Cellular Localization of Orexin 1 Receptor in Human Hypothalamus and Morphological Analysis of Neurons Expressing the Receptor
Abstract
:1. Introduction
2. Materials and Methods
3. Results
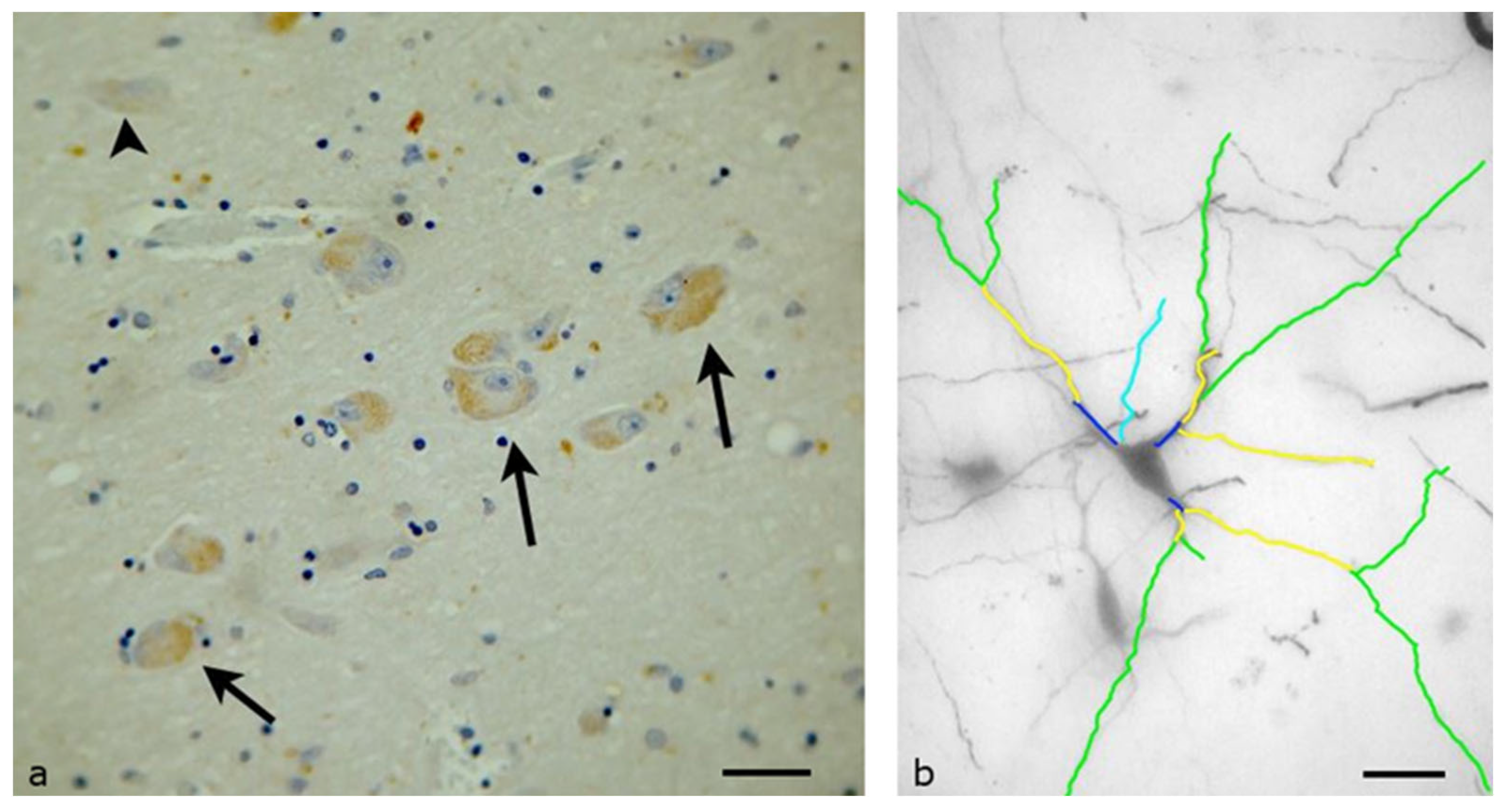
3.1. Lateral Hypothalamic Area
3.2. Lateral Preoptic Nucleus
3.3. Dorsomedial Nucleus
3.4. Ventromedial Nucleus
3.5. Supraoptic Nucleus
3.6. Paraventricular Nucleus
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors That Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef] [Green Version]
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.-B.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.F.; Gautvik, V.T.; Bartlett, F.S.; et al. The Hypocretins: Hypothalamus-Specific Peptides with Neuroexcitatory Activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyron, C.; Tighe, D.K.; van den Pol, A.N.; de Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, T.; Mieda, M.; Tsujino, N. The Orexin System: Roles in Sleep/Wake Regulation. Ann. N. Y. Acad. Sci. 2010, 1200, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T. The Neural Circuit of Orexin (Hypocretin): Maintaining Sleep and Wakefulness. Nat. Rev. Neurosci. 2007, 8, 171–181. [Google Scholar] [CrossRef]
- Putula, J.; Kukkonen, J.P. Mapping of the Binding Sites for the OX1 Orexin Receptor Antagonist, SB-334867, Using Orexin/Hypocretin Receptor Chimaeras. Neurosci. Lett. 2012, 506, 111–115. [Google Scholar] [CrossRef]
- Chieffi, S.; Carotenuto, M.; Monda, V.; Valenzano, A.; Villano, I.; Precenzano, F.; Tafuri, D.; Salerno, M.; Filippi, N.; Nuccio, F.; et al. Orexin System: The Key for a Healthy Life. Front. Physiol. 2017, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T. The Role of Orexin in Motivated Behaviours. Nat. Rev. Neurosci. 2014, 15, 719–731. [Google Scholar] [CrossRef]
- Sun, Y.; Tisdale, R.K.; Kilduff, T.S. Hypocretin/Orexin Receptor Pharmacology and Sleep Phases. Orexin System. Basic Sci. Role Sleep Pathol. 2021, 45, 22–37. [Google Scholar]
- Saper, C.B.; Lowell, B.B. The Hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, J.D.; Sporns, O.; Watts, A.G.; Swanson, L.W. Macroscale Intrinsic Network Architecture of the Hypothalamus. Proc. Natl. Acad. Sci. USA 2019, 116, 8018–8027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, P.; Yu, H.; MacNeil, D.J.; van der Ploeg, L.H.T.; Guan, X.-M. Distribution of Orexin Receptor MRNA in the Rat Brain. FEBS Lett. 1998, 438, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.-Y.; Bagnol, D.; Burke, S.; Akil, H.; Watson, S.J. Differential Distribution and Regulation of OX1 and OX2 Orexin/Hypocretin Receptor Messenger RNA in the Brain upon Fasting. Horm. Behav. 2000, 37, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Blache, D.; Vercoe, P.E.; Adam, C.L.; Blackberry, M.A.; Findlay, P.A.; Eidne, K.A.; Martin, G.B. Expression of Orexin Receptors in the Brain and Peripheral Tissues of the Male Sheep. Regul. Pept. 2005, 124, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, T.; Yan, L. A Comparison of the Orexin Receptor Distribution in the Brain between Diurnal Nile Grass Rats (Arvicanthis niloticus) and Nocturnal Mice (Mus musculus). Brain Res. 2018, 1690, 89–95. [Google Scholar] [CrossRef]
- Czerwinska, J.; Chojnowska, K.; Kaminski, T.; Bogacka, I.; Smolinska, N.; Kaminska, B. Orexin Receptor Expression in the Hypothalamic–Pituitary–Adrenal and Hypothalamic–Pituitary–Gonadal Axes of Free-Living European Beavers (Castor fiber L.) in Different Periods of the Reproductive Cycle. Gen. Comp. Endocrinol. 2017, 240, 103–113. [Google Scholar] [CrossRef]
- Blanco, M.; López, M.; GarcÍa-Caballero, T.; Gallego, R.; Vázquez-Boquete, Á.; Morel, G.; SeñarÍs, R.; Casanueva, F.; Diéguez, C.; Beiras, A. Cellular Localization of Orexin Receptors in Human Pituitary. J. Clin. Endocrinol. Metab. 2001, 86, 3444–3447. [Google Scholar] [CrossRef]
- Elahdadi Salmani, M.; Sarfi, M.; Goudarzi, I. Hippocampal Orexin Receptors: Localization and Function. Vitam. Horm. 2022, 118, 393–421. [Google Scholar]
- Marcus, J.N.; Aschkenasi, C.J.; Lee, C.E.; Chemelli, R.M.; Saper, C.B.; Yanagisawa, M.; Elmquist, J.K. Differential Expression of Orexin Receptors 1 and 2 in the Rat Brain. J. Comp. Neurol. 2001, 435, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Jöhren, O.; Neidert, S.J.; Kummer, M.; Dendorfer, A.; Dominiak, P. Prepro-Orexin and Orexin Receptor MRNAs Are Differentially Expressed in Peripheral Tissues of Male and Female Rats. Endocrinology 2001, 142, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Arihara, Z.; Suzuki, T.; Sone, M.; Kikuchi, K.; Sasano, H.; Murakami, O.; Totsune, K. Expression of Orexin-A and Orexin Receptors in the Kidney and the Presence of Orexin-A-like Immunoreactivity in Human Urine. Peptides 2006, 27, 871–877. [Google Scholar] [CrossRef]
- Karteris, E.; Chen, J.; Randeva, H.S. Expression of Human Prepro-Orexin and Signaling Characteristics of Orexin Receptors in the Male Reproductive System. J. Clin. Endocrinol. Metab. 2004, 89, 1957–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randeva, H.S.; Karteris, E.; Grammatopoulos, D.; Hillhouse, E.W. Expression of Orexin-A and Functional Orexin Type 2 Receptors in the Human Adult Adrenals: Implications for Adrenal Function and Energy Homeostasis. J. Clin. Endocrinol. Metab. 2001, 86, 4808–4813. [Google Scholar] [CrossRef] [PubMed]
- Digby, J.E.; Chen, J.; Tang, J.Y.; Lehnert, H.; Matthews, R.N.; Randeva, H.S. Orexin Receptor Expression in Human Adipose Tissue: Effects of Orexin-A and Orexin-B. J. Endocrinol. 2006, 191, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Valiante, S.; Liguori, G.; Tafuri, S.; Campese, R.; Monaco, R.; Paino, S.; Laforgia, V.; Staiano, N.; Vittoria, A. Expression of Orexin A and Its Receptor 1 in the Human Prostate. J. Anat. 2013, 222, 473–480. [Google Scholar] [CrossRef]
- Jöhren, O.; Brüggemann, N.; Dendorfer, A.; Dominiak, P. Gonadal Steroids Differentially Regulate the Messenger Ribonucleic Acid Expression of Pituitary Orexin Type 1 Receptors and Adrenal Orexin Type 2 Receptors. Endocrinology 2003, 144, 1219–1225. [Google Scholar] [CrossRef] [Green Version]
- Voisin, T.; el Firar, A.; Fasseu, M.; Rouyer-Fessard, C.; Descatoire, V.; Walker, F.; Paradis, V.; Bedossa, P.; Henin, D.; Lehy, T.; et al. Aberrant Expression of OX1 Receptors for Orexins in Colon Cancers and Liver Metastases: An Openable Gate to Apoptosis. Cancer Res. 2011, 71, 3341–3351. [Google Scholar] [CrossRef] [Green Version]
- Jöhren, O.; Neidert, S.J.; Kummer, M.; Dominiak, P. Sexually Dimorphic Expression of Prepro-Orexin MRNA in the Rat Hypothalamus. Peptides 2002, 23, 1177–1180. [Google Scholar] [CrossRef]
- Tsamis, K.; Mytilinaios, D.; Psaroulis, D.; Njau, S.N.; Costa, V.; Baloyannis, S.J. Reelin immunoreactivity and Morphological analysis of the human visual cortex. Int. J. Neurosci. 2007, 117, 25–46. [Google Scholar] [CrossRef]
- Garey, L.J.; Ong, W.Y.; Patel, T.S.; Kanani, M.; Davis, A.; Mortimer, A.M.; Barnes, T.R.E.; Hirsch, S.R. Reduced Dendritic Spine Density on Cerebral Cortical Pyramidal Neurons in Schizophrenia. J. Neurol. Neurosurg. Psychiatry 1998, 65, 446–453. [Google Scholar] [CrossRef]
- Papadopulos, F.; Spinelli, M.; Valente, S.; Foroni, L.; Orrico, C.; Alviano, F.; Pasquinelli, G. Common Tasks in Microscopic and Ultrastructural Image Analysis Using ImageJ. Ultrastruct. Pathol. 2007, 31, 401–407. [Google Scholar] [CrossRef]
- Tsamis, K.I.; Mytilinaios, D.G.; Njau, S.N.; Baloyannis, S.J. Glutamate Receptors in Human Caudate Nucleus in Normal Aging and Alzheimer’s Disease. Curr. Alzheimer Res. 2013, 10, 469–475. [Google Scholar] [CrossRef]
- Chen, L.; McKenna, J.T.; Bolortuya, Y.; Winston, S.; Thakkar, M.M.; Basheer, R.; Brown, R.E.; McCarley, R.W. Knockdown of Orexin Type 1 Receptor in Rat Locus Coeruleus Increases REM Sleep during the Dark Period. Eur. J. Neurosci. 2010, 32, 1528–1536. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Wang, S. Changes in the Orexin System in Rats Exhibiting Learned Helplessness Behaviors. Brain Sci. 2021, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, J.; Caverson, M.M. Hypothalamic Orexin-A (Hypocretin-1) Neuronal Projections to the Vestibular Complex and Cerebellum in the Rat. Brain Res. 2014, 1579, 20–34. [Google Scholar] [CrossRef] [PubMed]
- García-Cabezas, M.Á.; John, Y.J.; Barbas, H.; Zikopoulos, B. Distinction of Neurons, Glia and Endothelial Cells in the Cerebral Cortex: An Algorithm Based on Cytological Features. Front. Neuroanat. 2016, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Koutcherov, Y.; Mai, J.K.; Ashwell, K.W.S.; Paxinos, G. Organization of the Human Paraventricular Hypothalamic Nucleus. J. Comp. Neurol. 2000, 423, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yuan, K.; Zheng, Y.; Lu, L. Orexin Receptor Antagonists as Emerging Treatments for Psychiatric Disorders. Neurosci. Bull. 2020, 36, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Soya, S.; Sakurai, T. Evolution of Orexin Neuropeptide System: Structure and Function. Front. Neurosci. 2020, 14, 691. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Ng, S.Y.L.; Lee, L.T.O.; Ng, H.K.H.; Chow, B.K.C. Orexins and Their Receptors from Fish to Mammals: A Comparative Approach. Gen. Comp. Endocrinol. 2011, 171, 124–130. [Google Scholar] [CrossRef]
- Abounoori, M.; Maddah, M.M.; Ardeshiri, M.R. Orexin Neuropeptides Modulate the Hippocampal-Dependent Memory through Basolateral Amygdala Interconnections. Cereb. Circ. Cogn. Behav. 2022, 3, 100035. [Google Scholar] [CrossRef] [PubMed]
- Inutsuka, A.; Yamanaka, A. The Physiological Role of Orexin/Hypocretin Neurons in the Regulation of Sleep/Wakefulness and Neuroendocrine Functions. Front. Endocrinol. 2013, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- James, M.H.; Aston-Jones, G. Orexin Reserve: A Mechanistic Framework for the Role of Orexins (Hypocretins) in Addiction. Biol. Psychiatry 2022, 92, 836–844. [Google Scholar] [CrossRef]
- Toor, B.; Ray, L.B.; Pozzobon, A.; Fogel, S.M. Sleep, Orexin and Cognition. Orexin System. Basic Sci. Role Sleep Pathol. 2021, 45, 38–51. [Google Scholar]
- Gao, W.-R.; Hu, X.-H.; Yu, K.-Y.; Cai, H.-Y.; Wang, Z.-J.; Wang, L.; Wu, M.-N. Selective Orexin 1 Receptor Antagonist SB-334867 Aggravated Cognitive Dysfunction in 3xTg-AD Mice. Behav. Brain Res. 2023, 438, 114171. [Google Scholar] [CrossRef] [PubMed]
- Mahler, S.V.; Moorman, D.E.; Smith, R.J.; James, M.H.; Aston-Jones, G. Motivational Activation: A Unifying Hypothesis of Orexin/Hypocretin Function. Nat. Neurosci. 2014, 17, 1298–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirbolouk, B.; Rohampour, K.; Rostampour, M.; Jafari, A.; Khakpour-Taleghani, B. Chronic Orexin-1 Receptor Blockage Attenuates Depressive Behaviors and Provokes PSD-95 Expression in a Rat Model of Depression. Behav. Brain Res. 2023, 437, 114123. [Google Scholar] [CrossRef]
- Roberts, C.B.; Campbell, R.E.; Herbison, A.E.; Suter, K.J. Dendritic Action Potential Initiation in Hypothalamic Gonadotropin-Releasing Hormone Neurons. Endocrinology 2008, 149, 3355–3360. [Google Scholar] [CrossRef] [Green Version]
- Hodassman, S.; Vardi, R.; Tugendhaft, Y.; Goldental, A.; Kanter, I. Efficient Dendritic Learning as an Alternative to Synaptic Plasticity Hypothesis. Sci. Rep. 2022, 12, 6571. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Smith, R.J.; Sartor, G.C.; Moorman, D.E.; Massi, L.; Tahsili-Fahadan, P.; Richardson, K.A. Lateral Hypothalamic Orexin/Hypocretin Neurons: A Role in Reward-Seeking and Addiction. Brain Res. 2010, 1314, 74–90. [Google Scholar] [CrossRef] [Green Version]
- Hahn, J.D.; Swanson, L.W. Connections of the Lateral Hypothalamic Area Juxtadorsomedial Region in the Male Rat. J. Comp. Neurol. 2012, 520, 1831–1890. [Google Scholar] [CrossRef] [Green Version]
- Yousefvand, S.; Hamidi, F. Role of Lateral Hypothalamus Area in the Central Regulation of Feeding. Int. J. Pept. Res. 2022, 28, 83. [Google Scholar] [CrossRef]
- Carrive, P. Orexin, Orexin Receptor Antagonists and Central Cardiovascular Control. Front. Neurosci. 2013, 7, 257. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, A.; Tabuchi, S.; Tsunematsu, T.; Fukazawa, Y.; Tominaga, M. Orexin Directly Excites Orexin Neurons through Orexin 2 Receptor. J. Neurosci. 2010, 30, 12642–12652. [Google Scholar] [CrossRef] [Green Version]
- Bäckberg, M.; Hervieu, G.; Wilson, S.; Meister, B. Orexin Receptor-1 (OX-R1) Immunoreactivity in Chemically Identified Neurons of the Hypothalamus: Focus on Orexin Targets Involved in Control of Food and Water Intake. Eur. J. Neurosci. 2002, 15, 315–328. [Google Scholar] [CrossRef]
- Pow, D.V.; Morris, J.F. Dendrites of Hypothalamic Magnocellular Neurons Release Neurohypophysial Peptides by Exocytosis. Neuroscience 1989, 32, 435–439. [Google Scholar] [CrossRef]
- Morris, J.F.; Ludwig, M. Magnocellular Dendrites: Prototypic Receiver/Transmitters. J. Neuroendocr. 2004, 16, 403–408. [Google Scholar] [CrossRef]
- Kunii, K.; Yamanaka, A.; Nambu, T.; Matsuzaki, I.; Goto, K.; Sakurai, T. Orexins/Hypocretins Regulate Drinking Behaviour. Brain Res. 1999, 842, 256–261. [Google Scholar] [CrossRef]
- Henderson, L.A.; Macefield, V.G. The Role of the Dorsomedial and Ventromedial Hypothalamus in Regulating Behaviorally Coupled and Resting Autonomic Drive. Handb. Clin. Neurol. 2021, 180, 187–200. [Google Scholar] [PubMed]
- Brasil, T.F.S.; Lopes-Azevedo, S.; Belém-Filho, I.J.A.; Fortaleza, E.A.T.; Antunes-Rodrigues, J.; Corrêa, F.M.A. The Dorsomedial Hypothalamus Is Involved in the Mediation of Autonomic and Neuroendocrine Responses to Restraint Stress. Front Pharm. 2020, 10, 1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, D.; Sweeney, P.; Yang, Y. An Excitatory Ventromedial Hypothalamus to Paraventricular Thalamus Circuit That Suppresses Food Intake. Nat. Commun. 2020, 11, 6326. [Google Scholar] [CrossRef] [PubMed]
- Mieda, M.; Williams, S.C.; Richardson, J.A.; Tanaka, K.; Yanagisawa, M. The Dorsomedial Hypothalamic Nucleus as a Putative Food-Entrainable Circadian Pacemaker. Proc. Natl. Acad. Sci. USA 2006, 103, 12150–12155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavanji, V.; Georgopoulos, A.P.; Kotz, C.M. Orexin Enhances Neuronal Synchronization in Adult Rat Hypothalamic Culture: A Model to Study Hypothalamic Function. J. Neurophysiol. 2022, 127, 1221–1229. [Google Scholar] [CrossRef]
- Lin, C.; Huang, Y.; Quan, T.; Zhang, Y. Modelling Brain-Wide Neuronal Morphology via Rooted Cayley Trees. Sci. Rep. 2018, 8, 15666. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Patient | Gender | Age | Cause of Death |
|---|---|---|---|
| 1 | Male | 66 | Myocardial infarction |
| 2 | female | 29 | Traumatic brain injury—Car accident |
| 3 | Male | 32 | Respiratory infection |
| 4 | Male | 86 | Bolus aspiration |
| 5 | female | 74 | Myocardial infarction |
| 6 | Male | 72 | Pulmonary embolism |
| 7 | Male | 20 | Body injuries—Car accident |
| 8 | Male | 80 | Respiratory infection |
| 9 | Male | 17 | Traumatic brain injury—Car accident |
| 10 | female | 51 | Myocardial infarction |
| 11 | female | 73 | Pulmonary embolism |
| 12 | female | 51 | Respiratory infection |
| 13 | male | 69 | Myocardial infarction |
| 14 | male | 23 | Traumatic brain injury—Car accident |
| 15 | female | 62 | Rupture of ascending aorta |
| Lateral Hypothalamic Area | Lateral Preoptic Nucleus | Dorsomedial Nucleus | Ventromedial Nucleus | Supraoptic Nucleus SI/SII Type | Paraventricular Nucleus | |
|---|---|---|---|---|---|---|
| Number of Primary Dendrites (Average) | 2–3 (2.5) | 3–8 (4.8) | 2–3 † | 2–3 (2.42) | 2–5 (2.37)/2–4 (2.81) | 2–3 † |
| Range of length of Primary Dendrites (Average) | 11.8–88.1 (39.4) μm | 15.3–40.2 (30.1) μm | 11.8–67.4 μm † | 46.2–126.3 (101.3) μm | 16.4–49.1 (28.9)/16.1–79.4 (43.2) μm | 10.7–93.6 μm † |
| Range of total length of Primary dendrites (Average) | 76.2–199.3 (96.7) μm | 96.8–201.3 (138.4) μm | 76.2–203.3 μm † | 100.3–370.6 (243.9) μm | 32.8–123.7 (54.4)/64.4–312.5 (91.7) μm | 42.8–263.7 μm † |
| Number of Secondary Dendrites | 3–8 (5.6) | 3–9 (5.9) | 3–8 † | 2–6 (3.02) | 2–6 (3.46)/2–4 (2.9) | 3–9 † |
| Range of length of Secondary Dendrites (Average) | 23.5–185.6 (107.1) μm | 27.7–108.3 (69.8) μm | 49.1–176.2 μm † | 123.5–172.1 (131.9) μm | 14.7–71.3 (52.5)/36.2–102.3 (77.9) μm | 12.4–159.6 μm † |
| Range of total length of Secondary dendrites (Average) | 330.9–951.9 (580.9) μm | 305.6–912.9 (693.21) μm | 302.4–951.1 μm † | 247.1–1008.2 (501.9) μm | 88.2–369.6 (176.3)/72.4–396.9 (213.6) μm | 137.9–1138.3 μm † |
| Number of Tertiary Dendrites (Average) | 1–9 † | 3–9 (6.46) | 2–8 † | 0–2 † | 2–8 (4.21)/1–9 (4.65) | 2–9 † |
| Range of length of Tertiary Dendrites (Average) | 28.4–264.1 μm † | 24.5–297.9 (156.3) μm | 28.2–201.2 μm † | 0–108.7 μm † | 38.3–162.1 (96.8)/25.4–106.8 (64.2) μm | 15.2–169.0 μm † |
| Range of total length of Tertiary dendrites (Average) | 102.3–878.3 μm † | 102.0–889.2 μm † | 100.2–731.0 μm † | 0–217.6 μm † | 285.6–843.2 (501.1)/58.6–489.8 (265.1) μm | 65.1–972.8 μm † |
| Number of Quaternary Dendrites (Average) | 0–6 † | 0–5 † | - | - | 1–8 (4.67)/- | 0–7 † |
| Range of length of Quaternary Dendrites (Average) | 0–185.0 μm † | 0–143.2 (64.9) μm | - | - | 26.6–129.4 (76.2)/-μm | 0–83.1 μm † |
| Range of total length of Quaternary Dendrites (Average) | 0–813.1 μm † | 0–468.5 μm † | - | - | 123.1–583.2 (352.7)/-μm | 0–474.0 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vraka, K.; Mytilinaios, D.; Katsenos, A.P.; Serbis, A.; Baloyiannis, S.; Bellos, S.; Simos, Y.V.; Tzavellas, N.P.; Konitsiotis, S.; Vezyraki, P.; et al. Cellular Localization of Orexin 1 Receptor in Human Hypothalamus and Morphological Analysis of Neurons Expressing the Receptor. Biomolecules 2023, 13, 592. https://doi.org/10.3390/biom13040592
Vraka K, Mytilinaios D, Katsenos AP, Serbis A, Baloyiannis S, Bellos S, Simos YV, Tzavellas NP, Konitsiotis S, Vezyraki P, et al. Cellular Localization of Orexin 1 Receptor in Human Hypothalamus and Morphological Analysis of Neurons Expressing the Receptor. Biomolecules. 2023; 13(4):592. https://doi.org/10.3390/biom13040592
Chicago/Turabian StyleVraka, Konstantina, Dimitrios Mytilinaios, Andreas P. Katsenos, Anastasios Serbis, Stavros Baloyiannis, Stefanos Bellos, Yannis V. Simos, Nikolaos P. Tzavellas, Spyridon Konitsiotis, Patra Vezyraki, and et al. 2023. "Cellular Localization of Orexin 1 Receptor in Human Hypothalamus and Morphological Analysis of Neurons Expressing the Receptor" Biomolecules 13, no. 4: 592. https://doi.org/10.3390/biom13040592
APA StyleVraka, K., Mytilinaios, D., Katsenos, A. P., Serbis, A., Baloyiannis, S., Bellos, S., Simos, Y. V., Tzavellas, N. P., Konitsiotis, S., Vezyraki, P., Peschos, D., & Tsamis, K. I. (2023). Cellular Localization of Orexin 1 Receptor in Human Hypothalamus and Morphological Analysis of Neurons Expressing the Receptor. Biomolecules, 13(4), 592. https://doi.org/10.3390/biom13040592









