Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration
Abstract
:1. Introduction
2. dECM Derived from Different Sources
2.1. (Stem) Cell-Derived dECM (Decellularized Cell Sheet)
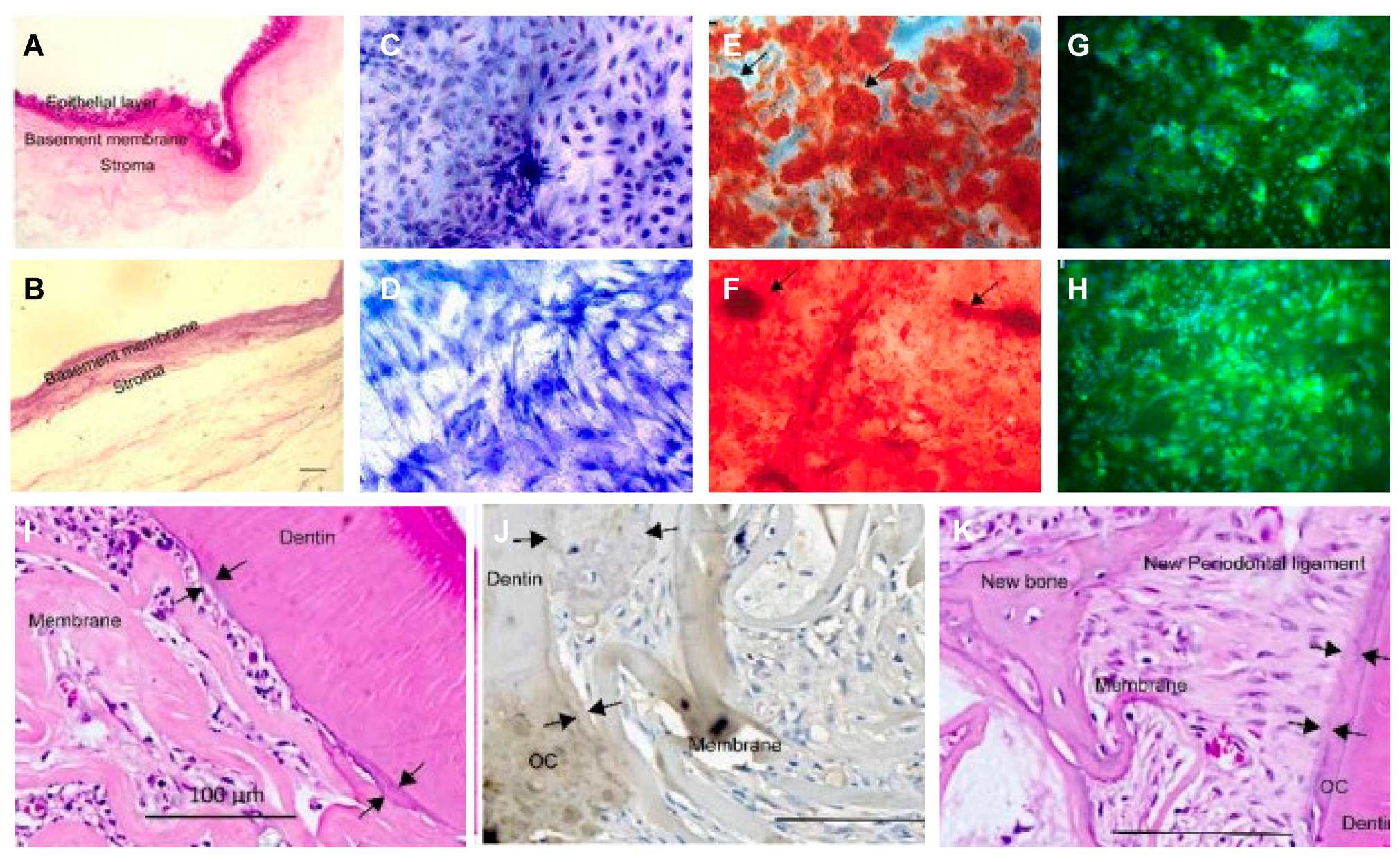
- (1)
- Periodontal ligament (stem) cell sheets
- (2)
- BMC/BMSC sheet:
- (3)
- Urogenic mesenchymal (stem) cell sheets:
| dECM Origin | Cell/Cytokine/Scaffold | Model | Outcome |
|---|---|---|---|
| PDLC sheet | 15-Deoxy-Δ (12,14)-prostaglandin J (2) nanoparticles/nanofiber scaffold | Rat periodontal defect model | Four weeks after surgery, newly formed bone, cementum, and periodontal membrane were observed [7]. |
| PDLSC sheet | PDLSC | In vitro | Promote periodontal membrane differentiation and osteogenic differentiation [14]. |
| PDLSC sheet | PCL scaffolds | In vitro | More new attachment formation was observed [12]. |
| UMSC sheet | PDLSC | In vitro | Promote cementogenic differentiation [16]. |
| UMSC sheet and PDLSC sheet | PDLSC | In vitro | Both UECM and PECM promoted hPDLSC proliferation, attachment, spreading, and differentiation. UECM showed advantages in enhancing proliferation, osteogenesis, and angiogenesis [17]. |
| BMSC sheet | BMSC | Rat calvarial critical size defect model | Fourteen days of dECM with a high content of type IV collagen chain enrichment can promote the osteogenic differentiation of bone marrow mesenchymal cells by the FAK/PI3K/AKT pathway [4]. |
| BMSC sheet | BMSC/Electrospun poly (e-caprolactone) (PCL) fifiber mesh scaffolds | In vitro | ECM-containing constructs maintained osteogenic differentiation ofMSCs [26]. |
| MC3T3-E1 cell sheet | BMSCs/GelMA | critical-sized segmental bone defects at rabbit radius | pODM can be effectively used as a biomimetic periosteum that preserves the desirable biological molecules of ECM, but without the concerns of using exogenous cells [27]. |
| BMSCs, MC3T3 osteoblasts, L929 fibroblasts | BMSCs/electrospun PLLA/gelatin fibrous meshes | C57BL mice Subcutaneous implantation | Different celltypes produce D-ECMs with different amounts of collagen, GAG and bioactive factors because of the differences in cell proliferation and differential potential. For the osteogenic differentiation of multipotential BMSCs, it was identified that dECM derived from cells originated from bone tissues showed the strongest promotion of the osteogenic differentiation of reseeded BMSCs [22]. |
2.2. Tissue-Derived Extracellular Matrix for Periodontal Tissue Engineering Constructs
2.2.1. Human (Allogenic) Tissue Derived dECMs
- (1)
- Dental (craniofacial)-related human tissues derived dECM
- (2)
- Non-dental-related tissue-derived dECM
- a.
- Human amnion dECM
- b.
- Human umbilical vein dECM
2.2.2. Heterogenous Tissue-Derived dECMs
- (1)
- Heterogenous dental (craniofacial)-related tissues derived from dECM
- a.
- Decellularized porcine dental matrix
- b.
- Decellularized matrix of dog periodontal ligament
- c.
- Decellularized rat mandible matrix
- (2)
- Heterogenous, non-dental-related tissue-derived dECM
- a.
- SIS
- b.
- Decellularized Amnion
- c.
- Decellularized pericardium
- d.
- To sum up, decellularization of natural tissues to produce extracellular matrix is a promising method for 3D scaffolding and for investigating cell-ECM interaction during regeneration of target tissue [3,51]. The fate and behavior of mesenchymal stem cells are influenced by the stem cell niches ideal biochemical and physical cues. Ana Rita Pereira et al. compared the biological behaviors of BMSCs when exposed to C-dECM and T-dECM, and better outcomes were observed in 3D decellularized bone tissue for greater architecture complexity and physicochemical properties [52]. To sum up, tissue-derived dECM has great potential in the context of endogenous periodontal regeneration, with a better effect on preserving the tissue niche intended for different tissues in periodontal defects.
| dECM Type | dECMOrigins | Host Species | Cell/Cytokines | Scaffold | Model | Outcome |
|---|---|---|---|---|---|---|
| Dental (cranial)-associated tissue derived-dECM | Swine deciduous incisor teeth dECM | Immunodeficien mice | hDFCs/rat DFCs/RSG | Cone shape scaffold, 10 mm in length, 4 mm in diameter | Dorsum transplanted in immunodeficien mice | Effectively decrease the expression of IL-1 and TNFα, and increase the expression of IL-10 and TGFβ to enable favorable immunomodulation and promote the soft/hard interface and their effective integration with host-local tissue by PPAR γ to induce alternatively activated macrophages [42]. |
| Mouse decellularized mandible bone with a PDL matrix | Rat | - | - | Implanted under the subrenal capsule in rat | Decellularized PDL matrix retained the collagen fiber structure and can reconstruct PDL tissue by controlling host cell migration, which could serve as a novel periodontal treatment approach [44]. | |
| Decellularized human tooth slices | - | PDLSCs | - | In vitro | A decellularized scaffold could support repopulation of PDL stem cells near the cementum and express cementum- and periodontal-ligament-related genes [30]. | |
| Decellularized Human Tooth Scaffold | Immunosuppressed mouse | PDLSCs DPSCs | Subcutaneously transplantation | A regeneration of the cementum/PDL complex could be expected [31]. | ||
| Porcine SIS dECM | Rat | PDLSCs | - | Rat cranial defect model | dECM promotes bone tissue regeneration, and the crosslinked dECM has a better effect on regenerating more bone tissue [45]. | |
| Non-dental(cranial)-associated tissue derived-dECM | Human Decellularized Amniotic Membrane | Rat | Adipose-derived stromal cells | Mineralized extracellular matrix | Rat periodontal furcation defect model | DAM promoted neovascularization and promoted osteoconduction DAM with ASC or without cells, and the ECM ensures bone tissue healing [13]. |
| Bovine Pericardium Membranes | - | Human periodontal ligament fibroblasts | - | In Vitro | Cellular migration along and within the layers of the membrane, binding with membrane fibers by means of filopodial extensions [48]. | |
| Porcine decellularized pericardial tissues | - | NIH3T3, C2C12, and mesenchymal stem cells | - | In Vitro | 3D-reconstructed decellularized pericardium with cells has the potential to be an attractive alternative to living tissues, such as ligament and tendon tissues [49]. | |
| Epiphyseal plate | Porcine | - | - | hBMSC | Support hBMSC proliferation and an increase in the expression of collagen types I, II, and X [8]. |
3. Application of dECM of Different Forms in Periodontal Regeneration
3.1. Solid Decellularized Extracellular Matrix (s-dECM)
- (1)
- Direct application of pure dECM
- (2)
- dECM-based hybrid scaffold
3.2. Soluble Decellularized Extracellular Matrix
3.2.1. Development of Soluble dECM
3.2.2. Application Practice of Soluble dECM
- (1)
- Grinded dECM powder/granule
- (2)
- Nanoparticles/microdroplets
- (3)
- Hydrogel
- a.
- 2D coating and hydrogel
- b.
- 3D hydrogel
4. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, Y.D.; Kim, K.H.; Lee, Y.M.; Ku, Y.; Seol, Y.J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Su, X.; Wang, T.; Guo, S. Identification of Biomarkers That Modulate Osteogenic Differentiation in Mesenchymal Stem Cells Related to Inflammation and Immunity: A Bioinformatics-Based Comprehensive Study. Pharmaceuticals 2022, 15, 1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pei, M.; Li, Q.; Zhang, Y. Decellularized extracellular matrix mediates tissue construction and regeneration. Front. Med. 2022, 16, 56–82. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yang, H.; Wu, J.; Wang, A.; Chen, X.; Hu, S.; Zhang, Y.; Bai, D.; Jin, Z. COL4A2 in the tissue-specific extracellular matrix plays important role on osteogenic differentiation of pessssriodontal ligament stem cells. Theranostics 2019, 9, 4265–4286. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Ventura, R.; Lee, B.T. Functionalization of porous BCP scaffold by generating cell-derived extracellular matrix from rat bone marrow stem cells culture for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e1256–e1267. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, G.Y.; Tang, C.; Wei, B.; Pei, X.; Gui, J.C.; Min, B.H.; Jin, C.Z.; Wang, L.M. Preparation and characterization of bone marrow mesenchymal stem cell-derived extracellular matrix scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 670–678. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.M.; Huang, J.P.; Lu, K.X.; Sun, W.L.; Tan, J.Y.; Li, B.X.; Chen, L.L.; Wu, Y. Regeneration potential of decellularized periodontal ligament cell sheets combined with 15-Deoxy-Δ12,14-prostaglandin J2 nanoparticles in a rat periodontal defect. Biomed. Mater. 2021, 16, 045008. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Tran Nguyen, D.H.; Nguyen, D.T.D.; Nguyen, D.H.; Truong, M.D. Decellularized Porcine Epiphyseal Plate-Derived Extracellular Matrix Powder: Synthesis and Characterization. Cells Tissues Organs 2020, 209, 101–109. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Mei, T.; Cao, H.; Hu, Y.; Jia, W.; Wang, J.; Zhang, Z.; Wang, Z.; Le, W.; et al. hESCs-Derived Early Vascular Cell Spheroids for Cardiac Tissue Vascular Engineering and Myocardial Infarction Treatment. Adv. Sci. (Weinh) 2022, 9, e2104299. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Liu, C.; Zhang, S.; Gao, F.; Guo, W.; Sun, X.; Zhang, C.; Li, H.; Rao, Z.; et al. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials 2021, 268, 120596. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.H.; Han, W.; Hwang, H.J.; Jang, J.; Kim, J.Y.; Cho, D.W. Extracellular matrix-based sticky sealants for scar-free corneal tissue reconstruction. Biomaterials 2023, 292, 121941. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J. Clin. Periodontol. 2018, 45, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Dziedzic, D.S.M.; Mogharbel, B.F.; Irioda, A.C.; Stricker, P.E.F.; Perussolo, M.C.; Franco, C.R.C.; Chang, H.W.; Abdelwahid, E.; de Carvalho, K.A.T. Adipose-Derived Stromal Cells and Mineralized Extracellular Matrix Delivery by a Human Decellularized Amniotic Membrane in Periodontal Tissue Engineering. Membranes 2021, 11, 606. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Volpato, F.Z.; Hutmacher, D.W.; Ivanovski, S. Assessment of static and perfusion methods for decellularization of PCL membrane-supported periodontal ligament cell sheet constructs. Arch. Oral Biol. 2018, 88, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Zheng, Z.; Yao, M.; Zhu, F.; Shen, T.; Li, J.; Zhu, C.; Yang, T.; Shao, M.; Wan, Z.; et al. A regulatory mechanism of a stepwise osteogenesis-mimicking decellularized extracellular matrix on the osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Mater. Chem. B 2022, 10, 6171–6180. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, X.; Zhou, W.; Feng, G.; Zhang, Y.; Dai, H.; Zhou, J. Effects of human urine-derived stem cells on the cementogenic differentiation of indirectly-cocultured periodontal ligament stem cells. Am. J. Transl. Res. 2020, 12, 361–378. [Google Scholar]
- Xiong, X.; Yang, X.; Dai, H.; Feng, G.; Zhang, Y.; Zhou, J.; Zhou, W. Extracellular matrix derived from human urine-derived stem cells enhances the expansion, adhesion, spreading, and differentiation of human periodontal ligament stem cells. Stem. Cell Res. Ther. 2019, 10, 396. [Google Scholar] [CrossRef] [Green Version]
- Hoshiba, T.; Lu, H.; Yamada, T.; Kawazoe, N.; Tateishi, T.; Chen, G. Effects of extracellular matrices derived from different cell sources on chondrocyte functions. Biotechnol. Prog. 2011, 27, 788–795. [Google Scholar] [CrossRef]
- Huang, J.P.; Wu, Y.M.; Liu, J.M.; Zhang, L.; Li, B.X.; Chen, L.L.; Ding, P.H.; Tan, J.Y. Decellularized matrix could affect the proliferation and differentiation of periodontal ligament stem cells in vitro. J. Periodontal. Res. 2021, 56, 929–939. [Google Scholar] [CrossRef]
- Junka, R.; Zhou, X.; Wang, W.; Yu, X. Albumin-Coated Polycaprolactone (PCL)-Decellularized Extracellular Matrix (dECM) Scaffold for Bone Regeneration. ACS Appl. Bio. Mater. 2022, 5, 5634–5644. [Google Scholar] [CrossRef]
- Ventura, R.D.; Padalhin, A.R.; Kim, B.; Park, M.; Lee, B.T. Evaluation of bone regeneration potential of injectable extracellular matrix (ECM) from porcine dermis loaded with biphasic calcium phosphate (BCP) powder. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110663. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Huang, Z.H.; Jing, W.; Wei, P.F.; Jin, L.; Zhang, X.H.; Cai, Q.; Deng, X.L.; Yang, X.P. Directing osteogenic differentiation of BMSCs by cell-secreted decellularized extracellular matrixes from different cell types. J. Mater. Chem. B 2018, 6, 7471–7485. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Fani, N.; Eglin, D.; Alini, M.; Stoddart, M.J.; Baghaban Eslaminejad, M. Human umbilical cord-derived scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. A 2019, 107, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Thibault, R.A.; Scott Baggett, L.; Mikos, A.G.; Kasper, F.K. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng. Part A 2010, 16, 431–440. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Zhang, W.; Wang, H.; Li, J.; Pan, L.; Han, F.; Li, B. Biomimetic periosteum-bone substitute composed of preosteoblast-derived matrix and hydrogel for large segmental bone defect repair. Acta. Biomater. 2020, 113, 317–327. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Bharadwaj, S.; Hammam, N.; Carnagey, K.; Myers, R.; Atala, A.; Van Dyke, M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 2009, 30, 4021–4028. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Danilova, T.I.; Kuznetsova, A.V.; Popova, O.P.; Yanushevich, O.O. Decellularized Matrix Induced Spontaneous Odontogenic and Osteogenic Differentiation in Periodontal Cells. Biomolecules 2023, 13, 122. [Google Scholar] [CrossRef]
- Son, H.; Jeon, M.; Choi, H.J.; Lee, H.S.; Kim, I.H.; Kang, C.M.; Song, J.S. Decellularized human periodontal ligament for periodontium regeneration. PLoS ONE 2019, 14, e0221236. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.H.; Jeon, M.; Cheon, K.; Kim, S.H.; Jung, H.S.; Shin, Y.; Kang, C.M.; Kim, S.O.; Choi, H.J.; Lee, H.S.; et al. In Vivo Evaluation of Decellularized Human Tooth Scaffold for Dental Tissue Regeneration. Appl. Sci. 2021, 11, 8472. [Google Scholar] [CrossRef]
- Iwasaki, K.; Peng, Y.; Kanda, R.; Umeda, M.; Ishikawa, I. Stem Cell Transplantation and Cell-Free Treatment for Periodontal Regeneration. Int. J. Mol. Sci. 2022, 23, 1011. [Google Scholar] [CrossRef]
- Liang, J.; Yi, P.; Wang, X.; Huang, F.; Luan, X.; Zhao, Z.; Liu, C. Acellular matrix hydrogel for repair of the temporomandibular joint disc. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2995–3007. [Google Scholar] [CrossRef]
- Lee, D.J.; Miguez, P.; Kwon, J.; Daniel, R.; Padilla, R.; Min, S.; Zalal, R.; Ko, C.C.; Shin, H.W. Decellularized pulp matrix as scaffold for mesenchymal stem cell mediated bone regeneration. J. Tissue Eng. 2020, 11, 2041731420981672. [Google Scholar] [CrossRef]
- Iwasaki, K.; Akazawa, K.; Nagata, M.; Komaki, M.; Honda, I.; Morioka, C.; Yokoyama, N.; Ayame, H.; Yamaki, K.; Tanaka, Y.; et al. The Fate of Transplanted Periodontal Ligament Stem Cells in Surgically Created Periodontal Defects in Rats. Int. J. Mol. Sci. 2019, 20, 192. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, N.; Lavu, V.; Balaji, S.K. Clinical efficacy of amniotic membrane with biphasic calcium phosphate in guided tissue regeneration of intrabony defects- a randomized controlled clinical trial. Biomater. Res. 2021, 25, 15. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef]
- Imamura, K.; Hamada, Y.; Yoshida, W.; Murakami, T.; Nakane-Koyachi, S.; Yoshikawa, K.; Saito, A. Investigating the Effects of Dehydrated Human Amnion-Chorion Membrane on Periodontal Healing. Biomolecules 2022, 12, 857. [Google Scholar] [CrossRef]
- Adachi, K.; Amemiya, T.; Nakamura, T.; Honjyo, K.; Kumamoto, S.; Yamamoto, T.; Bentley, A.J.; Fullwood, N.J.; Kinoshita, S.; Kanamura, N. Human periodontal ligament cell sheets cultured on amniotic membrane substrate. Oral Dis. 2014, 20, 582–590. [Google Scholar] [CrossRef]
- Goktas, S.; Pierre, N.; Abe, K.; Dmytryk, J.; McFetridge, P.S. Cellular interactions and biomechanical properties of a unique vascular-derived scaffold for periodontal tissue regeneration. Tissue Eng. Part A 2010, 16, 769–780. [Google Scholar] [CrossRef]
- Goktas, S.; Matuska, A.M.; Pierre, N.; Gibson, T.M.; Dmytryk, J.J.; McFetridge, P.S. Decellularization method influences early remodeling of an allogenic tissue scaffold. J. Biomed. Mater. Res. A 2014, 102, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liao, L.; Zhu, T.; Xu, Y.; Bi, F.; Xie, L.; Li, H.; Huo, F.; Tian, W.; Guo, W. Xenogeneic native decellularized matrix carrying PPARγ activator RSG regulating macrophage polarization to promote ligament-to-bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Nah, H.; Heo, D.N.; Kim, K.-H.; Seok, J.M.; Heo, M.; Moon, H.-J.; Lee, D.; Lee, J.S.; An, S.Y.; et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon 2020, 168, 264–277. [Google Scholar] [CrossRef]
- Nakamura, N.; Ito, A.; Kimura, T.; Kishida, A. Extracellular Matrix Induces Periodontal Ligament Reconstruction In Vivo. Int. J. Mol. Sci. 2019, 20, 3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, M.; Huang, Y.Z.; Hu, J.G.; Jiang, Y.L.; Zhang, X.Z.; Su, N.C.; Lei, Y.; Zhang, H.; Wang, H.; Xie, H.Q. Epigallocatechin-3-gallate Cross-Linked Small Intestinal Submucosa for Guided Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5024–5035. [Google Scholar] [CrossRef]
- Wilshaw, S.P.; Kearney, J.; Fisher, J.; Ingham, E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng. Part A 2008, 14, 463–472. [Google Scholar] [CrossRef]
- Semyari, H.; Rajipour, M.; Sabetkish, S.; Sabetkish, N.; Abbas, F.M.; Kajbafzadeh, A.M. Evaluating the bone regeneration in calvarial defect using osteoblasts differentiated from adipose-derived mesenchymal stem cells on three different scaffolds: An animal study. Cell Tissue Bank. 2016, 17, 69–83. [Google Scholar] [CrossRef]
- Bianchi, S.; Bernardi, S.; Simeone, D.; Torge, D.; Macchiarelli, G.; Marchetti, E. Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study. Materials 2022, 15, 8284. [Google Scholar] [CrossRef]
- Suzuki, M.; Kimura, T.; Yoshida, Y.; Kobayashi, M.; Hashimoto, Y.; Takahashi, H.; Shimizu, T.; Anzai, S.; Nakamura, N.; Kishida, A. In Vitro Tissue Reconstruction Using Decellularized Pericardium Cultured with Cells for Ligament Regeneration. Polymers 2022, 14, 2351. [Google Scholar] [CrossRef]
- Suzuki, M.; Kimura, T.; Nakano, Y.; Kobayashi, M.; Okada, M.; Matsumoto, T.; Nakamura, N.; Hashimoto, Y.; Kishida, A. Preparation of mineralized pericardium by alternative soaking for soft-hard interregional tissue application. J. Biomed. Mater. Res. A 2023, 111, 198–208. [Google Scholar] [CrossRef]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the retention of tissue niches. J. Tissue Eng. 2022, 13, 20417314221101151. [Google Scholar] [CrossRef]
- Pereira, A.R.; Trivanović, D.; Stahlhut, P.; Rudert, M.; Groll, J.; Herrmann, M. Preservation of the naïve features of mesenchymal stromal cells in vitro: Comparison of cell- and bone-derived decellularized extracellular matrix. J. Tissue Eng. 2022, 13. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Huang, J.J.; Liu, Y.; Chen, C.W.; Qu, G.W.; Wang, G.F.; Zhao, Y.; Wu, X.W.; Ren, J.A. Extracellular matrix bioink boosts stemness and facilitates transplantation of intestinal organoids as a biosafe Matrigel alternative. Bioeng. Transl. Med. 2023, 8, e10327. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L.; et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019, 10, 5658. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Liang, Z.; Lee, Y.W.; Yung, P.S.H.; Lui, P.P.Y. Bioactive Decellularized Tendon-Derived Stem Cell Sheet for Promoting Graft Healing After Anterior Cruciate Ligament Reconstruction. Am. J. Sport. Med. 2023, 51, 66–80. [Google Scholar] [CrossRef]
- Zhitkovich, A. Ascorbate: Antioxidant and biochemical activities and their importance for in vitro models. Arch. Toxicol. 2021, 95, 3623–3631. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Amirazad, H.; Dadashpour, M.; Zarghami, N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J. Biol. Eng. 2022, 16, 1. [Google Scholar] [CrossRef]
- Rothrauff, B.B.; Tuan, R.S. Decellularized bone extracellular matrix in skeletal tissue engineering. Biochem. Soc. Trans. 2020, 48, 755–764. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.; Tang, Y.; Qu, J.; Cao, Y.; Zheng, C.; Chen, Y.; Li, M.; Zhao, C.; Sun, L.; et al. Book-Shaped Acellular Fibrocartilage Scaffold with Cell-loading Capability and Chondrogenic Inducibility for Tissue-Engineered Fibrocartilage and Bone-Tendon Healing. ACS Appl. Mater. Interfaces 2019, 11, 2891–2907. [Google Scholar] [CrossRef]
- Meng, L.; Wei, Y.; Liang, Y.; Hu, Q.; Xie, H. Stem cell homing in periodontal tissue regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1017613. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Mizuno, N.; Nagahara, T.; Kaneda-Ikeda, E.; Kajiya, M.; Sasaki, S.; Takeda, K.; Kiyota, M.; Yagi, R.; Fujita, T.; et al. Cytokines regulate stemness of mesenchymal stem cells via miR-628-5p during periodontal regeneration. J. Periodontol. 2022, 93, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, K.; Iwasaki, K.; Nagata, M.; Yokoyama, N.; Ayame, H.; Yamaki, K.; Tanaka, Y.; Honda, I.; Morioka, C.; Kimura, T.; et al. Cell transfer technology for tissue engineering. Inflamm. Regen. 2017, 37, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorsand, A.; Eslaminejad, M.B.; Arabsolghar, M.; Paknejad, M.; Ghaedi, B.; Rokn, A.R.; Moslemi, N.; Nazarian, H.; Jahangir, S. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J. Oral Implantol. 2013, 39, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Seshima, F.; Bizenjima, T.; Aoki, H.; Imamura, K.; Kita, D.; Irokawa, D.; Matsugami, D.; Kitamura, Y.; Yamashita, K.; Sugito, H.; et al. Periodontal Regenerative Therapy Using rhFGF-2 and Deproteinized Bovine Bone Mineral versus rhFGF-2 Alone: 4-Year Extended Follow-Up of a Randomized Controlled Trial. Biomolecules 2022, 12, 1682. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Peterson, B.; Whang, P.G.; Iglesias, R.; Wang, J.C.; Lieberman, J.R. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J. Bone Joint Surg. Am. 2004, 86, 2243–2250. [Google Scholar] [CrossRef]
- Tabuchi, M.; Negishi, J.; Yamashita, A.; Higami, T.; Kishida, A.; Funamoto, S. Effect of decellularized tissue powders on a rat model of acute myocardial infarction. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 494–500. [Google Scholar] [CrossRef]
- Spang, M.T.; Christman, K.L. Extracellular matrix hydrogel therapies: In vivo applications and development. Acta. Biomater. 2018, 68, 1–14. [Google Scholar] [CrossRef]
- Choi, J.S.; Yang, H.J.; Kim, B.S.; Kim, J.D.; Kim, J.Y.; Yoo, B.; Park, K.; Lee, H.Y.; Cho, Y.W. Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J. Control. Release 2009, 139, 2–7. [Google Scholar] [CrossRef]
- Gao, C.; Sow, W.T.; Wang, Y.; Wang, Y.; Yang, D.; Lee, B.H.; Matičić, D.; Fang, L.; Li, H.; Zhang, C. Hydrogel composite scaffolds with an attenuated immunogenicity component for bone tissue engineering applications. J. Mater. Chem. B 2021, 9, 2033–2041. [Google Scholar] [CrossRef]
- Datta, S.; Rameshbabu, A.P.; Bankoti, K.; Roy, M.; Gupta, C.; Jana, S.; Das, A.K.; Sen, R.; Dhara, S. Decellularized bone matrix/oleoyl chitosan derived supramolecular injectable hydrogel promotes efficient bone integration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111604. [Google Scholar] [CrossRef]
- Link, P.A.; Ritchie, A.M.; Cotman, G.M.; Valentine, M.S.; Dereski, B.S.; Heise, R.L. Electrosprayed extracellular matrix nanoparticles induce a pro-regenerative cell response. J. Tissue Eng. Regen. Med. 2018, 12, 2331–2336. [Google Scholar] [CrossRef]
- Wang, X.; Ansari, A.; Pierre, V.; Young, K.; Kothapalli, C.R.; von Recum, H.A.; Senyo, S.E. Injectable Extracellular Matrix Microparticles Promote Heart Regeneration in Mice with Post-ischemic Heart Injury. Adv. Healthc. Mater. 2022, 11, e2102265. [Google Scholar] [CrossRef]
- Sivandzade, F.; Mashayekhan, S. Design and fabrication of injectable microcarriers composed of acellular cartilage matrix and chitosan. J. Biomater. Sci. Polym. Ed. 2018, 29, 683–700. [Google Scholar] [CrossRef]
- Ungerleider, J.L.; Johnson, T.D.; Rao, N.; Christman, K.L. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods 2015, 84, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, T.; Narayan, R.; Maji, S.; Ghosh, S.K.; Maiti, T.K. Decellularized caprine liver extracellular matrix as a 2D substrate coating and 3D hydrogel platform for vascularized liver tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e1678–e1690. [Google Scholar] [CrossRef]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [CrossRef]
- DeQuach, J.A.; Mezzano, V.; Miglani, A.; Lange, S.; Keller, G.M.; Sheikh, F.; Christman, K.L. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE 2010, 5, e13039. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, T.; Jiang, J.; Mao, Y.; Zhang, A.; Zhao, J. ECM coating modification generated by optimized decellularization process improves functional behavior of BMSCs. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110039. [Google Scholar] [CrossRef]
- Weigel, T.; Brennecke, J.; Hansmann, J. Improvement of the Electronic-Neuronal Interface by Natural Deposition of ECM. Materials 2021, 14, 1378. [Google Scholar] [CrossRef] [PubMed]
- Faulk, D.M.; Londono, R.; Wolf, M.T.; Ranallo, C.A.; Carruthers, C.A.; Wildemann, J.D.; Dearth, C.L.; Badylak, S.F. ECM hydrogel coating mitigates the chronic inflammatory response to polypropylene mesh. Biomaterials 2014, 35, 8585–8595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galili, U. Avoiding detrimental human immune response against Mammalian extracellular matrix implants. Tissue Eng. Part B Rev. 2015, 21, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Farnebo, S.; Woon, C.Y.; Schmitt, T.; Joubert, L.M.; Kim, M.; Pham, H.; Chang, J. Design and characterization of an injectable tendon hydrogel: A novel scaffold for guided tissue regeneration in the musculoskeletal system. Tissue Eng. Part A 2014, 20, 1550–1561. [Google Scholar] [CrossRef]
- Shen, X.; Li, S.; Zhao, X.; Han, J.; Chen, J.; Rao, Z.; Zhang, K.; Quan, D.; Yuan, J.; Bai, Y. Dual-crosslinked regenerative hydrogel for sutureless long-term repair of corneal defect. Bioact. Mater. 2023, 20, 434–448. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, J.; Heo, C.; Yoon, J.; Kim, H.; Lee, S.H.; Park, H.W.; Heo, M.S.; Moon, H.E.; Kim, C.; et al. 3D printed multi-growth factor delivery patches fabricated using dual-crosslinked decellularized extracellular matrix-based hybrid inks to promote cerebral angiogenesis. Acta. Biomater. 2022, 157, 137–148. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Wang, X.; Yuan, S.; Huo, F.; Yi, G.; Zhang, J.; Yang, B.; Tian, W. A 3D-Bioprinted Functional Module Based on Decellularized Extracellular Matrix Bioink for Periodontal Regeneration. Adv. Sci. (Weinh) 2022, 10, 2205041. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta. Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Z.; Jia, J.; Zhang, W.; Qin, L.; Zhang, W.; Lai, Y. Preparation and characterization of photocurable composite extracellular matrix-methacrylated hyaluronic acid bioink. J. Mater. Chem. B 2022, 10, 4242–4253. [Google Scholar] [CrossRef]
- Gu, R.; Liu, H.; Zhu, Y.; Liu, X.; Wang, S.; Liu, Y. Is extracellular matrix (ECM) a promising scaffold biomaterial for bone repair? Histol. Histopathol. 2021, 36, 1219–1234. [Google Scholar] [CrossRef]
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-Derived Decellularized Extracellular Matrix: A Game Changer for Bioink Manufacturing? Trends Biotechnol. 2018, 36, 787–805. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Vaquette, C.; Saifzadeh, S.; Farag, A.; Hutmacher, D.W.; Ivanovski, S. Periodontal Tissue Engineering with a Multiphasic Construct and Cell Sheets. J. Dent. Res. 2019, 98, 673–681. [Google Scholar] [CrossRef]
- Lee, J.; Hong, J.; Kim, W.; Kim, G.H. Bone-derived dECM/alginate bioink for fabricating a 3D cell-laden mesh structure for bone tissue engineering. Carbohydr. Polym. 2020, 250, 116914. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Zheng, K.; Li, X.; Li, L.; Xu, Y. 3D Polycaprolactone/Gelatin-Oriented Electrospun Scaffolds Promote Periodontal Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 46145–46160. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J.; Zhang, Y.; Liu, H.; Dong, F. Mineralization Induction of Gingival Fibroblasts and Construction of a Sandwich Tissue-Engineered Complex for Repairing Periodontal Defects. Med. Sci. Monit. 2018, 24, 1112–1123. [Google Scholar] [CrossRef]
- Park, C.H. Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. Int. J. Mol. Sci. 2019, 20, 4364. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.W. Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments. Chem. Rev. 2020, 120, 10608–10661. [Google Scholar] [CrossRef]
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef]
- Staples, R.; Ivanovski, S.; Vaquette, C. Fibre-guiding biphasic scaffold for perpendicular periodontal ligament attachment. Acta. Biomater. 2022, 150, 221–237. [Google Scholar] [CrossRef]
- Nobakht, S.; Milne, T.J.; Duncan, W.J.; Ram, A.; Tkatchenko, T.; Dong, Z.; Coates, D.E. Expression of the pleiotrophin-midkine axis in a sheep tooth socket model of bone healing. J. Periodontal. Res. 2023, 58, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.M.B.; Figadoli, A.L.F.; Alcantara, P.L.; Pomini, K.T.; Santos German, I.J.; Reis, C.H.B.; Rosa Júnior, G.M.; Rosso, M.P.O.; Santos, P.; Zangrando, M.S.R.; et al. Biological Behavior of Xenogenic Scaffolds in Alcohol-Induced Rats: Histomorphometric and Picrosirius Red Staining Analysis. Polymers 2022, 14, 584. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Jun, S.H.; Tallarico, M.; Park, J.B.; Park, D.H.; Hwang, K.G.; Park, C.J. A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials 2022, 15, 5294. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Zhao, X.Y.; Xiong, W.; Ji, L.F.; Jia, M.X.; Liu, Y.Y.; Guo, H.T.; Qu, F.; Cui, W.; Gu, Q.; et al. Cartilage Lacuna-inspired Microcarriers Drive Hyaline Neocartilage Regeneration. Adv. Mater. 2023, 6, e2212114. [Google Scholar] [CrossRef]
- Emara, A.; Shah, R. Recent update on craniofacial tissue engineering. J. Tissue Eng. 2021, 12, 20417314211003735. [Google Scholar] [CrossRef]
- Jamalpour, M.R.; Yadegari, A.; Vahdatinia, F.; Amirabad, L.M.; Jamshidi, S.; Shojaei, S.; Shokri, A.; Moeinifard, E.; Omidi, M.; Tayebi, L. 3D-printed bi-layered polymer/hydrogel construct for interfacial tissue regeneration in a canine model. Dent. Mater. 2022, 38, 1316–1329. [Google Scholar] [CrossRef]
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. 3 D nano bilayered spatially and functionally graded scaffold impregnated bromelain conjugated magnesium doped hydroxyapatite nanoparticle for periodontal regeneration. J. Mech. Behav. Biomed. Mater. 2020, 109, 103822. [Google Scholar] [CrossRef]
 : MSCs around T-dECM(dPDL).
: MSCs around T-dECM(dPDL).
 : MSCs around T-dECM(dPDL).
: MSCs around T-dECM(dPDL).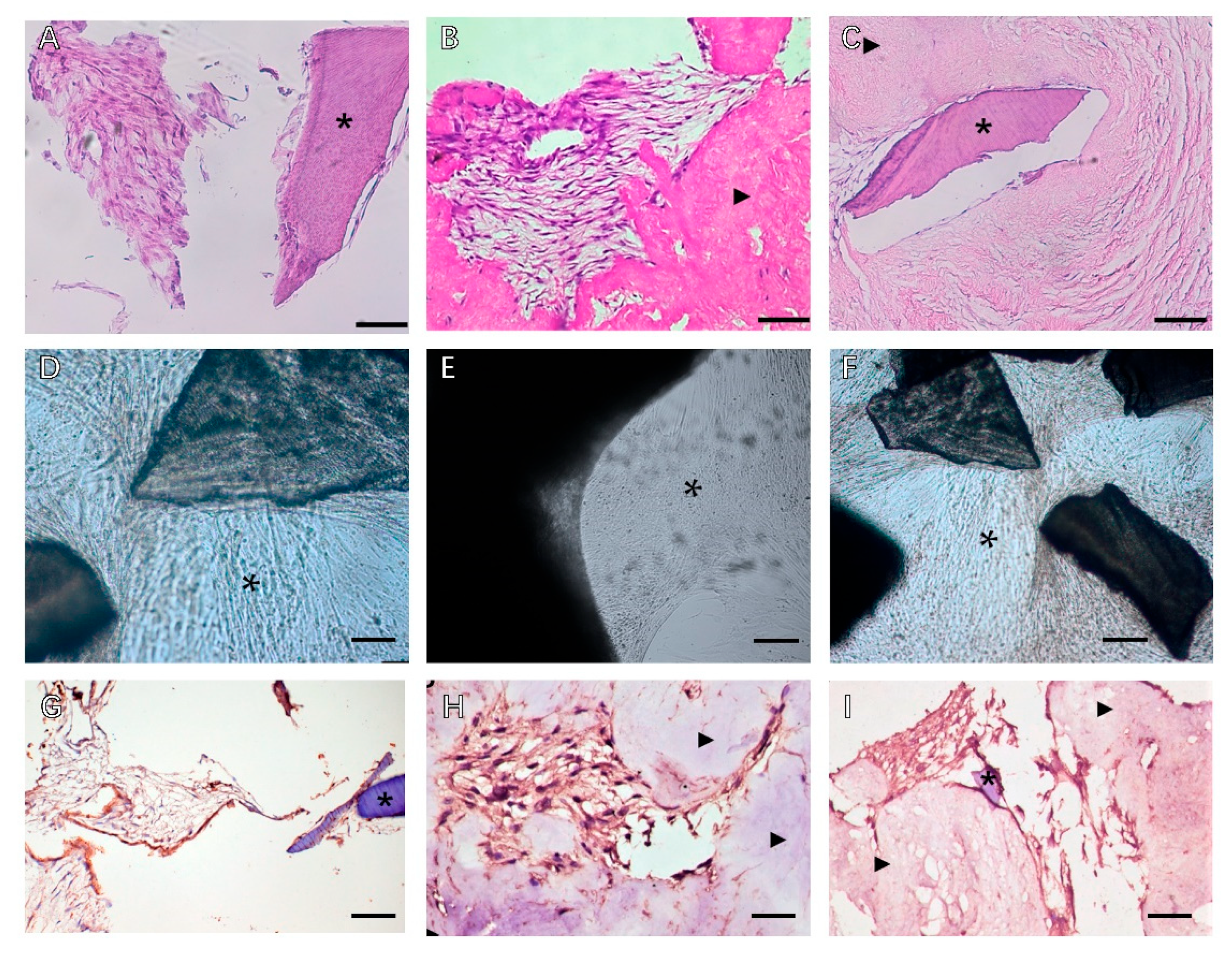
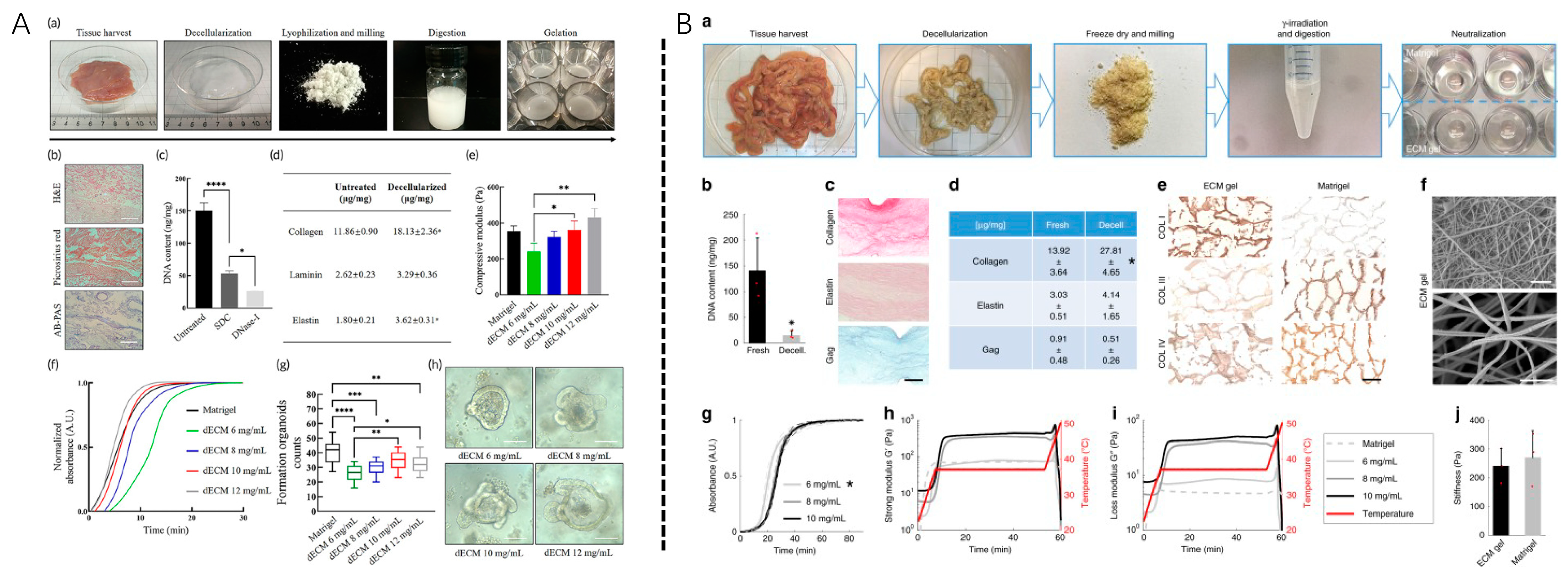
| Soluble | Soluble Degree | Cell/Cytokine/Scaffold | Model | Application |
|---|---|---|---|---|
| Particle/powder [8,70,71] | partial | Hydrogel/guiding tissue membrane [50,71] | Rat periodontal defect model; Calvarial defect model; subdermal implantation | Suspension [69] and scaffold loading [67,68] Bone powder [101,102,103]. |
| Nanoparticle/nanodrops/ | partial | - | In vivo; In vitro | Combined with electrojet technology or phase separation technology [73]. |
| 2D coating | completely | PDLSC | In vitro | Assessment of cellular responses to different dECM allows for parameters and evaluation of high-throughput analysis of rare or inaccessible pathological dECM in multiple cells [77]. |
| 2D hydrogel | completely | PDLSCs/DFCs | In vitro | With biochemical features of a tissue-specific extracellular matrix that guide the environment of cell growth [54]. |
| 3D hydrogel | completely | cytokines | Rat periodontal defect model; Cranial defect model; subdermal implantation | 3D printing technology is a useful method; multilayer dECM bioprinting scaffolds and multilayer hydrogel systems with spatial segmentation can provide controllable release of multiple growth factors to induce orderly regeneration of periodontal defects [85]. |
| Bio-inks | completely | DFCs+dECM+GelMA | Rat periodontal defect model +Beagle dog periodontal defect | With potent dECM immunomodulatory activity, ECM reduces proinflammatory factors released by M1 macrophages and reduces local inflammation in periodontal defects in SD rats; enhanced regeneration of beagle dog periodontal tissue, especially forming the anchor structure of the ideal bone-periodontal ligament interface, well-arranged periodontal fibers, and highly mineralized alveolar bone [87]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Liao, L.; Tian, W. Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration. Biomolecules 2023, 13, 673. https://doi.org/10.3390/biom13040673
Liang C, Liao L, Tian W. Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration. Biomolecules. 2023; 13(4):673. https://doi.org/10.3390/biom13040673
Chicago/Turabian StyleLiang, Chao, Li Liao, and Weidong Tian. 2023. "Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration" Biomolecules 13, no. 4: 673. https://doi.org/10.3390/biom13040673
APA StyleLiang, C., Liao, L., & Tian, W. (2023). Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration. Biomolecules, 13(4), 673. https://doi.org/10.3390/biom13040673








