Plant Polyphenol Gossypol Induced Cell Death and Its Association with Gene Expression in Mouse Macrophages
Abstract
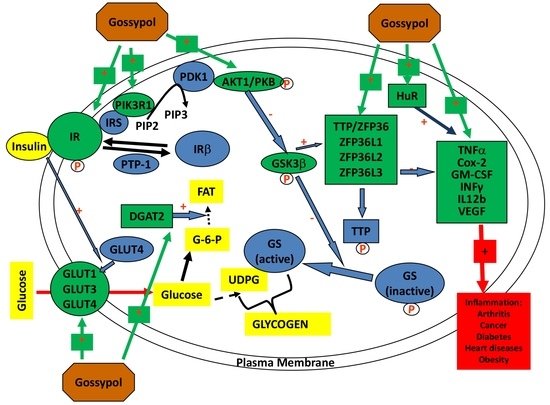
:1. Introduction
2. Materials and Methods
2.1. Cell Line, Chemicals and Reagents
2.2. Cell Culture and Treatment
2.3. Cell Toxicity Assay
2.4. Protein Determination
2.5. RNA Extraction, cDNA Synthesis and Real-Time qPCR Analysis
2.6. Statistics
3. Results
3.1. Gossypol Inhibited Mouse Macrophages Growth
3.2. Gossypol Reduced Soluble Protein Content in Mouse Macrophages
3.3. Relative Expression Levels of Selected Genes in Mouse Macrophages
3.4. Gossypol Increased TTP Family Gene Expression in Mouse Macrophages
3.5. Gossypol Increased Proinflammatory Cytokine Gene Expression in Mouse Macrophages
3.6. Gossypol Increased GLUT Family Gene Expression in Mouse Macrophages
3.7. Gossypol Increased Insulin Signaling Pathway Gene Expression in Mouse Macrophages
3.8. Gossypol Effect on APP and LEPR Gene Expression in Mouse Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, C.S.; Landau, J.M.; Huang, M.-T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A.; Xie, D.-Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Gu, L. Occurrence and biological significance of proanthocyanidins in the American diet. Phytochemistry 2005, 66, 2264–2280. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Rehman, K.-U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Guan, P.; Hu, X.; Yang, L.; He, L.; Lin, Q.; Luo, F.; Li, J.; He, X.; Du, Z.; et al. Natural Polyphenols as Targeted Modulators in Colon Cancer: Molecular Mechanisms and Applications. Front. Immunol. 2021, 12, 635484. [Google Scholar] [CrossRef]
- Kenar, J.A. Reaction chemistry of gossypol and its derivatives. J. Am. Oil Chem. Soc. 2006, 83, 269–302. [Google Scholar] [CrossRef]
- Coutinho, E.M. Gossypol: A contraceptive for men. Contraception 2002, 65, 259–263. [Google Scholar] [CrossRef]
- He, Z.; Zhang, D.; Cao, H. Protein profiling of water and alkali soluble cottonseed protein isolates. Sci. Rep. 2018, 8, 9306. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Zhang, H.; Olk, D.C. Chemical Composition of Defatted Cottonseed and Soy Meal Products. PLoS ONE 2015, 10, e0129933. [Google Scholar] [CrossRef]
- Zhong, S.; Leong, J.; Ye, W.; Xu, P.; Lin, S.-H.; Liu, J.-Y.; Lin, Y.C. (−)-Gossypol-enriched cottonseed oil inhibits proliferation and adipogenesis of human breast pre-adipocytes. Anticancer. Res. 2013, 33, 949–955. [Google Scholar]
- Chien, C.-C.; Ko, C.-H.; Shen, S.-C.; Yang, L.-Y.; Chen, Y.-C. The role of COX-2/PGE2 in gossypol-induced apoptosis of colorectal carcinoma cells. J. Cell. Physiol. 2012, 227, 3128–3137. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, A.J.; Castoreno, A.B.; Kuo, S.-Y.; Wang, Q.; Kuballa, P.; Xavier, R.; Shamji, A.F.; Schreiber, S.L.; Wagner, B.K. Gossypol and an HMT G9a inhibitor act in synergy to induce cell death in pancreatic cancer cells. Cell Death Dis. 2013, 4, e690. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.; Lum, L.G.; Schalk, D.; Azmi, A.; Banerjee, S.; Sarkar, F.H.; Mohommad, R. Pan-Bcl-2 Inhibitor AT-101 Enhances Tumor Cell Killing by EGFR Targeted T Cells. PLoS ONE 2012, 7, e47520. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Wu, Y.; Wu, Y.; Lu, B.; Chen, J.; Wang, J.; Yi, Z.; Qu, W.; Liu, M. (−)-Gossypol Suppresses the Growth of Human Prostate Cancer Xenografts via Modulating VEGF Signaling–Mediated Angiogenesis. Mol. Cancer Ther. 2011, 10, 795–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-W.; Wang, L.-S.; Dowd, M.K.; Wan, P.J.; Lin, Y.C. (−)-Gossypol reduces invasiveness in metastatic prostate cancer cells. Anticancer. Res. 2009, 29, 2179–2188. [Google Scholar] [PubMed]
- Huo, M.; Gao, R.; Jiang, L.; Cui, X.; Duan, L.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Suppression of LPS-induced inflammatory responses by gossypol in RAW 264.7 cells and mouse models. Int. Immunopharmacol. 2013, 15, 442–449. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Hendra, R.; Karimi, E. Bioactive Compounds, Antioxidant, Xanthine Oxidase Inhibitory, Tyrosinase Inhibitory and Anti-Inflammatory Activities of Selected Agro-Industrial By-products. Int. J. Mol. Sci. 2011, 12, 8610–8625. [Google Scholar] [CrossRef]
- Fu, M.; Blackshear, P.J. RNA-binding proteins in immune regulation: A focus on CCCH zinc finger proteins. Nat. Rev. Immunol. 2017, 17, 130–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patial, S.; Blackshear, P.J. Tristetraprolin as a Therapeutic Target in Inflammatory Disease. Trends Pharmacol. Sci. 2016, 37, 811–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carballo, E.; Lai, W.S.; Blackshear, P.J. Feedback Inhibition of Macrophage Tumor Necrosis Factor-α Production by Tristetraprolin. Science 1998, 281, 1001–1005. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.S.; Carballo, E.; Thorn, J.M.; Kennington, E.A.; Blackshear, P.J. Interactions of CCCH zinc finger proteins with mRNA. Binding of tristetraprolin-related zinc finger proteins to Au-rich elements and destabilization of mRNA. J. Biol. Chem. 2000, 275, 17827–17837. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.; Kedersha, N.; Shen, L.; Blackshear, P.J.; Anderson, P. Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor alpha, cyclooxygenase 2, and inflammatory arthritis. Proc. Natl. Acad. Sci. USA 2004, 101, 2011–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, G.A.; Carballo, E.; Lee, D.M.; Lai, W.S.; Thompson, M.J.; Patel, D.D.; I Schenkman, D.; Gilkeson, G.S.; E Broxmeyer, H.; Haynes, B.F.; et al. A Pathogenetic Role for TNFα in the Syndrome of Cachexia, Arthritis, and Autoimmunity Resulting from Tristetraprolin (TTP) Deficiency. Immunity 1996, 4, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, I.; Schaljo, B.; Vogl, C.; Gattermeier, I.; Kolbe, T.; Müller, M.; Blackshear, P.J.; Kovarik, P. Interferons limit inflammatory responses by induction of tristetraprolin. Blood 2006, 107, 4790–4797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Hininger-Favier, I.; Kelly, M.A.; Benaraba, R.; Dawson, H.D.; Coves, S.; Roussel, A.M.; Anderson, R.A. Green Tea Polyphenol Extract Regulates the Expression of Genes Involved in Glucose Uptake and Insulin Signaling in Rats Fed a High Fructose Diet. J. Agric. Food Chem. 2007, 55, 6372–6378. [Google Scholar] [CrossRef]
- Cao, H.; Kelly, M.; Kari, F.; Dawson, H.D.; Urban, J.F., Jr.; Coves, S.; Roussel, A.; Anderson, R. Green tea increases anti-inflammatory tristetraprolin and decreases pro-inflammatory tumor necrosis factor mRNA levels in rats. J. Inflamm. 2007, 4, 1. [Google Scholar] [CrossRef]
- Cao, H.; Polansky, M.M.; Anderson, R.A. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2007, 459, 214–222. [Google Scholar] [CrossRef]
- Cao, H.; Graves, D.J.; Anderson, R.A. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. Phytomedicine 2010, 17, 1027–1032. [Google Scholar] [CrossRef]
- Cao, H.; Urban, J.F.; Anderson, R.A. Cinnamon Polyphenol Extract Affects Immune Responses by Regulating Anti- and Proinflammatory and Glucose Transporter Gene Expression in Mouse Macrophages. J. Nutr. 2008, 138, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Anderson, R.A. Cinnamon Polyphenol Extract Regulates Tristetraprolin and Related Gene Expression in Mouse Adipocytes. J. Agric. Food Chem. 2011, 59, 2739–2744. [Google Scholar] [CrossRef]
- Blackshear, P.J. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 2002, 30, 945–952. [Google Scholar] [CrossRef]
- Blackshear, P.J.; Phillips, R.S.; Ghosh, S.; Ramos, S.V.; Richfield, E.K.; Lai, W.S. Zfp36l3, a Rodent X Chromosome Gene Encoding a Placenta-Specific Member of the Tristetraprolin Family of CCCH Tandem Zinc Finger Proteins. Biol. Reprod. 2005, 73, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Cha, H.J.; Lee, H.H.; Chae, S.W.; Cho, W.J.; Kim, Y.M.; Choi, H.-J.; Choi, D.H.; Jung, S.W.; Min, Y.J.; Lee, B.J.; et al. Tristetraprolin downregulates the expression of both VEGF and COX-2 in human colon cancer. Hepato-Gastroenterology 2011, 58, 790–795. [Google Scholar]
- Carballo, E.; Lai, W.S.; Blackshear, P. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood 2000, 95, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.; Boulougouris, G.; Manoloukos, M.; Armaka, M.; Apostolaki, M.; Pizarro, T.; Kotlyarov, A.; Forster, I.; Flavell, R.; Gaestel, M.; et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease. J. Exp. Med. 2002, 196, 1563–1574. [Google Scholar] [CrossRef] [Green Version]
- Molle, C.; Zhang, T.; De Lendonck, L.Y.; Gueydan, C.; Andrianne, M.; Sherer, F.; Van Simaeys, G.; Blackshear, P.; Leo, O.; Goriely, S. Tristetraprolin regulation of interleukin 23 mRNA stability prevents a spontaneous inflammatory disease. J. Exp. Med. 2013, 210, 1675–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamelli, R.L.; Liu, H.; He, L.-K.; Hofmann, C.A. Augmentations of glucose uptake and glucose transporter-1 in macrophages following thermal injury and sepsis in mice. J. Leukoc. Biol. 1996, 59, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Barbosa, V.M.; Calder, P. Olive oil in parenteral nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 165–174. [Google Scholar] [CrossRef]
- Püschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, 11, 4358. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K.; Bland, J.M. Isolation of Cottonseed Extracts That Affect Human Cancer Cell Growth. Sci. Rep. 2018, 8, 10458. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Sethumadhavan, K.; Cao, F.; Wang, T.T.Y. Gossypol decreased cell viability and down-regulated the expression of a number of genes in human colon cancer cells. Sci. Rep. 2021, 11, 5922. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Sethumadhavan, K. Identification of Bcl2 as a Stably Expressed qPCR Reference Gene for Human Colon Cancer Cells Treated with Cottonseed-Derived Gossypol and Bioactive Extracts and Bacteria-Derived Lipopolysaccharides. Molecules 2022, 27, 7560. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Tuttle, J.S.; Blackshear, P.J. Immunological Characterization of Tristetraprolin as a Low Abundance, Inducible, Stable Cytosolic Protein. J. Biol. Chem. 2004, 279, 21489–21499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H. Expression, Purification, and Biochemical Characterization of the Antiinflammatory Tristetraprolin: A Zinc-Dependent mRNA Binding Protein Affected by Posttranslational Modifications. Biochemistry 2004, 43, 13724–13738. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Shockey, J.M. Comparison of TaqMan and SYBR Green qPCR Methods for Quantitative Gene Expression in Tung Tree Tissues. J. Agric. Food Chem. 2012, 60, 12296–12303. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K. Cottonseed Extracts and Gossypol Regulate Diacylglycerol Acyltransferase Gene Expression in Mouse Macrophages. J. Agric. Food Chem. 2018, 66, 6022–6030. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cao, H.; Cao, F.; Roussel, A.-M.; Anderson, R.A. Quantitative PCR for glucose transporter and tristetraprolin family gene expression in cultured mouse adipocytes and macrophages. In Vitr. Cell. Dev. Biol.-Anim. 2013, 49, 759–770. [Google Scholar] [CrossRef]
- Fukuzumi, M.; Shinomiya, H.; Shimizu, Y.; Ohishi, K.; Utsumi, S. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT. Infect. Immun. 1996, 64, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Sethumadhavan, K. Gossypol but not cottonseed extracts or lipopolysaccharides stimulates HuR gene expression in mouse cells. J. Funct. Foods 2019, 59, 25–29. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K.; Wu, X.; Zeng, X. Cottonseed-derived gossypol and ethanol extracts differentially regulate cell viability and VEGF gene expression in mouse macrophages. Sci. Rep. 2021, 11, 15700. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Yuan, H.; Yi, J.; Lu, Y.; Wei, Q.; Guo, C.; Wu, J.; Yuan, L.; He, Z. Gossypol acetic acid induces apoptosis in RAW264.7 cells via a caspase-dependent mitochondrial signaling pathway. J. Vet. Sci. 2013, 14, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.-R.; Li, C.-G.; Zha, Q.-B.; Xu, L.-H.; Pan, H.; Zhao, G.-X.; Ouyang, D.-Y.; He, X.-H. Gossypol induces pyroptosis in mouse macrophages via a non-canonical inflammasome pathway. Toxicol. Appl. Pharmacol. 2016, 292, 56–64. [Google Scholar] [CrossRef]
- Lai, W.S.; Stumpo, D.J.; Blackshear, P.J. Rapid insulin-stimulated accumulation of an mRNA encoding a proline-rich protein. J. Biol. Chem. 1990, 265, 16556–16563. [Google Scholar] [CrossRef]
- DuBois, R.; McLane, M.; Ryder, K.; Lau, L.; Nathans, D. A growth factor-inducible nuclear protein with a novel cysteine/histidine repetitive sequence. J. Biol. Chem. 1990, 265, 19185–19191. [Google Scholar] [CrossRef]
- Varnum, B.C.; Lim, R.W.; Kujubu, D.A.; Luner, S.J.; Kaufman, S.E.; Greenberger, J.S.; Gasson, J.C.; Herschman, H.R. Granulocyte-macrophage colony-stimulating factor and tetradecanoyl phorbol acetate induce a distinct, restricted subset of primary- response TIS genes in both proliferating and terminally differentiated myeloid cells. Mol. Cell. Biol. 1989, 9, 3580–3583. [Google Scholar]
- Cousins, R.J.; Blanchard, R.K.; Popp, M.P.; Liu, L.; Cao, J.; Moore, J.B.; Green, C.L. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc. Natl. Acad. Sci. USA 2003, 100, 6952–6957. [Google Scholar] [CrossRef] [Green Version]
- Taddeo, B.; Zhang, W.; Roizman, B. The UL41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 2827–2832. [Google Scholar] [CrossRef] [Green Version]
- Stoecklin, G.; Stubbs, T.; Kedersha, N.; Wax, S.; Rigby, W.F.; Blackwell, T.K.; Anderson, P. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004, 23, 1313–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, B.; An, H.; Ra, J.-S.; Lim, J.-Y.; Lee, S.-H.; Yoo, C.-Y.; Lee, S.-H. Gossypol from Cottonseeds Ameliorates Glucose Uptake by Mimicking Insulin Signaling and Improves Glucose Homeostasis in Mice with Streptozotocin-Induced Diabetes. Oxid. Med. Cell. Longev. 2018, 2018, 5796102. [Google Scholar] [CrossRef] [PubMed]






| Gossypol Concentration (µg/mL) | A570 nm ± SD (2 h) (% of Control) | A570 nm ± SD (24 h) (% of Control) |
|---|---|---|
| 0 | 100.0 ± 8.1 | 100.0 ± 18.5 |
| 0.1 | 110.2 ± 9.0 | 92.8 ± 3.2 |
| 0.5 | 93.6 ± 4.8 | 90.4 ± 5.5 |
| 1 | 99.2 ± 1.3 | 88.3 ± 11.5 |
| 5 | 92.7 ± 4.9 | 7.6 ± 0.4 ** |
| 10 | 85.7 ± 7.4 * | 7.6 ± 0.4 ** |
| 50 | 69.3 ± 2.4 ** | 8.6 ± 0.5 ** |
| 100 | 20.0 ± 0.6 ** | 8.5 ± 3.2 ** |
| Treatment | Time (h) | Supernatant Protein | Pellet Protein | Total Protein | |||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (µg/µL) | Amount (mg) | Ratio (%) | Concentration (µg/µL) | Amount (mg) | Ratio (%) | Amount (mg) | Ratio (%) | ||
| control | 2 | 7.78 ± 0.36 | 3.89 | 100 | 22.05 ± 0.21 | 1.1 | 100 | 4.99 | 100 |
| gossypol | 2 | 6.56 ± 0.39 * | 3.28 | 84 | 23.67 ± 0.22 ** | 1.18 | 107 | 4.47 | 90 |
| gossypol | 4 | 6.45 ± 0.38 * | 3.22 | 83 | 31.47 ± 0.15 ** | 1.57 | 143 | 4.80 | 96 |
| gossypol | 8 | 4.85 ± 0.32 ** | 2.42 | 62 | 34.21 ± 0.45 ** | 1.71 | 155 | 4.13 | 83 |
| gossypol | 24 | 0.21 ± 0.18 ** | 0.11 | 3 | 22.12 ± 0.45 | 1.11 | 101 | 1.22 | 24 |
| mRNA | Cycle of Threshold (CT ± SD) | Cycle of Threshold (CT) | CTTarget − Ctref (ΔCT) | ΔCTTarget − ΔCTcal (ΔΔCT) | Fold (Ttp = 1) |
|---|---|---|---|---|---|
| Rpl32 | 17.82 ± 0.81 | 17.82 | 0.00 | ||
| Ttp/Zfp36/Tis11 | 24.76 ± 1.12 | 24.76 | 6.94 | 0.00 | 1.00 |
| Akt1 | 23.65 ± 1.69 | 23.65 | 5.83 | −1.11 | 2.16 |
| App | 22.51 ± 0.81 | 22.51 | 4.69 | −2.25 | 4.76 |
| Cox2 | 30.15 ± 2.38 | 30.15 | 12.33 | 5.39 | 0.02 |
| Glut1/Slc2a1 | 27.55 ± 1.49 | 27.55 | 9.73 | 2.79 | 0.14 |
| Glut3/Slc2a3 | 26.54 ± 0.75 | 26.54 | 8.72 | 1.78 | 0.29 |
| Glut4/Slc2a4 | 34.68 ± 1.20 | 34.68 | 16.86 | 9.92 | 0.001 |
| Gm-csf | 27.58 ± 2.06 | 27.58 | 9.76 | 2.82 | 0.14 |
| Ifnγ | 27.70 ± 2.42 | 27.70 | 9.88 | 2.94 | 0.13 |
| Il12b | 28.03 ± 1.89 | 28.03 | 10.21 | 3.27 | 0.10 |
| Insr | 26.39 ± 073 | 26.39 | 8.57 | 1.63 | 0.32 |
| Lepr | 28.33 ± 2.11 | 28.33 | 10.51 | 3.57 | 0.08 |
| Pik3r1 | 26.21 ± 0.58 | 26.21 | 8.39 | 1.45 | 0.37 |
| Tnf | 29.19 ± 2.68 | 29.19 | 11.37 | 4.43 | 0.05 |
| Zfp36l1/Tis11b | 26.07 ± 0.35 | 26.07 | 8.25 | 1.31 | 0.40 |
| Zfp36l2/Tis11d | 24.61 ± 1.03 | 24.61 | 6.79 | −0.15 | 1.11 |
| Zfp36l3 | 29.20 ± 1.13 | 29.20 | 11.38 | 4.44 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Sethumadhavan, K. Plant Polyphenol Gossypol Induced Cell Death and Its Association with Gene Expression in Mouse Macrophages. Biomolecules 2023, 13, 624. https://doi.org/10.3390/biom13040624
Cao H, Sethumadhavan K. Plant Polyphenol Gossypol Induced Cell Death and Its Association with Gene Expression in Mouse Macrophages. Biomolecules. 2023; 13(4):624. https://doi.org/10.3390/biom13040624
Chicago/Turabian StyleCao, Heping, and Kandan Sethumadhavan. 2023. "Plant Polyphenol Gossypol Induced Cell Death and Its Association with Gene Expression in Mouse Macrophages" Biomolecules 13, no. 4: 624. https://doi.org/10.3390/biom13040624
APA StyleCao, H., & Sethumadhavan, K. (2023). Plant Polyphenol Gossypol Induced Cell Death and Its Association with Gene Expression in Mouse Macrophages. Biomolecules, 13(4), 624. https://doi.org/10.3390/biom13040624