Trajectories of Allopregnanolone and Allopregnanolone to Progesterone Ratio across the Six Subphases of Menstrual Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample
2.3. Study Procedures
2.4. Sample Analysis
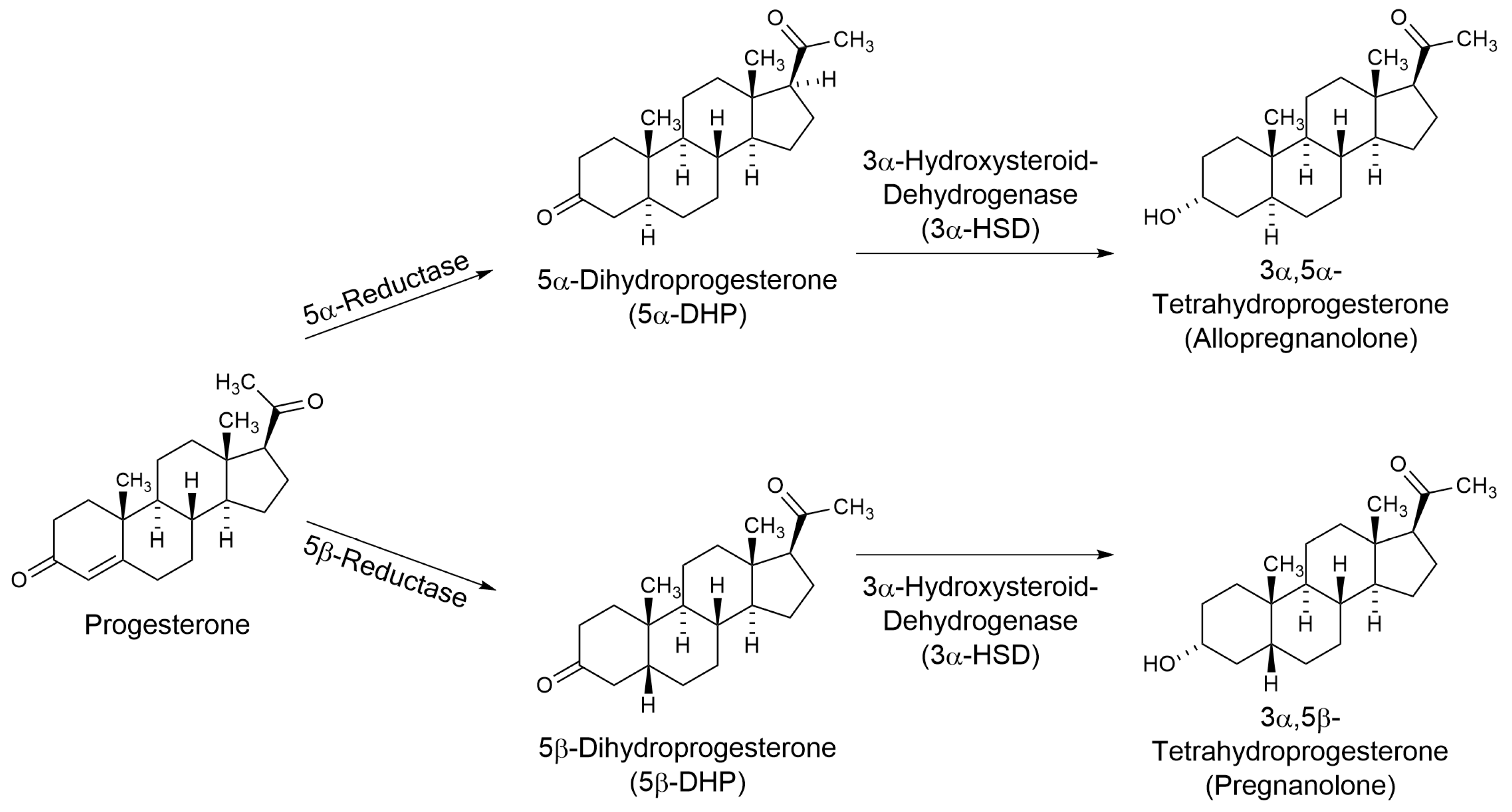
2.4.1. Progesterone and Allopregnanolone
2.4.2. Luteinizing Hormone
2.5. Power and Data Analyses
3. Results
3.1. Study Sample, Realignment, and Imputation Results
3.2. Study Participants
3.3. Allopregnanolone and Progesterone
3.4. Ratio of Allopregnanolone to Progesterone
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beall, D.; Reichstein, T. Isolation of Progesterone and Allopregnanolone from the Adrenal. Nature 1938, 142, 479. [Google Scholar] [CrossRef]
- King, S. Neurosteroids and the Nervous System; Springer: New York, NY, USA, 2013. [Google Scholar]
- Agís-Balboa, R.C.; Pinna, G.; Zhubi, A.; Maloku, E.; Veldic, M.; Costa, E.; Guidotti, A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14602–14607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensah-Nyagan, A.G.; Do-Rego, J.L.; Beaujean, D.; Luu-The, V.; Pelletier, G.; Vaudry, H. Neurosteroids: Expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol. Rev. 1999, 51, 63–81. [Google Scholar]
- Do Rego, J.L.; Seong, J.Y.; Burel, D.; Leprince, J.; Luu-The, V.; Tsutsui, K.; Tonon, M.C.; Pelletier, G.; Vaudry, H. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front. Neuroendocrinol. 2009, 30, 259–301. [Google Scholar] [CrossRef]
- Cai, H.; Zhou, X.; Dougherty, G.G.; Reddy, R.D.; Haas, G.L.; Montrose, D.M.; Keshavan, M.; Yao, J.K. Pregnenolone-progesterone-allopregnanolone pathway as a potential therapeutic target in first-episode antipsychotic-naïve patients with schizophrenia. Psychoneuroendocrinology 2018, 90, 43–51. [Google Scholar] [CrossRef]
- Dong, E.; Matsumoto, K.; Uzunova, V.; Sugaya, I.; Takahata, H.; Nomura, H.; Watanabe, H.; Costa, E.; Guidotti, A. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. USA 2001, 98, 2849–2854. [Google Scholar] [CrossRef] [Green Version]
- Agís-Balboa, R.C.; Pinna, G.; Pibiri, F.; Kadriu, B.; Costa, E.; Guidotti, A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 18736–18741. [Google Scholar] [CrossRef] [Green Version]
- Pinna, G.; Agis-Balboa, R.C.; Pibiri, F.; Nelson, M.; Guidotti, A.; Costa, E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem. Res. 2008, 33, 1990–2007. [Google Scholar] [CrossRef]
- Bortolato, M.; Devoto, P.; Roncada, P.; Frau, R.; Flore, G.; Saba, P.; Pistritto, G.; Soggiu, A.; Pisanu, S.; Zappala, A.; et al. Isolation rearing-induced reduction of brain 5α-reductase expression: Relevance to dopaminergic impairments. Neuropharmacology 2011, 60, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Follesa, P.; Serra, M.; Cagetti, E.; Pisu, M.G.; Porta, S.; Floris, S.; Massa, F.; Sanna, E.; Biggio, G. Allopregnanolone synthesis in cerebellar granule cells: Roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol. Pharmacol. 2000, 57, 1262–1270. [Google Scholar] [PubMed]
- Bäckström, T.; Bixo, M.; Johansson, M.; Nyberg, S.; Ossewaarde, L.; Ragagnin, G.; Savic, I.; Strömberg, J.; Timby, E.; van Broekhoven, F.; et al. Allopregnanolone and mood disorders. Prog. Neurobiol. 2014, 113, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Diviccaro, S.; Cioffi, L.; Falvo, E.; Giatti, S.; Melcangi, R.C. Allopregnanolone: An overview on its synthesis and effects. J. Neuroendocrinol. 2022, 34, e12996. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.G.; Frye, C.A. Allopregnanolone levels and seizure frequency in progesterone-treated women with epilepsy. Neurology 2014, 83, 345–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, P.E.; Rubinow, D.R.; Nieman, L.K.; Koziol, D.E.; Morrow, A.L.; Schiller, C.E.; Cintron, D.; Thompson, K.D.; Khine, K.K.; Schmidt, P.J. 5α-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder. Neuropsychopharmacology 2016, 41, 1093–1102. [Google Scholar] [CrossRef] [Green Version]
- Howards, P.P.; Schisterman, E.F.; Wactawski-Wende, J.; Reschke, J.E.; Frazer, A.A.; Hovey, K.M. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: The BioCycle Study. Am. J. Epidemiol. 2009, 169, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Mumford, S.; Schisterman, E.F.; Gaskins, A.J.; Pollack, A.Z.; Perkins, N.J.; Whitcomb, B.W.; Ye, A.; Wactawski-Wende, J. Realign ment and multiple imputation of longitudinal data: An application to menstrual cycle data. Paediatr. Perinat. Epidemiol. 2011, 25, 448–459. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass spectrometry and immunoassay: How to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef] [Green Version]
- Sohda, S.; Suzuki, K.; Igari, I. Relationship Between the Menstrual Cycle and Timing of Ovulation Revealed by New Protocols: Analysis of Data from a Self-Tracking Health App. J. Med. Internet Res. 2017, 19, e391. [Google Scholar] [CrossRef] [Green Version]
- Ney, L.J.; Felmingham, K.L.; Bruno, R.; Matthews, A.; Nichols, D.S. Simultaneous quantification of endocannabinoids, oleoylethanolamide and steroid hormones in human plasma and saliva. J. Chromatogr. B 2020, 1152, 122252. [Google Scholar] [CrossRef]
- Hamidovic, A.; Soumare, F.; Naveed, A.; Davis, J.; Sun, J.; Dang, N. Reduced Dehydroepiandrosterone-Sulfate Levels in the Mid-Luteal Subphase of the Menstrual Cycle: Implications to Women’s Health Research. Metabolites 2022, 12, 941. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Pirke, K.M.; Hellhammer, D.H. The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef]
- Leiva, R.A.; Bouchard, T.P.; Abdullah, S.H.; Ecochard, R. Urinary Luteinizing Hormone Tests: Which Concentration Threshold Best Predicts Ovulation? Front. Public Health 2017, 5, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ARUP Laboratories. Luteinizing Hormone, Serum: ARUP Laboratories Test Directory. Available online: https://ltd.aruplab.com/Tests/Pub/0070093 (accessed on 15 September 2022).
- Roche Diagnostics GmbH. Elecsys LH Assay; Roche Diagnostics GmbH: Indianapolis, IN, USA, 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.2.2); R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 15 November 2022).
- Van Buuren, S.; Brand, J.P.L.; Groothuis-Oudshoorn, C.G.M.; Rubin, D.B. Fully conditional specification in multivariate imputation. J. Stat. Comput. Simul. 2006, 76, 1049–1064. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Biagini, G.; Panuccio, G.; Avoli, M. Neurosteroids and epilepsy. Curr. Opin. Neurol. 2010, 23, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef]
- Tan, C.; Shard, C.; Ranieri, E.; Hynes, K.; Pham, D.H.; Leach, D.; Buchanan, G.; Corbett, M.; Shoubridge, C.; Kumar, R.; et al. Mutations of protocadherin 19 in female epilepsy (PCDH19-FE) lead to allopregnanolone deficiency. Hum. Mol. Genet. 2015, 24, 5250–5259. [Google Scholar] [CrossRef] [Green Version]
- Herzog, A.G. Catamenial epilepsy: Update on prevalence, pathophysiology and treatment from the findings of the NIH Progesterone Treatment Trial. Seizure 2015, 28, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Sun, H.; Rajasekaran, K.; Williamson, J.; Perez-Reyes, E.; Kapur, J. A novel therapeutic approach for treatment of catamenial epilepsy. Neurobiol. Dis. 2018, 111, 127–137. [Google Scholar] [CrossRef]
- Kimball, A.; Dichtel, L.E.; Nyer, M.B.; Mischoulon, D.; Fisher, L.B.; Cusin, C.; Dording, C.M.; Trinh, N.H.; Yeung, A.; Haines, M.S.; et al. The allopregnanolone to progesterone ratio across the menstrual cycle and in menopause. Psychoneuroendocrinology 2020, 112, 104512. [Google Scholar] [CrossRef]
- Dušková, M.; Simůnková, K.; Hill, M.; Velíková, M.; Kubátová, J.; Kancheva, L.; Kazihnitková, H.; Hruškovičová, H.; Pospíšilová, H.; Rácz, B.; et al. Chronic cigarette smoking alters circulating sex hormones and neuroactive steroids in premenopausal women. Physiol. Res. 2012, 61, 97–111. [Google Scholar] [CrossRef] [PubMed]




| Variable | Statistic |
|---|---|
| Age | 26.40 (4.86) |
| Race | |
| White | 12 |
| Black or African American | 6 |
| American Indian/Alaska Native | 1 |
| Asian | 7 |
| Native Hawaiian or other Pacific Islander | 0 |
| More than one race | 0 |
| Unknown/do not want to specify | 4 |
| Ethnicity | |
| Hispanic | 9 |
| Non-Hispanic | 19 |
| Do not know/do not want to specify | 2 |
| Student Status | |
| Yes | 16 |
| No | 14 |
| Marital Status | |
| Single, never married | 26 |
| Married | 3 |
| Divorced | 1 |
| Income | |
| Less than USD 20,000 | 14 |
| USD 20,000–34,999 | 5 |
| USD 35,000–49,999 | 4 |
| USD 50,000–74,999 | 4 |
| >USD 74,999 | 3 |
| Age of menarche | 11.85 (1.48) |
| BMI | 25.45 (4.80) |
| Timepoint Comparison | Class | t-Statistic | p-Value | Adjusted p-Value | Significance | |
|---|---|---|---|---|---|---|
| Early follicular | Mid-follicular | Imputed | −5.210 | 2.15 × 10−5 | 3.22 × 10−5 | *** |
| Early follicular | Periovulatory | Imputed | 1.028 | 0.3142 | 0.3142 | ns |
| Early follicular | Early luteal | Imputed | 17.201 | 1.61 × 10−15 | 8.05 × 10−15 | *** |
| Early follicular | Mid-luteal | Imputed | 23.709 | 4.85 × 10−18 | 7.28 × 10−17 | *** |
| Early follicular | Late luteal | Imputed | 6.045 | 3.88 × 10−6 | 7.28 × 10−6 | *** |
| Mid-follicular | Periovulatory | Imputed | 3.574 | 0.0015 | 0.0016 | ** |
| Mid-follicular | Early luteal | Imputed | 15.967 | 3.58 × 10−15 | 1.34 × 10−14 | *** |
| Mid-follicular | Mid-luteal | Imputed | 19.535 | 1.12 × 10−16 | 8.42 × 10−16 | *** |
| Mid-follicular | Late luteal | Imputed | 8.133 | 1.58 × 10−8 | 3.40 × 10−8 | *** |
| Periovulatory | Early luteal | Imputed | 14.474 | 3.87 × 10−11 | 1.16 × 10−10 | *** |
| Periovulatory | Mid-luteal | Imputed | 13.235 | 7.12 × 10−11 | 1.78 × 10−10 | *** |
| Periovulatory | Late luteal | Imputed | 4.126 | 0.0005 | 0.0006 | *** |
| Early luteal | Mid-luteal | Imputed | 3.686 | 0.0013 | 0.0015 | ** |
| Early luteal | Late luteal | Imputed | −4.128 | 0.0004 | 0.0005 | *** |
| Mid-luteal | Late luteal | Imputed | −5.995 | 7.28 × 10−6 | 1.21 × 10−5 | *** |
| Early follicular | Mid-follicular | Realigned | −4.580 | 0.0001 | 0.0002 | *** |
| Early follicular | Periovulatory | Realigned | 1.509 | 0.1450 | 0.1450 | ns |
| Early follicular | Early luteal | Realigned | 22.650 | 9.75 × 10−17 | 7.31 × 10−16 | *** |
| Early follicular | Mid-luteal | Realigned | 22.787 | 2.77 × 10−17 | 4.16 × 10−16 | *** |
| Early follicular | Late luteal | Realigned | 5.254 | 9.72 × 10−5 | 0.0002 | *** |
| Mid-follicular | Periovulatory | Realigned | 4.808 | 0.0001 | 0.0002 | *** |
| Mid-follicular | Early luteal | Realigned | 21.454 | 8.86 × 10−15 | 4.43 × 10−14 | *** |
| Mid-follicular | Mid-luteal | Realigned | 16.726 | 1.29 × 10−13 | 4.84 × 10−13 | *** |
| Mid-follicular | Late luteal | Realigned | 8.090 | 1.20 × 10−6 | 2.57 × 10−6 | *** |
| Periovulatory | Early luteal | Realigned | 14.575 | 4.08 × 10−12 | 1.13 × 10−11 | *** |
| Periovulatory | Mid-luteal | Realigned | 13.421 | 4.51 × 10−12 | 1.13 × 10−11 | *** |
| Periovulatory | Late luteal | Realigned | 2.743 | 0.0150 | 0.0171 | * |
| Early luteal | Mid-luteal | Realigned | 2.661 | 0.0160 | 0.0171 | * |
| Early luteal | Late luteal | Realigned | −3.165 | 0.0080 | 0.0100 | ** |
| Mid-luteal | Late luteal | Realigned | −4.915 | 0.0002 | 0.0002 | *** |
| Timepoint Comparison | Class | t-Statistic | p-Value | Adjusted p-Value | Significance | |
|---|---|---|---|---|---|---|
| Early follicular | Mid-follicular | Imputed | −5.210 | 2.15 × 10−5 | 3.22 × 10−5 | *** |
| Early follicular | Periovulatory | Imputed | 1.028 | 0.3142 | 0.3142 | ns |
| Early follicular | Early luteal | Imputed | 17.201 | 1.61 × 10−15 | 8.05 × 10−15 | *** |
| Early follicular | Mid-luteal | Imputed | 23.709 | 4.85 × 10−18 | 7.28 × 10−17 | *** |
| Early follicular | Late luteal | Imputed | 6.045 | 3.88 × 10−6 | 7.28 × 10−6 | *** |
| Mid-follicular | Periovulatory | Imputed | 3.574 | 0.0015 | 0.0016 | ** |
| Mid-follicular | Early luteal | Imputed | 15.967 | 3.58 × 10−15 | 1.34 × 10−14 | *** |
| Mid-follicular | Mid-luteal | Imputed | 19.535 | 1.12 × 10−16 | 8.42 × 10−16 | *** |
| Mid-follicular | Late luteal | Imputed | 8.133 | 1.58 × 10−8 | 3.40 × 10−8 | *** |
| Periovulatory | Early luteal | Imputed | 14.474 | 3.87 × 10−11 | 1.16 × 10−10 | *** |
| Periovulatory | Mid- luteal | Imputed | 13.235 | 7.12 × 10−11 | 1.78 × 10−10 | *** |
| Periovulatory | Late luteal | Imputed | 4.126 | 0.0005 | 0.0006 | *** |
| Early luteal | Mid- luteal | Imputed | 3.686 | 0.0013 | 0.0015 | ** |
| Early luteal | Late luteal | Imputed | −4.128 | 0.0004 | 0.0005 | *** |
| Mid-luteal | Late luteal | Imputed | −5.995 | 7.28 × 10−6 | 1.21 × 10−5 | *** |
| Early follicular | Mid-follicular | Realigned | −4.580 | 0.0001 | 0.0002 | *** |
| Early follicular | Periovulatory | Realigned | 1.509 | 0.1450 | 0.1450 | ns |
| Early follicular | Early luteal | Realigned | 22.650 | 9.75 × 10−17 | 7.31 × 10−16 | *** |
| Early follicular | Mid-luteal | Realigned | 22.787 | 2.77 × 10−17 | 4.16 × 10−16 | *** |
| Early follicular | Late luteal | Realigned | 5.254 | 9.72 × 10−5 | 0.0002 | *** |
| Mid-follicular | Periovulatory | Realigned | 4.808 | 0.0001 | 0.0002 | *** |
| Mid-follicular | Early luteal | Realigned | 21.454 | 8.86 × 10−15 | 4.43 × 10−14 | *** |
| Mid-follicular | Mid-luteal | Realigned | 16.726 | 1.29 × 10−13 | 4.84 × 10−13 | *** |
| Mid-follicular | Late luteal | Realigned | 8.090 | 1.20 × 10−6 | 2.57 × 10−6 | *** |
| Periovulatory | Early luteal | Realigned | 14.575 | 4.08 × 10−12 | 1.13 × 10−11 | *** |
| Periovulatory | Mid-luteal | Realigned | 13.421 | 4.51 × 10−12 | 1.13 × 10−11 | *** |
| Periovulatory | Late luteal | Realigned | 2.743 | 0.0150 | 0.0171 | * |
| Early luteal | Mid-luteal | Realigned | 2.661 | 0.0160 | 0.0171 | * |
| Early luteal | Late luteal | Realigned | −3.165 | 0.0080 | 0.0100 | ** |
| Mid-luteal | Late luteal | Realigned | −4.915 | 0.0002 | 0.0002 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidovic, A.; Davis, J.; Soumare, F.; Datta, A.; Naveed, A. Trajectories of Allopregnanolone and Allopregnanolone to Progesterone Ratio across the Six Subphases of Menstrual Cycle. Biomolecules 2023, 13, 652. https://doi.org/10.3390/biom13040652
Hamidovic A, Davis J, Soumare F, Datta A, Naveed A. Trajectories of Allopregnanolone and Allopregnanolone to Progesterone Ratio across the Six Subphases of Menstrual Cycle. Biomolecules. 2023; 13(4):652. https://doi.org/10.3390/biom13040652
Chicago/Turabian StyleHamidovic, Ajna, John Davis, Fatimata Soumare, Avisek Datta, and Aamina Naveed. 2023. "Trajectories of Allopregnanolone and Allopregnanolone to Progesterone Ratio across the Six Subphases of Menstrual Cycle" Biomolecules 13, no. 4: 652. https://doi.org/10.3390/biom13040652
APA StyleHamidovic, A., Davis, J., Soumare, F., Datta, A., & Naveed, A. (2023). Trajectories of Allopregnanolone and Allopregnanolone to Progesterone Ratio across the Six Subphases of Menstrual Cycle. Biomolecules, 13(4), 652. https://doi.org/10.3390/biom13040652







