The Roles of Antisense Long Noncoding RNAs in Tumorigenesis and Development through Cis-Regulation of Neighbouring Genes
Abstract
:1. Introduction
2. Classification of Antisense lncRNAs
2.1. Cis- Acting as-lncRNAs
2.2. Trans-Acting as-lncRNAs
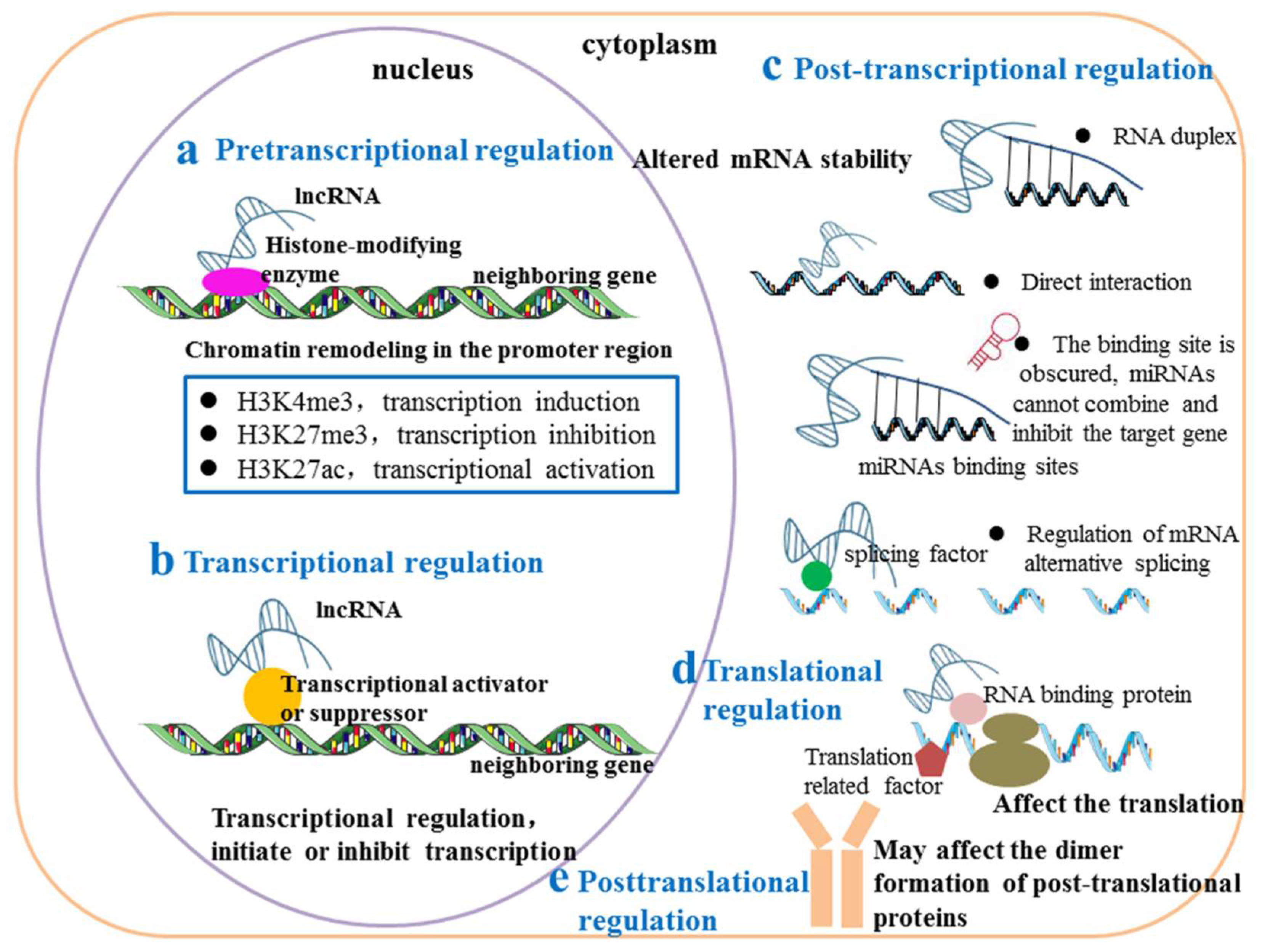
3. Regulation Mechanism of as-lncRNAs on Neighboring Genes
3.1. Regulation of Neighbouring Genes by as-lncRNAs at the Pretranscriptional Level
3.2. Regulation of Neighbouring Genes by as-lncRNAs at the Transcriptional Level
3.3. Regulation of Neighbouring Genes by as-lncRNAs at the Posttranscriptional Level
3.3.1. As-lncRNAs Regulate Neighbouring Genes by Affecting Their mRNA Stability
3.3.2. As-lncRNAs Regulate Adjacent Genes by Changing the Alternative Splicing of mRNA
3.3.3. As-lncRNAs Regulate Their Neighbouring Genes by Masking the Binding Sites of miRNAs or Adsorbing miRNAs as ceRNA Sponges
3.4. As-lncRNAs Regulate Neighbouring Genes at the Translation Level
3.5. Regulation of Neighbouring Genes by as-lncRNAs at the Posttranslational Level
4. Antisense lncRNAs Affect the Occurrence and Development of Tumours by Regulating Neighbouring Genes
5. Summary and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| As-lncRNA | Antisense long noncoding RNA |
| NATs | Natural antisense transcripts |
| ceRNA | competing endogenous RNA |
| ESCC | Esophageal squamous cell carcinoma |
| EMT | Epithelial-mesenchymal transition |
| HCC | Hepatocellular Carcinoma. |
References
- Meng, N.; Chen, M.; Chen, D.; Chen, X.H.; Wang, J.Z.; Zhu, S.; He, Y.T.; Zhang, X.L.; Lu, R.X.; Yan, G.R. Small Protein Hidden in lncRNA LOC90024 Promotes “Cancerous” RNA Splicing and Tumorigenesis. Adv. Sci. 2020, 7, 1903233. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, M.A.; Wahlestedt, C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Biol. 2009, 10, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Tomaru, Y.; Kasukawa, T.; Waki, K.; Nakanishi, M.; Nakamura, M.; Nishida, H.; Yap, C.C.; Suzuki, M.; Kawai, J.; et al. Antisense transcription in the mammalian transcriptome. Science 2005, 309, 1564–1566. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.V.; Vogt, P.K. Long antisense non-coding RNAs and their role in transcription and oncogenesis. Cell Cycle 2010, 9, 2544–2547. [Google Scholar] [CrossRef]
- Khorkova, O.; Myers, A.J.; Hsiao, J.; Wahlestedt, C. Natural antisense transcripts. Hum. Mol. Genet. 2014, 23, R54–R63. [Google Scholar] [CrossRef]
- Villegas, V.E.; Zaphiropoulos, P.G. Neighboring gene regulation by antisense long non-coding RNAs. Int. J. Mol. Sci. 2015, 16, 3251–3266. [Google Scholar] [CrossRef]
- Qi, W.; Li, Z.; Xia, L.; Dai, J.; Zhang, Q.; Wu, C.; Xu, S. LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci. Rep. 2019, 9, 16185. [Google Scholar] [CrossRef]
- Malhotra, S.; Freeberg, M.A.; Winans, S.J.; Taylor, J.; Beemon, K.L. A Novel Long Non-Coding RNA in the hTERT Promoter Region Regulates hTERT Expression. Noncoding RNA 2017, 4, 1. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Wight, M.; Werner, A. The functions of natural antisense transcripts. Essays Biochem. 2013, 54, 91–101. [Google Scholar] [CrossRef]
- Osato, N.; Suzuki, Y.; Ikeo, K.; Gojobori, T. Transcriptional interferences in cis natural antisense transcripts of humans and mice. Genetics 2007, 176, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Kiyosawa, H. Genome-wide impact of endogenous antisense transcripts in eukaryotes. Front. Biosci. (Landmark Ed.) 2012, 17, 300–315. [Google Scholar] [CrossRef]
- Werner, A. Biological functions of natural antisense transcripts. BMC Biol. 2013, 11, 31. [Google Scholar] [CrossRef]
- Wood, E.J.; Chin-Inmanu, K.; Jia, H.; Lipovich, L. Sense-antisense gene pairs: Sequence, transcription, and structure are not conserved between human and mouse. Front. Genet. 2013, 4, 183. [Google Scholar] [CrossRef]
- Solda, G.; Suyama, M.; Pelucchi, P.; Boi, S.; Guffanti, A.; Rizzi, E.; Bork, P.; Tenchini, M.L.; Ciccarelli, F.D. Non-random retention of protein-coding overlapping genes in Metazoa. BMC Genom. 2008, 9, 174. [Google Scholar] [CrossRef]
- Kong, F.; Deng, X.; Kong, X.; Du, Y.; Li, L.; Zhu, H.; Wang, Y.; Xie, D.; Guha, S.; Li, Z.; et al. ZFPM2-AS1, a novel lncRNA, attenuates the p53 pathway and promotes gastric carcinogenesis by stabilizing MIF. Oncogene 2018, 37, 5982–5996. [Google Scholar] [CrossRef]
- Wang, H.; Huo, X.; Yang, X.R.; He, J.; Cheng, L.; Wang, N.; Deng, X.; Jin, H.; Wang, N.; Wang, C.; et al. STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol. Cancer 2017, 16, 136. [Google Scholar] [CrossRef]
- Shuai, Y.; Ma, Z.; Liu, W.; Yu, T.; Yan, C.; Jiang, H.; Tian, S.; Xu, T.; Shu, Y. TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2. Mol. Cancer 2020, 19, 6. [Google Scholar] [CrossRef]
- Clark, B.S.; Blackshaw, S. Long non-coding RNA-dependent transcriptional regulation in neuronal development and disease. Front. Genet. 2014, 5, 164. [Google Scholar] [CrossRef]
- Batista, P.J.; Chang, H.Y. Long noncoding RNAs: Cellular address codes in development and disease. Cell 2013, 152, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Latge, G.; Poulet, C.; Bours, V.; Josse, C.; Jerusalem, G. Natural Antisense Transcripts: Molecular Mechanisms and Implications in Breast Cancers. Int. J. Mol. Sci. 2018, 19, 123. [Google Scholar] [CrossRef]
- Khorkova, O.; Stahl, J.; Joji, A.; Volmar, C.H.; Zeier, Z.; Wahlestedt, C. Natural antisense transcripts as drug targets. Front. Mol. Biosci. 2022, 9, 978375. [Google Scholar] [CrossRef]
- Zhang, A.; Xu, M.; Mo, Y.Y. Role of the lncRNA-p53 regulatory network in cancer. J. Mol. Cell Biol. 2014, 6, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Calle, A.; Kawamura, Y.; Yamamoto, Y.; Takeshita, F.; Ochiya, T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018, 109, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Guo, X.; Wang, M.; Qin, R. The patterns of antisense long non-coding RNAs regulating corresponding sense genes in human cancers. J. Cancer 2021, 12, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zuo, X.; Deng, H.; Liu, X.; Liu, L.; Ji, A. Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Res. Bull. 2013, 97, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Wanowska, E.; Kubiak, M.R.; Rosikiewicz, W.; Makalowska, I.; Szczesniak, M.W. Natural antisense transcripts in diseases: From modes of action to targeted therapies. Wiley Interdiscip. Rev. RNA 2018, 9, e1461. [Google Scholar] [CrossRef]
- Faust, T.; Frankel, A.; D’Orso, I. Transcription control by long non-coding RNAs. Transcription 2012, 3, 78–86. [Google Scholar] [CrossRef]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef]
- Wu, W.; Gao, H.; Li, X.; Zhu, Y.; Peng, S.; Yu, J.; Zhan, G.; Wang, J.; Liu, N.; Guo, X. LncRNA TPT1-AS1 promotes tumorigenesis and metastasis in epithelial ovarian cancer by inducing TPT1 expression. Cancer Sci. 2019, 110, 1587–1598. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhu, K.P.; Ma, X.L. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017, 396, 66–75. [Google Scholar] [CrossRef]
- Xiang, S.; Gu, H.; Jin, L.; Thorne, R.F.; Zhang, X.D.; Wu, M. LncRNA IDH1-AS1 links the functions of c-Myc and HIF1alpha via IDH1 to regulate the Warburg effect. Proc. Natl. Acad. Sci. USA 2018, 115, E1465–E1474. [Google Scholar] [CrossRef]
- Zhang, X.D.; Huang, G.W.; Xie, Y.H.; He, J.Z.; Guo, J.C.; Xu, X.E.; Liao, L.D.; Xie, Y.M.; Song, Y.M.; Li, E.M.; et al. The interaction of lncRNA EZR-AS1 with SMYD3 maintains overexpression of EZR in ESCC cells. Nucleic Acids Res. 2018, 46, 1793–1809. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, L.; Chi, W.; Cao, H.; Cui, W.; Meng, W. Aberrant methylation-mediated downregulation of lncRNA SSTR5-AS1 promotes progression and metastasis of laryngeal squamous cell carcinoma. Epigenetics Chromatin 2019, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liang, X.; Liu, L.; Guo, Y.; Shen, S.; Liang, J.; Dong, Z. MiR-6872 host gene SEMA3B and its antisense lncRNA SEMA3B-AS1 function synergistically to suppress gastric cardia adenocarcinoma progression. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2019, 22, 705–722. [Google Scholar] [CrossRef]
- Dhar, S.S.; Zhao, D.; Lin, T.; Gu, B.; Pal, K.; Wu, S.J.; Alam, H.; Lv, J.; Yun, K.; Gopalakrishnan, V.; et al. MLL4 Is Required to Maintain Broad H3K4me3 Peaks and Super-Enhancers at Tumor Suppressor Genes. Mol. Cell 2018, 70, 825–841.e6. [Google Scholar] [CrossRef]
- Ananthanarayanan, M.; Li, Y.; Surapureddi, S.; Balasubramaniyan, N.; Ahn, J.; Goldstein, J.A.; Suchy, F.J. Histone H3K4 trimethylation by MLL3 as part of ASCOM complex is critical for NR activation of bile acid transporter genes and is downregulated in cholestasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G771–G781. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Zhao, L.; Wang, L.; Ou-Yang, W.; Hu, S.S.; Li, W.L.; Ai, M.L.; Wang, Y.Q.; Han, Y.; Li, T.T.; et al. Nuclear lncRNA HOXD-AS1 suppresses colorectal carcinoma growth and metastasis via inhibiting HOXD3-induced integrin beta3 transcriptional activating and MAPK/AKT signalling. Mol. Cancer 2019, 18, 31. [Google Scholar] [CrossRef]
- Dong, Z.; Li, S.; Wu, X.; Niu, Y.; Liang, X.; Yang, L.; Guo, Y.; Shen, S.; Liang, J.; Guo, W. Aberrant hypermethylation-mediated downregulation of antisense lncRNA ZNF667-AS1 and its sense gene ZNF667 correlate with progression and prognosis of esophageal squamous cell carcinoma. Cell Death Dis. 2019, 10, 930. [Google Scholar] [CrossRef]
- Zhou, Y.; Huan, L.; Wu, Y.; Bao, C.; Chen, B.; Wang, L.; Huang, S.; Liang, L.; He, X. LncRNA ID2-AS1 suppresses tumor metastasis by activating the HDAC8/ID2 pathway in hepatocellular carcinoma. Cancer Lett. 2020, 469, 399–409. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Li, Y.; Lu, Z.; Che, Y.; Mao, S.; Lei, Y.; Zang, R.; Zheng, S.; Liu, C.; et al. Interferon-inducible lncRNA IRF1-AS represses esophageal squamous cell carcinoma by promoting interferon response. Cancer Lett. 2019, 459, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Cao, P.; Yan, Q.; Yang, L.; Wang, Y.; Yang, J.; Xin, S.; Zhang, J.; Li, J.; Long, S.; et al. IGFBP7-AS1 is a p53-responsive long noncoding RNA downregulated by Epstein-Barr virus that contributes to viral tumorigenesis. Cancer Lett. 2021, 523, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, X.; Fu, C.; Wang, X.; Zou, J.; Hua, H.; Bi, Z. Long noncoding RNA FGFR3-AS1 promotes osteosarcoma growth through regulating its natural antisense transcript FGFR3. Mol. Biol. Rep. 2016, 43, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lin, J.; Liu, J.; Huang, M.; Zhong, Y.; Liang, B.; Song, X.; Gu, S.; Chang, X.; Huang, D.; et al. A novel lncRNA NR4A1AS up-regulates orphan nuclear receptor NR4A1 expression by blocking UPF1-mediated mRNA destabilization in colorectal cancer. Clin. Sci. 2019, 133, 1457–1473. [Google Scholar] [CrossRef]
- Li, B.; Hu, Y.; Li, X.; Jin, G.; Chen, X.; Chen, G.; Chen, Y.; Huang, S.; Liao, W.; Liao, Y.; et al. Sirt1 Antisense Long Noncoding RNA Promotes Cardiomyocyte Proliferation by Enhancing the Stability of Sirt1. J. Am. Heart Assoc. 2018, 7, e009700. [Google Scholar] [CrossRef]
- Jadaliha, M.; Gholamalamdari, O.; Tang, W.; Zhang, Y.; Petracovici, A.; Hao, Q.; Tariq, A.; Kim, T.G.; Holton, S.E.; Singh, D.K.; et al. A natural antisense lncRNA controls breast cancer progression by promoting tumor suppressor gene mRNA stability. PLoS Genet. 2018, 14, e1007802. [Google Scholar] [CrossRef]
- Ma, J.; Chen, S.; Hao, L.; Sheng, W.; Chen, W.; Ma, X.; Zhang, B.; Ma, D.; Huang, G. Hypermethylation-mediated down-regulation of lncRNA TBX5-AS1:2 in Tetralogy of Fallot inhibits cell proliferation by reducing TBX5 expression. J. Cell. Mol. Med. 2020, 24, 6472–6484. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, L.; Dai, W.; Han, L.; Wang, R.; Xiang, W.; Wang, Z.; Li, Q.; Yu, J.; Yuan, J.; et al. Antisense lncRNA LDLRAD4-AS1 promotes metastasis by decreasing the expression of LDLRAD4 and predicts a poor prognosis in colorectal cancer. Cell Death Dis. 2020, 11, 155. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Lin, L.; Huang, Q.; He, W.; Zhang, S.; Dong, S.; Wen, Z.; Rao, J.; Liao, W.; et al. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol. Cancer 2018, 17, 69. [Google Scholar] [CrossRef]
- Han, S.; Cao, D.; Sha, J.; Zhu, X.; Chen, D. LncRNA ZFPM2-AS1 promotes lung adenocarcinoma progression by interacting with UPF1 to destabilize ZFPM2. Mol. Oncol. 2020, 14, 1074–1088. [Google Scholar] [CrossRef]
- Gupta, P.; Li, Y.R. Upf proteins: Highly conserved factors involved in nonsense mRNA mediated decay. Mol. Biol. Rep. 2018, 45, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Cammas, A.; Sanchez, B.J.; Lian, X.J.; Dormoy-Raclet, V.; van der Giessen, K.; Lopez de Silanes, I.; Ma, J.; Wilusz, C.; Richardson, J.; Gorospe, M.; et al. Destabilization of nucleophosmin mRNA by the HuR/KSRP complex is required for muscle fibre formation. Nat. Commun. 2014, 5, 4190. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kuwano, Y.; Srikantan, S.; Lee, E.K.; Martindale, J.L.; Gorospe, M. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 2009, 23, 1743–1748. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, Q.; Qi, Y.; Ruan, X.; Wu, F.; Li, L.; Zhou, J.; Liu, W.; Jiang, T.; Zhang, J.; et al. PTB-AS, a Novel Natural Antisense Transcript, Promotes Glioma Progression by Improving PTBP1 mRNA Stability with SND1. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Yang, W.Z.; Chen, Y.P.; Yuan, H.S. Structural and functional insights into human Tudor-SN, a key component linking RNA interference and editing. Nucleic Acids Res. 2008, 36, 3579–3589. [Google Scholar] [CrossRef]
- Paukku, K.; Kalkkinen, N.; Silvennoinen, O.; Kontula, K.K.; Lehtonen, J.Y. p100 increases AT1R expression through interaction with AT1R 3’-UTR. Nucleic Acids Res. 2008, 36, 4474–4487. [Google Scholar] [CrossRef] [PubMed]
- Niehus, S.E.; Allister, A.B.; Hoffmann, A.; Wiehlmann, L.; Tamura, T.; Tran, D.D.H. Myc/Max dependent intronic long antisense noncoding RNA, EVA1A-AS, suppresses the expression of Myc/Max dependent anti-proliferating gene EVA1A in a U2 dependent manner. Sci. Rep. 2019, 9, 17319. [Google Scholar] [CrossRef]
- Yuan, J.H.; Liu, X.N.; Wang, T.T.; Pan, W.; Tao, Q.F.; Zhou, W.P.; Wang, F.; Sun, S.H. The MBNL3 splicing factor promotes hepatocellular carcinoma by increasing PXN expression through the alternative splicing of lncRNA-PXN-AS1. Nat. Cell Biol. 2017, 19, 820–832. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, X.; You, H.; Zheng, X.; Ruan, X. LncRNA SOCS2-AS1 inhibits progression and metastasis of colorectal cancer through stabilizing SOCS2 and sponging miR-1264. Aging 2020, 12, 10517–10526. [Google Scholar] [CrossRef]
- Guo, M.; Liu, T.; Zhang, S.; Yang, L. RASSF1-AS1, an antisense lncRNA of RASSF1A, inhibits the translation of RASSF1A to exacerbate cardiac fibrosis in mice. Cell Biol. Int. 2019, 43, 1163–1173. [Google Scholar] [CrossRef]
- Reddy, N.S.; Roth, W.W.; Bragg, P.W.; Wahba, A.J. Isolation and mapping of a gene for protein synthesis initiation factor 4A and its expression during differentiation of murine erythroleukemia cells. Gene 1988, 70, 231–243. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Ding, Y.; Zhou, J.; Liu, G.; Ji, Z. lncRNA ZEB1-AS1 promotes migration and metastasis of bladder cancer cells by post-transcriptional activation of ZEB1. Int. J. Mol. Med. 2019, 44, 196–206. [Google Scholar] [CrossRef]
- Wu, S.; Ding, L.; Xu, H.; Gao, J.; Shao, Y.; Zhang, S.; Wei, Z. The Long Non-Coding RNA IDH1-AS1 Promotes Prostate Cancer Progression by Enhancing IDH1 Enzyme Activity. OncoTargets Ther. 2020, 13, 7897–7906. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.P.; Chen, P.P.; Koeffler, H.P.; Tong, X.J.; Xie, D. Involvement of IFN regulatory factor (IRF)-1 and IRF-2 in the formation and progression of human esophageal cancers. Cancer Res. 2007, 67, 2535–2543. [Google Scholar] [CrossRef]
- Khanna, C.; Wan, X.; Bose, S.; Cassaday, R.; Olomu, O.; Mendoza, A.; Yeung, C.; Gorlick, R.; Hewitt, S.M.; Helman, L.J. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat. Med. 2004, 10, 182–186. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Zhao, C.Y. Expression of ezrin and moesin related to invasion, metastasis and prognosis of laryngeal squamous cell carcinoma. Genet. Mol. Res. GMR 2014, 13, 8002–8013. [Google Scholar] [CrossRef]
- Hyun, S.; Lee, J.H.; Jin, H.; Nam, J.; Namkoong, B.; Lee, G.; Chung, J.; Kim, V.N. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell 2009, 139, 1096–1108. [Google Scholar] [CrossRef]
- Bommer, U.A.; Iadevaia, V.; Chen, J.; Knoch, B.; Engel, M.; Proud, C.G. Growth-factor dependent expression of the translationally controlled tumour protein TCTP is regulated through the PI3-K/Akt/mTORC1 signalling pathway. Cell. Signal. 2015, 27, 1557–1568. [Google Scholar] [CrossRef]
- Bommer, U.A. The Translational Controlled Tumour Protein TCTP: Biological Functions and Regulation. Results Probl. Cell Differ. 2017, 64, 69–126. [Google Scholar] [CrossRef]
- Das, R.; Gregory, P.A.; Fernandes, R.C.; Denis, I.; Wang, Q.; Townley, S.L.; Zhao, S.G.; Hanson, A.R.; Pickering, M.A.; Armstrong, H.K.; et al. MicroRNA-194 Promotes Prostate Cancer Metastasis by Inhibiting SOCS2. Cancer Res. 2017, 77, 1021–1034. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Ji, W. miR-196b is a prognostic factor of human laryngeal squamous cell carcinoma and promotes tumor progression by targeting SOCS2. Biochem. Biophys. Res. Commun. 2018, 501, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tong, D.; Guo, Q.; Wang, X.; Wu, F.; Li, Q.; Yang, J.; Zhao, L.; Qin, Y.; Liu, Y.; et al. HOXD3 targeted by miR-203a suppresses cell metastasis and angiogenesis through VEGFR in human hepatocellular carcinoma cells. Sci. Rep. 2018, 8, 2431. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Hamada, J.; Tada, M.; Aoyama, T.; Furuuchi, K.; Takahashi, Y.; Totsuka, Y.; Moriuchi, T. HOXD3-overexpression increases integrin alpha v beta 3 expression and deprives E-cadherin while it enhances cell motility in A549 cells. Clin. Exp. Metastasis 2006, 23, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Drabsch, Y.; Dekker, T.J.; de Vinuesa, A.G.; Li, Y.; Hawinkels, L.J.; Sheppard, K.A.; Goumans, M.J.; Luwor, R.B.; de Vries, C.J.; et al. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-beta signalling. Nat. Commun. 2014, 5, 3388. [Google Scholar] [CrossRef]
- Wu, D.W.; Chuang, C.Y.; Lin, W.L.; Sung, W.W.; Cheng, Y.W.; Lee, H. Paxillin promotes tumor progression and predicts survival and relapse in oral cavity squamous cell carcinoma by microRNA-218 targeting. Carcinogenesis 2014, 35, 1823–1829. [Google Scholar] [CrossRef]
- Zhao, C.J.; Du, S.K.; Dang, X.B.; Gong, M. Expression of Paxillin is Correlated with Clinical Prognosis in Colorectal Cancer Patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1989–1995. [Google Scholar] [CrossRef]
- Niu, Y.; Shao, Z.; Wang, H.; Yang, J.; Zhang, F.; Luo, Y.; Xu, L.; Ding, Y.; Zhao, L. LASP1-S100A11 axis promotes colorectal cancer aggressiveness by modulating TGFbeta/Smad signaling. Sci. Rep. 2016, 6, 26112. [Google Scholar] [CrossRef]
- Yin, L.; Chen, Y.; Zhou, Y.; Deng, G.; Han, Y.; Guo, C.; Li, Y.; Zeng, S.; Shen, H. Increased long noncoding RNA LASP1-AS is critical for hepatocellular carcinoma tumorigenesis via upregulating LASP1. J. Cell. Physiol. 2019, 234, 13493–13509. [Google Scholar] [CrossRef]
- Rothschild, G.; Zhao, X.; Iavarone, A.; Lasorella, A. E Proteins and Id2 converge on p57Kip2 to regulate cell cycle in neural cells. Mol. Cell. Biol. 2006, 26, 4351–4361. [Google Scholar] [CrossRef]
- Coma, S.; Amin, D.N.; Shimizu, A.; Lasorella, A.; Iavarone, A.; Klagsbrun, M. Id2 promotes tumor cell migration and invasion through transcriptional repression of semaphorin 3F. Cancer Res. 2010, 70, 3823–3832. [Google Scholar] [CrossRef]
- Tsunedomi, R.; Iizuka, N.; Tamesa, T.; Sakamoto, K.; Hamaguchi, T.; Somura, H.; Yamada, M.; Oka, M. Decreased ID2 promotes metastatic potentials of hepatocellular carcinoma by altering secretion of vascular endothelial growth factor. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Cmarik, J.L.; Min, H.; Hegamyer, G.; Zhan, S.; Kulesz-Martin, M.; Yoshinaga, H.; Matsuhashi, S.; Colburn, N.H. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc. Natl. Acad. Sci. USA 1999, 96, 14037–14042. [Google Scholar] [CrossRef] [PubMed]
- Stein, U.; Walther, W.; Arlt, F.; Schwabe, H.; Smith, J.; Fichtner, I.; Birchmeier, W.; Schlag, P.M. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat. Med. 2009, 15, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Kannius-Janson, M. Forkhead Box F1 promotes breast cancer cell migration by upregulating lysyl oxidase and suppressing Smad2/3 signaling. BMC Cancer 2016, 16, 142. [Google Scholar] [CrossRef]
- Fulford, L.; Milewski, D.; Ustiyan, V.; Ravishankar, N.; Cai, Y.; Le, T.; Masineni, S.; Kasper, S.; Aronow, B.; Kalinichenko, V.V.; et al. The transcription factor FOXF1 promotes prostate cancer by stimulating the mitogen-activated protein kinase ERK5. Sci. Signal. 2016, 9, ra48. [Google Scholar] [CrossRef]
- Kun-Peng, Z.; Chun-Lin, Z.; Xiao-Long, M. Antisense lncRNA FOXF1-AS1 Promotes Migration and Invasion of Osteosarcoma Cells through the FOXF1/MMP-2/-9 Pathway. Int. J. Biol. Sci. 2017, 13, 1180–1191. [Google Scholar] [CrossRef]
- Bi, Y.; Jing, Y.; Cao, Y. Overexpression of miR-100 inhibits growth of osteosarcoma through FGFR3. Tumour Biol. J. Int. Soc. Oncodevelop. Biol. Med. 2015, 36, 8405–8411. [Google Scholar] [CrossRef]

| As-lncRNAs | Regulatory Mechanism | Influence on Tumour Progression |
|---|---|---|
| ZFPM2-AS1 | Binds MIF and protects the stability of its protein | Promotes the progression of gastric cancer [16] |
| HOXD-AS1 | Prevents the degradation of SOX4 mediated by miR-130a-3p | Facilitates liver cancer metastasis [17] |
| MNX1-AS1 | Sponges miR-6785-5p and upregulates the expression of Bcl-2; inhibitesBTG2 expression through EZH2-induced H3K27me3 modification in the BTG2 promoter region | Contributes to gastric cancer progression [18] |
| As-lncRNA | Regulatory Level | Regulatory Mechanism | Effects on Neighbouring Genes | Influence on Tumour Progression |
|---|---|---|---|---|
| ZNF667-AS1 | Pretranscriptional level | Interacts with demethylase (TET1) and histone demethylase (UTX) | Transcription of ZNF667 is activated | Inhibits the progression of oesophageal squamous cell carcinoma [39] |
| IRF-AS | Transcriptional level | Forms a complex with RNA binding proteins ILF3 and DHX9 | Transcription of IRF1 is activated | Inhibits the progression of oesophageal squamous cell carcinoma [41] |
| EZR-AS1 | Pretranscriptional level | SMYD3 (lysine methyltransferase) is recruited and bound to the SBS-1 site in the EZR promoter region, causing H3K4 methylation in the EZR promoter region | Transcription of EZR is upregulated | Promotes the migration and invasiveness of oesophageal squamous cell carcinoma cells [33] |
| ZFPM2-AS1 | post-transcriptional level | ZFPM2-AS1, ZFPM2 mRNA and UFP1 protein form a binding complex, which makes ZFPM2 mRNA unstable | Expression of ZFPM2 is downregulated | Promotes the proliferation, invasion, and EMT of lung adenocarcinoma cells [16] |
| TPT1-AS1 | Transcriptional level | The transcriptional activity of TPT1 promoter is directly induced, but the degradation of TPT1 mRNA is not inhibited | Promotes the transcription of TPT1 | Promotes proliferation, invasion, and metastasis of epithelial ovarian cancer [30] |
| SOCS2-AS1 | Post-transcriptional level | Competitive a binding of miR-1264 | Expression of SOCS2 is upregulated | Inhibits the proliferation and metastasis of colorectal cancer cells [59] |
| NR4A1AS | Post-transcriptional level | The direct binding of UPF1 protein to NR4A1 mRNA 3′UTR damages the binding of UPF1 protein to NR4A1 mRNA and prevents UPF1-mediated mRNA degradation | NR4A1 mRNA is more stable | Promotes the proliferation, migration, and invasion of colorectal cancer cells [44] |
| LDLRAD4-AS1 | Post-transcriptional level | It directly interacts with LDLRAD4 mRNA and reduces the stability of LDLRAD4 mRNA mainly through its 1-1098 bp sequence region | Expression of LDLRAD4 is decreased | Promotes metastasis of colorectal cancer [48] |
| HOXD-AS1 | Pretranscriptional level | Recruits PRC2 (histone methyltransferase) complex to bind to the promoter region of HOXD3 and induces the accumulation of inhibitory marker H3K27me3 | Transcription of HOXD3 is inhibited | Inhibits the growth and metastasis of colorectal cancer [38] |
| PXN-AS1 | Post-transcriptional level | The inclusion of exon 4 of PXN-AS1 is transcribed into a PXN-AS-L transcript containing exon 4 through splicing factor MBNL3 | Promotes the expression of PXN mRNA and protein | Promotes the occurrence of liver cancer [58] |
| ID2-AS1 | Pretranscriptional level | By blocking the binding of histone deacetylase 8 (HDAC8) to the ID2 enhancer, and increasing the accumulation of H3K27ac in the ID2 enhancer region | Promotes the transcription of ID2 | Inhibits metastasis of liver cancer [40] |
| PTB-AS | Post-transcriptional level | By competitive combination the binding site of miR-9 in PTBP1-3 ‘UTR, miR-9 could not mediate negative regulation of PTBP1 | The stability of PTBP1 mRNA is maintained | Promotes the occurrence of glioma [54] |
| PDCD4-AS1 | Post-transcriptional level | Negatively regulates the binding of HuR to PDCD4 mRNA | Promotes the stability of PDCD4 mRNA | Inhibits the proliferation and migration of breast cancer cells [46] |
| MACC1-AS1 | Post-transcriptional level | Promotes phosphorylation of AMPK, resulting in translocation of the RNA binding protein Lin28 from the nucleus to the cytoplasm | Enhances MACC1 mRNA stability | Promotes malignant phenotype of gastric cancer cells [49] |
| FGFR3-AS1 | Post-transcriptional level | Forms RNA–RNA double strands with FGFR3 mRNA | Makes FGFR3 mRNA more stable | Promotes the progression of osteosarcoma [43] |
| IGFBP7-AS1 | Post-transcriptional level | Forms RNA–RNA double strands with IGFBP7 mRNA | Makes IGFBP7 mRNA more stable | Inhibits the progression B-cell lymphoma [42] |
| ZEB1-AS1 | Translation level | Recruits AUF1 and activates the translation of ZEB1 mRNA | Activates the translation of ZEB1 mRNA | Promotes bladder cancer cells migration and invasion [62] |
| IDH1-AS1 | Posttranslational level | Alters IDH1enzyme activity | Enhances the enzyme activity of IDH1 | Promotes prostate cancer progression [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Yuan, Y.; Yi, T.; Dang, W. The Roles of Antisense Long Noncoding RNAs in Tumorigenesis and Development through Cis-Regulation of Neighbouring Genes. Biomolecules 2023, 13, 684. https://doi.org/10.3390/biom13040684
Jiang B, Yuan Y, Yi T, Dang W. The Roles of Antisense Long Noncoding RNAs in Tumorigenesis and Development through Cis-Regulation of Neighbouring Genes. Biomolecules. 2023; 13(4):684. https://doi.org/10.3390/biom13040684
Chicago/Turabian StyleJiang, Binyuan, Yeqin Yuan, Ting Yi, and Wei Dang. 2023. "The Roles of Antisense Long Noncoding RNAs in Tumorigenesis and Development through Cis-Regulation of Neighbouring Genes" Biomolecules 13, no. 4: 684. https://doi.org/10.3390/biom13040684






