The N-Glycosylation of Total Plasma Proteins and IgG in Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Isolation of IgG from Human Plasma
2.3. Enzymatic Release and Fluorescent Labeling of N-Glycans from IgG and Total Plasma Proteins
2.4. Hydrophilic Interaction Liquid Chromatography of Labeled N-Glycans
2.5. Data Analysis
3. Results
3.1. Associations of Plasma and IgG N-Glycans with AF
3.2. Associations of Total Plasma Protein N-Glycans with AF Recurrence after Catheter Ablation
3.3. Differences in IgG N-Glycome following Catheter Ablation
3.4. Association of IgG N-Glycans with the CHA2DS2-VASc Score
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults: National Implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.H.; Franco, O.H.; Hofman, A.; Witteman, J.C.M.; Stricker, B.H.; Heeringa, J. Projections on the Number of Individuals with Atrial Fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilaveris, P.E.; Kennedy, H.L. Silent Atrial Fibrillation: Epidemiology, Diagnosis, and Clinical Impact. Clin. Cardiol. 2017, 40, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Markides, V.; Schilling, R.J. Atrial Fibrillation: Classification, Pathophysiology, Mechanisms and Drug Treatment. Heart 2003, 89, 939–943. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort: The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef]
- Lau, D.H.; Nattel, S.; Kalman, J.M.; Sanders, P. Modifiable Risk Factors and Atrial Fibrillation. Circulation 2017, 136, 583–596. [Google Scholar] [CrossRef]
- Hendriks, J.M.; Gallagher, C.; Middeldorp, M.E.; Lau, D.H.; Sanders, P. Risk Factor Management and Atrial Fibrillation. EP Eur. 2021, 23, ii52–ii60. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.; Qu, Z.; Weiss, J.N. Cardiac Fibrosis and Arrhythmogenesis: The Road to Repair Is Paved with Perils. J. Mol. Cell. Cardiol. 2014, 70, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Harada, M.; Nattel, S. Implications of Inflammation and Fibrosis in Atrial Fibrillation Pathophysiology. Card. Electrophysiol. Clin. 2021, 13, 25–35. [Google Scholar] [CrossRef]
- Aviles, R.J.; Martin, D.O.; Apperson-Hansen, C.; Houghtaling, P.L.; Rautaharju, P.; Kronmal, R.A.; Tracy, R.P.; Van Wagoner, D.R.; Psaty, B.M.; Lauer, M.S.; et al. Inflammation as a Risk Factor for Atrial Fibrillation. Circulation 2003, 108, 3006–3010. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, E.; Zografos, T.; Papamikroulis, G.-A.; Siasos, G.; Vogiatzi, G.; Theofilis, P.; Briasoulis, A.; Papaioannou, S.; Vavuranakis, M.; Gennimata, V.; et al. Biomarkers in Atrial Fibrillation and Heart Failure. Curr. Med. Chem. 2019, 26, 873–887. [Google Scholar] [CrossRef]
- Radovani, B.; Gudelj, I. N-Glycosylation and Inflammation; the Not-So-Sweet Relation. Front. Immunol. 2022, 13, 893365. [Google Scholar] [CrossRef]
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the Frequency of Protein Glycosylation, as Deduced from Analysis of the SWISS-PROT Database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, P.; Hennet, T.; Varki, A. Biological Functions of Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; ISBN 978-1-62182-421-3. [Google Scholar]
- Zhao, Y.-Y.; Takahashi, M.; Gu, J.-G.; Miyoshi, E.; Matsumoto, A.; Kitazume, S.; Taniguchi, N. Functional Roles of N-Glycans in Cell Signaling and Cell Adhesion in Cancer. Cancer Sci. 2008, 99, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Novokmet, M.; Lukić, E.; Vučković, F.; Ðurić, Ž.; Keser, T.; Rajšl, K.; Remondini, D.; Castellani, G.; Gašparović, H.; Gornik, O.; et al. Changes in IgG and Total Plasma Protein Glycomes in Acute Systemic Inflammation. Sci. Rep. 2014, 4, 4347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.J.; Kronewitter, S.R.; de Leoz, M.L.A.; Lebrilla, C.B. Glycomics and Disease Markers. Curr. Opin. Chem. Biol. 2009, 13, 601–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashti, H.; Pabon Porras, M.A.; Mora, S. Glycosylation and Cardiovascular Diseases. Adv. Exp. Med. Biol. 2021, 1325, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Ząbczyńska, M.; Link-Lenczowski, P.; Pocheć, E. Glycosylation in Autoimmune Diseases. Adv. Exp. Med. Biol. 2021, 1325, 205–218. [Google Scholar] [CrossRef]
- Quast, I.; Peschke, B.; Lünemann, J.D. Regulation of Antibody Effector Functions through IgG Fc N-Glycosylation. Cell. Mol. Life Sci. CMLS 2017, 74, 837–847. [Google Scholar] [CrossRef]
- Anthony, R.M.; Nimmerjahn, F. The Role of Differential IgG Glycosylation in the Interaction of Antibodies with FcγRs in Vivo. Curr. Opin. Organ Transplant. 2011, 16, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Akinkuolie, A.O.; Buring, J.E.; Ridker, P.M.; Mora, S. A Novel Protein Glycan Biomarker and Future Cardiovascular Disease Events. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2014, 3, e001221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukov, A.; Plavša, B.; Eichelmann, F.; Kuxhaus, O.; Hoshi, R.A.; Rudman, N.; Štambuk, T.; Trbojević-Akmačić, I.; Schiborn, C.; Morze, J.; et al. Immunoglobulin G N-Glycosylation Signatures in Incident Type 2 Diabetes and Cardiovascular Disease. Diabetes Care 2022, 45, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Wittenbecher, C.; Štambuk, T.; Kuxhaus, O.; Rudman, N.; Vučković, F.; Štambuk, J.; Schiborn, C.; Rahelić, D.; Dietrich, S.; Gornik, O.; et al. Plasma N-Glycans as Emerging Biomarkers of Cardiometabolic Risk: A Prospective Investigation in the EPIC-Potsdam Cohort Study. Diabetes Care 2020, 43, 661–668. [Google Scholar] [CrossRef]
- Menni, C.; Gudelj, I.; Macdonald-Dunlop, E.; Mangino, M.; Zierer, J.; Bešić, E.; Joshi, P.K.; Trbojević-Akmačić, I.; Chowienczyk, P.J.; Spector, T.D.; et al. Glycosylation Profile of Immunoglobulin G Is Cross-Sectionally Associated With Cardiovascular Disease Risk Score and Subclinical Atherosclerosis in Two Independent Cohorts. Circ. Res. 2018, 122, 1555–1564. [Google Scholar] [CrossRef]
- Kifer, D.; Louca, P.; Cvetko, A.; Deriš, H.; Cindrić, A.; Grallert, H.; Peters, A.; Polašek, O.; Gornik, O.; Mangino, M.; et al. N-Glycosylation of Immunoglobulin G Predicts Incident Hypertension. J. Hypertens. 2021, 39, 2527–2533. [Google Scholar] [CrossRef] [PubMed]
- Rudman, N.; Kifer, D.; Kaur, S.; Simunović, V.; Cvetko, A.; Pociot, F.; Morahan, G.; Gornik, O. Children at Onset of Type 1 Diabetes Show Altered N-Glycosylation of Plasma Proteins and IgG. Diabetologia 2022, 65, 1315–1327. [Google Scholar] [CrossRef]
- Lemmers, R.F.H.; Vilaj, M.; Urda, D.; Agakov, F.; Šimurina, M.; Klaric, L.; Rudan, I.; Campbell, H.; Hayward, C.; Wilson, J.F.; et al. IgG Glycan Patterns Are Associated with Type 2 Diabetes in Independent European Populations. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2240–2249. [Google Scholar] [CrossRef]
- Cvetko, A.; Mangino, M.; Tijardović, M.; Kifer, D.; Falchi, M.; Keser, T.; Perola, M.; Spector, T.D.; Lauc, G.; Menni, C.; et al. Plasma N-Glycome Shows Continuous Deterioration as the Diagnosis of Insulin Resistance Approaches. BMJ Open Diabetes Res. Care 2021, 9, e002263. [Google Scholar] [CrossRef]
- Nossan, J.S.; Šesto, I.; Štambuk, K.; Šipić, T.; Bernat, R.; Gudelj, I.; Rotkvić, L.; Žulj, M.; Mirat, J. Is the Extent of Left Atrial Fibrosis Associated with Body Mass Index in Patients Undergoing Pulmonary Vein Isolation for Atrial Fibrillation? Kardiol. Pol. 2021, 79, 531–538. [Google Scholar] [CrossRef]
- Pucić, M.; Knezević, A.; Vidic, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Supraha-Goreta, S.; Wormald, M.R.; Redzić, I.; et al. High Throughput Isolation and Glycosylation Analysis of IgG-Variability and Heritability of the IgG Glycome in Three Isolated Human Populations. Mol. Cell. Proteom. MCP 2011, 10, M111.010090. [Google Scholar] [CrossRef] [Green Version]
- Akmačić, I.T.; Ugrina, I.; Štambuk, J.; Gudelj, I.; Vučković, F.; Lauc, G.; Pučić-Baković, M. High-Throughput Glycomics: Optimization of Sample Preparation. Biochem. Biokhimiia 2015, 80, 934–942. [Google Scholar] [CrossRef]
- Saldova, R.; Asadi Shehni, A.; Haakensen, V.D.; Steinfeld, I.; Hilliard, M.; Kifer, I.; Helland, A.; Yakhini, Z.; Børresen-Dale, A.-L.; Rudd, P.M. Association of N-Glycosylation with Breast Carcinoma and Systemic Features Using High-Resolution Quantitative UPLC. J. Proteome Res. 2014, 13, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, A.; Gornik, O.; Polasek, O.; Pucic, M.; Redzic, I.; Novokmet, M.; Rudd, P.M.; Wright, A.F.; Campbell, H.; Rudan, I.; et al. Effects of Aging, Body Mass Index, Plasma Lipid Profiles, and Smoking on Human Plasma N-Glycans. Glycobiology 2010, 20, 959–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotz, V.; Wuhrer, M. N-Glycome Signatures in Human Plasma: Associations with Physiology and Major Diseases. FEBS Lett. 2019, 593, 2966–2976. [Google Scholar] [CrossRef] [Green Version]
- Lip, G.Y.H.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach: The Euro Heart Survey on Atrial Fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ji, L. Adjusting Multiple Testing in Multilocus Analyses Using the Eigenvalues of a Correlation Matrix. Heredity 2005, 95, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxley, R.R.; Alonso, A.; Lopez, F.L.; Filion, K.B.; Agarwal, S.K.; Loehr, L.R.; Soliman, E.Z.; Pankow, J.S.; Selvin, E. Type 2 Diabetes, Glucose Homeostasis and Incident Atrial Fibrillation: The Atherosclerosis Risk in Communities Study. Heart Br. Card. Soc. 2012, 98, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Clerc, F.; Reiding, K.R.; Jansen, B.C.; Kammeijer, G.S.M.; Bondt, A.; Wuhrer, M. Human Plasma Protein N-Glycosylation. Glycoconj. J. 2016, 33, 309–343. [Google Scholar] [CrossRef] [Green Version]
- Šoić, D.; Keser, T.; Štambuk, J.; Kifer, D.; Pociot, F.; Lauc, G.; Morahan, G.; Novokmet, M.; Gornik, O. High-Throughput Human Complement C3 N-Glycoprofiling Identifies Markers of Early Onset Type 1 Diabetes Mellitus in Children. Mol. Cell. Proteom. MCP 2022, 21, 100407. [Google Scholar] [CrossRef]
- Scott, D.W.; Patel, R.P. Endothelial Heterogeneity and Adhesion Molecules N-Glycosylation: Implications in Leukocyte Trafficking in Inflammation. Glycobiology 2013, 23, 622–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Jiao, H.; Zhao, D.; Teng, J. Association between Serum Apolipoprotein B and Atrial Fibrillation: A Case–Control Study. Sci. Rep. 2022, 12, 9597. [Google Scholar] [CrossRef]
- Davies, J.; Jiang, L.; Pan, L.-Z.; LaBarre, M.J.; Anderson, D.; Reff, M. Expression of GnTIII in a Recombinant Anti-CD20 CHO Production Cell Line: Expression of Antibodies with Altered Glycoforms Leads to an Increase in ADCC through Higher Affinity for FCγRIII. Biotechnol. Bioeng. 2001, 74, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Poljičanin, T.; Metelko, Ž. Epidemiology of Diabetes Mellitus in Croatia and Worldwide. Medix Spec. Med. Dvomjesečnik 2009, 80–81, 82–88. [Google Scholar]
- Liu, T.; Li, G.; Li, L.; Korantzopoulos, P. Association between C-Reactive Protein and Recurrence of Atrial Fibrillation after Successful Electrical Cardioversion: A Meta-Analysis. J. Am. Coll. Cardiol. 2007, 49, 1642–1648. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-Inflammatory Activity of Immunoglobulin G Resulting from Fc Sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, R.; Wormald, M.R.; Rudd, P.M.; Fischer, P.B.; Dwek, R.A.; Sim, R.B. Glycosylation Changes of IgG Associated with Rheumatoid Arthritis Can Activate Complement via the Mannose-Binding Protein. Nat. Med. 1995, 1, 237–243. [Google Scholar] [CrossRef]
- Karsten, C.M.; Pandey, M.K.; Figge, J.; Kilchenstein, R.; Taylor, P.R.; Rosas, M.; McDonald, J.U.; Orr, S.J.; Berger, M.; Petzold, D.; et al. Galactosylated IgG1 Links FcγRIIB and Dectin-1 to Block Complement-Mediated Inflammation. Nat. Med. 2012, 18, 1401–1406. [Google Scholar] [CrossRef] [Green Version]

| Atrial Fibrillation | Control | ||
|---|---|---|---|
| N | 172 | 54 | |
| Age (years) | 64 (57–69) | 64 (57–69) | |
| Female sex | 63 (36%) | 19 (35%) | |
| BMI | 28.9 (4.37) | ||
| AF classification | |||
| Paroxysmal | 135 (78%) | ||
| Persistent | 37 (22%) | ||
| CHA2DS2-VASc | |||
| 0 | 33 (19%) | ||
| 1 | 48 (28%) | ||
| 2 | 45 (26%) | ||
| 3 | 36 (21%) | ||
| <4 | 10 (6%) | ||
| Hypertension | |||
| No | 58 (34%) | ||
| Yes | 114 (66%) | ||
| Diabetes melitus | |||
| No | 159 (92%) | ||
| Yes | 13 (8%) | ||
| Coronary disease | |||
| No | 153 (89%) | ||
| Yes | 19 (11%) | ||
| Stroke or TIA | |||
| No | 165 (96%) | ||
| Yes | 7 (4%) | ||
| AF recurrence (6 months) | |||
| No | 120 (70%) | ||
| Yes | 50 (29%) | ||
| N/A | 2 (1%) | ||
| Atrial fibrosis # | |||
| No | 21 (12%) | ||
| Mild | 43 (25%) | ||
| Extensive | 103 (60%) | ||
| N/A | 5 (3%) |
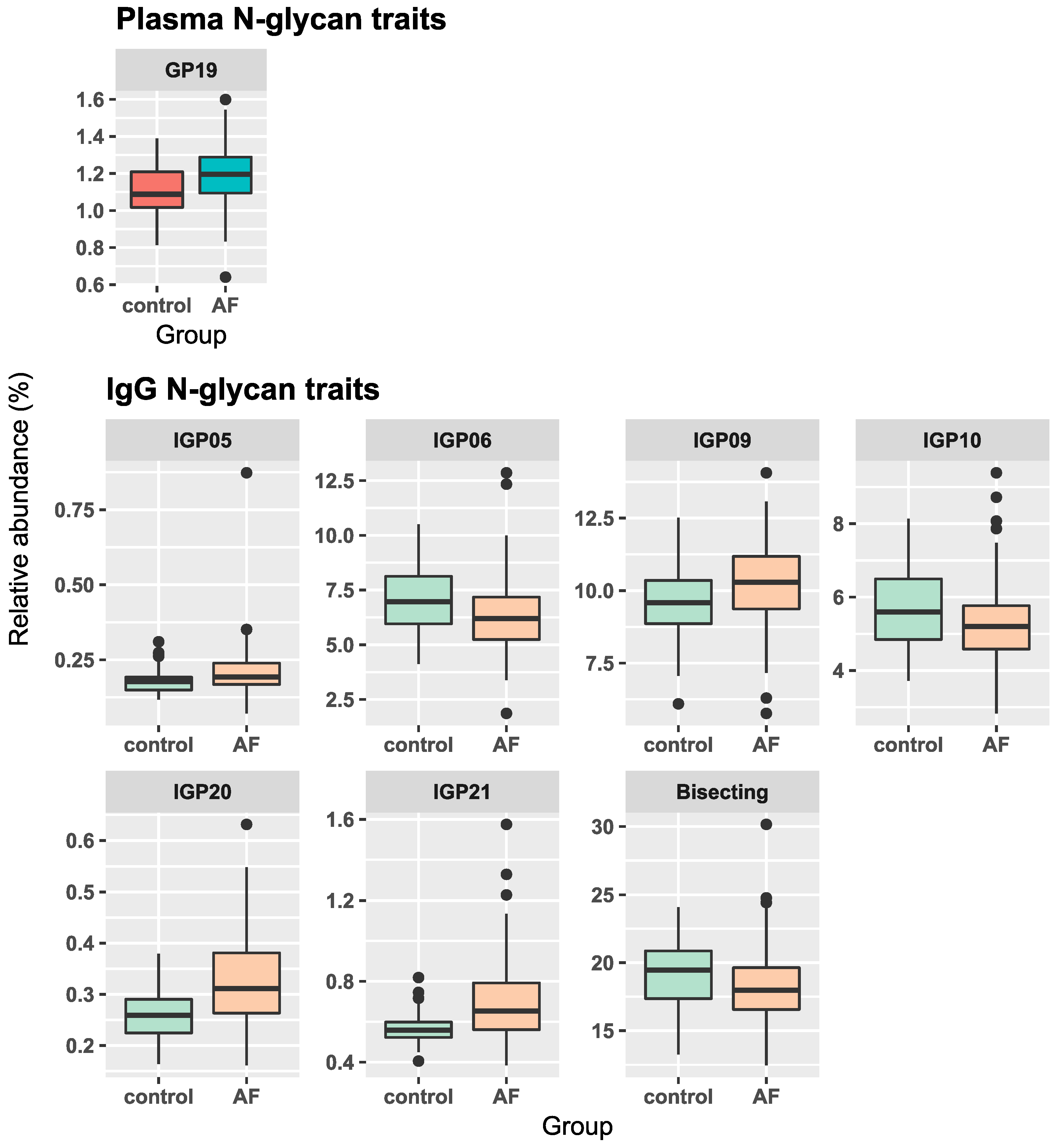
| Sample | Glycan Trait | β-Coefficient (95% Confidence Interval) * | p-Value | Adjusted p-Value # |
|---|---|---|---|---|
| Plasma | GP19 | 0.075 (0.036, 0.114) | 0.0002 | 0.004 |
| IgG | IGP5 | 0.123 (0.042, 0.204) | 0.003 | 0.012 |
| IGP6 | −0.093 (−0.167, −0.019) | 0.0134 | 0.022 | |
| IGP9 | 0.065 (0.02, 0.109) | 0.0048 | 0.047 | |
| IGP10 | −0.083 (−0.143, −0.024) | 0.0062 | 0.029 | |
| IGP20 | 0.221 (0.149, 0.294) | 8 × 10−9 | 1 × 10−7 | |
| IGP21 | 0.166 (0.095, 0.237) | 6 × 10−6 | 6 × 10−5 | |
| Bisecting | −0.055 (−0.096, −0.014) | 0.0084 | 0.037 |
| Sample | Association | Glycan Trait | β-Coefficient (95% Confidence Interval) * | p-Value | Adjusted p-Value # |
|---|---|---|---|---|---|
| Plasma | AF recurrence | GP2 | 0.117 (0.038, 0.196) | 0.0041 | 0.034 |
| GP7 | 0.094 (0.028, 0.159) | 0.0053 | 0.037 | ||
| GP9 | 0.123 (0.039, 0.207) | 0.0043 | 0.034 | ||
| GP15 | 0.12 (0.046, 0.195) | 0.0017 | 0.024 | ||
| OligoMan | 0.069 (0.03, 0.107) | 0.0006 | 0.012 | ||
| IgG | Pre- and post-procedure | IGP05 | −0.108 (−0.166, −0.05) | 0.0003 | 0.002 |
| IGP17 | −0.086 (−0.143, −0.029) | 0.0031 | 0.018 | ||
| IGP20 | −0.19 (−0.249, −0.13) | 2 × 10−9 | 2 × 10−8 | ||
| IGP21 | −0.172 (−0.227, −0.118) | 2 × 10−9 | 2 × 10−8 |
| Glycan Trait | Linear β-Coefficient (95% Confidence Interval) | p-Value | Adjusted p-Value # |
|---|---|---|---|
| IGP4 | 0.179 (0.04, 0.319) | 0.0118 | 0.034 |
| IGP6 | 0.355 (0.201, 0.508) | 1 × 10−5 | 1 × 10−4 |
| IGP8 | −0.067 (−0.119, −0.015) | 0.0123 | 0.034 |
| IGP14 | −0.213 (−0.352, −0.074) | 0.0028 | 0.014 |
| IGP18 | −0.186 (−0.323, −0.048) | 0.0086 | 0.029 |
| IGP23 | −0.275 (−0.475, −0.076) | 0.007 | 0.027 |
| Bisecting | 0.132 (0.049, 0.215) | 0.0019 | 0.013 |
| G0 | 0.214 (0.087, 0.341) | 0.0011 | 0.011 |
| G2 | -0.184 (−0.315, −0.053) | 0.006 | 0.026 |
| S | -0.152 (−0.248, −0.055) | 0.0023 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plavša, B.; Szavits-Nossan, J.; Blivajs, A.; Rapčan, B.; Radovani, B.; Šesto, I.; Štambuk, K.; Mustapić, V.; Đerek, L.; Rudan, D.; et al. The N-Glycosylation of Total Plasma Proteins and IgG in Atrial Fibrillation. Biomolecules 2023, 13, 605. https://doi.org/10.3390/biom13040605
Plavša B, Szavits-Nossan J, Blivajs A, Rapčan B, Radovani B, Šesto I, Štambuk K, Mustapić V, Đerek L, Rudan D, et al. The N-Glycosylation of Total Plasma Proteins and IgG in Atrial Fibrillation. Biomolecules. 2023; 13(4):605. https://doi.org/10.3390/biom13040605
Chicago/Turabian StylePlavša, Branimir, Janko Szavits-Nossan, Aleksandar Blivajs, Borna Rapčan, Barbara Radovani, Igor Šesto, Krešimir Štambuk, Vito Mustapić, Lovorka Đerek, Diana Rudan, and et al. 2023. "The N-Glycosylation of Total Plasma Proteins and IgG in Atrial Fibrillation" Biomolecules 13, no. 4: 605. https://doi.org/10.3390/biom13040605
APA StylePlavša, B., Szavits-Nossan, J., Blivajs, A., Rapčan, B., Radovani, B., Šesto, I., Štambuk, K., Mustapić, V., Đerek, L., Rudan, D., Lauc, G., & Gudelj, I. (2023). The N-Glycosylation of Total Plasma Proteins and IgG in Atrial Fibrillation. Biomolecules, 13(4), 605. https://doi.org/10.3390/biom13040605







