Glaucoma and Myopia: Diagnostic Challenges
Abstract
:1. Introduction
2. Anatomical Basis for Disease and Corresponding Clinical Findings
3. Diagnostic Challenges
3.1. Visual Field
3.2. Intraocular Pressure
3.3. Imaging
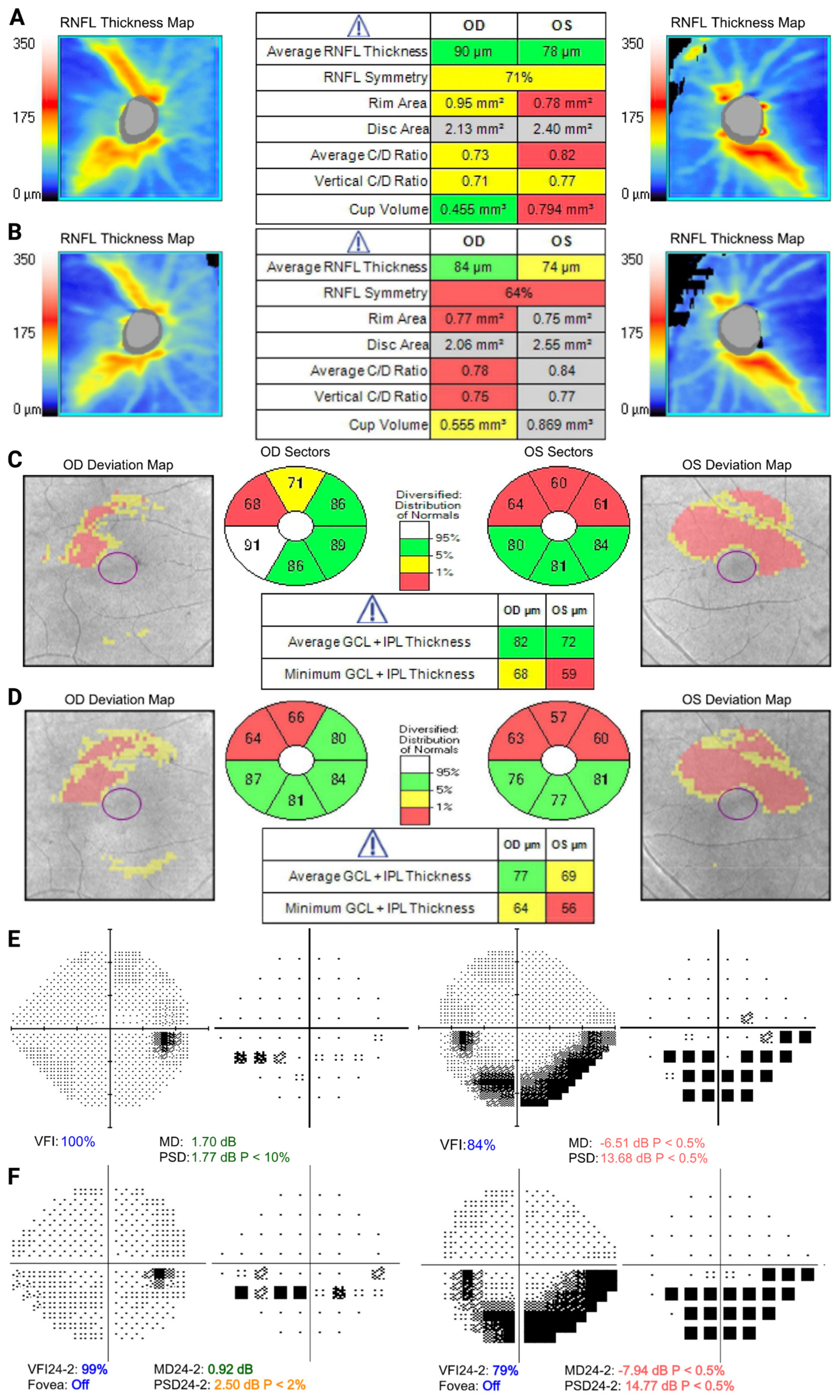
4. Case Discussion
4.1. Case 1
4.2. Case 2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [Green Version]
- Pärssinen, O.; Kauppinen, M. Associations of near Work Time, Watching TV, Outdoors Time, and Parents’ Myopia with Myopia among School Children Based on 38-Year-Old Historical Data. Acta Ophthalmol. 2022, 100, e430–e438. [Google Scholar] [CrossRef]
- Pärssinen, O. The Increased Prevalence of Myopia in Finland. Acta Ophthalmol. 2012, 90, 497–502. [Google Scholar] [CrossRef]
- Lam, C.S.-Y.; Lam, C.-H.; Cheng, S.C.-K.; Chan, L.Y.-L. Prevalence of Myopia among Hong Kong Chinese Schoolchildren: Changes over Two Decades. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. Optom. 2012, 32, 17–24. [Google Scholar] [CrossRef]
- Castagno, V.D.; Fassa, A.G.; Carret, M.L.V.; Vilela, M.A.P.; Meucci, R.D. Hyperopia: A Meta-Analysis of Prevalence and a Review of Associated Factors among School-Aged Children. BMC Ophthalmol. 2014, 14, 163. [Google Scholar] [CrossRef] [Green Version]
- Pärssinen, O.; Soh, Z.D.; Tan, C.-S.; Lanca, C.; Kauppinen, M.; Saw, S.-M. Comparison of Myopic Progression in Finnish and Singaporean Children. Acta Ophthalmol. 2021, 99, 171–180. [Google Scholar] [CrossRef]
- Lin, L.L.K.; Shih, Y.F.; Hsiao, C.K.; Chen, C.J. Prevalence of Myopia in Taiwanese Schoolchildren: 1983 to 2000. Ann. Acad. Med. Singapore 2004, 33, 27–33. [Google Scholar] [PubMed]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.-M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef]
- Knapp, A. Glaucoma in Myopic Eyes. Trans. Am. Ophthalmol. Soc. 1925, 23, 61–70. [Google Scholar] [PubMed]
- Moller, H.U. Excessive Myopia and Glaucoma. Acta Ophthalmol. 1948, 26, 185–193. [Google Scholar]
- Fong, D.S.; Epstein, D.L.; Allingham, R.R. Glaucoma and Myopia: Are They Related? Int. Ophthalmol. Clin. 1990, 30, 215–218. [Google Scholar] [CrossRef]
- Daubs, J.G.; Crick, R.P. Effect of Refractive Error on the Risk of Ocular Hypertension and Open Angle Glaucoma. Trans. Ophthalmol. Soc. U. K. 1981, 101, 121–126. [Google Scholar]
- Xu, L.; Wang, Y.; Wang, S.; Wang, Y.; Jonas, J.B. High Myopia and Glaucoma Susceptibility the Beijing Eye Study. Ophthalmology 2007, 114, 216–220. [Google Scholar] [CrossRef]
- Mitchell, P.; Hourihan, F.; Sandbach, J.; Wang, J.J. The Relationship between Glaucoma and Myopia: The Blue Mountains Eye Study. Ophthalmology 1999, 106, 2010–2015. [Google Scholar] [CrossRef]
- Kuzin, A.A.; Varma, R.; Reddy, H.S.; Torres, M.; Azen, S.P. Ocular Biometry and Open Angle Glaucoma: The Los Angeles Latino Eye Study. Ophthalmology 2010, 117, 1713–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, S.A.; Wong, T.Y.; Tay, W.-T.; Foster, P.J.; Saw, S.-M.; Aung, T. Refractive Error, Axial Dimensions, and Primary Open-Angle Glaucoma: The Singapore Malay Eye Study. Arch. Ophthalmol. 2010, 128, 900–905. [Google Scholar] [CrossRef] [Green Version]
- Ha, A.; Kim, C.Y.; Shim, S.R.; Chang, I.B.; Kim, Y.K. Degree of Myopia and Glaucoma Risk: A Dose-Response Meta-Analysis. Am. J. Ophthalmol. 2022, 236, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Martus, P.; Budde, W.M. Anisometropia and Degree of Optic Nerve Damage in Chronic Open-Angle Glaucoma. Am. J. Ophthalmol. 2002, 134, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; et al. The Ocular Hypertension Treatment Study: Baseline Factors That Predict the Onset of Primary Open-Angle Glaucoma. Arch. Ophthalmol. 2002, 120, 714–720, discussion 829–830. [Google Scholar] [CrossRef]
- Chao, D.L.; Shrivastava, A.; Kim, D.H.; Lin, H.; Singh, K. Axial Length Does Not Correlate with Degree of Visual Field Loss in Myopic Chinese Individuals with Glaucomatous Appearing Optic Nerves. J. Glaucoma 2010, 19, 509–513. [Google Scholar] [CrossRef]
- Scott, R.; Grosvenor, T. Structural Model for Emmetropic and Myopic Eyes. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. Optom. 1993, 13, 41–47. [Google Scholar] [CrossRef]
- Saw, S.-M.; Gazzard, G.; Shih-Yen, E.C.; Chua, W.-H. Myopia and Associated Pathological Complications. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. Optom. 2005, 25, 381–391. [Google Scholar] [CrossRef]
- Cahane, M.; Bartov, E. Axial Length and Scleral Thickness Effect on Susceptibility to Glaucomatous Damage: A Theoretical Model Implementing Laplace’s Law. Ophthalmic Res. 1992, 24, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Kim, M.; Weinreb, R.N.; Woo, S.J.; Park, K.H.; Hwang, J.-M. Optic Disc Change with Incipient Myopia of Childhood. Ophthalmology 2012, 119, 21–26.e3. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Jonas, R.A.; Bikbov, M.M.; Wang, Y.X.; Panda-Jonas, S. Myopia: Histology, Clinical Features, and Potential Implications for the Etiology of Axial Elongation. Prog. Retin. Eye Res. 2022, 101156. [Google Scholar] [CrossRef]
- Lee, S.; Han, S.X.; Young, M.; Beg, M.F.; Sarunic, M.V.; Mackenzie, P.J. Optic Nerve Head and Peripapillary Morphometrics in Myopic Glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4378–4393. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.H.; Chung, S.K.; Lee, N.Y. Topographical Analysis of Non-Glaucomatous Myopic Optic Discs Using a Confocal Scanning Laser Ophthalmoscope (TopSS). Semin. Ophthalmol. 2015, 30, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Wang, N.; Li, B.; Li, L.; Gao, F.; Xu, X.; Jonas, J.B. Lamina Cribrosa and Peripapillary Sclera Histomorphometry in Normal and Advanced Glaucomatous Chinese Eyes with Various Axial Length. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2175–2184. [Google Scholar] [CrossRef] [Green Version]
- Jonas, J.B.; Ohno-Matsui, K.; Panda-Jonas, S. Optic Nerve Head Histopathology in High Axial Myopia. J. Glaucoma 2017, 26, 187–193. [Google Scholar] [CrossRef]
- Jonas, J.B. Clinical Implications of Peripapillary Atrophy in Glaucoma. Curr. Opin. Ophthalmol. 2005, 16, 84–88. [Google Scholar] [CrossRef]
- Zhou, D.; Cao, M.; Duan, X. Prevalence and Diagnostic Ability of β-Zone Parapapillary Atrophy in Open-Angle Glaucoma: A Systematic Review and Meta-Analysis. BMC Ophthalmol. 2022, 22, 72. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Li, J.; Wang, J.-D.; Xiong, Y.; Cao, K.; Hou, S.-M.; Yusufu, M.; Wang, K.-J.; Li, M.; Mao, Y.-Y.; et al. The Association of Myopia Progression with the Morphological Changes of Optic Disc and β-Peripapillary Atrophy in Primary School Students. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2022, 260, 677–687. [Google Scholar] [CrossRef]
- Teng, C.C.; De Moraes, C.G.V.; Prata, T.S.; Tello, C.; Ritch, R.; Liebmann, J.M. Beta-Zone Parapapillary Atrophy and the Velocity of Glaucoma Progression. Ophthalmology 2010, 117, 909–915. [Google Scholar] [CrossRef]
- Jonas, J.B.; Dichtl, A. Optic Disc Morphology in Myopic Primary Open-Angle Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Jonas, S.B.; Jonas, R.A.; Holbach, L.; Dai, Y.; Sun, X.; Panda-Jonas, S. Parapapillary Atrophy: Histological Gamma Zone and Delta Zone. PLoS ONE 2012, 7, e47237. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Ohno-Matsui, K.; Spaide, R.F.; Holbach, L.; Panda-Jonas, S. Macular Bruch’s Membrane Defects and Axial Length: Association with Gamma Zone and Delta Zone in Peripapillary Region. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1295–1302. [Google Scholar] [CrossRef] [Green Version]
- Jonas, J.B.; Weber, P.; Nagaoka, N.; Ohno-Matsui, K. Glaucoma in High Myopia and Parapapillary Delta Zone. PLoS ONE 2017, 12, e0175120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, J.B.; Wang, Y.X.; Dong, L.; Guo, Y.; Panda-Jonas, S. Advances in Myopia Research Anatomical Findings in Highly Myopic Eyes. Eye Vis. 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Papastathopoulos, K.I. Optic Disc Shape in Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234 (Suppl. S1), S167–S173. [Google Scholar] [CrossRef]
- Doshi, A.; Kreidl, K.O.; Lombardi, L.; Sakamoto, D.K.; Singh, K. Nonprogressive Glaucomatous Cupping and Visual Field Abnormalities in Young Chinese Males. Ophthalmology 2007, 114, 472–479. [Google Scholar] [CrossRef]
- Ding, X.; Chang, R.T.; Guo, X.; Liu, X.; Johnson, C.A.; Holden, B.A.; He, M. Visual Field Defect Classification in the Zhongshan Ophthalmic Center-Brien Holden Vision Institute High Myopia Registry Study. Br. J. Ophthalmol. 2016, 100, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Chen, S.; Song, Y.; Li, F.; Wang, W.; Zhao, Z.; Gao, X.; Wang, P.; Jin, L.; Liu, Y.; et al. Classification of Visual Field Abnormalities in Highly Myopic Eyes without Pathologic Change. Ophthalmology 2022, 129, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Baskaran, M.; Singh, K.; Aung, T. Clinical Characterization of Young Chinese Myopes with Optic Nerve and Visual Field Changes Resembling Glaucoma. J. Glaucoma 2012, 21, 281–286. [Google Scholar] [CrossRef]
- Park, H.-Y.L.; Hong, K.E.; Park, C.K. Impact of Age and Myopia on the Rate of Visual Field Progression in Glaucoma Patients. Medicine 2016, 95, e3500. [Google Scholar] [CrossRef] [PubMed]
- Karmel, M. Myopia and Glaucoma: Sorting Out the Diagnosis. Available online: https://www.aao.org/eyenet/article/myopia-glaucoma-sorting-out-diagnosis (accessed on 24 October 2022).
- Shen, S.Y.; Wong, T.Y.; Foster, P.J.; Loo, J.-L.; Rosman, M.; Loon, S.-C.; Wong, W.L.; Saw, S.-M.; Aung, T. The Prevalence and Types of Glaucoma in Malay People: The Singapore Malay Eye Study. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3846–3851. [Google Scholar] [CrossRef] [Green Version]
- Dandona, L.; Dandona, R.; Srinivas, M.; Mandal, P.; John, R.K.; McCarty, C.A.; Rao, G.N. Open-Angle Glaucoma in an Urban Population in Southern India: The Andhra Pradesh Eye Disease Study. Ophthalmology 2000, 107, 1702–1709. [Google Scholar] [CrossRef]
- He, M.; Foster, P.J.; Ge, J.; Huang, W.; Zheng, Y.; Friedman, D.S.; Lee, P.S.; Khaw, P.T. Prevalence and Clinical Characteristics of Glaucoma in Adult Chinese: A Population-Based Study in Liwan District, Guangzhou. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2782–2788. [Google Scholar] [CrossRef] [Green Version]
- Iwase, A.; Suzuki, Y.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. The Prevalence of Primary Open-Angle Glaucoma in Japanese: The Tajimi Study. Ophthalmology 2004, 111, 1641–1648. [Google Scholar] [CrossRef]
- Kim, C.; Seong, G.J.; Lee, N.; Song, K.; Namil Study Group. Korean Glaucoma Society Prevalence of Primary Open-Angle Glaucoma in Central South Korea the Namil Study. Ophthalmology 2011, 118, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.B.; Friedman, D.S.; Zhou, Q.; Yang, X.; Sun, L.P.; Guo, L.X.; Tao, Q.S.; Chang, D.S.; Wang, N.L.; Handan Eye Study Group. Prevalence of Primary Open Angle Glaucoma in a Rural Adult Chinese Population: The Handan Eye Study. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8250–8257. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Shan, L.; Cheng, F.; Fan, P.; Zhang, L.; Qu, W.; Zhang, Q.; Yuan, H. Prevalence of Glaucoma in a Rural Northern China Adult Population: A Population-Based Survey in Kailu County, Inner Mongolia. Ophthalmology 2011, 118, 1982–1988. [Google Scholar] [CrossRef]
- Jonas, J.B.; Nagaoka, N.; Fang, Y.X.; Weber, P.; Ohno-Matsui, K. Intraocular Pressure and Glaucomatous Optic Neuropathy in High Myopia. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5897–5906. [Google Scholar] [CrossRef]
- Chong, R.S.; Li, H.; Cheong, A.J.Y.; Fan, Q.; Koh, V.; Raghavan, L.; Nongpiur, M.E.; Cheng, C.-Y. Mendelian Randomization Implicates Bidirectional Association between Myopia and Primary Open-Angle Glaucoma or Intraocular Pressure. Ophthalmology 2022. [Google Scholar] [CrossRef] [PubMed]
- Choquet, H.; Khawaja, A.P.; Jiang, C.; Yin, J.; Melles, R.B.; Glymour, M.M.; Hysi, P.G.; Jorgenson, E. Association Between Myopic Refractive Error and Primary Open-Angle Glaucoma: A 2-Sample Mendelian Randomization Study. JAMA Ophthalmol. 2022, 140, 864–871. [Google Scholar] [CrossRef]
- Abdalla, M.I.; Hamdi, M. Applanation Ocular Tension in Myopia and Emmetropia. Br. J. Ophthalmol. 1970, 54, 122–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, A.; Phillips, C.I. Applanation Tension and Axial Length of the Eyeball. Br. J. Ophthalmol. 1970, 54, 548–553. [Google Scholar] [CrossRef] [Green Version]
- David, R.; Zangwill, L.M.; Tessler, Z.; Yassur, Y. The Correlation between Intraocular Pressure and Refractive Status. Arch. Ophthalmol. 1985, 103, 1812–1815. [Google Scholar] [CrossRef]
- Liu, J.H.K.; Kripke, D.F.; Twa, M.D.; Gokhale, P.A.; Jones, E.I.; Park, E.-H.; Meehan, J.E.; Weinreb, R.N. Twenty-Four-Hour Pattern of Intraocular Pressure in Young Adults with Moderate to Severe Myopia. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2351–2355. [Google Scholar]
- Yang, Y.; Li, Z.; Wang, N.; Wu, L.; Zhen, Y.; Wang, T.; Ren, C.; Peng, X.; Hao, J.; Xia, Y. Intraocular Pressure Fluctuation in Patients with Primary Open-Angle Glaucoma Combined with High Myopia. J. Glaucoma 2014, 23, 19–22. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kook, M.S.; Lee, K.S.; Lee, J.R.; Han, S. Circadian Pattern of Intraocular Pressure Fluctuations in Young Myopic Eyes with Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2148–2156. [Google Scholar] [CrossRef] [Green Version]
- Vincent, S.J.; Collins, M.J.; Read, S.A.; Carney, L.G. Retinal and Choroidal Thickness in Myopic Anisometropia. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2445–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBrien, N.A.; Cornell, L.M.; Gentle, A. Structural and Ultrastructural Changes to the Sclera in a Mammalian Model of High Myopia. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2179–2187. [Google Scholar]
- Costa, V.P.; Arcieri, E.S. Hypotony Maculopathy. Acta Ophthalmol. Scand. 2007, 85, 586–597. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.W.; Im, S.K.; Lee, S.H.; Ahn, M.D. Effect of Myopia on the Thickness of the Retinal Nerve Fiber Layer Measured by Cirrus HD Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4075–4083. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.K.-S.; Mohamed, S.; Leung, K.S.; Cheung, C.Y.-L.; Chan, S.L.; Cheng, D.K.; Lee, A.K.; Leung, G.Y.; Rao, S.K.; Lam, D.S.C. Retinal Nerve Fiber Layer Measurements in Myopia: An Optical Coherence Tomography Study. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5171–5176. [Google Scholar] [CrossRef] [Green Version]
- Mwanza, J.-C.; Sayyad, F.E.; Aref, A.A.; Budenz, D.L. Rates of Abnormal Retinal Nerve Fiber Layer and Ganglion Cell Layer OCT Scans in Healthy Myopic Eyes: Cirrus versus RTVue. Ophthalmic Surg. Lasers Imaging Off. J. Int. Soc. Imaging Eye 2012, 43, S67–S74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuman, J.S. Optical Coherence Tomography in High Myopia. JAMA Ophthalmol. 2016, 134, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, D.C.; De Moraes, C.G. Challenges to the Common Clinical Paradigm for Diagnosis of Glaucomatous Damage with OCT and Visual Fields. Investig. Ophthalmol. Vis. Sci. 2018, 59, 788–791. [Google Scholar] [CrossRef]
- Zemborain, Z.Z.; Jarukasetphon, R.; Tsamis, E.; De Moraes, C.G.; Ritch, R.; Hood, D.C. Optical Coherence Tomography Can Be Used to Assess Glaucomatous Optic Nerve Damage in Most Eyes with High Myopia. J. Glaucoma 2020, 29, 833–845. [Google Scholar] [CrossRef]
- Akagi, T.; Hangai, M.; Kimura, Y.; Ikeda, H.O.; Nonaka, A.; Matsumoto, A.; Akiba, M.; Yoshimura, N. Peripapillary Scleral Deformation and Retinal Nerve Fiber Damage in High Myopia Assessed with Swept-Source Optical Coherence Tomography. Am. J. Ophthalmol. 2013, 155, 927–936.e1. [Google Scholar] [CrossRef]
- Ang, M.; Sng, C.; Milea, D. Optical Coherence Tomography Angiography in Dural Carotid-Cavernous Sinus Fistula. BMC Ophthalmol. 2016, 16, 93. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Foo, L.-L.; Wong, C.W.; Li, J.; Hoang, Q.V.; Schmetterer, L.; Ting, D.S.W.; Ang, M. Pathologic Myopia: Advances in Imaging and the Potential Role of Artificial Intelligence. Br. J. Ophthalmol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Yuan, M.-Z.; Zhao, X.-Y.; Yu, W.-H.; Chen, Y.-X. Wide-Field Swept Source Optical Coherence Tomography Evaluation of Posterior Segment Changes in Highly Myopic Eyes. Eur. J. Ophthalmol. 2022, 32, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jia, Y.; Takusagawa, H.L.; Pechauer, A.D.; Edmunds, B.; Lombardi, L.; Davis, E.; Morrison, J.C.; Huang, D. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol. 2015, 133, 1045–1052. [Google Scholar] [CrossRef]
- Lim, W.S.; Ho, H.-Y.; Ho, H.-C.; Chen, Y.-W.; Lee, C.-K.; Chen, P.-J.; Lai, F.; Jang, J.-S.R.; Ko, M.-L. Use of Multimodal Dataset in AI for Detecting Glaucoma Based on Fundus Photographs Assessed with OCT: Focus Group Study on High Prevalence of Myopia. BMC Med. Imaging 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, W.; Sun, X. Comparison of Several Parameters in Two Optical Coherence Tomography Systems for Detecting Glaucomatous Defects in High Myopia. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4910–4915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.-W.; Wang, H.-Z.; Liu, J.-R.; Zhang, X.-F.; Li, M.; Huo, Y.-J.; Yang, X.-G. Diagnostic Ability of Ganglion Cell Complex Thickness to Detect Glaucoma in High Myopia Eyes by Fourier Domain Optical Coherence Tomography. Int. J. Ophthalmol. 2018, 11, 791–796. [Google Scholar] [CrossRef]
- Shoji, T.; Sato, H.; Ishida, M.; Takeuchi, M.; Chihara, E. Assessment of Glaucomatous Changes in Subjects with High Myopia Using Spectral Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1098–1102. [Google Scholar] [CrossRef] [Green Version]
- Shoji, T.; Nagaoka, Y.; Sato, H.; Chihara, E. Impact of High Myopia on the Performance of SD-OCT Parameters to Detect Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Zhang, Y.; Zhang, T.; Sun, X. Consistency between Optical Coherence Tomography and Humphrey Visual Field for Evaluating Glaucomatous Defects in High Myopic Eyes. BMC Ophthalmol. 2020, 20, 460. [Google Scholar] [CrossRef]
- Nakano, N.; Hangai, M.; Noma, H.; Nukada, M.; Mori, S.; Morooka, S.; Takayama, K.; Kimura, Y.; Ikeda, H.O.; Akagi, T.; et al. Macular Imaging in Highly Myopic Eyes with and without Glaucoma. Am. J. Ophthalmol. 2013, 156, 511–523.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansal, V.; Armstrong, J.J.; Pintwala, R.; Hutnik, C. Optical Coherence Tomography for Glaucoma Diagnosis: An Evidence Based Meta-Analysis. PLoS ONE 2018, 13, e0190621. [Google Scholar] [CrossRef]
- Shin, J.W.; Song, M.K.; Sung, K.R. Longitudinal Macular Ganglion Cell-Inner Plexiform Layer Measurements to Detect Glaucoma Progression in High Myopia. Am. J. Ophthalmol. 2021, 223, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Hopf, S.; Korb, C.; Nickels, S.; Schulz, A.; Münzel, T.; Wild, P.S.; Michal, M.; Schmidtmann, I.; Lackner, K.J.; Pfeiffer, N.; et al. Prevalence of Myopic Maculopathy in the German Population: Results from the Gutenberg Health Study. Br. J. Ophthalmol. 2020, 104, 1254–1259. [Google Scholar] [CrossRef]
- Wong, Y.-L.; Sabanayagam, C.; Ding, Y.; Wong, C.-W.; Yeo, A.C.-H.; Cheung, Y.-B.; Cheung, G.; Chia, A.; Ohno-Matsui, K.; Wong, T.-Y.; et al. Prevalence, Risk Factors, and Impact of Myopic Macular Degeneration on Visual Impairment and Functioning Among Adults in Singapore. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4603–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.T.; Tran, M.; Singh, K.; Chang, R.; Wang, H.; Sun, Y. Glaucoma and Myopia: Diagnostic Challenges. Biomolecules 2023, 13, 562. https://doi.org/10.3390/biom13030562
Sun MT, Tran M, Singh K, Chang R, Wang H, Sun Y. Glaucoma and Myopia: Diagnostic Challenges. Biomolecules. 2023; 13(3):562. https://doi.org/10.3390/biom13030562
Chicago/Turabian StyleSun, Michelle T., Matthew Tran, Kuldev Singh, Robert Chang, Huaizhou Wang, and Yang Sun. 2023. "Glaucoma and Myopia: Diagnostic Challenges" Biomolecules 13, no. 3: 562. https://doi.org/10.3390/biom13030562




