Modulation of Allosteric Control and Evolution of Hemoglobin
Abstract
:1. Preamble
2. The Swim Bladder
3. Trout HbI, a Bare Cooperative Hemoglobin
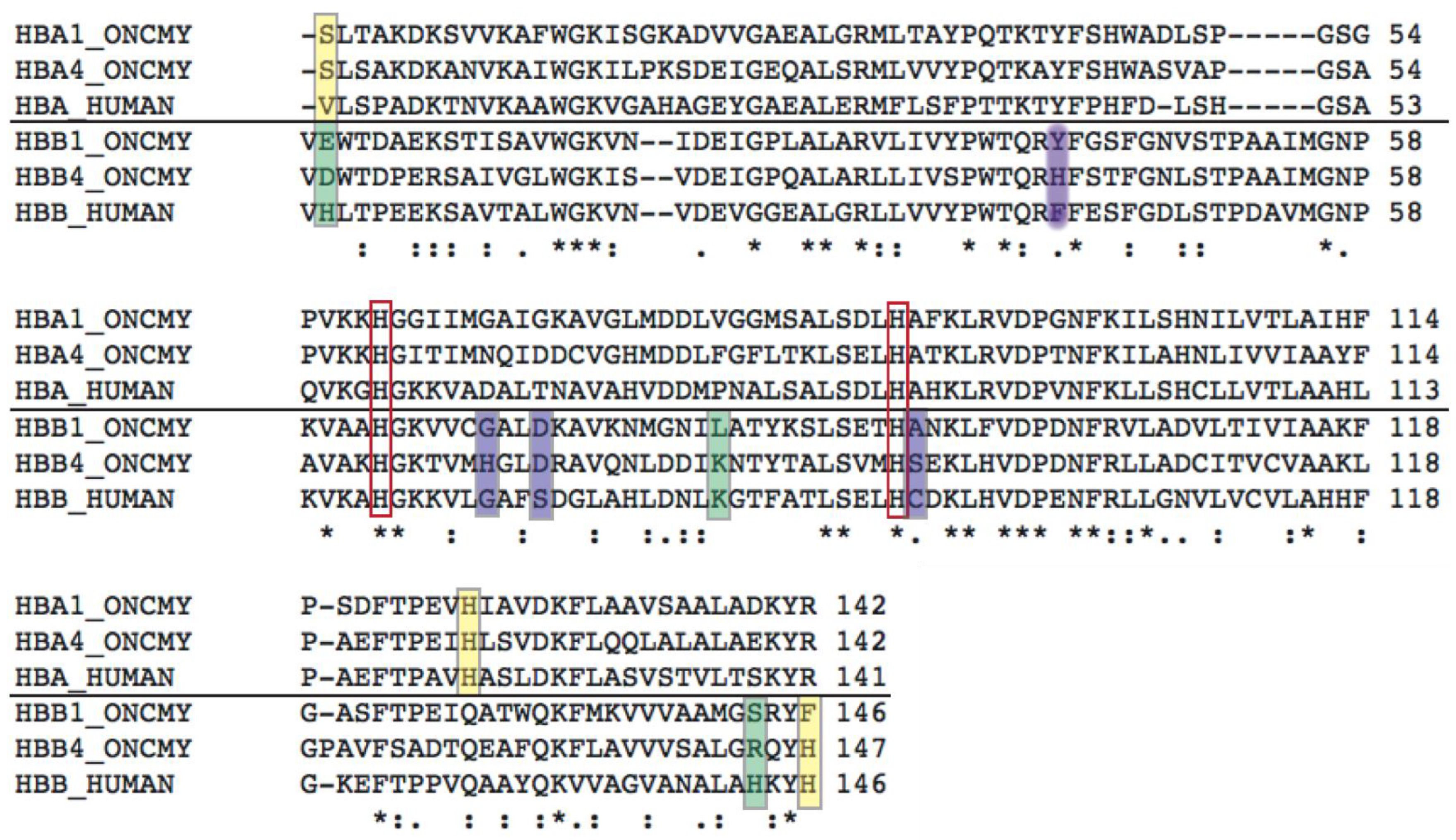
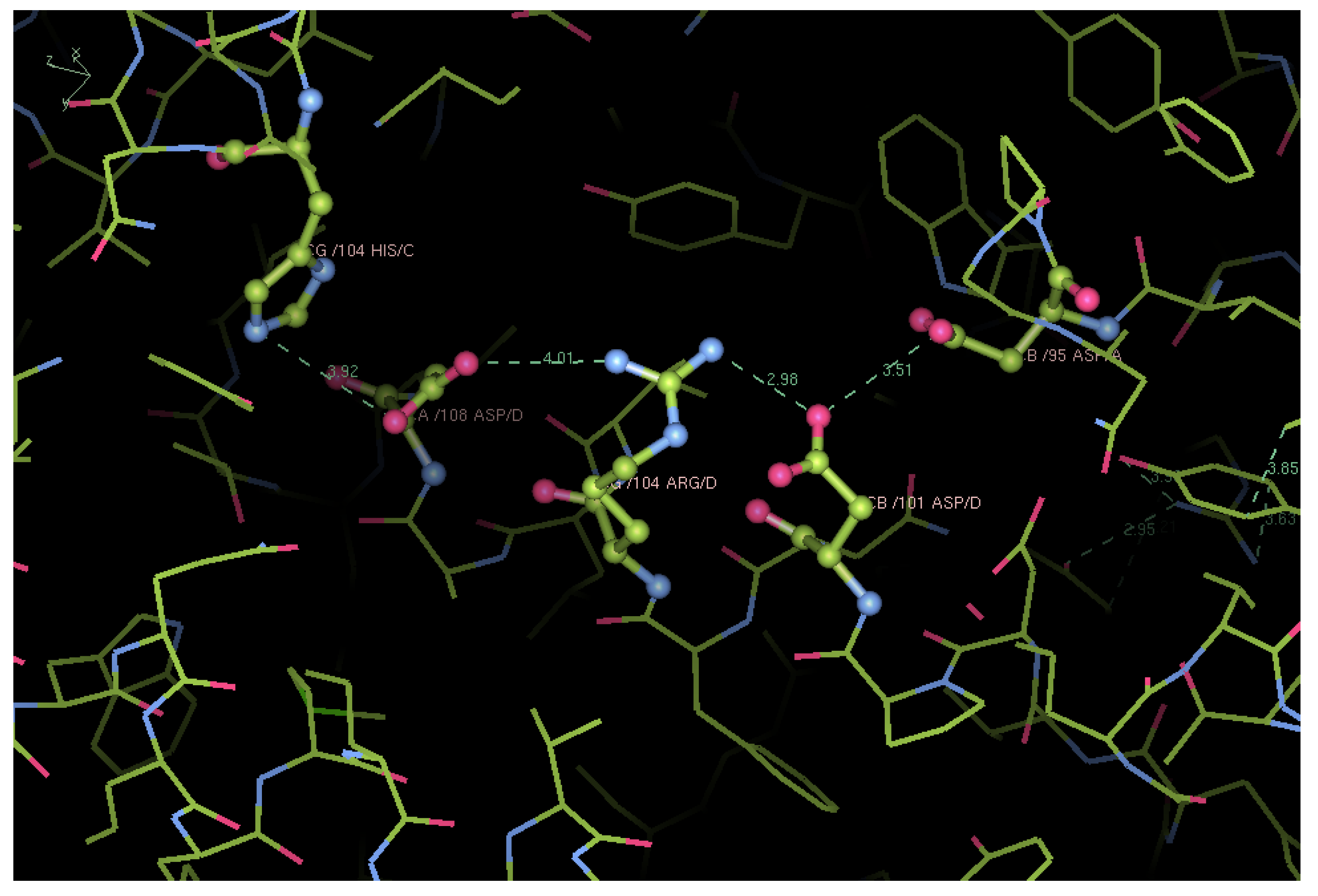
Structural Analysis of Trout HbI
4. Trout HbIV, a Molecular Transducer
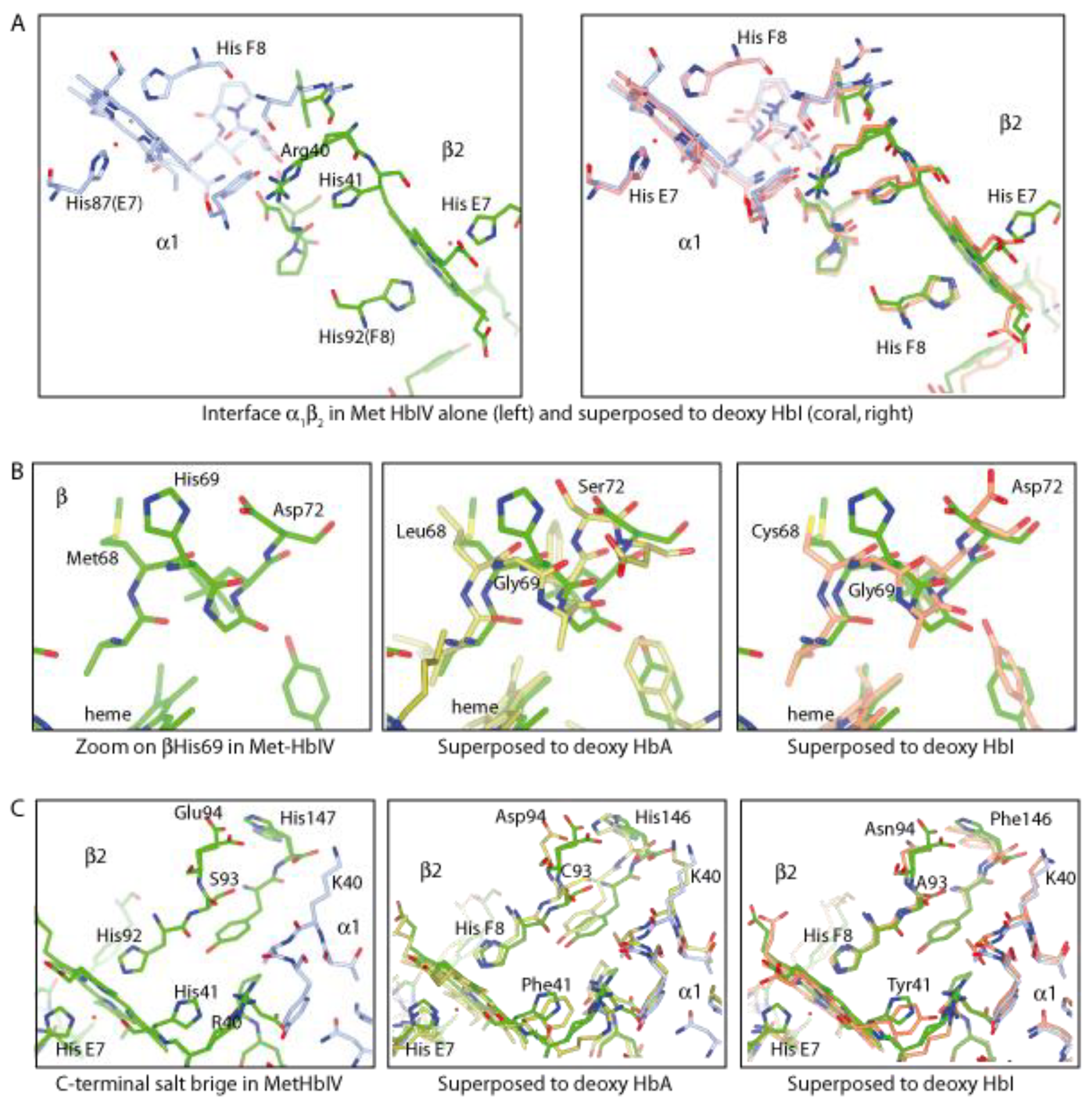
Structural Analysis of Trout HbIV
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Riggs, A. The Alpha Helix Expedition to the Amazon for the study of fish bloods and hemoglobins. Comp. Biochem. Physiol. 1979, 62A, 1–272. [Google Scholar]
- Wyman, J. Variations on the theme: A comparative study of fish hemoglobins. Comp. Biochem. Physiol. 1979, 62A, 9–12. [Google Scholar] [CrossRef]
- Monod, J.; Wyman, J.; Changeux, J.-P. On the Nature of Allosteric Transitions: A Plausible Model. J. Mol. Biol. 1965, 12, 88–118. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Karplus, M. Allostery and cooperativity revisited. Protein Sci. 2008, 17, 1295–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buc, H. Interactions between Jacques Monod and Jeffries Wyman (or the burdens of co-authorship). Rend. Lincei 2006, 17, 31–49. [Google Scholar] [CrossRef]
- Denton, E.J. The buoyancy of fish and cephalophods. Prog. Biophys. Biophys. Chem. 1961, 11, 177–234. [Google Scholar] [CrossRef]
- Root, R.W. The respiratory function of the blood of marine fishes. Biol. Bull. Mar. Biol. Lab. Woods Hole 1931, 61, 427–456. [Google Scholar] [CrossRef]
- Riggs, A. Properties of fish hemoglobins. In Fish Physiology; Academic Press: Cambridge, MA, USA, 1970; Volume 4, pp. 209–252. [Google Scholar]
- Noble, R.W.; Russel, R.; Pennelly, R.; Riggs, A. Studies of the functional properties of the hemoglobin from the benthic fish, Antimora Rostrata. Comp. Biochem. Physiol. 1975, 52B, 75–81. [Google Scholar] [CrossRef]
- Rossi Fanelli, A.; Antonini, E. Oxygen equilibrium of Haemoglobin from Thunnus thynnus. Nature 1960, 186, 895–898. [Google Scholar] [CrossRef]
- Wyman, J. Linked functions and reciprocal effects in hemoglobin: A second look. Adv. Prot. Chem. 1964, 19, 223–228. [Google Scholar]
- Binotti, I.; Giovenco, S.; Giardina, B.; Antonini, E.; Brunori, M.; Wyman, J. Studies on the functional properties of fish hemoglobins. II. The oxygen equilibrium of the isolated hemoglobin components from trout blood. Arch. Biochem. Biophys. 1971, 142, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Brunori, M. Molecular adaptation to physiological requirements: The hemoglobin system of trout. Curr. Top. Cell. Regul. 1975, 9, 1–39. [Google Scholar] [PubMed]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reactions with Ligands; North Holland Pub. Co.: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Imai, K. Allosteric Effects in Haemoglobin; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Brunori, M.; Giardina, B.; Colosimo, A.; Coletta, M.; Falcioni, G.; Gill, S. Thermodynamics and kinetics of the reactions of trout HbI with O2 and CO. In Hemoglobin and Oxygen Binding; Ho, C., Ed.; Elsevier: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Barisas, B.G.; Gill, S.J. Thermodynamic analysis of carbon monoxide binding by hemoglobin trout I. Biophys. Chem. 1979, 9, 235–244. [Google Scholar] [CrossRef]
- Brunori, M.; Coletta, M.; Giardina, B.; Wyman, J. A macromolecular transducer as illustrated by trout hemoglobin IV. Proc. Natl. Acad. Sci. USA 1978, 75, 4310–4312. [Google Scholar] [CrossRef] [Green Version]
- Miele, A.E.; Bellelli, A.; Brunori, M. Hemoglobin allostery: New views on old players. J. Mol. Biol. 2013, 425, 1515–1526. [Google Scholar] [CrossRef]
- Tame, J.R.H.; Wilson, J.C.; Weber, R. The crystal structures of Trout Hb I in the deoxy and carbonmonoxy forms. J. Mol. Biol. 1996, 259, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Perutz, M.F. Stereochemistry of cooperative effects in haemoglobin. Nature 1970, 228, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Karplus, M. A mathematical model for structure-function relations in hemoglobin. J. Mol. Biol. 1972, 72, 163–197. [Google Scholar] [CrossRef]
- Brunori, M.; Giardina, B.; Colosimo, A.; Falcioni, G.; Gill, S.J. Temperature perturbation of the allosteric equilibrium in trout hemoglobin. J. Biol. Chem. 1980, 255, 3841–3843. [Google Scholar] [CrossRef]
- Barra, D.; Bossa, F.; Brunori, M. Structure of binding sites for heterotropic effectors in fish haemoglobins. Nature 1981, 293, 587–588. [Google Scholar] [CrossRef]
- Powers, D.A. Hemoglobin adaptation for fast and slow water habitats in sympatric catostomid fishes. Science 1972, 177, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
- Casañal, A.; Lohkamp, B.; Emsley, P. Current developments in Coot for macromolecular model building of Electron Cryo-microscopy and Crystallographic Data. Protein Sci. 2020, 29, 1069–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Yokoyama, T.; Shibayama, N.; Shiro, Y.; Tame, J.R. 1.25 Å resolution crystal structures of human haemoglobin in the oxy, deoxy and carbonmonoxy forms. J. Mol. Biol. 2006, 360, 690–701. [Google Scholar] [CrossRef]
- Giardina, B.; Ascoli, F.; Brunori, M. Spectral changes and allosteric transition in trout haemoglobin. Nature 1975, 256, 761–762. [Google Scholar] [CrossRef]
- Bellelli, A.; Brunori, M. Optical measurements of quaternary structural changes in hemoglobin. Methods Enzymol. 1994, 232, 56–71. [Google Scholar]
- Martino, A.J.; Ferrone, F.A. Rate of allosteric change in hemoglobin measured by modulated excitation using fluorescence detection. Biophys. J. 1989, 56, 781–794. [Google Scholar] [CrossRef] [Green Version]
- Perutz, M.F.; Brunori, M. Stereochemistry of cooperative effects in fish and amphibian haemoglobins. Nature 1982, 299, 421–426. [Google Scholar] [CrossRef]
- Yokoyama, T.; Chong, K.T.; Miyazaki, G.; Morimoto, H.; Shih, D.T.; Unzai, S.; Tame, J.R.; Park, S.Y. Novel mechanisms of pH sensitivity in tuna hemoglobin: A structural explanation of the Root effect. J. Biol. Chem. 2004, 279, 28632–28640. [Google Scholar] [CrossRef] [Green Version]
- Coletta, M.; Santucci, R.; Focesi, A., Jr.; Ascoli, F.; Brunori, M. Redox properties of components I and IV of trout hemoglobins: Kinetic and potentiometric studies. Biochim. Biophys. Acta 1987, 915, 415–419. [Google Scholar] [CrossRef]
- Giacometti, G.M.; Giardina, B.; Brunori, M.; Giacometti, G.; Rigatti, G. Observations on CO trout hemoglobins by 13C NMR. FEBS Lett. 1976, 62, 157–160. [Google Scholar] [CrossRef]
- Aranda, R., 4th; Cai, H.; Worley, C.E.; Levin, E.J.; Li, R.; Olson, J.S.; Phillips, G.N., Jr.; Richards, M.P. Structural analysis of fish versus mammalian hemoglobins: Effect of the heme pocket environment on autooxidation and hemin loss. Proteins 2009, 75, 217–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicholas, S.; Potterton, E.; Wilson, K.S.; Noble, M.E. Presenting your structures: The CCP4mg molecular-graphics software. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Bellelli, A.; Brunori, M.; Miele, A.E.; Panetta, G.; Vallone, B. The allosteric properties of hemoglobin: Insights from natural and site directed mutants. Curr. Protein Pept. Sci. 2006, 7, 17–45. [Google Scholar] [CrossRef]
- Miele, A.E.; Draghi, F.; Vallone, B.; Boffi, A. The carbon monoxide derivative of human hemoglobin carrying the double mutation LeuB10-->Tyr and HisE7-->Gln on alpha and beta chains probed by infrared spectroscopy. Arch. Biochem. Biophys. 2002, 402, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Miele, A.E.; Santanché, S.; Travaglini-Allocatelli, C.; Vallone, B.; Brunori, M.; Bellelli, A. Modulation of ligand binding in engineered human hemoglobin distal pocket. J. Mol. Biol. 1999, 290, 515–524. [Google Scholar] [CrossRef]
- Milvaganam, S.E.; Bonaventura, C.; Bonaventura, J.; Getzoff, E.D. Structural basis for the Root effect in haemoglobin. Nat. Struct. Biol. 1996, 3, 275–283. [Google Scholar] [CrossRef]
- Nagai, K.; Luisi, B.; Shih, D.; Miyazaki, G.; Imai, K.; Poyart, C.; De Young, A.; Kwiatkowsky, L.; Noble, R.W.; Lin, S.H.; et al. Distal residues in the oxygen binding site of haemoglobin studied by protein engineering. Nature 1987, 329, 858–860. [Google Scholar] [CrossRef]
- Olson, J.S.; Mathews, A.J.; Rohlfs, R.J.; Springer, B.A.; Egeberg, K.D.; Sligar, S.G.; Tame, J.; Renaud, J.P.; Nagai, K. The role of the distal histidine in myoglobin and haemoglobin. Nature 1988, 336, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Springer, B.A.; Sligar, S.G.; Olson, J.S.; Phillips, G.N., Jr. Mechanisms of ligand recognition in Myoglobin. Chem. Rev. 1994, 94, 699–714. [Google Scholar] [CrossRef]
- Eaton, W.A. A retrospective on statistical mechanical models for hemoglobin allostery. J. Chem. Phys. 2022, 157, 184104. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.R.; Mozzarelli, A.; Viappiani, C.; Abbruzzetti, S.; Bettati, S.; Ronda, L.; Bruno, S.; Eaton, W.A. Experiments on Hemoglobin in Single Crystals and Silica Gels Distinguish among Allosteric Models. Biophys. J. 2015, 109, 1264–1272. [Google Scholar] [CrossRef] [Green Version]
- Airoldi, L.; Brunori, M.; Giardina, B. Properties of trout HbI in water and ligand linked binding of Na. FEBS Lett. 1981, 129, 273–276. [Google Scholar] [CrossRef]
- Lee, A.W.; Karplus, M. Structure-specific model of hemoglobin cooperativity. Proc. Natl. Acad. Sci. USA 1983, 80, 7055–7059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Schaeffer, R.D.; Liao, Y.; Kinch, L.N.; Pei, J.; Shi, S.; Kim, B.H.; Grishin, N.V. ECOD: An evolutionary classification of protein domains. PLoS Comput. Biol. 2014, 10, e1003926. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Norn, C.; Wicky, B.I.M.; Juergens, D.; Liu, S.; Kim, D.; Tischer, D.; Koepnick, B.; Anishchenko, I.; Players, F.; Baker, D.; et al. Protein sequence design by conformational landscape optimization. Proc. Natl. Acad. Sci. USA 2021, 118, e2017228118. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunori, M.; Miele, A.E. Modulation of Allosteric Control and Evolution of Hemoglobin. Biomolecules 2023, 13, 572. https://doi.org/10.3390/biom13030572
Brunori M, Miele AE. Modulation of Allosteric Control and Evolution of Hemoglobin. Biomolecules. 2023; 13(3):572. https://doi.org/10.3390/biom13030572
Chicago/Turabian StyleBrunori, Maurizio, and Adriana Erica Miele. 2023. "Modulation of Allosteric Control and Evolution of Hemoglobin" Biomolecules 13, no. 3: 572. https://doi.org/10.3390/biom13030572
APA StyleBrunori, M., & Miele, A. E. (2023). Modulation of Allosteric Control and Evolution of Hemoglobin. Biomolecules, 13(3), 572. https://doi.org/10.3390/biom13030572








