Beneficial Effect of Bee Venom and Its Major Components on Facial Nerve Injury Induced in Mice
Abstract
:1. Introduction
- -
- To analyze the effect of BV therapy on behavioral recovery (whisker movement and nasal deviation) after distal section-suture of the facial nerve.
- -
- To analyze the recovery of facial motoneuron retrograde FG labeling in treated and control mice after distal section-suture of the facial nerve.
- -
- To compare the BV and betamethasone/or the BV and mixture of the bvPLA2 and melittin effects on both functional and neuronal recovery.
2. Materials and Methods
2.1. Bee Venom and Its Major Components
2.2. UHPLC Peptide Analysis of Honeybee Venom
2.3. Animals
2.4. Surgical Procedure
2.5. Treatments
2.6. Functional Analysis of Nerve Regeneration
2.6.1. Whisker Movements
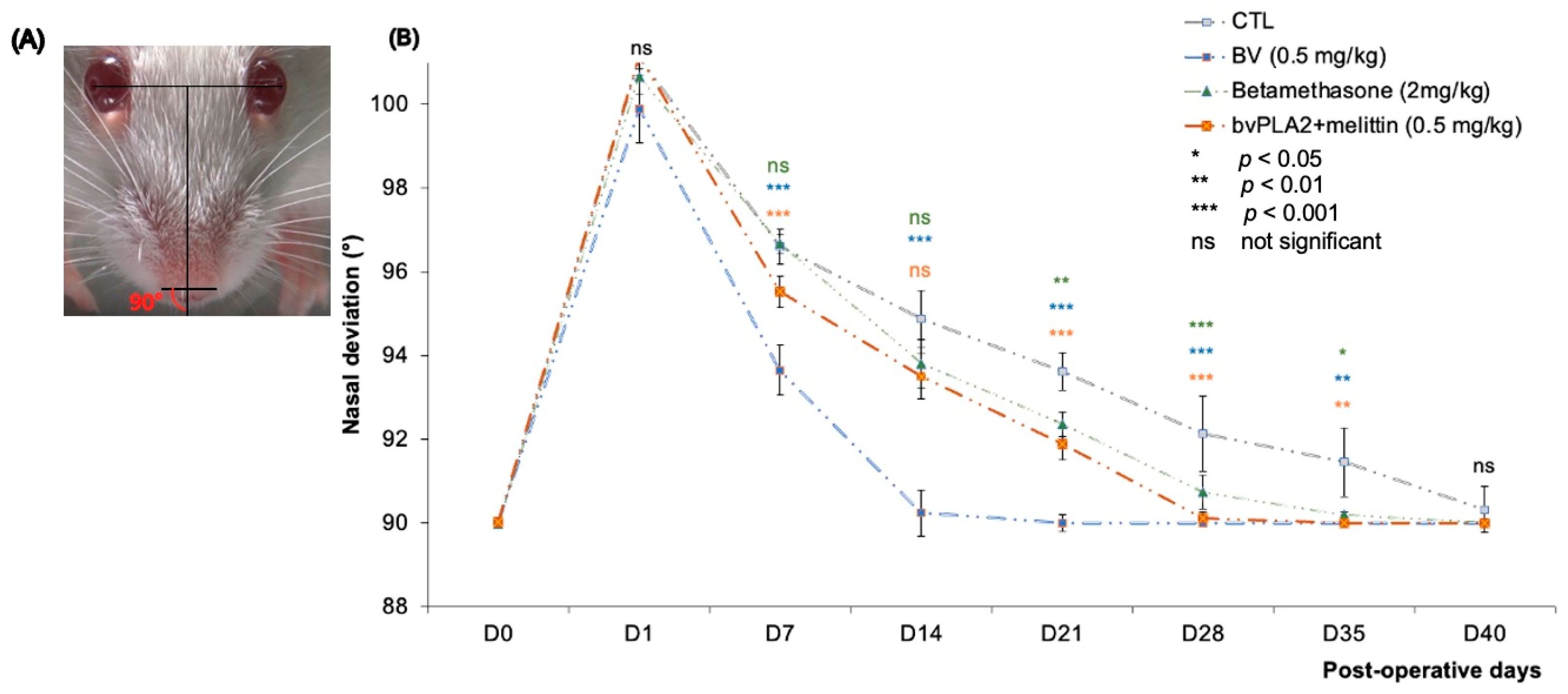
2.6.2. Measurement of Nasal Deviation
- The inter-iris line was drawn linking the center of the right and left pupil.
- A perpendicular line to the first was then drawn midway between the irises.
- A line was added connecting the outer edges of the two nostrils.
2.7. Retrograde Labeling of Regenerated Facial Motoneurons
2.8. Statistical Analysis
3. Results
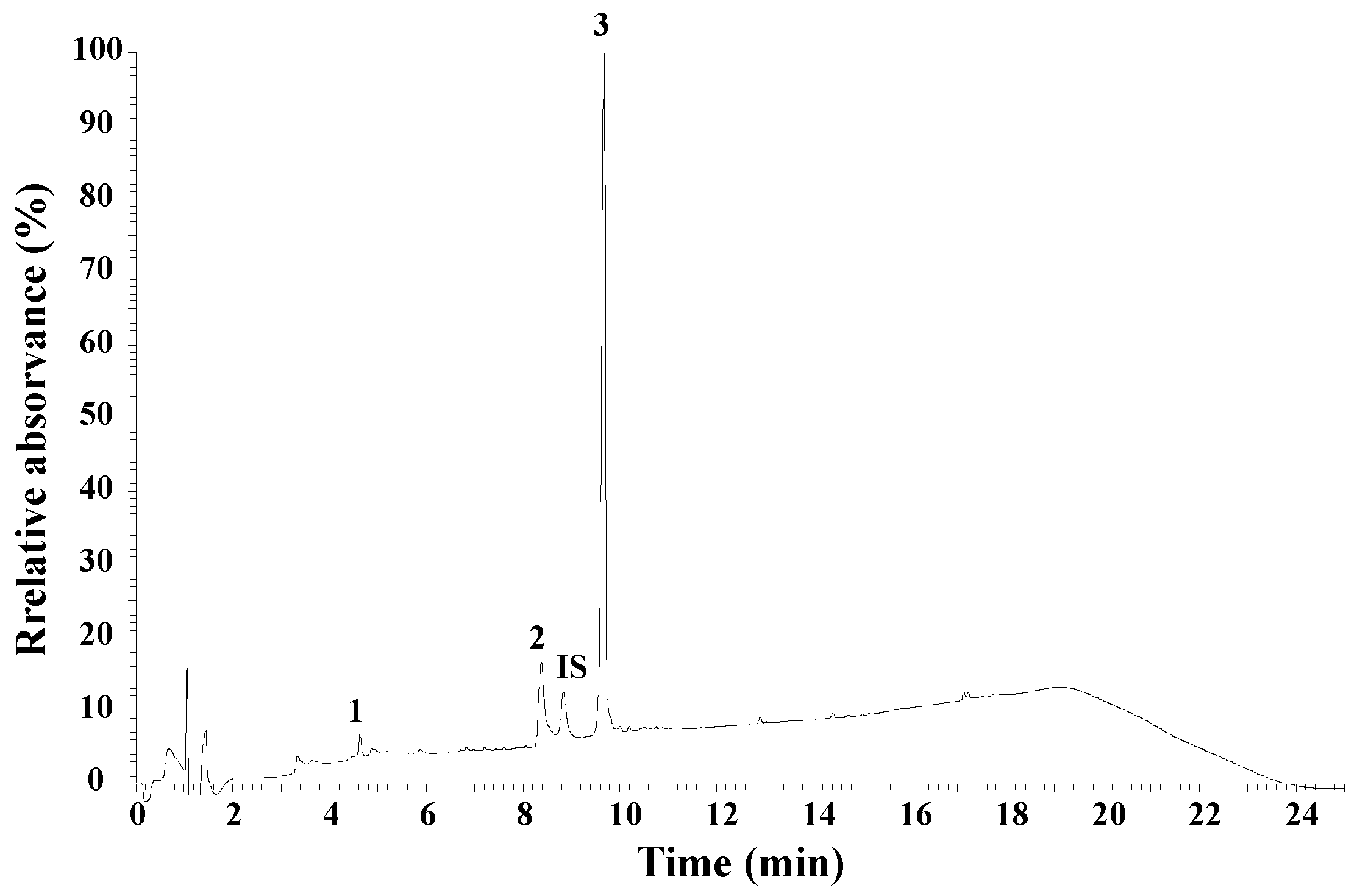
3.1. UHPLC Peptide Analysis of Honeybee Venom
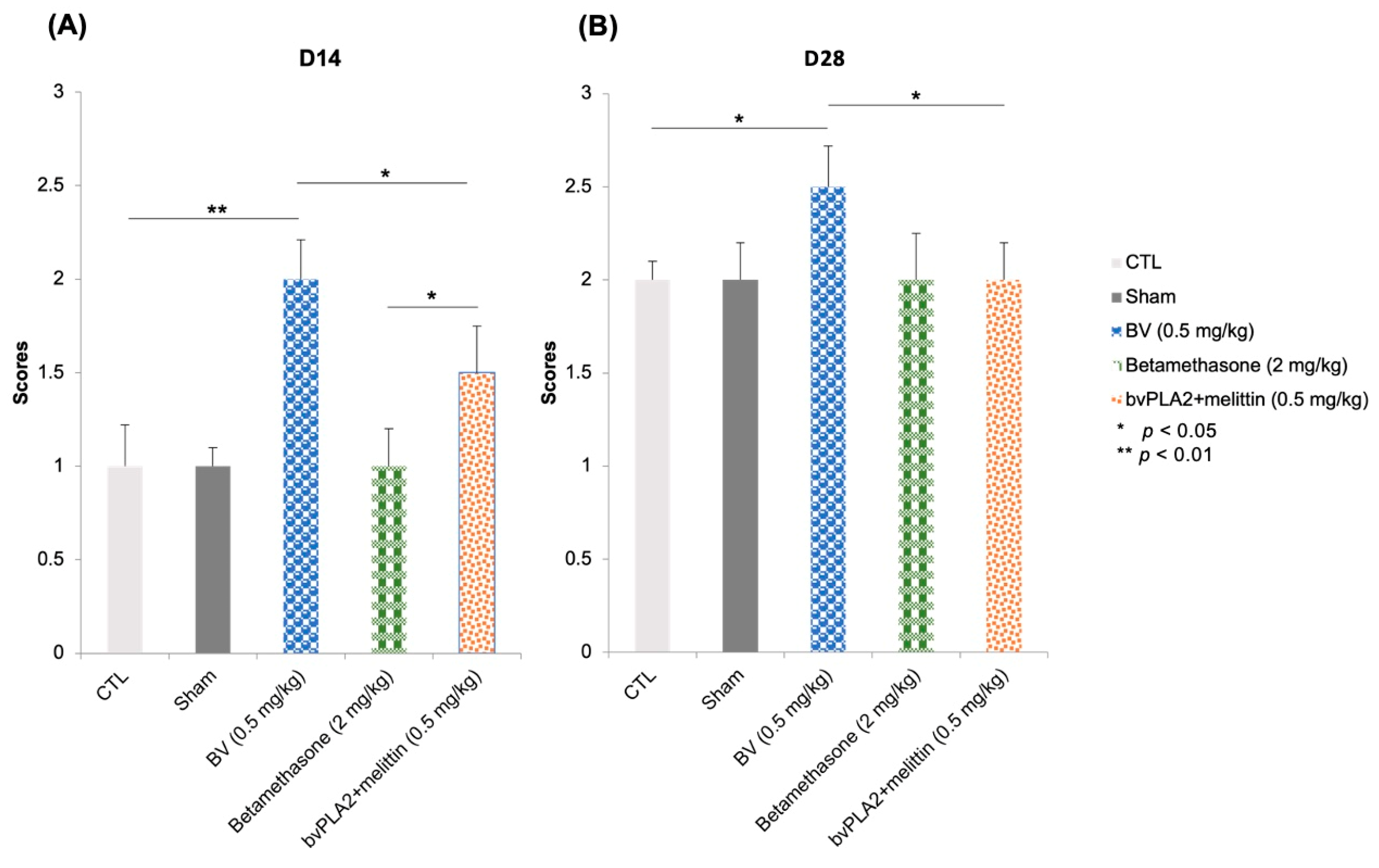
3.2. Vibrissae Mouvments
3.3. Nasal Deviation
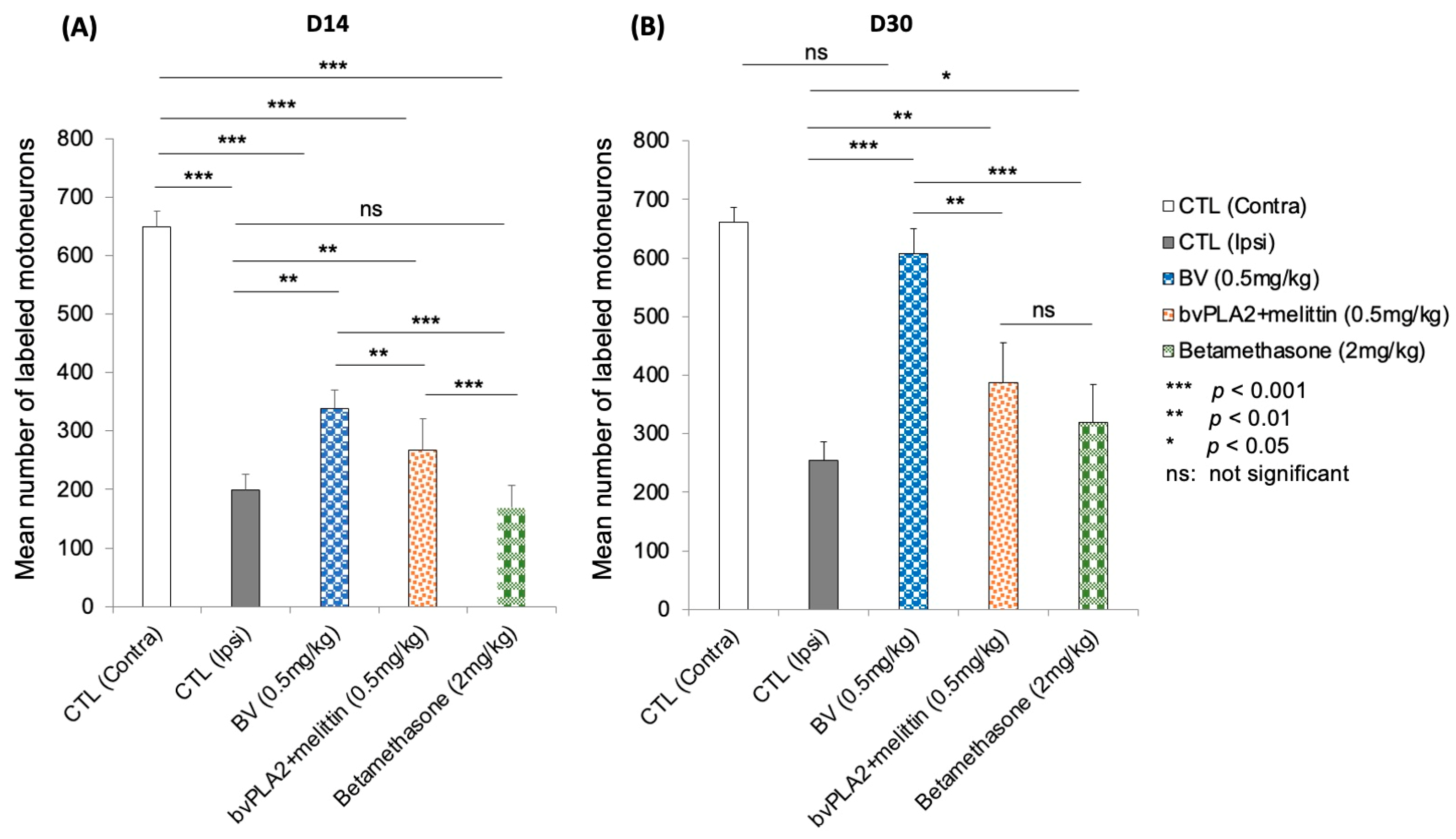
3.4. Fluorescent Retrograde Labeling of Regenerated Motoneurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kingham, P.J.; Terenghi, G. Bioengineered nerve regeneration and muscle reinnervation. J. Anat. 2006, 209, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Belkas, J.S.; Shoichet, M.S.; Midha, R. Peripheral nerve regeneration through guidance tubes. Neurol. Res. 2004, 26, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, S.; Inada, Y.; Nakamura, T. Artificial nerve tubes and their application for repair of peripheral nerve injury: An update of current concepts. Injury 2008, 39, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, H.; Huang, S. Role of NGF and its receptors in wound healing. Exp. Ther. Med. 2021, 21, 599. [Google Scholar] [CrossRef]
- Raza, C.; Riaz, H.A.; Anjum, R. Repair strategies for injured peripheral nerve. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef]
- Lee, J.I.; Hur, J.M.; You, J.; Lee, D.H. Functional recovery with histomorphometric analysis of nerves and muscles after combination treatment with erythropoietin and dexamethasone in acute peripheral nerve injury. PLoS ONE 2020, 15, e0238208. [Google Scholar] [CrossRef]
- Yildiran, H.; Macit, M.S.; Özata Uyar, G. New approach to peripheral nerve injury: Nutritional therapy. Nutr. Neurosci. 2020, 23, 744–755. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef]
- Angelov, D.N.; Ceynowa, M.; Guntinas-Lichius, O.; Streppel, M.; Grosheva, M.; Kiryakova, S.I.; Skouras, E.; Maegele, M.; Irintchev, A.; Neiss, W.F.; et al. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiol. Dis. 2007, 26, 229–242. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Neumann, C.M.; Brushart, T.M.; Gordon, T. Brief Electrical Stimulation Promotes the Speed and Accuracy of Motor Axonal Regeneration. J. Neurosci. 2000, 20, 2602–2608. [Google Scholar] [CrossRef]
- Wang, S.-M.; Tsai, H.-P.; Huang, J.-J.; Huang, H.-C.; Lin, J.-L.; Liu, P.-H. Inhibition of nitric oxide synthase promotes facial axonal regeneration following neurorrhaphy. Exp. Neurol. 2009, 216, 499–510. [Google Scholar] [CrossRef]
- Ruan, R.-S.; Leong, S.-K.; Yeoh, K.-H. The role of nitric oxide in facial motoneuronal death. Brain Res. 1995, 698, 163–168. [Google Scholar] [CrossRef]
- Varathan, V.; Shigenaga, Y.; Takemura, M. Nitric oxide synthase/nicotinamide adenine dinucleotide phosphate-diaphorase in the brainstem trigeminal nuclei after transection of the masseteric nerve in rats. J. Neurosci. Res. 2001, 66, 428–438. [Google Scholar] [CrossRef]
- Wu, W.; Li, L.; Yick, L.-W.; Chai, H.; Xie, Y.; Yang, Y.; Prevette, D.M.; Oppenheim, R.W. GDNF and BDNF alter the expression of neuronal NOS, c-Jun, and p75 and prevent motoneuron death following spinal root avulsion in adult rats. J. Neurotrauma 2003, 20, 603–612. [Google Scholar] [CrossRef]
- Zhang, L.; Johnson, D.; Johnson, J.A. Deletion of Nrf2 impairs functional recovery, reduces clearance of myelin debris and decreases axonal remyelination after peripheral nerve injury. Neurobiol. Dis. 2013, 54, 329–338. [Google Scholar] [CrossRef]
- Lanza, C.; Raimondo, S.; Vergani, L.; Catena, N.; Sénès, F.; Tos, P.; Geuna, S. Expression of antioxidant molecules after peripheral nerve injury and regeneration. J. Neurosci. Res. 2012, 90, 842–848. [Google Scholar] [CrossRef]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 2090. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Anti-Inflammatory Applications of Melittin, a Major Component of Bee Venom: Detailed Mechanism of Action and Adverse Effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Lee, G. Neuroprotective Activity of Melittin—The Main Component of Bee Venom—Against Oxidative Stress Induced by Aβ25–35 in In Vitro and In Vivo Models. Antioxidants 2021, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ke, T.; He, C.; Cao, W.; Wei, M.; Zhang, L.; Zhang, J.-X.; Wang, W.; Ma, J.; Wang, Z.-R.; et al. The Anti-Arthritic Effects of Synthetic Melittin on the Complete Freund’s Adjuvant-Induced Rheumatoid Arthritis Model in Rats. Am. J. Chin. Med. 2010, 38, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.; Shapla, U.; Gan, S.; Khalil, M.I. Impact of Bee Venom Enzymes on Diseases and Immune Responses. Molecules 2016, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Valentin, E.; Ghomashchi, F.; Gelb, M.H.; Lazdunski, M.; Lambeau, G. Novel Human Secreted Phospholipase A2 with Homology to the Group III Bee Venom Enzyme. J. Biol. Chem. 2000, 275, 7492–7496. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Chung, H.-S.; Lee, C.; Yoon, M.S.; Yu, A.R.; Kim, J.S.; Hwang, D.-S.; Shim, I.; Bae, H. Neuroprotective effects of bee venom phospholipase A2 in the 3xTg AD mouse model of Alzheimer’s disease. J. Neuroinflamm. 2016, 13, 10. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Proulx, É.; Power, S.K.; Oliver, D.K.; Sargin, D.; McLaurin, J.; Lambe, E.K. Apamin Improves Prefrontal Nicotinic Impairment in Mouse Model of Alzheimer’s Disease. Cereb. Cortex 2019, 30, 563–574. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.Y.; Lee, J.; Jeon, W.-J.; Ha, I.-H. Apamin Enhances Neurite Outgrowth and Regeneration after Laceration Injury in Cortical Neurons. Toxins 2021, 13, 603. [Google Scholar] [CrossRef]
- Ahmed-Farid, O.A.; Taha, M.; Bakeer, R.M.; Radwan, O.K.; Hendawy, H.A.M.; Soliman, A.S.; Yousef, E. Effects of bee venom and dopamine-loaded nanoparticles on reserpine-induced Parkinson’s disease rat model. Sci. Rep. 2021, 11, 21141. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, S.-M.; Yang, E.J. Melittin Ameliorates the Inflammation of Organs in an Amyotrophic Lateral Sclerosis Animal Model. Exp. Neurobiol. 2014, 23, 86–92. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.-R.; Lin, L.-T.; Xiao, L.-Y.; Zhou, P.; Shi, G.-X.; Liu, C.-Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef]
- Mehdi, I.E.; Falcão, S.I.; Boujraf, S.; Mustapha, H.; Campos, M.G.; Vilas-Boas, M. Analytical methods for honeybee venom characterization. J. Adv. Pharm. Technol. Res. 2022, 13, 154–160. [Google Scholar]
- Janvier-Labs. Available online: https://janvier-labs.com/en/fiche_produit/swiss_mouse/ (accessed on 19 February 2023).
- Semba, K.; Egger, M.D. The facial? motor? nerve of the rat: Control of vibrissal movement and examination of motor and sensory components. J. Comp. Neurol. 1986, 247, 144–158. [Google Scholar] [CrossRef]
- Er-Rouassi, H.; Benichou, L.; Lyoussi, B.; Vidal, C. Efficacy of LED Photobiomodulation for Functional and Axonal Regeneration After Facial Nerve Section-Suture. Front. Neurol. 2022, 13, 827218. [Google Scholar] [CrossRef]
- Abercrombie, M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946, 94, 239–247. [Google Scholar] [CrossRef]
- Ashwell, K.W. The adult mouse facial nerve nucleus: Morphology and musculotopic organization. J. Anat. 1982, 135, 531–538. [Google Scholar]
- Badawi, H.; Abdel-Salam, R.; Abdel-Salam, O.; Youness, E.; Shaffie, N.; Eldenshary, E.D. Bee venom attenuates neurodegeneration and motor impairment and modulates the response to L-dopa or rasagiline in a mice model of Parkinson’s disease. Iran. J. Basic Med. Sci. 2020, 23, 1628–1638. [Google Scholar] [CrossRef]
- Hwang, D.-S.; Kim, S.; Bae, H. Therapeutic Effects of Bee Venom on Immunological and Neurological Diseases. Toxins 2015, 7, 2413–2421. [Google Scholar] [CrossRef]
- Szokan, G.; Horvath, J.; Almas, M.; Saftics, G.; Palocz, A. Liquid Chromatographic Analysis and Separation of Polipeptide Components from Honey Bee Venoms. J. Liq. Chromatogr. 1994, 17, 3333–3349. [Google Scholar] [CrossRef]
- Rybak-Chmielewska, H.; Szczêsna, T. Hplc Study of Chemical Composition of Honeybee (Apis mellifera L.) Venom. J. Apic. Sci. 2004, 48, 103–109. [Google Scholar]
- Somwongin, S.; Chantawannakul, P.; Chaiyana, W. Antioxidant activity and irritation property of venoms from Apis species. Toxicon 2018, 145, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.A.; Ribchester, R.R. Persistent polyneuronal innervation in partially denervated rat muscle after reinnervation and recovery from prolonged nerve conduction block. J. Neurosci. 1995, 15, 6327–6339. [Google Scholar] [CrossRef] [PubMed]
- Eleore, L.; Vassias, I.; Vidal, P.; Triller, A.; De Waele, C. Modulation of glycine receptor subunits and gephyrin expression in the rat facial nucleus after axotomy. Eur. J. Neurosci. 2005, 21, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Raslan, A.; Ernst, P.; Werle, M.; Thieme, H.; Szameit, K.; Finkensieper, M.; Guntinas-Lichius, O.; Irintchev, A. Reduced cholinergic and glutamatergic synaptic input to regenerated motoneurons after facial nerve repair in rats: Potential implications for recovery of motor function. Brain Struct. Funct. 2014, 219, 891–909. [Google Scholar] [CrossRef]
- Kou, S.; Chiu, A.Y.; Patterson, P.H. Differential regulation of motor neuron survival and choline acetyltransferase expression following axotomy. J. Neurobiol. 1995, 27, 561–572. [Google Scholar] [CrossRef]
- Nishimura, Y.; Asahara, T.; Yamamoto, T.; Tanaka, T. Observations on morphology and electrophysiological properties of the normal and axotomlzed facial motoneurons in the cat. Brain Res. 1992, 596, 305–310. [Google Scholar] [CrossRef]
- Shi, S.; Xu, L.; Li, J.; Han, Y.; Wang, H. Different discharge properties of facial nucleus motoneurons following neurotmesis in a rat model. Neurosci. Lett. 2016, 629, 180–185. [Google Scholar] [CrossRef]
- Rochkind, S. Photobiomodulation in Neuroscience: A Summary of Personal Experience. Photomed. Laser Surg. 2017, 35, 604–615. [Google Scholar] [CrossRef]
- Sasso, L.L.; de Souza, L.G.; Girasol, C.E.; Marcolino, A.M.; de Jesus Guirro, R.R.; Barbosa, R.I. Photobiomodulation in Sciatic Nerve Crush Injuries in Rodents: A Systematic Review of the Literature and Perspectives for Clinical Treatment. J. Lasers Med. Sci. 2020, 11, 332–344. [Google Scholar] [CrossRef]
- Cajal, Y.; Jain, M.K. Synergism between Mellitin and Phospholipase A 2 from Bee Venom: Apparent Activation by Intervesicle Exchange of Phospholipids. Biochemistry 1997, 36, 3882–3893. [Google Scholar] [CrossRef]
- Tender, T.; Rahangdale, R.R.; Balireddy, S.; Nampoothiri, M.; Sharma, K.K.; Raghu Chandrashekar, H. Melittin, a honeybee venom derived peptide for the treatment of chemotherapy-induced peripheral neuropathy. Med. Oncol. 2021, 38, 52. [Google Scholar] [CrossRef]
- Li, D.; Kim, W.; Shin, D.; Jung, Y.; Bae, H.; Kim, S. Preventive Effects of Bee Venom Derived Phospholipase A2 on Oxaliplatin-Induced Neuropathic Pain in Mice. Toxins 2016, 8, 27. [Google Scholar] [CrossRef]
- Masuda, S.; Yamamoto, K.; Hirabayashi, T.; Ishikawa, Y.; Ishii, T.; Kudo, I.; Murakami, M. Human group III secreted phospholipase A2 promotes neuronal outgrowth and survival. Biochem. J. 2008, 409, 429–438. [Google Scholar] [CrossRef]
- De, S.; Trigueros, M.A.; Kalyvas, A.; David, S. Phospholipase A 2 plays an important role in myelin breakdown and phagocytosis during wallerian degeneration. Mol. Cell. Neurosci. 2003, 24, 753–765. [Google Scholar] [CrossRef]
- Stoll, G.; Jander, S.; Myers, R.R. Degeneration and regeneration of the peripheral nervous system: From Augustus Waller’s observations to neuroinflammation. J. Peripher. Nerv. Syst. 2002, 7, 13–27. [Google Scholar] [CrossRef]
- Ousman, S.S.; David, S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. GLIA 2000, 30, 92–104. [Google Scholar] [CrossRef]
- Ousman, S.S.; David, S. MIP-1α, MCP-1, GM-CSF, and TNF-α Control the Immune Cell Response That Mediates Rapid Phagocytosis of Myelin from the Adult Mouse Spinal Cord. J. Neurosci. 2001, 21, 4649–4656. [Google Scholar] [CrossRef]
- Saada, A.; Reichert, F.; Rotshenker, S. Granulocyte Macrophage Colony Stimulating Factor Produced in Lesioned Peripheral Nerves Induces the Up-Regulation of Cell Surface Expression of MAC-2 by Macrophages and Schwann Cells. J. Cell Biol. 1996, 133, 159–167. [Google Scholar] [CrossRef]
- Sommer, C.; Schafers, M. Painful mononeuropathy in C57BLrWld mice with delayed Wallerian degeneration: Differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998, 784, 154–162. [Google Scholar] [CrossRef]
- Shamash, S.; Reichert, F.; Rotshenker, S. The Cytokine Network of Wallerian Degeneration: Tumor Necrosis Factor-α, Interleukin-1α, and Interleukin-1β. J. Neurosci. 2002, 22, 3052–3060. [Google Scholar] [CrossRef]
- Boivin, A. Role des Recepteurs Toll-Like Dans la Régénérescence D’axones Périphériques. Master’s Thesis, Laval University, Quebec City, QC, Canada, 2007; p. 101. [Google Scholar]
- Scheinman, R.I.; Gualberto, A.; Jewell, C.M.; Cidlowski, J.A.; Baldwin, A.S. Characterization of Mechanisms Involved in Transrepression of NF-kappa B by Activated Glucocorticoid Receptors. Mol. Cell. Biol. 1995, 15, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Patko, T.; Vassias, I.; Vidal, P.P.; De Waele, C. Modulation of the voltage-gated sodium- and calcium-dependent potassium channels in rat vestibular and facial nuclei after unilateral labyrinthectomy and facial nerve transsection: An in situ hybridization study. Neuroscience 2003, 117, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.T. Small Conductance Ca2+-Activated K+ Channel Knock-Out Mice Reveal the Identity of Calcium-Dependent Afterhyperpolarization Currents. J. Neurosci. 2004, 24, 5301–5306. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Leonard, J.; Mourre, C.; Chabbert, C. Apamin treatment accelerates equilibrium recovery and gaze stabilization in unilateral vestibular neurectomized cats: Cellular and behavioral aspects. Neuropharmacology 2019, 144, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Monge-Fuentes, V.; Gomes, F.; Lopes, K.; Anjos, L.; Campos, G.; Arenas, C.; Biolchi, A.; Gonçalves, J.; Galante, P.; et al. Pharmacological Alternatives for the Treatment of Neurodegenerative Disorders: Wasp and Bee Venoms and Their Components as New Neuroactive Tools. Toxins 2015, 7, 3179–3209. [Google Scholar] [CrossRef]
- Moran, L.B.; Graeber, M.B. The facial nerve axotomy model. Brain Res. Rev. 2004, 44, 154–178. [Google Scholar] [CrossRef]
- Rodella, U.; Scorzeto, M.; Duregotti, E.; Negro, S.; Dickinson, B.C.; Chang, C.J.; Yuki, N.; Rigoni, M.; Montecucco, C. An animal model of Miller Fisher syndrome: Mitochondrial hydrogen peroxide is produced by the autoimmune attack of nerve terminals and activates Schwann cells. Neurobiol. Dis. 2016, 96, 95–104. [Google Scholar] [CrossRef]
- Llobet Rosell, A.; Neukomm, L.J. Axon death signalling in Wallerian degeneration among species and in disease. Open Biol. 2019, 9, 190118. [Google Scholar] [CrossRef]
- Duregotti, E.; Negro, S.; Scorzeto, M.; Zornetta, I.; Dickinson, B.C.; Chang, C.J.; Montecucco, C.; Rigoni, M. Mitochondrial alarmins released by degenerating motor axon terminals activate perisynaptic Schwann cells. Proc. Natl. Acad. Sci. USA 2015, 112, E497–E505. [Google Scholar] [CrossRef]
- Lv, W.; Deng, B.; Duan, W.; Li, Y.; Liu, Y.; Li, Z.; Xia, W.; Li, C. Schwann Cell Plasticity is Regulated by a Weakened Intrinsic Antioxidant Defense System in Acute Peripheral Nerve Injury. Neuroscience 2018, 382, 1–13. [Google Scholar] [CrossRef]
- Adham, E.K.E. Evaluating the role of propolis and bee venom on the oxidative stress induced by gamma rays in rats. Sci. Rep. 2022, 22, 2656. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; Queiroz, M.J.R.P.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef]
- Rieger, S.; Sagasti, A. Hydrogen Peroxide Promotes Injury-Induced Peripheral Sensory Axon Regeneration in the Zebrafish Skin. PLoS Biol. 2011, 9, e1000621. [Google Scholar] [CrossRef]


| Groups | Treatment | Behavioral Study | Retrograde Fluorogold Labeling | |
|---|---|---|---|---|
| D1 to D30 | D14 | D30 | ||
| Control | Injury without treatment | n = 6 | n = 6 | n = 6 |
| Sham group (saline) | Injury + normal saline injection at the injury site (0.5 mg/kg) | n = 6 | / | / |
| BV | Injury + BV injection at the injury site (0.5 mg/kg) | n = 6 | n = 6 | n = 6 |
| bvPLA2 + melittin | Injury + bvPLA2 + melittin injection at the injury site (0.5 mg/kg) | n = 6 | n = 6 | n = 6 |
| Betamethasone | Injury + betamethasone injection at the injury site (2 mg/kg) | n = 6 | n = 6 | n = 6 |
| Rota Rod Score | Bar Score | Head-Down Score | Tunnel Score | Final Score |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0: No movement |
| 1 | 1 | 1 | 1 | 1: Slight vibrissae movements |
| 2 | 2 | 1 | 1 | 1.5: Slight fibrillations on the injured side compared to the intact side on some devices |
| 2 | 2 | 2 | 2 | 2: Net and asymmetrical voluntary movement of the lesioned side (smaller amplitude) relative to the intact side on all devices |
| 3 | 3 | 2 | 3 | 2.5: Net and asymmetrical voluntary movement of the lesioned side relative to the intact side for some, but not on all devices (incomplete amplitude) |
| 3 | 3 | 3 | 3 | 3: Normal and symmetrical movement in the injured side compared to the intact side (complete amplitude and frequency) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Er-Rouassi, H.; Bakour, M.; Touzani, S.; Vilas-Boas, M.; Falcão, S.; Vidal, C.; Lyoussi, B. Beneficial Effect of Bee Venom and Its Major Components on Facial Nerve Injury Induced in Mice. Biomolecules 2023, 13, 680. https://doi.org/10.3390/biom13040680
Er-Rouassi H, Bakour M, Touzani S, Vilas-Boas M, Falcão S, Vidal C, Lyoussi B. Beneficial Effect of Bee Venom and Its Major Components on Facial Nerve Injury Induced in Mice. Biomolecules. 2023; 13(4):680. https://doi.org/10.3390/biom13040680
Chicago/Turabian StyleEr-Rouassi, Hafsa, Meryem Bakour, Soumaya Touzani, Miguel Vilas-Boas, Soraia Falcão, Catherine Vidal, and Badiaa Lyoussi. 2023. "Beneficial Effect of Bee Venom and Its Major Components on Facial Nerve Injury Induced in Mice" Biomolecules 13, no. 4: 680. https://doi.org/10.3390/biom13040680









