The Ubiquitin-like Proteins of Saccharomyces cerevisiae
Abstract
1. Introduction
| S. cerevisiae Ubls | E1 | E2 | E3 | Substrate | References |
|---|---|---|---|---|---|
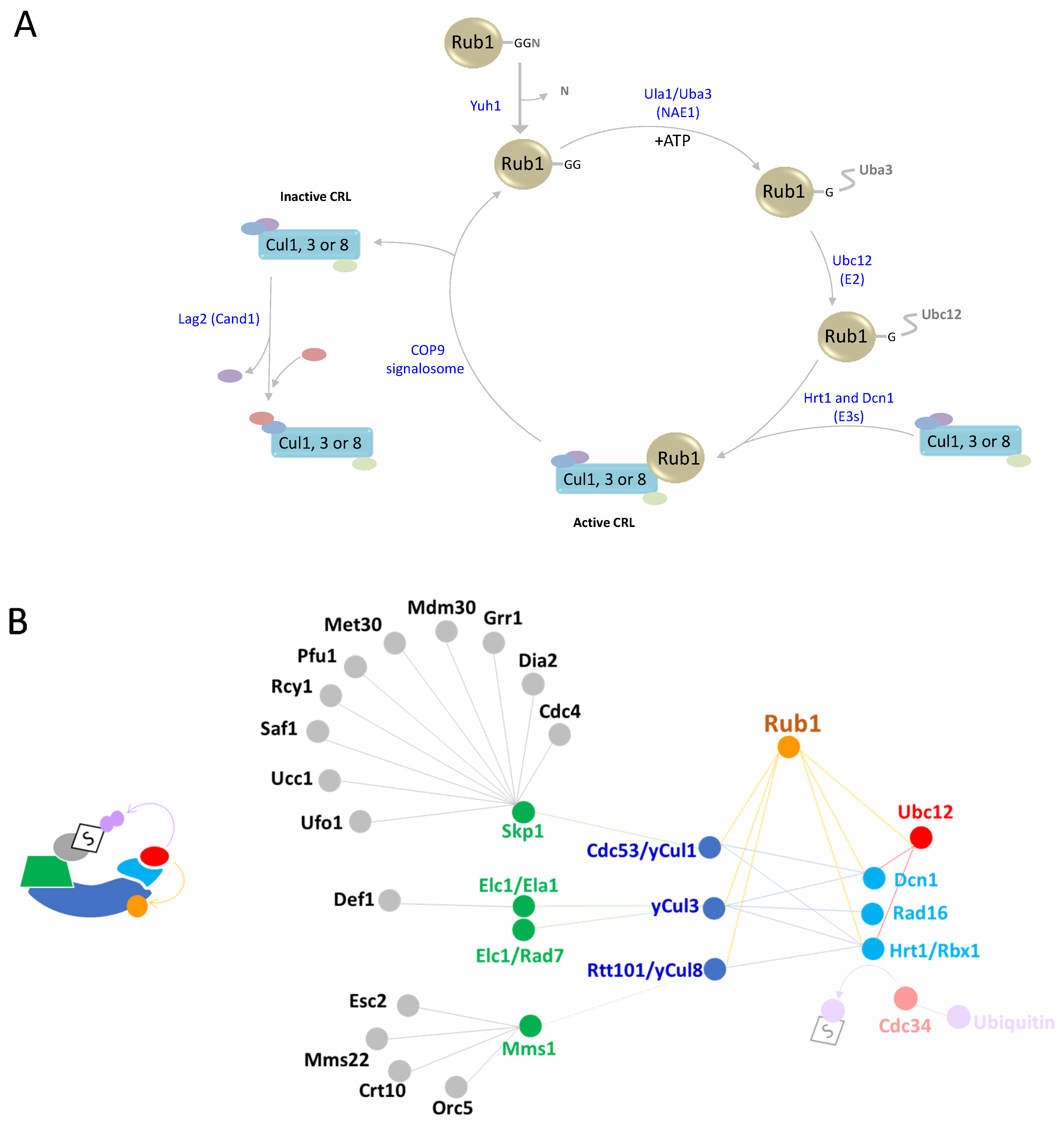
| Rub1 (YDR139C) | A dimer of Ula1(YPL003W) Uba3 (YPR066W) | Ubc12 (YLR306W) | Dcn1 (YLR128W) Hrt1 (YOL133W) Rad16 (YBR114W) | Cdc53 (YDL132W) yCul3 (YGR003W) Rtt101 (YJL047C) | [10,11,12] |
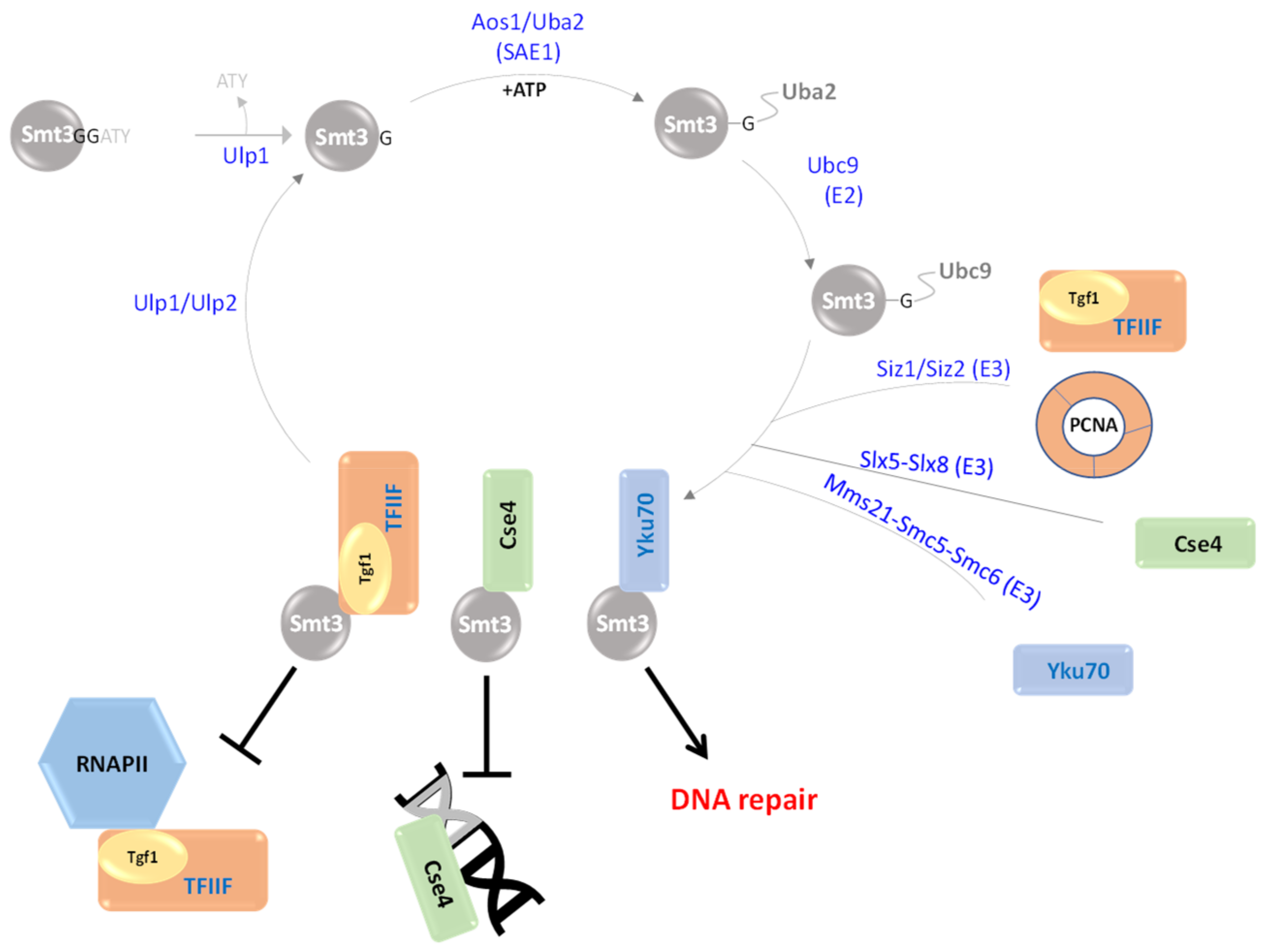
| Smt3 (YDR510W) | A dimer of Aos1 (YPR180W) Uba2 (YDR390C) | Ubc9 (YDL064W) | Siz1(YDR409W), Siz2/Nfi1 (YOR156C), Mms21/Nse2 (YEL019C) Slx5(YDL013W)/ Slx8(YER116C) | Tfg1 (YGR186W) PCNA(YBR088C) Cse4(YKL049C) Yku70(YMR284W) | [13,14,15,16,17,18,19] |
| Atg8 (YBL078C) | Atg7 (YHR171W) | Atg3 (YNR007C) | A complex of Atg12 (YBR217W) Atg5 (YPL149W) Atg16(YMR159C) | PE * | [20,21,22,23] |
| Atg12 (YBR217W) | Atg7 (YHR171W) | Atg10 (YLL042C) | — | Atg5 (YPL149W) | [24,25] |
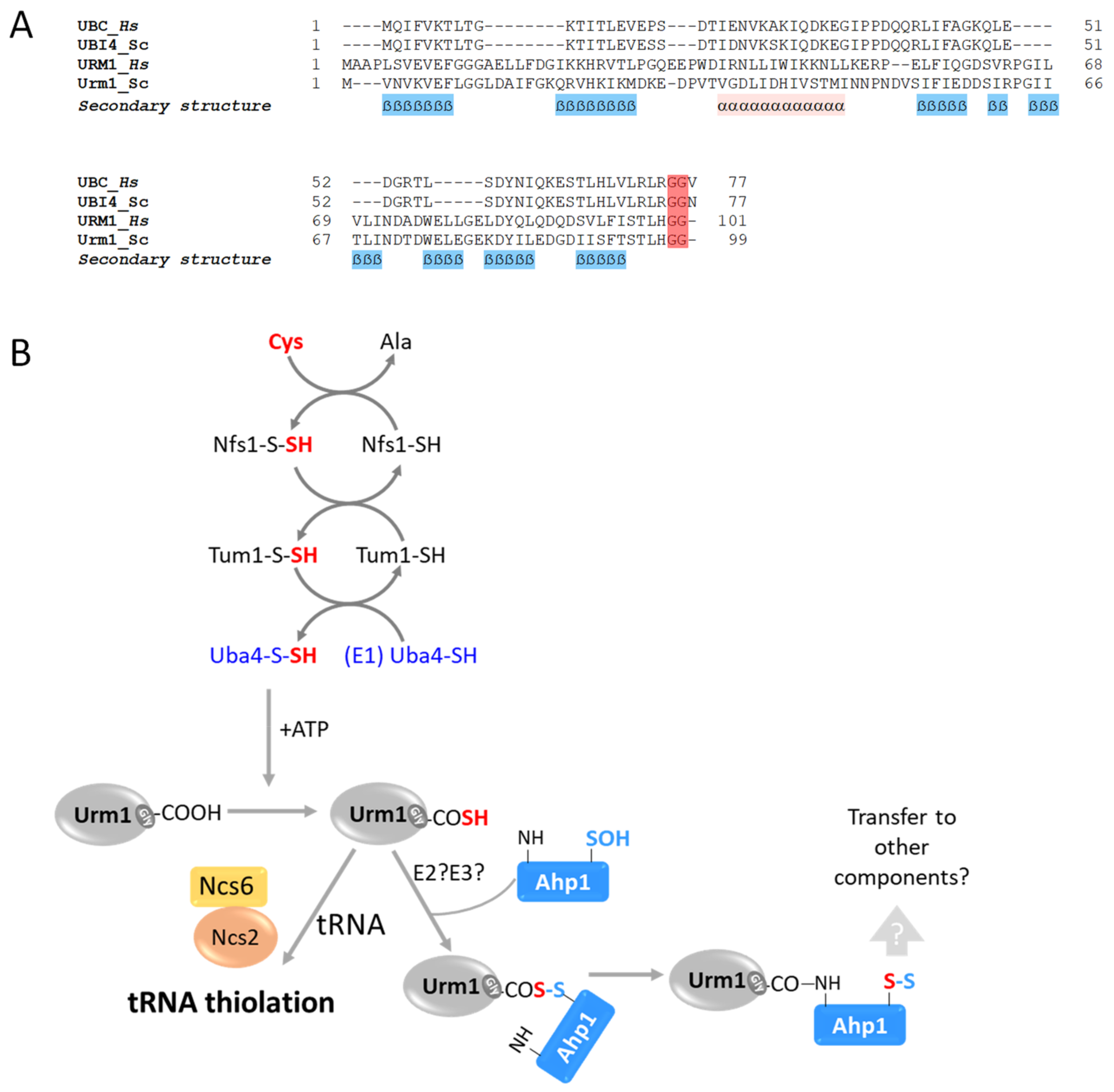
| Urm1 (YIL008W) | Uba4 (YHR111W) | — | — | tRNA ** Ahp1 (YLR109W) | [26,27,28] |
| Hub1 (YNR032C-A) | — | — | — | Snu66 (YOR308C) | [29,30] |
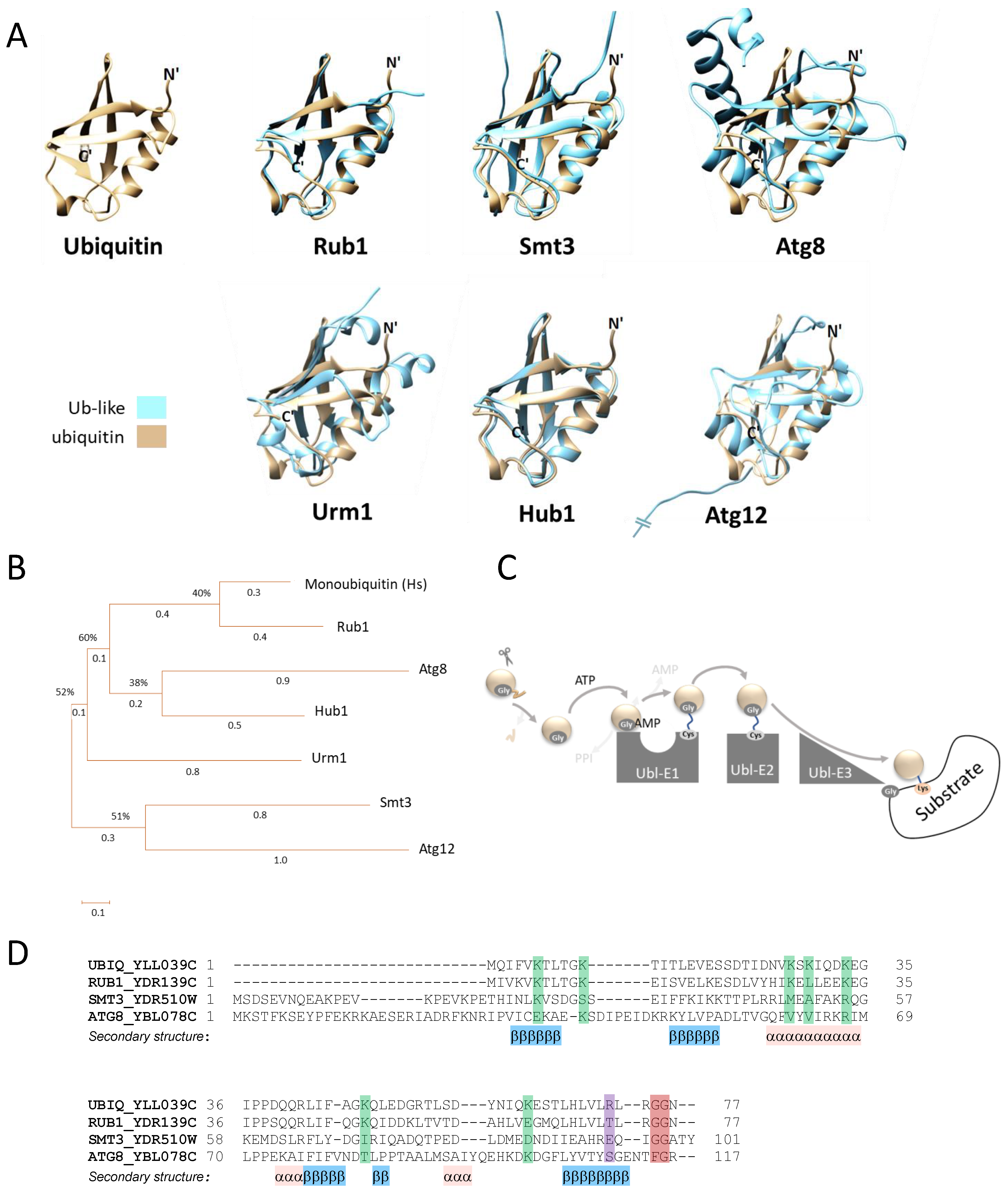
2. Rub1 Is the Closest Paralog of Ubiquitin
3. Smt3 Is a Vital Ubl
4. Atg8 and Atg12: Two Ubls in the Same Pathway
5. Urm1 Is a bi-Functional Ubl
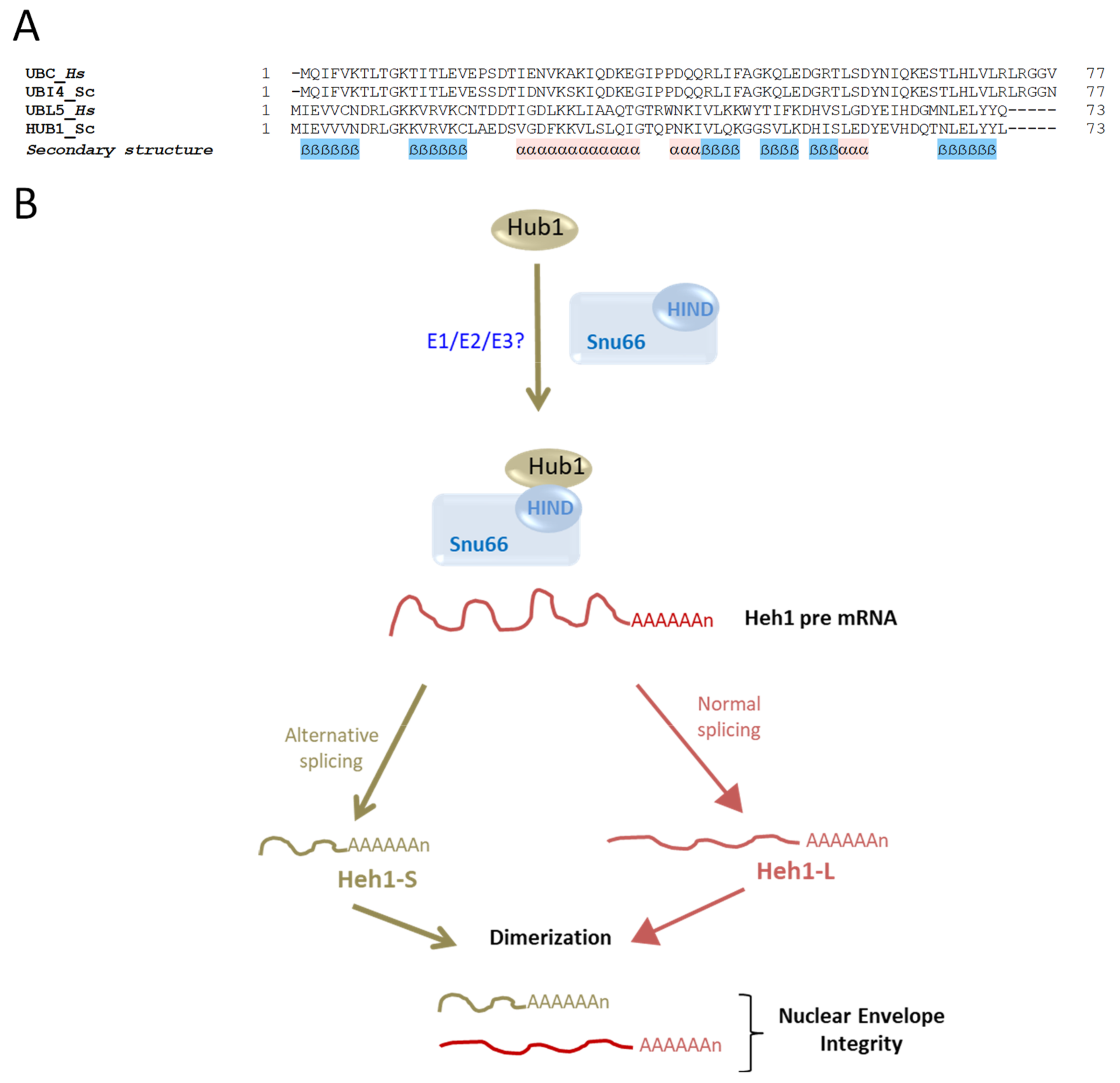
6. Hub1: A Ubl That Lacks a Carboxylic Terminal Glycine
7. Summary and Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.A.; Cole, P.A. The Chemical Biology of Reversible Lysine Post-translational Modifications. Cell Chem. Biol. 2020, 27, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. The age of crosstalk: Phosphorylation, ubiquitination, and beyond. Mol. Cell 2007, 28, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto, G.J., Jr. Protein Posttranslational Modifications: The Chemistry of Proteome Diversifications. Angew. Chem. Int. Ed. 2005, 44, 7342–7372. [Google Scholar] [CrossRef]
- Pickart, C.M.; Fushman, D. Polyubiquitin chains: Polymeric protein signals. Curr. Opin. Chem. Biol. 2004, 8, 610–616. [Google Scholar] [CrossRef]
- Cappadocia, L.; Lima, C.D. Ubiquitin-like Protein Conjugation: Structures, Chemistry, and Mechanism. Chem. Rev. 2017, 118, 889–918. [Google Scholar] [CrossRef]
- Hu, H.Y. Protein Ubiquitination and Deubiquitination. Curr. Protein Pept. Sci. 2012, 13, 413. [Google Scholar] [CrossRef]
- Ziv, I.; Matiuhin, Y.; Kirkpatrick, D.S.; Erpapazoglou, Z.; Leon, S.; Pantazopoulou, M.; Kim, W.; Gygi, S.P.; Haguenauer-Tsapis, R.; Reis, N.; et al. A Perturbed Ubiquitin Landscape Distinguishes Between Ubiquitin in Trafficking and in Proteolysis. Mol. Cell. Proteom. 2011, 10, M111.009753. [Google Scholar] [CrossRef]
- Clague, M.J.; Heride, C.; Urbé, S. The demographics of the ubiquitin system. Trends Cell Biol. 2015, 25, 417–426. [Google Scholar] [CrossRef]
- Silva, G.M.; Finley, D.; Vogel, C. K63 polyubiquitination is a new modulator of the oxidative stress response. Nat. Struct. Mol. Biol. 2015, 22, 116–123. [Google Scholar] [CrossRef]
- Liakopoulos, D.; Doenges, G.; Matuschewski, K.; Jentsch, S. A novel protein modification pathway related to the ubiquitin system. EMBO J. 1998, 17, 2208–2214. [Google Scholar] [CrossRef]
- Scott, D.C.; Monda, J.K.; Grace, C.R.; Duda, D.M.; Kriwacki, R.W.; Kurz, T.; Schulman, B.A. A dual E3 mechanism for Rub1 ligation to Cdc53. Mol. Cell 2010, 39, 784–796. [Google Scholar] [CrossRef]
- Laplaza, J.M.; Bostick, M.; Scholes, D.T.; Curcio, M.J.; Callis, J. Saccharomyces cerevisiae ubiquitin-like protein Rub1 conjugates to cullin proteins Rtt101 and Cul3 in vivo. Biochem. J. 2004, 377, 459–467. [Google Scholar] [CrossRef]
- Johnson, E.S.; Schwienhorst, I.; Dohmen, R.J.; Blobel, G. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 1997, 18, 5509–5519. [Google Scholar] [CrossRef]
- Schwarz, S.E.; Matuschewski, K.; Liakopoulos, D.; Scheffner, M.; Jentsch, S. The ubiquitin-like proteins SMT3 and SUMO-1 are conjugated by the UBC9 E2 enzyme. Proc. Natl. Acad. Sci. USA 1998, 95, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S.; Gupta, K. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 2001, 106, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Blobel, G. A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc. Natl. Acad. Sci. USA 2005, 102, 4777–4782. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.S.; Dou, Y.; Bergey, B.G.; Bahar, R.; Burgener, J.M.; Moallem, M.; McNeil, J.B.; Akhter, A.; Burke, G.L.; Sri Theivakadadcham, V.S.; et al. Dynamic sumoylation of promoter-bound general transcription factors facilitates transcription by RNA polymerase II. PLoS Genet. 2021, 17, e1009828. [Google Scholar] [CrossRef]
- Armstrong, A.A.; Mohideen, F.; Lima, C.D. Recognition of SUMO-modified PCNA requires tandem receptor motifs in Srs2. Nature 2012, 483, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ohkuni, K.; Takahashi, Y.; Fulp, A.; Lawrimore, J.; Au, W.C.; Pasupala, N.; Levy-Myers, R.; Warren, J.; Strunnikov, A.; Baker, R.E.; et al. SUMO-Targeted Ubiquitin Ligase (STUbL) Slx5 regulates proteolysis of centromeric histone H3 variant Cse4 and prevents its mislocalization to euchromatin. Mol. Biol. Cell 2016, 27, 1500–1510. [Google Scholar] [CrossRef]
- Tanida, I.; Mizushima, N.; Kiyooka, M.; Ohsumi, M.; Ueno, T.; Ohsumi, Y.; Kominami, E. Apg7p/Cvt2p: A novel protein-activating enzyme essential for autophagy. Mol. Biol. Cell 1999, 10, 1367–1379. [Google Scholar] [CrossRef]
- Shpilka, T.; Weidberg, H.; Pietrokovski, S.; Elazar, Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Noda, N.N.; Satomi, Y.; Ichimura, Y.; Fujioka, Y.; Takao, T.; Inagaki, F.; Ohsumi, Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007, 282, 37298–37302. [Google Scholar] [CrossRef] [PubMed]
- Schlumpberger, M.; Schaeffeler, E.; Straub, M.; Bredschneider, M.; Wolf, D.H.; Thumm, M. AUT1, a gene essential for autophagocytosis in the yeast Saccharomyces cerevisiae. J. Bacteriol. 1997, 179, 1068–1076. [Google Scholar] [CrossRef]
- Mizushima, N.; Noda, T.; Yoshimori, T.; Tanaka, Y.; Ishii, T.; George, M.D.; Klionsky, D.J.; Ohsumi, M.; Ohsumi, Y. A protein conjugation system essential for autophagy. Nature 1998, 395, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Mizushima, N.; Ogawa, Y.; Matsuura, A.; Noda, T.; Ohsumi, Y. Apg10p, a novel protein-conjugating enzyme essential for autophagy in yeast. EMBO J. 1999, 18, 5234–5241. [Google Scholar] [CrossRef] [PubMed]
- Goehring, A.S.; Rivers, D.M.; Sprague, G.F., Jr. Urmylation: A ubiquitin-like pathway that functions during invasive growth and budding in yeast. Mol. Biol. Cell 2003, 14, 4329–4341. [Google Scholar] [CrossRef] [PubMed]
- Leidel, S.; Pedrioli, P.G.; Bucher, T.; Brost, R.; Costanzo, M.; Schmidt, A.; Aebersold, R.; Boone, C.; Hofmann, K.; Peter, M. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature 2009, 458, 228–232. [Google Scholar] [CrossRef]
- Brachmann, C.; Kaduhr, L.; Jüdes, A.; Ravichandran, K.E.; West, J.D.; Glatt, S.; Schaffrath, R. Redox requirements for ubiquitin-like urmylation of Ahp1, a 2-Cys peroxiredoxin from yeast. Redox Biol. 2020, 30, 101438. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, G.A.; Wilkinson, C.R.; Jedrzejewski, P.T.; Finley, D. Role of a ubiquitin-like modification in polarized morphogenesis. Science 2002, 295, 2442–2446. [Google Scholar] [CrossRef]
- Mishra, S.K.; Ammon, T.; Popowicz, G.M.; Krajewski, M.; Nagel, R.J.; Ares, M., Jr.; Holak, T.A.; Jentsch, S. Role of the ubiquitin-like protein Hub1 in splice-site usage and alternative splicing. Nature 2011, 474, 173–178. [Google Scholar] [CrossRef]
- Lambies, G.; Garcia de Herreros, A.; Diaz, V.M. The role of DUBs in the post-translational control of cell migration. Essays Biochem. 2019, 63, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Geurink, P.P.; van der Heden van Noort, G.J.; Mulder, M.P.C.; Knaap, R.C.M.; Kikkert, M.; Ovaa, H. Profiling DUBs and Ubl-specific proteases with activity-based probes. Methods Enzymol. 2019, 618, 357–387. [Google Scholar] [CrossRef] [PubMed]
- Pruneda, J.N.; Komander, D. Evaluating enzyme activities and structures of DUBs. Methods Enzymol. 2019, 618, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, T.; Pichlo, C.; Woiwode, I.; Klopffleisch, K.; Witting, K.F.; Ovaa, H.; Baumann, U.; Hofmann, K. A family of unconventional deubiquitinases with modular chain specificity determinants. Nat. Commun. 2018, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Whitby, F.G.; Xia, G.; Pickart, C.M.; Hill, C.P. Crystal structure of the human ubiquitin-like protein NEDD8 and interactions with ubiquitin pathway enzymes. J. Biol. Chem. 1998, 273, 34983–34991. [Google Scholar] [CrossRef] [PubMed]
- Sela, N.; Atir-Lande, A.; Kornitzer, D. Neddylation and CAND1 independently stimulate SCF ubiquitin ligase activity in Candida albicans. Eukaryot. Cell 2012, 11, 42–52. [Google Scholar] [CrossRef]
- Pick, E. The necessity of NEDD8/Rub1 for vitality and its association with mitochondria-derived oxidative stress. Redox Biol. 2020, 37, 101765. [Google Scholar] [CrossRef]
- Walden, H.; Podgorski, M.S.; Schulman, B.A. Insights into the ubiquitin transfer cascade from the structure of the activating enzyme for NEDD8. Nature 2003, 422, 330–334. [Google Scholar] [CrossRef]
- Kurz, T.; Ozlu, N.; Rudolf, F.; O’Rourke, S.M.; Luke, B.; Hofmann, K.; Hyman, A.A.; Bowerman, B.; Peter, M. The conserved protein DCN-1/Dcn1p is required for cullin neddylation in C. elegans and S. cerevisiae. Nature 2005, 435, 1257–1261. [Google Scholar] [CrossRef]
- Hotton, S.K.; Callis, J. Regulation of cullin RING ligases. Annu. Rev. Plant Biol. 2008, 59, 467–489. [Google Scholar] [CrossRef] [PubMed]
- Linghu, B.; Callis, J.; Goebl, M.G. Rub1p Processing by Yuh1p Is Required for Wild-Type Levels of Rub1p Conjugation to Cdc53p. Eukaryot. Cell 2002, 1, 491–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, P.; Zhang, M.; He, S.; Lu, K.; Chen, Y.; Xing, G.; Lu, Y.; Liu, P.; Li, Y.; Wang, S.; et al. The covalent modifier Nedd8 is critical for the activation of Smurf1 ubiquitin ligase in tumorigenesis. Nat Commun. 2014, 5, 3733. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, R.N.; Haas, A.L. Conservation in the mechanism of Nedd8 activation by the human AppBp1-Uba3 heterodimer. J. Biol. Chem. 2003, 278, 26823–26830. [Google Scholar] [CrossRef] [PubMed]
- Walden, H.; Podgorski, M.S.; Huang, D.T.; Miller, D.W.; Howard, R.J.; Minor, D.L., Jr.; Holton, J.M.; Schulman, B.A. The structure of the APPBP1-UBA3-NEDD8-ATP complex reveals the basis for selective ubiquitin-like protein activation by an E1. Mol. Cell 2003, 12, 1427–1437. [Google Scholar] [CrossRef]
- Souphron, J.; Waddell, M.B.; Paydar, A.; Tokgoz-Gromley, Z.; Roussel, M.F.; Schulman, B.A. Structural dissection of a gating mechanism preventing misactivation of ubiquitin by NEDD8’s E1. Biochemistry 2008, 47, 8961–8969. [Google Scholar] [CrossRef]
- Gurevich, S.Z.; Sinha, A.; Longworth, J.; Singh, R.K.; Lemma, B.E.; Thakur, A.; Popp, O.; Kornitzer, D.; Reis, N.; Scheffner, M.; et al. Rub1/NEDD8, a ubiquitin-like modifier, is also a ubiquitin modifier. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hjerpe, R.; Thomas, Y.; Chen, J.; Zemla, A.; Curran, S.; Shpiro, N.; Dick, L.R.; Kurz, T. Changes in the ratio of free NEDD8 to ubiquitin triggers NEDDylation by ubiquitin enzymes. Biochem. J. 2012, 441, 927–936. [Google Scholar] [CrossRef]
- Leidecker, O.; Matic, I.; Mahata, B.; Pion, E.; Xirodimas, D.P. The ubiquitin E1 enzyme Ube1 mediates NEDD8 activation under diverse stress conditions. Cell Cycle 2012, 11, 1142–1150. [Google Scholar] [CrossRef]
- Singh, R.K.; Zerath, S.; Kleifeld, O.; Scheffner, M.; Glickman, M.H.; Fushman, D. Recognition and cleavage of related to ubiquitin 1 (Rub1) and Rub1-ubiquitin chains by components of the ubiquitin-proteasome system. Mol. Cell Proteom. 2012, 11, 1595–1611. [Google Scholar] [CrossRef]
- Skaar, J.R.; Pagan, J.K.; Pagano, M. Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Anderica-Romero, A.C.; Gonzalez-Herrera, I.G.; Santamaria, A.; Pedraza-Chaverri, J. Cullin 3 as a novel target in diverse pathologies. Redox Biol. 2013, 1, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Sen, P.; Hofmann, K.; Ma, L.; Goebl, M.; Harper, J.W.; Elledge, S.J. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 1996, 86, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Ribar, B.; Prakash, L.; Prakash, S. ELA1 and CUL3 are required along with ELC1 for RNA polymerase II polyubiquitylation and degradation in DNA-damaged yeast cells. Mol. Cell Biol. 2007, 27, 3211–3216. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.D.; Harreman, M.; Taschner, M.; Reid, J.; Walker, J.; Erdjument-Bromage, H.; Tempst, P.; Svejstrup, J.Q. Proteasome-mediated processing of Def1, a critical step in the cellular response to transcription stress. Cell 2013, 154, 983–995. [Google Scholar] [CrossRef]
- Lejeune, D.; Chen, X.; Ruggiero, C.; Berryhill, S.; Ding, B.; Li, S. Yeast Elc1 plays an important role in global genomic repair but not in transcription coupled repair. DNA Repair 2009, 8, 40–50. [Google Scholar] [CrossRef]
- Wilson, M.D.; Harreman, M.; Svejstrup, J.Q. Ubiquitylation and degradation of elongating RNA polymerase II: The last resort. Biochim. Biophys. Acta 2013, 1829, 151–157. [Google Scholar] [CrossRef]
- Liu, L.; Huo, Y.; Li, J.; Jiang, T. Crystal structure of the yeast Rad7-Elc1 complex and assembly of the Rad7-Rad16-Elc1-Cul3 complex. DNA Repair 2019, 77, 1–9. [Google Scholar] [CrossRef]
- Zaidi, I.W.; Rabut, G.; Poveda, A.; Scheel, H.; Malmstrom, J.; Ulrich, H.; Hofmann, K.; Pasero, P.; Peter, M.; Luke, B. Rtt101 and Mms1 in budding yeast form a CUL4(DDB1)-like ubiquitin ligase that promotes replication through damaged DNA. EMBO Rep. 2008, 9, 1034–1040. [Google Scholar] [CrossRef]
- Mimura, S.; Yamaguchi, T.; Ishii, S.; Noro, E.; Katsura, T.; Obuse, C.; Kamura, T. Cul8/Rtt101 forms a variety of protein complexes that regulate DNA damage response and transcriptional silencing. J. Biol. Chem. 2010, 285, 9858–9867. [Google Scholar] [CrossRef]
- Collins, S.R.; Miller, K.M.; Maas, N.L.; Roguev, A.; Fillingham, J.; Chu, C.S.; Schuldiner, M.; Gebbia, M.; Recht, J.; Shales, M.; et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 2007, 446, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Duro, E.; Vaisica, J.A.; Brown, G.W.; Rouse, J. Budding yeast Mms22 and Mms1 regulate homologous recombination induced by replisome blockage. DNA Repair 2008, 7, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Luke, B.; Versini, G.; Jaquenoud, M.; Zaidi, I.W.; Kurz, T.; Pintard, L.; Pasero, P.; Peter, M. The cullin Rtt101p promotes replication fork progression through damaged DNA and natural pause sites. Curr. Biol. 2006, 16, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Lyapina, S.; Cope, G.; Shevchenko, A.; Serino, G.; Tsuge, T.; Zhou, C.; Wolf, D.A.; Wei, N.; Shevchenko, A.; Deshaies, R.J. Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science 2001, 292, 1382–1385. [Google Scholar] [CrossRef]
- Zhou, C.; Seibert, V.; Geyer, R.; Rhee, E.; Lyapina, S.; Cope, G.; Deshaies, R.J.; Wolf, D.A. The fission yeast COP9/signalosome is involved in cullin modification by ubiquitin-related Ned8p. BMC Biochem. 2001, 2, 7. [Google Scholar] [CrossRef]
- Scheel, H.; Hofmann, K. Prediction of a common structural scaffold for proteasome lid, COP9-signalosome and eIF3 complexes. BMC Bioinform. 2005, 6, 71. [Google Scholar] [CrossRef]
- Pick, E.; Hofmann, K.; Glickman, M.H. PCI complexes: Beyond the proteasome, CSN, and eIF3 Troika. Mol. Cell 2009, 35, 260–264. [Google Scholar] [CrossRef]
- Maytal-Kivity, V.; Pick, E.; Piran, R.; Hofmann, K.; Glickman, M.H. The COP9 signalosome-like complex in S. cerevisiae and links to other PCI complexes. Int. J. Biochem. Cell Biol. 2003, 35, 706–715. [Google Scholar] [CrossRef]
- Yu, Z.; Kleifeld, O.; Lande-Atir, A.; Bsoul, M.; Kleiman, M.; Krutauz, D.; Book, A.; Vierstra, R.D.; Hofmann, K.; Reis, N.; et al. Dual function of Rpn5 in two PCI complexes, the 26S proteasome and COP9 signalosome. Mol. Biol. Cell 2011, 22, 911–920. [Google Scholar] [CrossRef]
- Pick, E.; Golan, A.; Zimbler, J.Z.; Guo, L.; Sharaby, Y.; Tsuge, T.; Hofmann, K.; Wei, N. The Minimal Deneddylase Core of the COP9 Signalosome Excludes the Csn6 MPN(−) Domain. PLoS ONE 2012, 7, e43980. [Google Scholar] [CrossRef]
- Sinha, A.; Israeli, R.; Cirigliano, A.; Gihaz, S.; Trabelcy, B.; Braus, G.H.; Gerchman, Y.; Fishman, A.; Negri, R.; Rinaldi, T.; et al. The COP9 signalosome mediates the Spt23 regulated fatty acid desaturation and ergosterol biosynthesis. FASEB J. 2020, 34, 4870–4889. [Google Scholar] [CrossRef] [PubMed]
- Meluh, P.B.; Koshland, D. Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the mammalian centromere protein CENP-C. Mol. Biol. Cell 1995, 6, 793–807. [Google Scholar] [CrossRef]
- Mannen, H.; Tseng, H.M.; Cho, C.L.; Li, S.S. Cloning and expression of human homolog HSMT3 to yeast SMT3 suppressor of MIF2 mutations in a centromere protein gene. Biochem. Biophys. Res. Commun. 1996, 222, 178–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGrath, J.P.; Jentsch, S.; Varshavsky, A. UBA 1: An essential yeast gene encoding ubiquitin-activating enzyme. EMBO J. 1991, 10, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Biggins, S.; Bhalla, N.; Chang, A.; Smith, D.L.; Murray, A.W. Genes involved in sister chromatid separation and segregation in the budding yeast Saccharomyces cerevisiae. Genetics 2001, 159, 453–470. [Google Scholar] [CrossRef]
- Takahashi, Y.; Iwase, M.; Konishi, M.; Tanaka, M.; Toh-e, A.; Kikuchi, Y. Smt3, a SUMO-1 homolog, is conjugated to Cdc3, a component of septin rings at the mother-bud neck in budding yeast. Biochem. Biophys. Res. Commun. 1999, 259, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Dieckhoff, P.; Bolte, M.; Sancak, Y.; Braus, G.H.; Irniger, S. Smt3/SUMO and Ubc9 are required for efficient APC/C-mediated proteolysis in budding yeast. Mol. Microbiol. 2004, 51, 1375–1387. [Google Scholar] [CrossRef]
- Newman, H.A.; Meluh, P.B.; Lu, J.; Vidal, J.; Carson, C.; Lagesse, E.; Gray, J.J.; Boeke, J.D.; Matunis, M.J. A high throughput mutagenic analysis of yeast sumo structure and function. PLoS Genet. 2017, 13, e1006612. [Google Scholar] [CrossRef]
- Johnson, E.S.; Blobel, G. Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J. Biol. Chem. 1997, 272, 26799–26802. [Google Scholar] [CrossRef]
- Minty, A.; Dumont, X.; Kaghad, M.; Caput, D. Covalent modification of p73alpha by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif. J. Biol. Chem. 2000, 275, 36316–36323. [Google Scholar] [CrossRef]
- Hochstrasser, M. SP-RING for SUMO: New functions bloom for a ubiquitin-like protein. Cell 2001, 107, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Harris, B.; Wang, F.; Seidel, C.; McCroskey, S.; Gerton, J.L. Mms21 SUMO Ligase Activity Promotes Nucleolar Function in Saccharomyces cerevisiae. Genetics 2016, 204, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, J.; Yang, W.C.; Spear, E.D.; Michaelis, S.; Matunis, M.J. Analysis of a degron-containing reporter protein GFP-CL1 reveals a role for SUMO1 in cytosolic protein quality control. J. Biol. Chem. 2023, 299, 102851. [Google Scholar] [CrossRef]
- Klionsky, D.J. Autophagy. Curr. Biol. 2005, 15, R282–R283. [Google Scholar] [CrossRef] [PubMed]
- Downes, B.; Vierstra, R.D. Post-translational regulation in plants employing a diverse set of polypeptide tags. Biochem. Soc. Trans. 2005, 33, 393–399. [Google Scholar] [CrossRef][Green Version]
- Kirisako, T.; Ichimura, Y.; Okada, H.; Kabeya, Y.; Mizushima, N.; Yoshimori, T.; Ohsumi, M.; Takao, T.; Noda, T.; Ohsumi, Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 2000, 151, 263–276. [Google Scholar] [CrossRef]
- Ichimura, Y.; Kirisako, T.; Takao, T.; Satomi, Y.; Shimonishi, Y.; Ishihara, N.; Mizushima, N.; Tanida, I.; Kominami, E.; Ohsumi, M.; et al. A ubiquitin-like system mediates protein lipidation. Nature 2000, 408, 488–492. [Google Scholar] [CrossRef]
- Sakoh-Nakatogawa, M.; Matoba, K.; Asai, E.; Kirisako, H.; Ishii, J.; Noda, N.N.; Inagaki, F.; Nakatogawa, H.; Ohsumi, Y. Atg12-Atg5 conjugate enhances E2 activity of Atg3 by rearranging its catalytic site. Nat. Struct. Mol. Biol. 2013, 20, 433–439. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Ma, C. Atg3 promotes Atg8 lipidation via altering lipid diffusion and rearrangement. Protein Sci. 2020, 29, 1511–1523. [Google Scholar] [CrossRef]
- Popelka, H.; Klionsky, D.J. Multiple structural rearrangements mediated by high-plasticity regions in Atg3 are key for efficient conjugation of Atg8 to PE during autophagy. Autophagy 2021, 17, 1805–1808. [Google Scholar] [CrossRef]
- Furukawa, K.; Mizushima, N.; Noda, T.; Ohsumi, Y. A protein conjugation system in yeast with homology to biosynthetic enzyme reaction of prokaryotes. J. Biol. Chem. 2000, 275, 7462–7465. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, A.G.; Schorpp, K.; Schlieker, C.; Buti, L.; Damon, J.R.; Spooner, E.; Ploegh, H.L.; Jentsch, S. Role of the ubiquitin-like protein Urm1 as a noncanonical lysine-directed protein modifier. Proc. Natl. Acad. Sci. USA 2011, 108, 1763–1770. [Google Scholar] [CrossRef]
- Termathe, M.; Leidel, S.A. The Uba4 domain interplay is mediated via a thioester that is critical for tRNA thiolation through Urm1 thiocarboxylation. Nucleic Acids Res. 2018, 46, 5171–5181. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.E.; Kaduhr, L.; Skupien-Rabian, B.; Shvetsova, E.; Sokołowski, M.; Krutyhołowa, R.C.; Kwasna, D.; Brachmann, C.; Lin, S.; Guzman Perez, S.; et al. E2/E3-independent ubiquitin-like protein conjugation by Urm1 is directly coupled to cysteine persulfidation. EMBO J. 2022, 41, e111318. [Google Scholar] [CrossRef] [PubMed]
- Chanarat, S. UBL5/Hub1: An Atypical Ubiquitin-Like Protein with a Typical Role as a Stress-Responsive Regulator. Int. J. Mol. Sci. 2021, 22, 9384. [Google Scholar] [CrossRef] [PubMed]
- Ramelot, T.A.; Cort, J.R.; Yee, A.A.; Semesi, A.; Edwards, A.M.; Arrowsmith, C.H.; Kennedy, M.A. Solution structure of the yeast ubiquitin-like modifier protein Hub1. J. Struct. Funct. Genom. 2003, 4, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Navarro, S.; Igual, J.C.; Pérez-Ortín, J.E. SRC1: An intron-containing yeast gene involved in sister chromatid segregation. Yeast 2002, 19, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Capella, M.; Martín Caballero, L.; Pfander, B.; Braun, S.; Jentsch, S. ESCRT recruitment by the S. cerevisiae inner nuclear membrane protein Heh1 is regulated by Hub1-mediated alternative splicing. J. Cell. Sci. 2020, 133, jcs250688. [Google Scholar] [CrossRef]
- Gu, M.; LaJoie, D.; Chen, O.S.; von Appen, A.; Ladinsky, M.S.; Redd, M.J.; Nikolova, L.; Bjorkman, P.J.; Sundquist, W.I.; Ullman, K.S.; et al. LEM2 recruits CHMP7 for ESCRT-mediated nuclear envelope closure in fission yeast and human cells. Proc. Natl. Acad. Sci. USA 2017, 114, E2166–E2175. [Google Scholar] [CrossRef]
- Webster, B.M.; Colombi, P.; Jäger, J.; Lusk, C.P. Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4. Cell 2014, 159, 388–401. [Google Scholar] [CrossRef]
- Willan, J.; Cleasby, A.J.; Flores-Rodriguez, N.; Stefani, F.; Rinaldo, C.; Pisciottani, A.; Grant, E.; Woodman, P.; Bryant, H.E.; Ciani, B. ESCRT-III is necessary for the integrity of the nuclear envelope in micronuclei but is aberrant at ruptured micronuclear envelopes generating damage. Oncogenesis 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Karaduman, R.; Chanarat, S.; Pfander, B.; Jentsch, S. Error-Prone Splicing Controlled by the Ubiquitin Relative Hub1. Mol. Cell 2017, 67, 423–432.e424. [Google Scholar] [CrossRef] [PubMed]
- Ammon, T.; Mishra, S.K.; Kowalska, K.; Popowicz, G.M.; Holak, T.A.; Jentsch, S. The conserved ubiquitin-like protein Hub1 plays a critical role in splicing in human cells. J. Mol. Cell Biol. 2014, 6, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kago, G.; Yellman, C.M.; Matouschek, A. Ubiquitin-like domains can target to the proteasome but proteolysis requires a disordered region. EMBO J. 2016, 35, 1522–1536. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengupta, S.; Pick, E. The Ubiquitin-like Proteins of Saccharomyces cerevisiae. Biomolecules 2023, 13, 734. https://doi.org/10.3390/biom13050734
Sengupta S, Pick E. The Ubiquitin-like Proteins of Saccharomyces cerevisiae. Biomolecules. 2023; 13(5):734. https://doi.org/10.3390/biom13050734
Chicago/Turabian StyleSengupta, Swarnab, and Elah Pick. 2023. "The Ubiquitin-like Proteins of Saccharomyces cerevisiae" Biomolecules 13, no. 5: 734. https://doi.org/10.3390/biom13050734
APA StyleSengupta, S., & Pick, E. (2023). The Ubiquitin-like Proteins of Saccharomyces cerevisiae. Biomolecules, 13(5), 734. https://doi.org/10.3390/biom13050734







