]
- Xue, T.; Qiu, X.; Liu, H.; Gan, C.; Tan, Z.; Xie, Y.; Wang, Y.; Ye, T. Epigenetic regulation in fibrosis progress. Pharmacol. Res. 2021, 173, 105910. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, J.; Deng, H.; Ma, R.; Liao, J.Y.; Liang, H.; Hu, J.; Li, J.; Guo, Z.; Cai, J.; et al. Targeting Mitochondria-Located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell 2020, 183, 76–93.e22. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Reviews. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Hey, J.; Paulsen, M.; Toth, R.; Weichenhan, D.; Butz, S.; Schatterny, J.; Liebers, R.; Lutsik, P.; Plass, C.; Mall, M.A. Epigenetic reprogramming of airway macrophages promotes polarization and inflammation in muco-obstructive lung disease. Nat. Commun. 2021, 12, 6520. [Google Scholar] [CrossRef]
- Shenderov, K.; Collins, S.L.; Powell, J.D.; Horton, M.R. Immune dysregulation as a driver of idiopathic pulmonary fibrosis. J. Clin. Investig. 2021, 131, e143226. [Google Scholar] [CrossRef] [PubMed]
- Ptasinski, V.A.; Stegmayr, J.; Belvisi, M.G.; Wagner, D.E.; Murray, L.A. Targeting Alveolar Repair in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2021, 65, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Shin, Y.J.; Go, R.E.; Bae, S.H.; Kim, C.W.; Kim, S.; Kim, M.S.; Choi, K.C. Inhalation exposure by cigarette smoke: Effects on the progression of bleomycin- and lipopolysaccharide-induced lung injuries in rat models. Toxicology 2021, 451, 152695. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef]
- Ramallal, R.; Toledo, E.; Martinez, J.A.; Shivappa, N.; Hebert, J.R.; Martinez-Gonzalez, M.A.; Ruiz-Canela, M. Inflammatory potential of diet, weight gain, and incidence of overweight/obesity: The SUN cohort. Obesity 2017, 25, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, K.; Konzack, A.; Pihlajaniemi, T.; Heljasvaara, R.; Kietzmann, T. Redox-fibrosis: Impact of TGFbeta1 on ROS generators, mediators and functional consequences. Redox Biol. 2015, 6, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. AMS 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- O’Rourke, R.W. Inflammation in obesity-related diseases. Surgery 2009, 145, 255–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Reviews. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Samokhin, A.O.; Buhling, F.; Theissig, F.; Bromme, D. ApoE-deficient mice on cholate-containing high-fat diet reveal a pathology similar to lung sarcoidosis. Am. J. Pathol. 2010, 176, 1148–1156. [Google Scholar] [CrossRef] [Green Version]
- Stylianou, E. Epigenetics of chronic inflammatory diseases. J. Inflamm. Res. 2019, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bayarsaihan, D. Epigenetic mechanisms in inflammation. J. Dent. Res. 2011, 90, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chen, Z.; Shen, W.; Huang, G.; Sedivy, J.M.; Wang, H.; Ju, Z. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: The regulation and intervention. Signal Transduct. Target. Ther. 2021, 6, 245. [Google Scholar] [CrossRef]
- Ahmed, M.; de Winther, M.P.J.; Van den Bossche, J. Epigenetic mechanisms of macrophage activation in type 2 diabetes. Immunobiology 2017, 222, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jaramillo, V.; Portilla-Fernandez, E.; Glisic, M.; Voortman, T.; Ghanbari, M.; Bramer, W.; Chowdhury, R.; Nijsten, T.; Dehghan, A.; Franco, O.H.; et al. Epigenetics and Inflammatory Markers: A Systematic Review of the Current Evidence. Int. J. Inflamm. 2019, 2019, 6273680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermsdorff, H.H.; Mansego, M.L.; Campion, J.; Milagro, F.I.; Zulet, M.A.; Martinez, J.A. TNF-alpha promoter methylation in peripheral white blood cells: Relationship with circulating TNFalpha, truncal fat and n-6 PUFA intake in young women. Cytokine 2013, 64, 265–271. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Q.; Yu, L.; Shi, H.; Xue, B.; Shi, H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight 2016, 1, e87748. [Google Scholar] [CrossRef] [Green Version]
- Kamei, Y.; Suganami, T.; Ehara, T.; Kanai, S.; Hayashi, K.; Yamamoto, Y.; Miura, S.; Ezaki, O.; Okano, M.; Ogawa, Y. Increased expression of DNA methyltransferase 3a in obese adipose tissue: Studies with transgenic mice. Obesity 2010, 18, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, X.; Liu, D.; Yu, L.; Xue, B.; Shi, H. Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol. Endocrinol. 2014, 28, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Raciti, G.A.; Spinelli, R.; Desiderio, A.; Longo, M.; Parrillo, L.; Nigro, C.; D’Esposito, V.; Mirra, P.; Fiory, F.; Pilone, V.; et al. Specific CpG hyper-methylation leads to Ankrd26 gene down-regulation in white adipose tissue of a mouse model of diet-induced obesity. Sci. Rep. 2017, 7, 43526. [Google Scholar] [CrossRef] [Green Version]
- Desiderio, A.; Longo, M.; Parrillo, L.; Campitelli, M.; Cacace, G.; de Simone, S.; Spinelli, R.; Zatterale, F.; Cabaro, S.; Dolce, P.; et al. Epigenetic silencing of the ANKRD26 gene correlates to the pro-inflammatory profile and increased cardio-metabolic risk factors in human obesity. Clin. Epigenetics 2019, 11, 181. [Google Scholar] [CrossRef]
- Shanaki, M.; Omidifar, A.; Shabani, P.; Toolabi, K. Association between HDACs and pro-inflammatory cytokine gene expressions in obesity. Arch. Physiol. Biochem. 2022, 128, 880–886. [Google Scholar] [CrossRef]
- Nuno, D.W.; Lamping, K.G. Dietary Fatty Acid Saturation Modulates Sphingosine-1-Phosphate-Mediated Vascular Function. J. Diabetes Res. 2019, 2019, 2354274. [Google Scholar] [CrossRef]
- Fu, P.; Ebenezer, D.L.; Ha, A.W.; Suryadevara, V.; Harijith, A.; Natarajan, V. Nuclear lipid mediators: Role of nuclear sphingolipids and sphingosine-1-phosphate signaling in epigenetic regulation of inflammation and gene expression. J. Cell. Biochem. 2018, 119, 6337–6353. [Google Scholar] [CrossRef]
- Suryadevara, V.; Ramchandran, R.; Kamp, D.W.; Natarajan, V. Lipid Mediators Regulate Pulmonary Fibrosis: Potential Mechanisms and Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4257. [Google Scholar] [CrossRef]
- Huang, L.S.; Berdyshev, E.; Mathew, B.; Fu, P.; Gorshkova, I.A.; He, D.; Ma, W.; Noth, I.; Ma, S.F.; Pendyala, S.; et al. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1749–1760. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Zhang, Q.; Mul, J.D.; Yu, M.; Xu, J.; Qi, C.; Wang, T.; Xiao, X. Maternal high-calorie diet is associated with altered hepatic microRNA expression and impaired metabolic health in offspring at weaning age. Endocrine 2016, 54, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, J.; Liu, S.; Zhang, L.; Xiao, H.; Li, J.; Chen, H.; Petersen, R.B.; Huang, K.; Zheng, L. DNA hypomethylation of inflammation-associated genes in adipose tissue of female mice after multigenerational high fat diet feeding. Int. J. Obes. 2014, 38, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Guo, L.; Chi, M.H.; Sun, H.M.; Chen, X.W. Identification of active miRNA and transcription factor regulatory pathways in human obesity-related inflammation. BMC Bioinform. 2015, 16, 76. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, J.G.; Diehl, K.J.; Bammert, T.D.; Kavlich, P.J.; Lincenberg, G.M.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Influence of Overweight and Obesity on Circulating Inflammation-Related microRNA. MicroRNA 2018, 7, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hui, X.; Hoo, R.L.C.; Ye, D.; Chan, C.Y.C.; Feng, T.; Wang, Y.; Lam, K.S.L.; Xu, A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Mei, H.; Chang, X.; Chen, F.; Zhu, Y.; Han, X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 2016, 8, 505–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, K.; Yang, X.; Bam, M.; Murphy, E.A.; Nagarkatti, P.S.; Nagarkatti, M. MicroRNA-30 modulates metabolic inflammation by regulating Notch signaling in adipose tissue macrophages. Int. J. Obes. 2018, 42, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Wu, G.; Xiong, W.; Gu, W.; Wang, C.Y. Macrophages: Friend or foe in idiopathic pulmonary fibrosis? Respir. Res. 2018, 19, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyob, K.M.; Mieth, S.; Sugar, S.S.; Graf, A.E.; Lallier, S.W.; Britt, R.D., Jr.; Rogers, L.K. Maternal high-fat diet alters lung development and function in the offspring. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L167–L174. [Google Scholar] [CrossRef]
- Snow, S.J.; Phillips, P.M.; Ledbetter, A.; Johnstone, A.F.M.; Schladweiler, M.C.; Gordon, C.J.; Kodavanti, U.P. The influence of maternal and perinatal high-fat diet on ozone-induced pulmonary responses in offspring. J. Toxicol. Environ. Health. Part A 2019, 82, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Alfaradhi, M.Z.; Kusinski, L.C.; Fernandez-Twinn, D.S.; Pantaleao, L.C.; Carr, S.K.; Ferland-McCollough, D.; Yeo, G.S.; Bushell, M.; Ozanne, S.E. Maternal Obesity in Pregnancy Developmentally Programs Adipose Tissue Inflammation in Young, Lean Male Mice Offspring. Endocrinology 2016, 157, 4246–4256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Q.; Wei, M.; Kang, X.; Liu, D.; Quan, Y.; Pan, X.; Liu, X.; Liao, D.; Liu, J.; Zhang, B. Reciprocal inhibition between miR-26a and NF-kappaB regulates obesity-related chronic inflammation in chondrocytes. Biosci. Rep. 2015, 35, e00204. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Liu, J.; Xu, H.; Li, Y.; Tao, R.; Zhang, Z. Pirfenidone inhibits cell fibrosis in connective tissue disease-associated interstitial lung disease by targeting the TNF-alpha/STAT3/KL6 pathway. J. Thorac. Dis. 2022, 14, 2089–2102. [Google Scholar] [CrossRef]
- Nakatsuka, Y.; Handa, T.; Kokosi, M.; Tanizawa, K.; Puglisi, S.; Jacob, J.; Sokai, A.; Ikezoe, K.; Kanatani, K.T.; Kubo, T.; et al. The Clinical Significance of Body Weight Loss in Idiopathic Pulmonary Fibrosis Patients. Respir. Int. Rev. Thorac. Dis. 2018, 96, 338–347. [Google Scholar] [CrossRef]
- Dai, H.; Xiang, J.; Hou, Y.; Xuan, L.; Wang, T.; Li, M.; Zhao, Z.; Xu, Y.; Lu, J.; Chen, Y.; et al. Fat mass to fat-free mass ratio and the risk of non-alcoholic fatty liver disease and fibrosis in non-obese and obese individuals. Nutr. Metab. 2021, 18, 21. [Google Scholar] [CrossRef]
- Lee, D.H.; Keum, N.; Hu, F.B.; Orav, E.J.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: Prospective US cohort study. Bmj 2018, 362, k2575. [Google Scholar] [CrossRef] [Green Version]
- Iliodromiti, S.; Celis-Morales, C.A.; Lyall, D.M.; Anderson, J.; Gray, S.R.; Mackay, D.F.; Nelson, S.M.; Welsh, P.; Pell, J.P.; Gill, J.M.R.; et al. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: A cohort study of 296 535 adults of white European descent. Eur. Heart J. 2018, 39, 1514–1520. [Google Scholar] [CrossRef]
- Ionescu, A.A.; Evans, W.D.; Pettit, R.J.; Nixon, L.S.; Stone, M.D.; Shale, D.J. Hidden depletion of fat-free mass and bone mineral density in adults with cystic fibrosis. Chest 2003, 124, 2220–2228. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.F.; Qiu, Y.Q.; Wang, L.; Gao, K.G.; Jiang, Z.Y. A high-fat diet increases body fat mass and up-regulates expression of genes related to adipogenesis and inflammation in a genetically lean pig. J. Zhejiang Univ. Sci. B 2018, 19, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.; Gede, N.; Ocskay, K.; Dobai, B.M.; Abada, A.; Vereczkei, Z.; Pazmany, P.; Kato, D.; Hegyi, P.; Parniczky, A. Association of Body Mass Index with Clinical Outcomes in Patients with Cystic Fibrosis: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e220740. [Google Scholar] [CrossRef] [PubMed]
- Monnard, C.R.; Dulloo, A.G. Polyunsaturated fatty acids as modulators of fat mass and lean mass in human body composition regulation and cardiometabolic health. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021, 22 (Suppl. S2), e13197. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.I., Jr.; Chandler, D.B.; Fulmer, J.D.; Wert, M.B.; Grizzle, W.E. Dietary fish oil inhibits bleomycin-induced pulmonary fibrosis in the rat. Exp. Lung Res. 1989, 15, 315–329. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, T.; Zhao, X.; Xiea, K.; Bi, Y.; Zhong, Z.; Zhao, X. Docosahexaenoic acid (DHA) ameliorates paraquat-induced pulmonary fibrosis in rats possibly through up-regulation of Smad 7 and SnoN. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 57, 330–337. [Google Scholar] [CrossRef]
- Lawrenz, J.; Herndon, B.; Kamal, A.; Mehrer, A.; Dim, D.C.; Baidoo, C.; Gasper, D.; Nitz, J.; Molteni, A.; Baybutt, R.C. Dietary Flaxseed Oil Protects against Bleomycin-Induced Pulmonary Fibrosis in Rats. Pulm. Med. 2012, 2012, 457031. [Google Scholar] [CrossRef]
- Abidi, A.; Kourda, N.; Feki, M.; Ben Khamsa, S. Protective Effect of Tunisian Flaxseed Oil against Bleomycin-Induced Pulmonary Fibrosis in Rats. Nutr. Cancer 2020, 72, 226–238. [Google Scholar] [CrossRef]
- Velten, M.; Britt, R.D., Jr.; Heyob, K.M.; Tipple, T.E.; Rogers, L.K. Maternal dietary docosahexaenoic acid supplementation attenuates fetal growth restriction and enhances pulmonary function in a newborn mouse model of perinatal inflammation. J. Nutr. 2014, 144, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Wang, Q.; D’Souza, V.; Bartis, D.; Dancer, R.; Parekh, D.; Gao, F.; Lian, Q.; Jin, S.; Thickett, D.R. ResolvinD(1) stimulates epithelial wound repair and inhibits TGF-beta-induced EMT whilst reducing fibroproliferation and collagen production. Lab. Investig. A J. Tech. Methods Pathol. 2018, 98, 130–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercader-Barcelo, J.; Truyols-Vives, J.; Rio, C.; Lopez-Safont, N.; Sala-Llinas, E.; Chaplin, A. Insights into the Role of Bioactive Food Ingredients and the Microbiome in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 6051. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Yokoyama, T.; Chida, K.; Azuma, A.; Suda, T.; Kudoh, S.; Sakamoto, N.; Okamoto, K.; Kobashi, G.; et al. Vegetable, fruit, and cereal intake and risk of idiopathic pulmonary fibrosis in Japan. Ann. Nutr. Metab. 2004, 48, 390–397. [Google Scholar] [CrossRef]
- Wang, X.; Yi, X.; Tang, D. Aerobic Exercise Improves Pulmonary Fibrosis by Improving Insulin Resistance and Inflammation in Obese Mice. Front. Physiol. 2021, 12, 785117. [Google Scholar] [CrossRef]
- Tang, X.; Peng, R.; Phillips, J.E.; Deguzman, J.; Ren, Y.; Apparsundaram, S.; Luo, Q.; Bauer, C.M.; Fuentes, M.E.; DeMartino, J.A.; et al. Assessment of Brd4 inhibition in idiopathic pulmonary fibrosis lung fibroblasts and in vivo models of lung fibrosis. Am. J. Pathol. 2013, 183, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, K.; Bialas, A.J.; Kotecki, M.J.; Gorski, P.; Piotrowski, W.J. More than a Genetic Code: Epigenetics of Lung Fibrosis. Mol. Diagn. Ther. 2020, 24, 665–681. [Google Scholar] [CrossRef]
- Davies, E.R.; Haitchi, H.M.; Thatcher, T.H.; Sime, P.J.; Kottmann, R.M.; Ganesan, A.; Packham, G.; O’Reilly, K.M.; Davies, D.E. Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2012, 46, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Chioccioli, M.; Roy, S.; Newell, R.; Pestano, L.; Dickinson, B.; Rigby, K.; Herazo-Maya, J.; Jenkins, G.; Ian, S.; Saini, G.; et al. A lung targeted miR-29 mimic as a therapy for pulmonary fibrosis. EBioMedicine 2022, 85, 104304. [Google Scholar] [CrossRef]
- Montgomery, R.L.; Yu, G.; Latimer, P.A.; Stack, C.; Robinson, K.; Dalby, C.M.; Kaminski, N.; van Rooij, E. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol. Med. 2014, 6, 1347–1356. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Liang, C.; Liu, L.; Wang, L.; Yu, G. High-Fat Diet Related Lung Fibrosis-Epigenetic Regulation Matters. Biomolecules 2023, 13, 558. https://doi.org/10.3390/biom13030558
Yang J, Liang C, Liu L, Wang L, Yu G. High-Fat Diet Related Lung Fibrosis-Epigenetic Regulation Matters. Biomolecules. 2023; 13(3):558. https://doi.org/10.3390/biom13030558
Chicago/Turabian StyleYang, Juntang, Chenxi Liang, Lulu Liu, Lan Wang, and Guoying Yu. 2023. "High-Fat Diet Related Lung Fibrosis-Epigenetic Regulation Matters" Biomolecules 13, no. 3: 558. https://doi.org/10.3390/biom13030558
APA StyleYang, J., Liang, C., Liu, L., Wang, L., & Yu, G. (2023). High-Fat Diet Related Lung Fibrosis-Epigenetic Regulation Matters. Biomolecules, 13(3), 558. https://doi.org/10.3390/biom13030558







