Role of Sirtuin 3 in Degenerative Diseases of the Central Nervous System
Abstract
1. Introduction
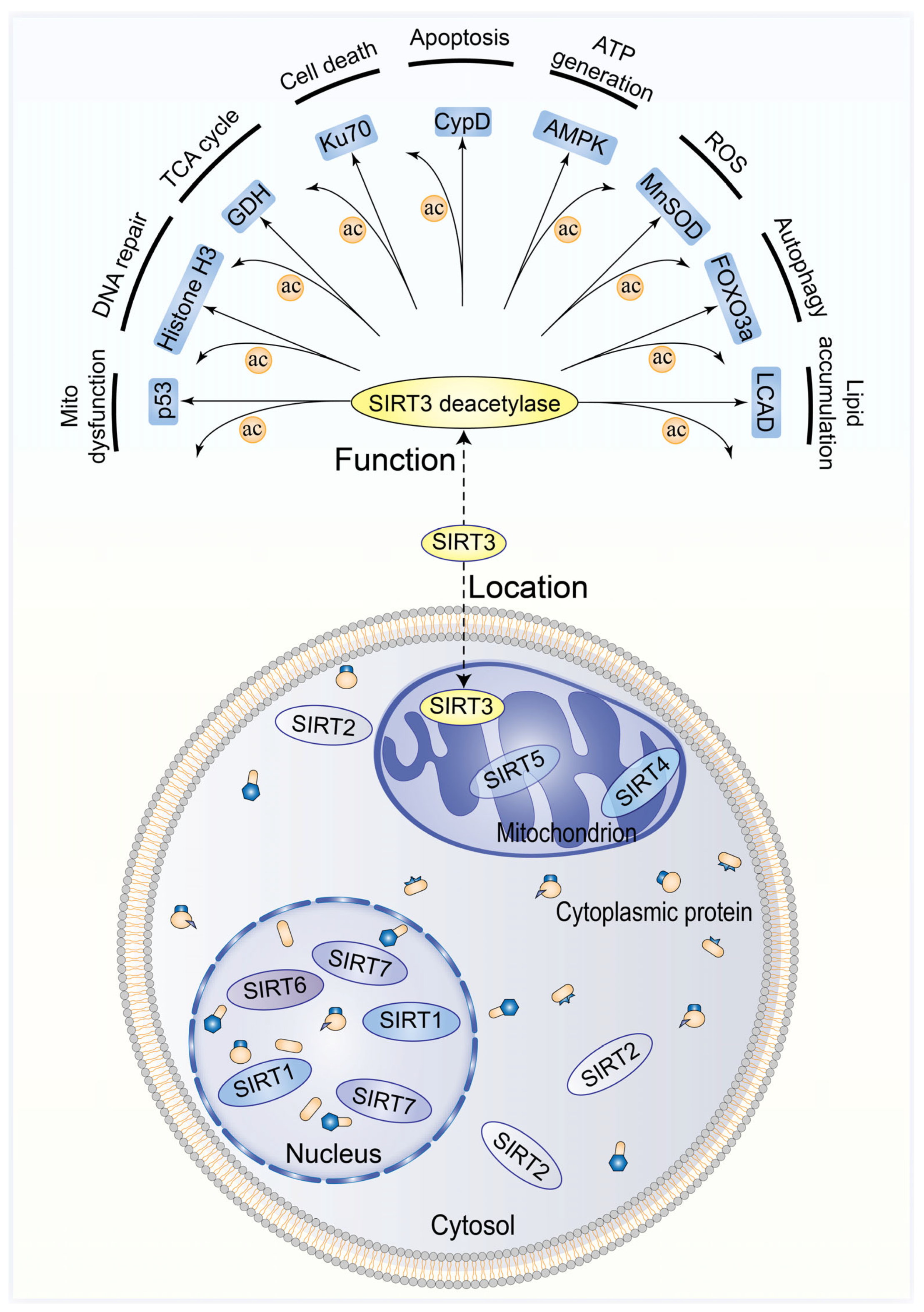
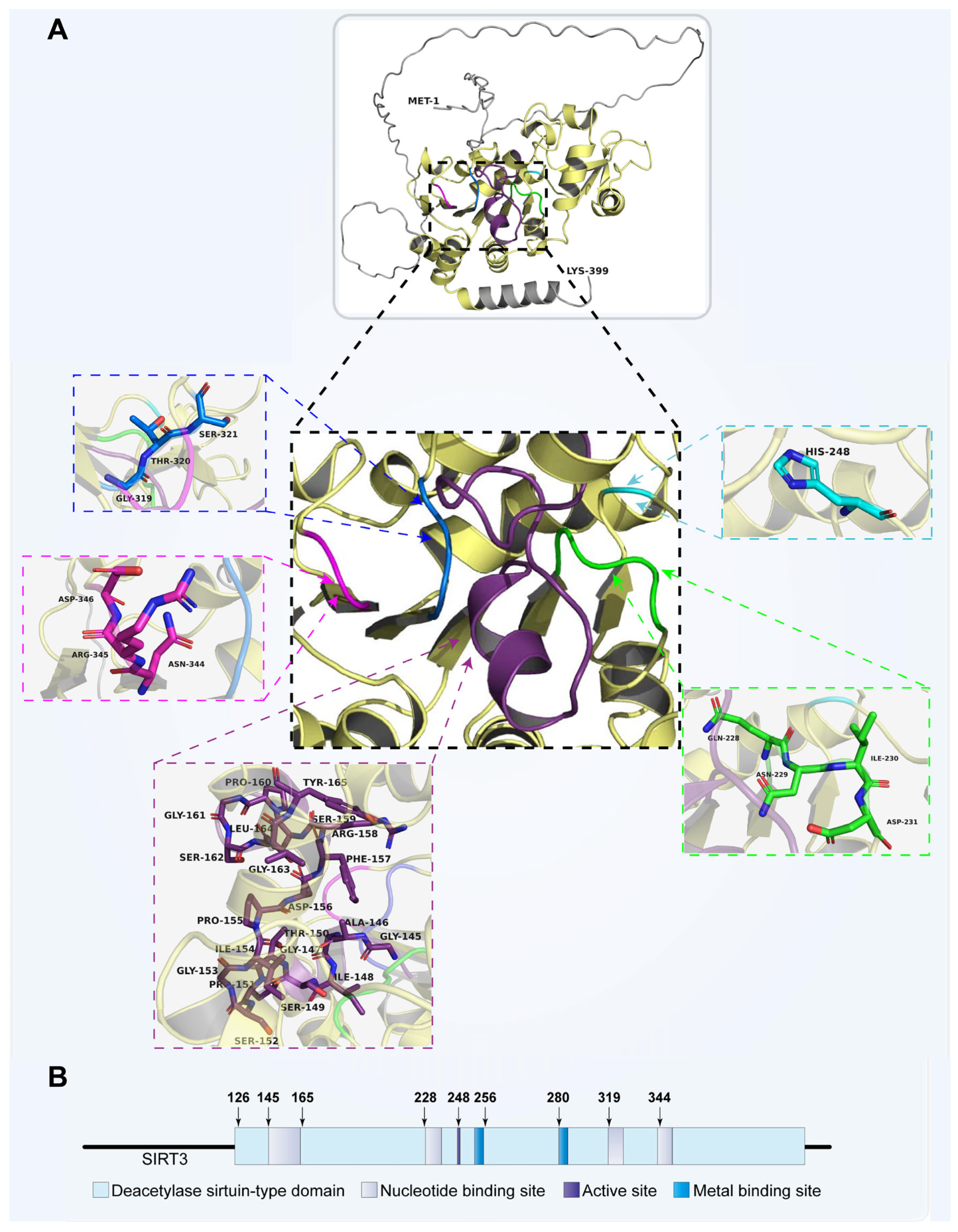
2. Molecular Structure and Function of Sirt3
2.1. Sirtuin Family and Sirt3
2.2. Molecular Function of Sirt3
3. Cellular Function of Sirt3 in the CNS
3.1. Sirt3 and Neurons
3.2. Sirt3 and Astrocytes
3.3. Sirt3 and Microglia
4. Sirt3 and Neurodegenerative Diseases
4.1. Sirt3 and Alzheimer’s Disease
4.2. Sirt3 and Parkinson’s Disease
4.3. Sirt3 and Huntington’s Disease
4.4. Sirt3 and Amyotrophic Lateral Sclerosis
4.5. Sirt3 and Multiple Sclerosis
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Du, W.; Zhao, Y.; Lim, K.; Lu, L.; Zhang, C.; Li, L. Mitochondria targeting drugs for neurodegenerative diseases-Design, mechanism and application. Acta Pharm. Sin. B 2022, 12, 2778–2789. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Joshi, A.U.; Minhas, P.S.; Liddelow, S.A.; Haileselassie, B.; Andreasson, K.I.; Dorn, G.W., II; Mochly-Rosen, D. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat. Neurosci. 2019, 22, 1635–1648. [Google Scholar] [CrossRef]
- Malpartida, A.B.; Williamson, M.; Narendra, D.P.; Wade-Martins, R.; Ryan, B.J. Mitochondrial Dysfunction and Mitophagy in Parkinson′s Disease: From Mechanism to Therapy. Trends Biochem. Sci. 2021, 46, 329–343. [Google Scholar] [CrossRef]
- Diao, Z.; Ji, Q.; Wu, Z.; Zhang, W.; Cai, Y.; Wang, Z.; Hu, J.; Liu, Z.; Wang, Q.; Bi, S.; et al. Sirt3 consolidates heterochromatin and counteracts senescence. Nucleic Acids Res. 2021, 49, 4203–4219. [Google Scholar] [CrossRef]
- Li, M.; Chiang, Y.L.; Lyssiotis, C.A.; Teater, M.R.; Hong, J.Y.; Shen, H.; Wang, L.; Hu, J.; Jing, H.; Chen, Z.; et al. Non-oncogene Addiction to Sirt3 Plays a Critical Role in Lymphomagenesis. Cancer Cell 2019, 35, 916–931.e9. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Pandey, A.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Billings, F.T.t.; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef][Green Version]
- Jiang, B.; Tian, Q.; Shu, C.; Zhao, J.; Xue, M.; Zhu, S. Resveratrol Enhances the Anti-Cancer Effects of Cis-Platinum on Human Cervical Cancer Cell Lines by Activating the Sirt3 Relative Anti-Oxidative Pathway. Front. Pharmacol. 2022, 13, 916876. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, R.; Wang, G.; Chen, Z.; Li, Y.; Zhao, Y.; Liu, D.; Zhao, H.; Zhang, F.; Yao, J.; et al. Sirt3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol. 2020, 28, 101343. [Google Scholar] [CrossRef]
- Yi, X.; Guo, W.; Shi, Q.; Yang, Y.; Zhang, W.; Chen, X.; Kang, P.; Chen, J.; Cui, T.; Ma, J.; et al. Sirt3-Dependent Mitochondrial Dynamics Remodeling Contributes to Oxidative Stress-Induced Melanocyte Degeneration in Vitiligo. Theranostics 2019, 9, 1614–1633. [Google Scholar] [CrossRef]
- Koentges, C.; Cimolai, M.C.; Pfeil, K.; Wolf, D.; Marchini, T.; Tarkhnishvili, A.; Hoffmann, M.M.; Odening, K.E.; Diehl, P.; von Zur Muhlen, C.; et al. Impaired Sirt3 activity mediates cardiac dysfunction in endotoxemia by calpain-dependent disruption of ATP synthesis. J. Mol. Cell Cardiol. 2019, 133, 138–147. [Google Scholar] [CrossRef]
- Watroba, M.; Szukiewicz, D. The role of sirtuins in aging and age-related diseases. Adv. Med. Sci. 2016, 61, 52–62. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, J.C.; Jiang, Q.; Lee, W.Y. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging Cell 2021, 20, e13301. [Google Scholar] [CrossRef]
- Heinonen, T.; Ciarlo, E.; Theroude, C.; Pelekanou, A.; Herderschee, J.; Le Roy, D.; Roger, T. Sirtuin 5 Deficiency Does Not Compromise Innate Immune Responses to Bacterial Infections. Front. Immunol. 2018, 9, 2675. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Canto, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef][Green Version]
- Gao, P.; You, M.; Li, L.; Zhang, Q.; Fang, X.; Wei, X.; Zhou, Q.; Zhang, H.; Wang, M.; Lu, Z.; et al. Salt-Induced Hepatic Inflammatory Memory Contributes to Cardiovascular Damage Through Epigenetic Modulation of Sirt3. Circulation 2022, 145, 375–391. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Liu, T.; Hwang, Y.J.; Hyeon, S.J.; Im, H.; Lee, K.; Alvarez, V.E.; McKee, A.C.; Um, S.J.; et al. Sirt3 deregulation is linked to mitochondrial dysfunction in Alzheimer′s disease. Aging Cell 2018, 17, e12679. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Liu, L.; Chen, W.C.; Wang, F.; Cheng, Y.R.; Liu, Y.M.; Lai, Y.F.; Zhang, R.J.; Qiao, Y.N.; Yuan, Y.Y.; et al. Gestational Leucylation Suppresses Embryonic T-Box Transcription Factor 5 Signal and Causes Congenital Heart Disease. Adv. Sci. 2022, 9, e2201034. [Google Scholar] [CrossRef]
- Vatner, D.E.; Zhang, J.; Oydanich, M.; Guers, J.; Katsyuba, E.; Yan, L.; Sinclair, D.; Auwerx, J.; Vatner, S.F. Enhanced longevity and metabolism by brown adipose tissue with disruption of the regulator of G protein signaling 14. Aging Cell 2018, 17, e12751. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, L.L.; Wen, X.; Wang, X.Y.; Liu, J.; Cheng, Y.; Huang, J. Sirtuin-3 (Sirt3), a therapeutic target with oncogenic and tumor-suppressive function in cancer. Cell Death Dis. 2014, 5, e1047. [Google Scholar] [CrossRef][Green Version]
- Jin, L.; Wei, W.; Jiang, Y.; Peng, H.; Cai, J.; Mao, C.; Dai, H.; Choy, W.; Bemis, J.E.; Jirousek, M.R.; et al. Crystal structures of human Sirt3 displaying substrate-induced conformational changes. J. Biol. Chem. 2009, 284, 24394–24405. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Nagasawa, K.; Munch, C.; Xu, Y.; Satterstrom, K.; Jeong, S.; Hayes, S.D.; Jedrychowski, M.P.; Vyas, F.S.; Zaganjor, E.; et al. Mitochondrial Sirtuin Network Reveals Dynamic Sirt3-Dependent Deacetylation in Response to Membrane Depolarization. Cell 2016, 167, 985–1000.e21. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.; Liu, J.; Shen, S.; Tong, Q.; Ma, X.; Lin, L. Sirt3 promotes lipophagy and chaperon-mediated autophagy to protect hepatocytes against lipotoxicity. Cell Death Differ. 2020, 27, 329–344. [Google Scholar] [CrossRef][Green Version]
- Zou, X.; Zhu, Y.; Park, S.H.; Liu, G.; O′Brien, J.; Jiang, H.; Gius, D. Sirt3-Mediated Dimerization of IDH2 Directs Cancer Cell Metabolism and Tumor Growth. Cancer Res. 2017, 77, 3990–3999. [Google Scholar] [CrossRef][Green Version]
- Li, S.T.; Huang, D.; Shen, S.; Cai, Y.; Xing, S.; Wu, G.; Jiang, Z.; Hao, Y.; Yuan, M.; Wang, N.; et al. Myc-mediated SDHA acetylation triggers epigenetic regulation of gene expression and tumorigenesis. Nat. Metab. 2020, 2, 256–269. [Google Scholar] [CrossRef]
- Masgras, I.; Cannino, G.; Ciscato, F.; Sanchez-Martin, C.; Darvishi, F.B.; Scantamburlo, F.; Pizzi, M.; Menga, A.; Fregona, D.; Castegna, A.; et al. Tumor growth of neurofibromin-deficient cells is driven by decreased respiration and hampered by NAD(+) and Sirt3. Cell Death Differ. 2022, 29, 1996–2008. [Google Scholar] [CrossRef]
- Wei, T.; Gao, J.; Huang, C.; Song, B.; Sun, M.; Shen, W. Sirt3 (Sirtuin-3) Prevents Ang II (Angiotensin II)-Induced Macrophage Metabolic Switch Improving Perivascular Adipose Tissue Function. Arter. Thromb. Vasc. Biol. 2021, 41, 714–730. [Google Scholar] [CrossRef]
- Zhou, W.; Nie, Z.W.; Zhou, D.J.; Cui, X.S. Acetyl-CoA synthases are essential for maintaining histone acetylation under metabolic stress during zygotic genome activation in pigs. J. Cell Physiol. 2021, 236, 6948–6962. [Google Scholar] [CrossRef]
- Hallows, W.C.; Yu, W.; Smith, B.C.; Devries, M.K.; Ellinger, J.J.; Someya, S.; Shortreed, M.R.; Prolla, T.; Markley, J.L.; Smith, L.M.; et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol. Cell 2011, 41, 139–149. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Cimen, H.; Han, M.J.; Shi, T.; Deng, J.H.; Koc, H.; Palacios, O.M.; Montier, L.; Bai, Y.; Tong, Q.; et al. NAD+-dependent deacetylase Sirt3 regulates mitochondrial protein synthesis by deacetylation of the ribosomal protein MRPL10. J. Biol. Chem. 2010, 285, 7417–7429. [Google Scholar] [CrossRef][Green Version]
- Si, Y.; Bao, H.; Han, L.; Chen, L.; Zeng, L.; Jing, L.; Xing, Y.; Geng, Y. Dexmedetomidine attenuation of renal ischaemia-reperfusion injury requires sirtuin 3 activation. Br. J. Anaesth. 2018, 121, 1260–1271. [Google Scholar] [CrossRef][Green Version]
- Karvinen, S.; Silvennoinen, M.; Vainio, P.; Sistonen, L.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Effects of intrinsic aerobic capacity, aging and voluntary running on skeletal muscle sirtuins and heat shock proteins. Exp. Gerontol. 2016, 79, 46–54. [Google Scholar] [CrossRef][Green Version]
- Docrat, T.F.; Nagiah, S.; Naicker, N.; Baijnath, S.; Singh, S.; Chuturgoon, A.A. The protective effect of metformin on mitochondrial dysfunction and endoplasmic reticulum stress in diabetic mice brain. Eur. J. Pharmacol. 2020, 875, 173059. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Jiao, X.; Wang, G.; Wang, R.; Wu, Y. Sirt3 alleviates neuropathic pain by deacetylating FoxO3a in the spinal dorsal horn of diabetic model rats. Reg. Anesth. Pain Med. 2021, 46, 49–56. [Google Scholar] [CrossRef]
- Ren, J.H.; Hu, J.L.; Cheng, S.T.; Yu, H.B.; Wong, V.K.W.; Law, B.Y.K.; Yang, Y.F.; Huang, Y.; Liu, Y.; Chen, W.X.; et al. Sirt3 restricts hepatitis B virus transcription and replication through epigenetic regulation of covalently closed circular DNA involving suppressor of variegation 3–9 homolog 1 and SET domain containing 1A histone methyltransferases. Hepatology 2018, 68, 1260–1276. [Google Scholar] [CrossRef][Green Version]
- Shi, K.; Tian, D.C.; Li, Z.G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Nebie, O.; Carvalho, K.; Barro, L.; Delila, L.; Faivre, E.; Renn, T.Y.; Chou, M.L.; Wu, Y.W.; Nyam-Erdene, A.; Chou, S.Y.; et al. Human platelet lysate biotherapy for traumatic brain injury: Preclinical assessment. Brain 2021, 144, 3142–3158. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Wang, T.; Cao, Y.; Zheng, Q.; Tu, J.; Zhou, W.; He, J.; Zhong, J.; Chen, Y.; Wang, J.; Cai, R.; et al. SENP1-Sirt3 Signaling Controls Mitochondrial Protein Acetylation and Metabolism. Mol. Cell 2019, 75, 823–834.e5. [Google Scholar] [CrossRef]
- Hilton, B.J.; Husch, A.; Schaffran, B.; Lin, T.C.; Burnside, E.R.; Dupraz, S.; Schelski, M.; Kim, J.; Muller, J.A.; Schoch, S.; et al. An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury. Neuron 2022, 110, 51–69.e7. [Google Scholar] [CrossRef]
- Sun, Q.; Kang, R.R.; Chen, K.G.; Liu, K.; Ma, Z.; Liu, C.; Deng, Y.; Liu, W.; Xu, B. Sirtuin 3 is required for the protective effect of Resveratrol on Manganese-induced disruption of mitochondrial biogenesis in primary cultured neurons. J. Neurochem. 2021, 156, 121–135. [Google Scholar] [CrossRef]
- Ye, J.S.; Chen, L.; Lu, Y.Y.; Lei, S.Q.; Peng, M.; Xia, Z.Y. Sirt3 activator honokiol ameliorates surgery/anesthesia-induced cognitive decline in mice through anti-oxidative stress and anti-inflammatory in hippocampus. CNS Neurosci. Ther. 2019, 25, 355–366. [Google Scholar] [CrossRef][Green Version]
- Yu, W.; Lyu, J.; Jia, L.; Sheng, M.; Yu, H.; Du, H. Dexmedetomidine Ameliorates Hippocampus Injury and Cognitive Dysfunction Induced by Hepatic Ischemia/Reperfusion by Activating Sirt3-Mediated Mitophagy and Inhibiting Activation of the NLRP3 Inflammasome in Young Rats. Oxid. Med. Cell. Longev. 2020, 2020, 7385458. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, A.; Li, Y.J.; Yang, Y.; Kishimoto, Y.; Zhang, S.; Wang, Y.; Wan, R.; Raefsky, S.M.; Lu, D.; et al. Sirt3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun. 2019, 10, 1886. [Google Scholar] [CrossRef][Green Version]
- Zhou, Z.D.; Tan, E.K. Oxidized nicotinamide adenine dinucleotide-dependent mitochondrial deacetylase sirtuin-3 as a potential therapeutic target of Parkinson′s disease. Ageing Res. Rev. 2020, 62, 101107. [Google Scholar] [CrossRef]
- He, J.; Shangguan, X.; Zhou, W.; Cao, Y.; Zheng, Q.; Tu, J.; Hu, G.; Liang, Z.; Jiang, C.; Deng, L.; et al. Glucose limitation activates AMPK coupled SENP1-Sirt3 signalling in mitochondria for T cell memory development. Nat. Commun. 2021, 12, 4371. [Google Scholar] [CrossRef]
- Cai, H.; Bian, X.; Chen, L.; Zhang, N.; Li, L.; Tang, W.; Liu, X.; Li, Z. Selective intra-arterial brain cooling induces cerebral protection against ischemia/reperfusion injury through SENP1-Sirt3 signaling. Free Radic. Biol. Med. 2021, 171, 272–283. [Google Scholar] [CrossRef]
- Dai, S.H.; Chen, T.; Li, X.; Yue, K.Y.; Luo, P.; Yang, L.K.; Zhu, J.; Wang, Y.H.; Fei, Z.; Jiang, X.F. Sirt3 confers protection against neuronal ischemia by inducing autophagy: Involvement of the AMPK-mTOR pathway. Free Radic. Biol. Med. 2017, 108, 345–353. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, X.; Wang, J.; Shi, Y.; Hu, Q.; Cui, W.; Bai, H.; Zhou, J.; Du, Y.; Han, L.; et al. Adiponectin/AdiopR1 signaling prevents mitochondrial dysfunction and oxidative injury after traumatic brain injury in a Sirt3 dependent manner. Redox Biol. 2022, 54, 102390. [Google Scholar] [CrossRef]
- Chen, T.; Liu, W.B.; Qian, X.; Xie, K.L.; Wang, Y.H. The AMPAR antagonist perampanel protects the neurovascular unit against traumatic injury via regulating Sirt3. CNS Neurosci. Ther. 2021, 27, 134–144. [Google Scholar] [CrossRef]
- Yin, J.; Li, S.; Nielsen, M.; Carcione, T.; Liang, W.S.; Shi, J. Sirtuin 3 attenuates amyloid-beta induced neuronal hypometabolism. Aging 2018, 10, 2874–2883. [Google Scholar] [CrossRef]
- Dai, S.H.; Chen, T.; Wang, Y.H.; Zhu, J.; Luo, P.; Rao, W.; Yang, Y.F.; Fei, Z.; Jiang, X.F. Sirt3 protects cortical neurons against oxidative stress via regulating mitochondrial Ca2+ and mitochondrial biogenesis. Int. J. Mol. Sci. 2014, 15, 14591. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Lu, J.; Cao, X.; Zhao, H.; Gao, L.; Xia, P.; Pei, G. A Newly Synthesized Rhamnoside Derivative Alleviates Alzheimer′s Amyloid-beta-Induced Oxidative Stress, Mitochondrial Dysfunction, and Cell Senescence through Upregulating Sirt3. Oxid. Med. Cell. Longev. 2020, 2020, 7698560. [Google Scholar] [CrossRef][Green Version]
- Dai, S.H.; Chen, T.; Wang, Y.H.; Zhu, J.; Luo, P.; Rao, W.; Yang, Y.F.; Fei, Z.; Jiang, X.F. Sirt3 attenuates hydrogen peroxide-induced oxidative stress through the preservation of mitochondrial function in HT22 cells. Int. J. Mol. Med. 2014, 34, 1159–1168. [Google Scholar] [CrossRef][Green Version]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Geng, K.; Yang, K.; Shao, J.; Xia, W. Sirt3 Protects Against Ischemic Stroke Injury by Regulating HIF-1alpha/VEGF Signaling and Blood-Brain Barrier Integrity. Cell Mol. Neurobiol. 2021, 41, 1203–1215. [Google Scholar] [CrossRef]
- Chen, T.; Dai, S.H.; Li, X.; Luo, P.; Zhu, J.; Wang, Y.H.; Fei, Z.; Jiang, X.F. Sirt1-Sirt3 axis regulates human blood-brain barrier permeability in response to ischemia. Redox Biol. 2018, 14, 229–236. [Google Scholar] [CrossRef]
- Xie, X.; Yu, C.; Zhou, J.; Xiao, Q.; Shen, Q.; Xiong, Z.; Li, Z.; Fu, Z. Nicotinamide mononucleotide ameliorates the depression-like behaviors and is associated with attenuating the disruption of mitochondrial bioenergetics in depressed mice. J. Affect. Disord. 2020, 263, 166–174. [Google Scholar] [CrossRef]
- Lee, S.; Jeon, Y.M.; Jo, M.; Kim, H.J. Overexpression of Sirt3 Suppresses Oxidative Stress-induced Neurotoxicity and Mitochondrial Dysfunction in Dopaminergic Neuronal Cells. Exp. Neurobiol. 2021, 30, 341–355. [Google Scholar] [CrossRef]
- Gao, J.M.; Zhang, X.; Shu, G.T.; Chen, N.N.; Zhang, J.Y.; Xu, F.; Li, F.; Liu, Y.G.; Wei, Y.; He, Y.Q.; et al. Trilobatin rescues cognitive impairment of Alzheimer′s disease by targeting HMGB1 through mediating Sirt3/SOD2 signaling pathway. Acta Pharmacol. Sin. 2022, 43, 2482–2494. [Google Scholar] [CrossRef]
- Mira, R.G.; Lira, M.; Cerpa, W. Traumatic Brain Injury: Mechanisms of Glial Response. Front. Physiol. 2021, 12, 740939. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.M.; Huang, H.; Chen, C.; Wan, J.; Ma, L.H.; Sun, Y.Y.; Miao, H.H.; Wu, Y.Q. Sirtuin 3 protects against anesthesia/surgery-induced cognitive decline in aged mice by suppressing hippocampal neuroinflammation. J. Neuroinflammation 2021, 18, 41. [Google Scholar] [CrossRef]
- Tyagi, A.; Mirita, C.; Shah, I.; Reddy, P.H.; Pugazhenthi, S. Effects of Lipotoxicity in Brain Microvascular Endothelial Cells During Sirt3 Deficiency-Potential Role in Comorbid Alzheimer′s Disease. Front. Aging Neurosci. 2021, 13, 716616. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Ryu, J.K.; Bardehle, S.; Meyer-Franke, A.; Ang, K.K.; Wilson, C.; Baeten, K.M.; Hanspers, K.; Merlini, M.; Thomas, S.; et al. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol. 2020, 21, 513–524. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, S.J.; Liu, X.Y.; Zhao, H.Y.; Yang, M.G.; Xu, D.X.; Guo, J.; Li, J.H.; Li, J.J. Expression of Sirt3 in various glial cell types in the periventricular white matter in the neonatal rat brain after hypoxia. Tissue Cell 2018, 52, 1–8. [Google Scholar] [CrossRef]
- Jiang, D.Q.; Wang, Y.; Li, M.X.; Ma, Y.J.; Wang, Y. Sirt3 in Neural Stem Cells Attenuates Microglia Activation-Induced Oxidative Stress Injury Through Mitochondrial Pathway. Front. Cell Neurosci. 2017, 11, 7. [Google Scholar] [CrossRef][Green Version]
- Guo, J.; Zhang, X.L.; Bao, Z.R.; Yang, X.K.; Li, L.S.; Zi, Y.; Li, F.; Wu, C.Y.; Li, J.J.; Yuan, Y. Gastrodin Regulates the Notch Signaling Pathway and Sirt3 in Activated Microglia in Cerebral Hypoxic-Ischemia Neonatal Rats and in Activated BV-2 Microglia. Neuromolecular Med. 2021, 23, 348–362. [Google Scholar] [CrossRef]
- Thangaraj, A.; Chivero, E.T.; Tripathi, A.; Singh, S.; Niu, F.; Guo, M.L.; Pillai, P.; Periyasamy, P.; Buch, S. HIV TAT-mediated microglial senescence: Role of Sirt3-dependent mitochondrial oxidative stress. Redox Biol. 2021, 40, 101843. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L. Circulating Exosomal miRNA as Diagnostic Biomarkers of Neurodegenerative Diseases. Front. Mol. Neurosci. 2020, 13, 53. [Google Scholar] [CrossRef][Green Version]
- Jang, M.H.; Piao, X.L.; Kim, H.Y.; Cho, E.J.; Baek, S.H.; Kwon, S.W.; Park, J.H. Resveratrol oligomers from Vitis amurensis attenuate beta-amyloid-induced oxidative stress in PC12 cells. Biol. Pharm. Bull. 2007, 30, 1130–1134. [Google Scholar] [CrossRef][Green Version]
- Weir, H.J.; Murray, T.K.; Kehoe, P.G.; Love, S.; Verdin, E.M.; O′Neill, M.J.; Lane, J.D.; Balthasar, N. CNS Sirt3 expression is altered by reactive oxygen species and in Alzheimer′s disease. PLoS ONE 2012, 7, e48225. [Google Scholar] [CrossRef][Green Version]
- Hou, M.; Bao, W.; Gao, Y.; Chen, J.; Song, G. Honokiol improves cognitive impairment in APP/PS1 mice through activating mitophagy and mitochondrial unfolded protein response. Chem. Biol. Interact. 2022, 351, 109741. [Google Scholar] [CrossRef]
- Yin, J.; Han, P.; Song, M.; Nielsen, M.; Beach, T.G.; Serrano, G.E.; Liang, W.S.; Caselli, R.J.; Shi, J. Amyloid-beta Increases Tau by Mediating Sirtuin 3 in Alzheimer′s Disease. Mol. Neurobiol. 2018, 55, 8592–8601. [Google Scholar] [CrossRef]
- Yin, J.; Nielsen, M.; Carcione, T.; Li, S.; Shi, J. Apolipoprotein E regulates mitochondrial function through the PGC-1alpha-sirtuin 3 pathway. Aging 2019, 11, 11148–11156. [Google Scholar] [CrossRef]
- Yin, J.; Nielsen, M.; Li, S.; Shi, J. Ketones improves Apolipoprotein E4-related memory deficiency via sirtuin 3. Aging 2019, 11, 4579–4586. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Wang, Y. Curcumin Alleviates Abeta42-Induced Neuronal Metabolic Dysfunction via the Thrb/Sirt3 Axis and Improves Cognition in APPTG Mice. Neurochem. Res. 2021, 46, 3166–3178. [Google Scholar] [CrossRef]
- Han, P.; Tang, Z.; Yin, J.; Maalouf, M.; Beach, T.G.; Reiman, E.M.; Shi, J. Pituitary adenylate cyclase-activating polypeptide protects against beta-amyloid toxicity. Neurobiol. Aging 2014, 35, 2064–2071. [Google Scholar] [CrossRef]
- Hu, W.; Guan, L.S.; Dang, X.B.; Ren, P.Y.; Zhang, Y.L. Small-molecule inhibitors at the PSD-95/nNOS interface attenuate MPP+-induced neuronal injury through Sirt3 mediated inhibition of mitochondrial dysfunction. Neurochem. Int. 2014, 79, 57–64. [Google Scholar] [CrossRef]
- Liu, L.; Peritore, C.; Ginsberg, J.; Kayhan, M.; Donmez, G. Sirt3 attenuates MPTP-induced nigrostriatal degeneration via enhancing mitochondrial antioxidant capacity. Neurochem. Res. 2015, 40, 600–608. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Deng, Y.N.; Zhang, M.; Su, H.; Qu, Q.M. Sirt3 Acts as a Neuroprotective Agent in Rotenone-Induced Parkinson Cell Model. Neurochem. Res. 2016, 41, 1761–1773. [Google Scholar] [CrossRef]
- Gleave, J.A.; Arathoon, L.R.; Trinh, D.; Lizal, K.E.; Giguere, N.; Barber, J.H.M.; Najarali, Z.; Khan, M.H.; Thiele, S.L.; Semmen, M.S.; et al. Sirtuin 3 rescues neurons through the stabilisation of mitochondrial biogenetics in the virally-expressing mutant alpha-synuclein rat model of parkinsonism. Neurobiol. Dis. 2017, 106, 133–146. [Google Scholar] [CrossRef]
- Ma, Z.K.; Li, M.L.; Bai, H.Y. Effects of curcumin on the levels of reactive oxygen species clusters in PD model cells and the expression of silencing information regulator 3. Shandong Med. J. 2017, 57, 50–52. [Google Scholar] [CrossRef]
- Geng, L.; Zhang, T.; Liu, W.; Chen, Y. miR-494-3p modulates the progression of in vitro and in vivo Parkinson′s disease models by targeting Sirt3. Neurosci. Lett. 2018, 675, 23–30. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.B.; Chen, D.J. Neuroprotective Efects of Saikosaponin-d on MPP+-induced Cytotoxicity in SH-SY5Y cells via Regulating Sirt3. J. Hubei Univ. Med. 2018, 37, 29–34. [Google Scholar] [CrossRef]
- Duan, W.J.; Liang, L.; Pan, M.H.; Lu, D.H.; Wang, T.M.; Li, S.B.; Zhong, H.B.; Yang, X.J.; Cheng, Y.; Liu, B.; et al. Theacrine, a purine alkaloid from kucha, protects against Parkinson′s disease through Sirt3 activation. Phytomedicine 2020, 77, 153281. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Q.; Shi, J.; Zhou, S. Regulation of Sirt3 on mitochondrial functions and oxidative stress in Parkinson′s disease. Biomed. Pharmacother. 2020, 132, 110928. [Google Scholar] [CrossRef]
- Luo, H.; Peng, C.; Xu, X.; Peng, Y.; Shi, F.; Li, Q.; Dong, J.; Chen, M. The Protective Effects of Mogroside V Against Neuronal Damages by Attenuating Mitochondrial Dysfunction via Upregulating Sirtuin3. Mol. Neurobiol. 2022, 59, 2068–2084. [Google Scholar] [CrossRef]
- Fu, J.; Jin, J.; Cichewicz, R.H.; Hageman, S.A.; Ellis, T.K.; Xiang, L.; Peng, Q.; Jiang, M.; Arbez, N.; Hotaling, K.; et al. trans-(-)-epsilon-Viniferin increases mitochondrial sirtuin 3 (Sirt3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J. Biol. Chem. 2012, 287, 24460–24472. [Google Scholar] [CrossRef][Green Version]
- Ribeiro, M.; Silva, A.C.; Rodrigues, J.; Naia, L.; Rego, A.C. Oxidizing effects of exogenous stressors in Huntington′s disease knock-in striatal cells--protective effect of cystamine and creatine. Toxicol. Sci. 2013, 136, 487–499. [Google Scholar] [CrossRef][Green Version]
- Ribeiro, M.; Rosenstock, T.R.; Oliveira, A.M.; Oliveira, C.R.; Rego, A.C. Insulin and IGF-1 improve mitochondrial function in a PI-3K/Akt-dependent manner and reduce mitochondrial generation of reactive oxygen species in Huntington′s disease knock-in striatal cells. Free Radic. Biol. Med. 2014, 74, 129–144. [Google Scholar] [CrossRef]
- Hong, C.; Seo, H.; Kwak, M.; Jeon, J.; Jang, J.; Jeong, E.M.; Myeong, J.; Hwang, Y.J.; Ha, K.; Kang, M.J.; et al. Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington′s disease. Brain 2015, 138, 3030–3047. [Google Scholar] [CrossRef][Green Version]
- Cheng, A.; Yang, Y.; Zhou, Y.; Maharana, C.; Lu, D.; Peng, W.; Liu, Y.; Wan, R.; Marosi, K.; Misiak, M.; et al. Mitochondrial Sirt3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016, 23, 128–142. [Google Scholar] [CrossRef][Green Version]
- Naia, L.; Carmo, C.; Campesan, S.; Fao, L.; Cotton, V.E.; Valero, J.; Lopes, C.; Rosenstock, T.R.; Giorgini, F.; Rego, A.C. Mitochondrial Sirt3 confers neuroprotection in Huntington′s disease by regulation of oxidative challenges and mitochondrial dynamics. Free Radic. Biol. Med. 2021, 163, 163–179. [Google Scholar] [CrossRef]
- Song, W.; Song, Y.; Kincaid, B.; Bossy, B.; Bossy-Wetzel, E. Mutant SOD1G93A triggers mitochondrial fragmentation in spinal cord motor neurons: Neuroprotection by Sirt3 and PGC-1alpha. Neurobiol. Dis. 2013, 51, 72–81. [Google Scholar] [CrossRef][Green Version]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef][Green Version]
- Magnifico, S.; Saias, L.; Deleglise, B.; Duplus, E.; Kilinc, D.; Miquel, M.C.; Viovy, J.L.; Brugg, B.; Peyrin, J.M. NAD+ acts on mitochondrial SirT3 to prevent axonal caspase activation and axonal degeneration. FASEB J. 2013, 27, 4712–4722. [Google Scholar] [CrossRef]
- Nagai, M.; Re, D.B.; Nagata, T.; Chalazonitis, A.; Jessell, T.M.; Wichterle, H.; Przedborski, S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 2007, 10, 615–622. [Google Scholar] [CrossRef][Green Version]
- Harlan, B.A.; Pehar, M.; Sharma, D.R.; Beeson, G.; Beeson, C.C.; Vargas, M.R. Enhancing NAD+ Salvage Pathway Reverts the Toxicity of Primary Astrocytes Expressing Amyotrophic Lateral Sclerosis-linked Mutant Superoxide Dismutase 1 (SOD1). J. Biol. Chem. 2016, 291, 10836–10846. [Google Scholar] [CrossRef][Green Version]
- Khodaei, F.; Rashedinia, M.; Heidari, R.; Rezaei, M.; Khoshnoud, M.J. Ellagic acid improves muscle dysfunction in cuprizone-induced demyelinated mice via mitochondrial Sirt3 regulation. Life Sci. 2019, 237, 116954. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer′s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Austad, S.N.; Ballinger, S.; Buford, T.W.; Carter, C.S.; Smith, D.L., Jr.; Darley-Usmar, V.; Zhang, J. Targeting whole body metabolism and mitochondrial bioenergetics in the drug development for Alzheimer′s disease. Acta Pharm. Sin. B 2022, 12, 511–531. [Google Scholar] [CrossRef]
- Klimova, N.; Long, A.; Kristian, T. Nicotinamide mononucleotide alters mitochondrial dynamics by Sirt3-dependent mechanism in male mice. J. Neurosci. Res. 2019, 97, 975–990. [Google Scholar] [CrossRef][Green Version]
- Ying, Y.; Lu, C.; Chen, C.; Liu, Y.; Liu, Y.U.; Ruan, X.; Yang, Y. Sirt3 Regulates Neuronal Excitability of Alzheimer′s Disease Models in an Oxidative Stress-Dependent Manner. Neuromolecular Med. 2022, 24, 261–267. [Google Scholar] [CrossRef]
- Pi, T.; Lang, G.; Liu, B.; Shi, J. Protective Effects of Dendrobium nobile Lindl. Alkaloids on Alzheimer′s Disease-like Symptoms Induced by High-methionine Diet. Curr. Neuropharmacol. 2022, 20, 983–997. [Google Scholar] [CrossRef]
- Guo, X.; Tian, Y.; Yang, Y.; Li, S.; Guo, L.; Shi, J. Pituitary Adenylate Cyclase-Activating Polypeptide Protects Against Cognitive Impairment Caused by Chronic Cerebral Hypoperfusion. Mol. Neurobiol. 2021, 58, 4309–4322. [Google Scholar] [CrossRef]
- Zheng, J.; Shi, L.; Liang, F.; Xu, W.; Li, T.; Gao, L.; Sun, Z.; Yu, J.; Zhang, J. Sirt3 Ameliorates Oxidative Stress and Mitochondrial Dysfunction After Intracerebral Hemorrhage in Diabetic Rats. Front. Neurosci. 2018, 12, 414. [Google Scholar] [CrossRef]
- Polito, L.; Kehoe, P.G.; Davin, A.; Benussi, L.; Ghidoni, R.; Binetti, G.; Quadri, P.; Lucca, U.; Tettamanti, M.; Clerici, F.; et al. The SIRT2 polymorphism rs10410544 and risk of Alzheimer′s disease in two Caucasian case-control cohorts. Alzheimer′s Dement. 2013, 9, 392–399. [Google Scholar] [CrossRef]
- Yin, J.; Reiman, E.M.; Beach, T.G.; Serrano, G.E.; Sabbagh, M.N.; Nielsen, M.; Caselli, R.J.; Shi, J. Effect of ApoE isoforms on mitochondria in Alzheimer disease. Neurology 2020, 94, e2404–e2411. [Google Scholar] [CrossRef]
- Li, Y.F.; Ouyang, S.H.; Tu, L.F.; Wang, X.; Yuan, W.L.; Wang, G.E.; Wu, Y.P.; Duan, W.J.; Yu, H.M.; Fang, Z.Z.; et al. Caffeine Protects Skin from Oxidative Stress-Induced Senescence through the Activation of Autophagy. Theranostics 2018, 8, 5713–5730. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson′s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Park, J.S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson′s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef][Green Version]
- Raza, C.; Anjum, R.; Shakeel, N.U.A. Parkinson′s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson′s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Ghosh, R.; Leavitt, B.R. Huntingtin Lowering Strategies for Disease Modification in Huntington′s Disease. Neuron 2019, 101, 801–819. [Google Scholar] [CrossRef][Green Version]
- Hu, D.; Sun, X.; Magpusao, A.; Fedorov, Y.; Thompson, M.; Wang, B.; Lundberg, K.; Adams, D.J.; Qi, X. Small-molecule suppression of calpastatin degradation reduces neuropathology in models of Huntington′s disease. Nat. Commun. 2021, 12, 5305. [Google Scholar] [CrossRef]
- Lombard, D.B.; Alt, F.W.; Cheng, H.L.; Bunkenborg, J.; Streeper, R.S.; Mostoslavsky, R.; Kim, J.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; et al. Mammalian Sir2 homolog Sirt3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 2007, 27, 8807–8814. [Google Scholar] [CrossRef][Green Version]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Feng, J. Neuroprotective mechanisms of epsilon-viniferin in a rotenone-induced cell model of Parkinson′s disease: Significance of Sirt3-mediated FOXO3 deacetylation. Neural Regen. Res. 2020, 15, 2143–2153. [Google Scholar] [CrossRef]
- Freischmidt, A.; Muller, K.; Ludolph, A.C.; Weishaupt, J.H.; Andersen, P.M. Association of Mutations in TBK1 With Sporadic and Familial Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. JAMA Neurol. 2017, 74, 110–113. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Tang, L.; Fan, D. Amyotrophic lateral sclerosis: New era, new challenges. Lancet Neurol. 2022, 21, 400–401. [Google Scholar] [CrossRef]
- Buck, E.; Bayer, H.; Lindenberg, K.S.; Hanselmann, J.; Pasquarelli, N.; Ludolph, A.C.; Weydt, P.; Witting, A. Comparison of Sirtuin 3 Levels in ALS and Huntington′s Disease-Differential Effects in Human Tissue Samples vs. Transgenic Mouse Models. Front. Mol. Neurosci. 2017, 10, 156. [Google Scholar] [CrossRef][Green Version]
- Hor, J.H.; Santosa, M.M.; Lim, V.J.W.; Ho, B.X.; Taylor, A.; Khong, Z.J.; Ravits, J.; Fan, Y.; Liou, Y.C.; Soh, B.S.; et al. ALS motor neurons exhibit hallmark metabolic defects that are rescued by Sirt3 activation. Cell Death Differ. 2021, 28, 1379–1397. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; Marchio, P.; Lopez-Blanch, R.; Jihad-Jebbar, A.; Rivera, P.; Valles, S.L.; Banacloche, S.; Alcacer, J.; Colomer, N.; et al. Nicotinamide Riboside and Pterostilbene Cooperatively Delay Motor Neuron Failure in ALS SOD1(G93A) Mice. Mol. Neurobiol. 2021, 58, 1345–1371. [Google Scholar] [CrossRef]
- Harlan, B.A.; Killoy, K.M.; Pehar, M.; Liu, L.; Auwerx, J.; Vargas, M.R. Evaluation of the NAD(+) biosynthetic pathway in ALS patients and effect of modulating NAD(+) levels in hSOD1-linked ALS mouse models. Exp. Neurol. 2020, 327, 113219. [Google Scholar] [CrossRef]
- Albani, D.; Pupillo, E.; Bianchi, E.; Chierchia, A.; Martines, R.; Forloni, G.; Beghi, E. The role of single-nucleotide variants of the energy metabolism-linked genes Sirt3, PPARGC1A and APOE in amyotrophic lateral sclerosis risk. Genes Genet. Syst. 2017, 91, 301–309. [Google Scholar] [CrossRef][Green Version]
- Perez-Torres, E.J.; Utkina-Sosunova, I.; Mishra, V.; Barbuti, P.; De Planell-Saguer, M.; Dermentzaki, G.; Geiger, H.; Basile, A.O.; Robine, N.; Fagegaltier, D.; et al. Retromer dysfunction in amyotrophic lateral sclerosis. Proc. Natl. Acad Sci. USA 2022, 119, e2118755119. [Google Scholar] [CrossRef]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Front. Immunol. 2020, 11, 186. [Google Scholar] [CrossRef][Green Version]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef][Green Version]
- Jastorff, A.M.; Haegler, K.; Maccarrone, G.; Holsboer, F.; Weber, F.; Ziemssen, T.; Turck, C.W. Regulation of proteins mediating neurodegeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Proteom. Clin. Appl. 2009, 3, 1273–1287. [Google Scholar] [CrossRef]
- Inkster, B.; Strijbis, E.M.; Vounou, M.; Kappos, L.; Radue, E.W.; Matthews, P.M.; Uitdehaag, B.M.; Barkhof, F.; Polman, C.H.; Montana, G.; et al. Histone deacetylase gene variants predict brain volume changes in multiple sclerosis. Neurobiol. Aging 2013, 34, 238–247. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Chen, H.T.; Liang, Y.C.; Wang, T.E.; Huang, K.H.; Hsu, C.C.; Liang, H.J.; Huang, C.H.; Jan, T.R. Development of Nanosome-Encapsulated Honokiol for Intravenous Therapy Against Experimental Autoimmune Encephalomyelitis. Int. J. Nanomed. 2020, 15, 17–29. [Google Scholar] [CrossRef][Green Version]
- Hares, K.; Kemp, K.; Rice, C.; Gray, E.; Scolding, N.; Wilkins, A. Reduced axonal motor protein expression in non-lesional grey matter in multiple sclerosis. Mult. Scler. J. 2014, 20, 812–821. [Google Scholar] [CrossRef]
- Leite, J.A.; Ghirotto, B.; Targhetta, V.P.; de Lima, J.; Camara, N.O.S. Sirtuins as pharmacological targets in neurodegenerative and neuropsychiatric disorders. Br. J. Pharmacol. 2022, 179, 1496–1511. [Google Scholar] [CrossRef]


| Neurodegenerative Disease | Mechanism | Experimental Setting | Research |
|---|---|---|---|
| AD | ROS in mitochondria increase Sirt3 expression. | Cell model | [73] |
| Pharmacological enhancement of mitochondrial ROS increases the expression of Sirt3 in primary hippocampal cultures. | AD mouse model and cell model | [74] | |
| PACAP stimulates the production of mitochondrial Sirt3 and reduces neuronal death. | Postmortem human tissue, triple transgenic mouse model, and cell model | [75] | |
| Amyloid-β increases levels of total tau and acetylated tau through its modulation of Sirt3. | Postmortem human tissue | [76] | |
| APOE4 reduces ATP production by modulating the PGC-1α-Sirt3 signaling pathway, triggering mitochondrial oxidative stress and disrupting synaptic function. | Postmortem human tissue | [77] | |
| Sirt3 may mediate the neuroprotection of ketones by increasing neuronal energy metabolism. | APOE4 mouse model | [78] | |
| Alleviation of Aβ 42-induced neuronal metabolic dysfunction occurs via the THRB/Sirt3 axis and improves cognition. | APPTG mouse model | [79] | |
| Activation of mitophagy and mitochondrial unfolded protein response occurs. | APP/PS1 mouse model | [80] | |
| PD | IC87201 and ZL006 reduce ROS production and improve mitochondrial dysfunction by increasing the expression of Sirt3 after MPP+ exposure. | MPP+-induced primary cortical neuron cell models | [81] |
| Sirt3 has a possible role in MPTP-induced neurodegeneration by preserving the free radical scavenging capacity of mitochondria. | Sirt3 null mouse model | [82] | |
| Sirt3 overexpression dramatically increases cell viability, decreases cell apoptosis, prevents the accumulation of α-synuclein, suppresses the reduction of SOD and glutathione, decreases ROS generation, and alleviates MMP collapse induced by rotenone. | PD cell model | [83] | |
| Sirt3 rescues neurons through the stabilization of mitochondrial biogenetics. | Virally expressed mutant α-synuclein rat model of parkinsonism | [84] | |
| Curcumin lowers ROS levels in SH-SY5Y cells and upregulates Sirt3 expression. | SH-SY5Y cell models | [85] | |
| miR-494-3p downregulation increases Sirt3 expression, reduces oxidative stress, and improves dyskinesia. | MPTP-induced PD mouse model and SH-SY5Y cell model | [86] | |
| Saikosaponin-d exerts a neuroprotective effect by upregulating Sirt3 expression and alleviating oxidative stress damage. | MPP+-induced SH-SY5Y cell models | [87] | |
| Sirt3 mediates SOD2 deacetylation to reduce ROS accumulation and to restore mitochondrial function, thereby preventing apoptosis. | 6-OHDA-treated rat, MPTP-treated mouse, and zebrafish models | [88] | |
| Regulation of Sirt3 in mitochondrial functions and oxidative stress occurs in PD. | Sirt3 null mouse and PD mouse models | [89] | |
| Upregulated Sirt3 mitigates the protective effect of mitochondrial dysfunction on neuronal damage. | SH-SY5Y cell models | [90] | |
| HD | Knockdown of Sirt3 significantly inhibits viniferin-mediated AMP-activated kinase activation and diminishes the neuroprotective effects of viniferin. | Mutant HTT cell model | [91] |
| Increased Sirt3 levels and/or activity reduce oxidative damage. | Cell model, HD knockin mouse model, and Huntington’s disease transgenic (YAC128) mouse model | [92,93,94] | |
| Sirt3 protects neurons against metabolic and oxidative stress by reducing mitochondrial superoxide levels, stabilizing cellular and mitochondrial Ca2+ homeostasis, and inhibiting mitochondrial membrane permeability transition pore formation to prevent apoptosis. | Cell model and HD mouse model | [95] | |
| Sirt3 overexpression promotes the antioxidant effect of cells expressing mutant HTT, leading to enhanced mitochondrial function and balanced dynamics. | Postmortem human tissue and primary striatal neuron cell model | [96] | |
| ALS | Sirt3 protects against mitochondrial fragmentation and neuronal cell death with mutant SOD1 (G93A). | SOD1G93A transgenic mouse model and primary cortical neuronal cell model | [97] |
| Overexpression of Sirt3 increases NADPH levels and protects from oxidative-stress-induced cell death. | Sirt3 mouse model | [98] | |
| Grape wine polyphenols prevent axonal apoptosis and act via mitochondrial Sirt3 activation in axons. | Primary cortical neuronal cell model | [99] | |
| Sirt3 can restore neuronal mitochondrial fragmentation and transport disorders, reducing neuronal death, and protects against mitochondrial alterations. | SOD1-mutant cell model | [100,101] | |
| MS | The EA protects muscle tissue from cuprizone-induced demyelination by overexpressing Sirt3 to protect mitochondria and to reduce oxidative stress. | Mouse model | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Dai, S.; Yang, Y.; Wei, J.; Li, X.; Luo, P.; Jiang, X. Role of Sirtuin 3 in Degenerative Diseases of the Central Nervous System. Biomolecules 2023, 13, 735. https://doi.org/10.3390/biom13050735
Zhang H, Dai S, Yang Y, Wei J, Li X, Luo P, Jiang X. Role of Sirtuin 3 in Degenerative Diseases of the Central Nervous System. Biomolecules. 2023; 13(5):735. https://doi.org/10.3390/biom13050735
Chicago/Turabian StyleZhang, Haofuzi, Shuhui Dai, Yuefan Yang, Jialiang Wei, Xin Li, Peng Luo, and Xiaofan Jiang. 2023. "Role of Sirtuin 3 in Degenerative Diseases of the Central Nervous System" Biomolecules 13, no. 5: 735. https://doi.org/10.3390/biom13050735
APA StyleZhang, H., Dai, S., Yang, Y., Wei, J., Li, X., Luo, P., & Jiang, X. (2023). Role of Sirtuin 3 in Degenerative Diseases of the Central Nervous System. Biomolecules, 13(5), 735. https://doi.org/10.3390/biom13050735





