Natural Killer Cells, as the Rising Point in Tissues, Are Forgotten in the Kidney
Abstract
1. Introduction
2. Natural Killers Cells
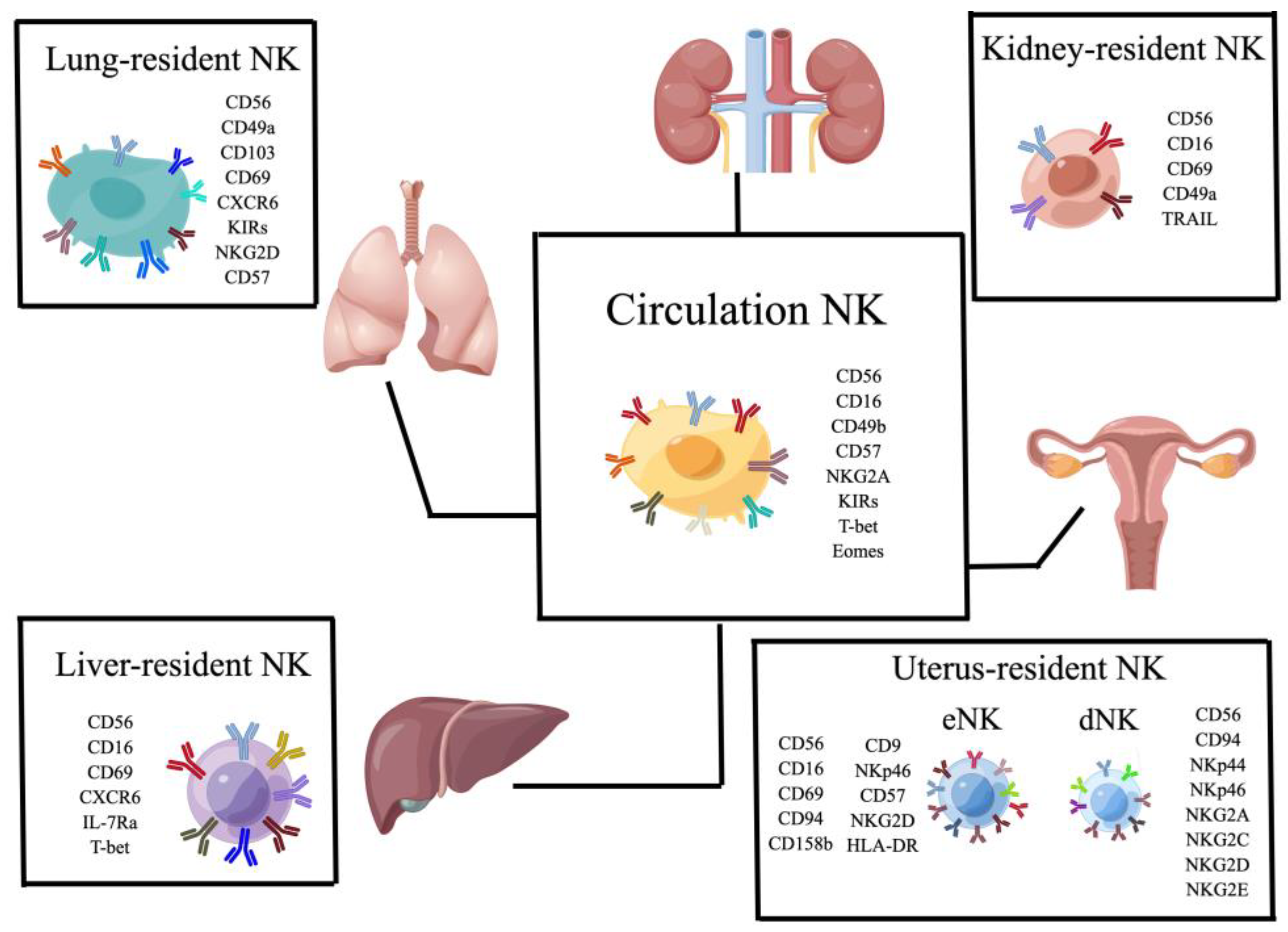
3. The Role of NK Cells in Different Tissues
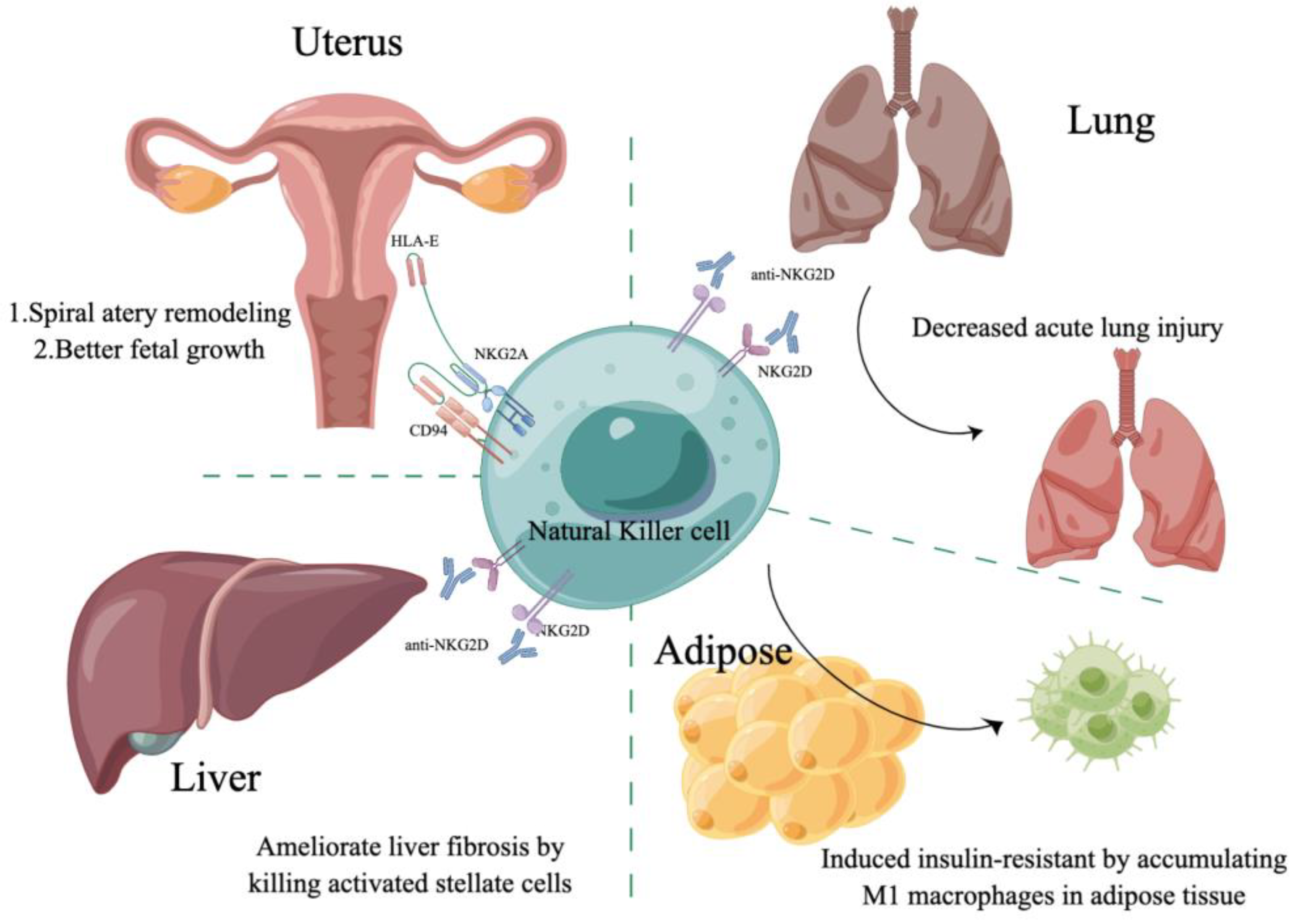
3.1. NK Cells in the Uterus
3.2. NK Cells in the Liver
3.3. NK Cells in the Lung
3.4. NK Cells in Adipose Tissue
4. Natural Killer Cells in the Kidney
4.1. NK Cells in a Steady Kidney
4.2. NK Cells in Acute Kidney Injury
4.3. NK Cells in Adriamycin Nephropathy
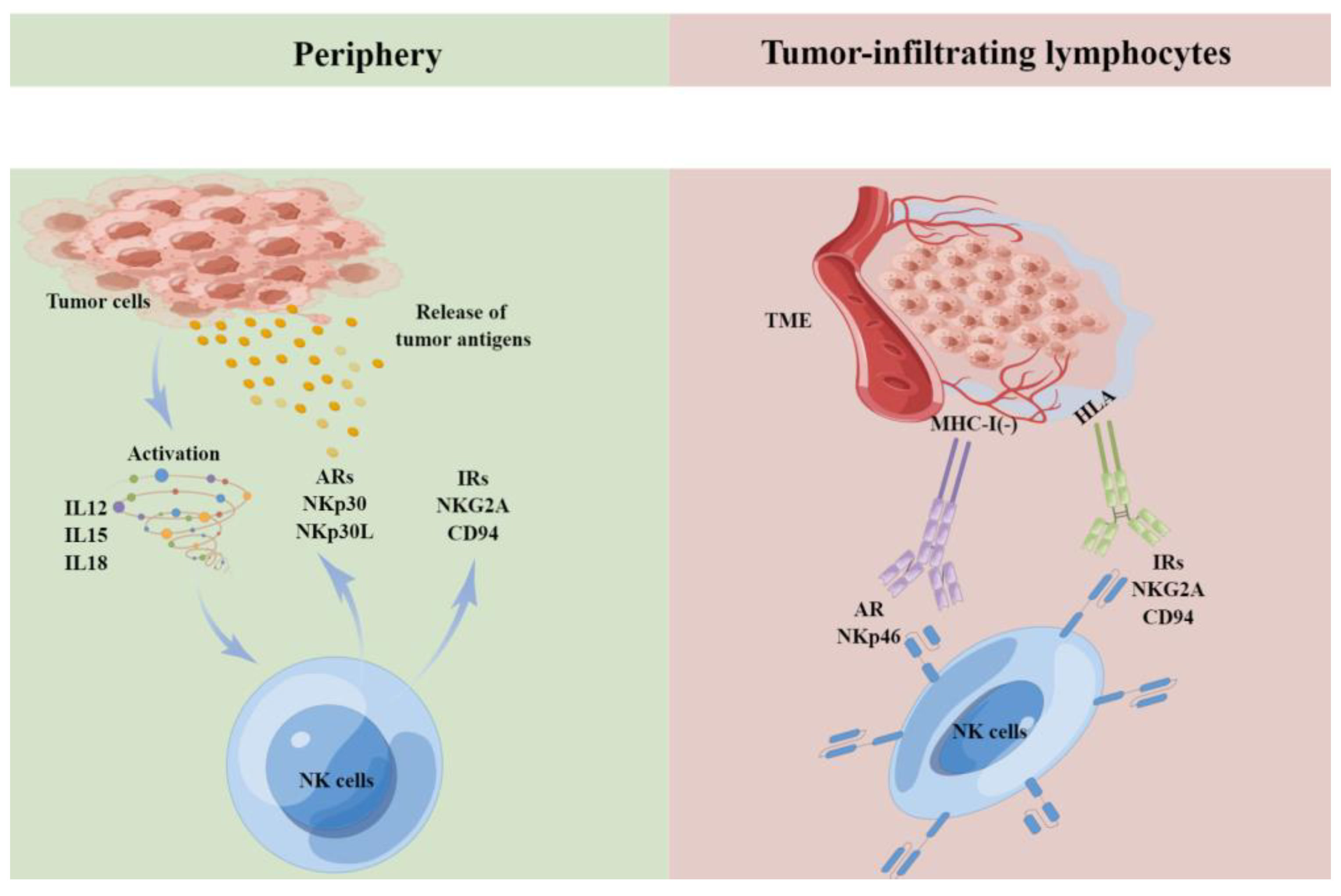
4.4. NK Cells in Renal Cell Carcinoma
5. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berhani, O.; Glasner, A.; Kahlon, S.; Duev-Cohen, A.; Yamin, R.; Horwitz, E.; Enk, J.; Moshel, O.; Varvak, A.; Porgador, A.; et al. Human anti-NKp46 antibody for studies of NKp46-dependent NK cell function and its applications for type 1 diabetes and cancer research. Eur. J. Immunol. 2019, 49, 228–241. [Google Scholar] [CrossRef]
- Hudspeth, K.; Pontarini, E.; Tentorio, P.; Cimino, M.; Donadon, M.; Torzilli, G.; Lugli, E.; Della Bella, S.; Gershwin, M.E.; Mavilio, D. The role of natural killer cells in autoimmune liver disease: A comprehensive review. J. Autoimmun. 2013, 46, 55–65. [Google Scholar] [CrossRef]
- Herberman, R.B.; Nunn, M.E.; Holden, H.T.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer 1975, 16, 230–239. [Google Scholar] [CrossRef]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Nagler, A.; Lanier, L.L.; Cwirla, S.; Phillips, J.H. Comparative studies of human FcRIII-positive and negative natural killer cells. J. Immunol. 1989, 143, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Tambussi, G.; Bottino, C.; Tripodi, G.; Merli, A.; Ciccone, E.; Pantaleo, G.; Moretta, L. A novel surface antigen expressed by a subset of human CD3- CD16+ natural killer cells. Role in cell activation and regulation of cytolytic function. J. Exp. Med. 1990, 171, 695–714. [Google Scholar] [CrossRef]
- Pang, G.; Buret, A.; Batey, R.T.; Chen, Q.Y.; Couch, L.; Cripps, A.; Clancy, R. Morphological, phenotypic and functional characteristics of a pure population of CD56+ CD16- CD3- large granular lymphocytes generated from human duodenal mucosa. Immunology 1993, 79, 498–505. [Google Scholar]
- Carson, W.; Caligiuri, M. Natural Killer Cell Subsets and Development. Methods 1996, 9, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Kasaian, M.T.; Whitters, M.J.; Carter, L.L.; Lowe, L.D.; Jussif, J.M.; Deng, B.; Johnson, K.A.; Witek, J.S.; Senices, M.; Konz, R.F.; et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: A mediator of the transition from innate to adaptive immunity. Immunity 2002, 16, 559–569. [Google Scholar] [CrossRef]
- Koka, R.; Burkett, P.R.; Chien, M.; Chai, S.; Chan, F.; Lodolce, J.P.; Boone, D.L.; Ma, A. Interleukin (IL)-15R [alpha]-deficient natural killer cells survive in normal but not IL-15R [alpha]-deficient mice. J. Exp. Med. 2003, 197, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Koka, R.; Burkett, P.; Chien, M.; Chai, S.; Boone, D.L.; Ma, A. Cutting edge: Murine dendritic cells require IL-15R alpha to prime NK cells. J. Immunol. 2004, 173, 3594–3598. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Schachterle, W.; Oberle, K.; Aichele, P.; Diefenbach, A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 2007, 26, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O. Negative signaling by inhibitory receptors: The NK cell paradigm. Immunol. Rev. 2008, 224, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Marcenaro, E.; Parolini, S.; Ferlazzo, G.; Moretta, L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008, 15, 226–233. [Google Scholar] [CrossRef]
- Gordon, S.M.; Chaix, J.; Rupp, L.J.; Wu, J.; Madera, S.; Sun, J.C.; Lindsten, T.; Reiner, S.L. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 2012, 36, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Parham, P.; Norman, P.J.; Abi-Rached, L.; Guethlein, L.A. Human-specific evolution of killer cell immunoglobulin-like receptor recognition of major histocompatibility complex class I molecules. Philos. Trans. R Soc. Lond B Biol. Sci. 2012, 367, 800–811. [Google Scholar] [CrossRef]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Daussy, C.; Faure, F.; Mayol, K.; Viel, S.; Gasteiger, G.; Charrier, E.; Bienvenu, J.; Henry, T.; Debien, E.; Hasan, U.A.; et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 2014, 211, 563–577. [Google Scholar] [CrossRef]
- Kaminski, H.; Couzi, L.; Eberl, M. Unconventional T cells and kidney disease. Nat. Rev. Nephrol. 2021, 17, 795–813. [Google Scholar] [CrossRef]
- Callemeyn, J.; Lamarthee, B.; Koenig, A.; Koshy, P.; Thaunat, O.; Naesens, M. Allorecognition and the spectrum of kidney transplant rejection. Kidney Int. 2022, 101, 692–710. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Harris, D.C.H.; Cao, Q. Innate lymphoid cells in kidney diseases. Kidney Int. 2021, 99, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liang, S.; Zhang, C. NK Cells in Autoimmune Diseases: Protective or Pathogenic? Front. Immunol. 2021, 12, 624687. [Google Scholar] [CrossRef] [PubMed]
- Segerberg, F.; Lundtoft, C.; Reid, S.; Hjorton, K.; Leonard, D.; Nordmark, G.; Carlsten, M.; Hagberg, N. Autoantibodies to Killer Cell Immunoglobulin-Like Receptors in Patients with Systemic Lupus Erythematosus Induce Natural Killer Cell Hyporesponsiveness. Front. Immunol. 2019, 10, 2164. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lu, Y.; Wang, B.; Jiao, P.; Ma, J. Analysis of immune cell components and immune-related gene expression profiles in peripheral blood of patients with type 1 diabetes mellitus. J. Transl. Med. 2021, 19, 319. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.S.; Holloman, B.L.; Wilson, K.; Miranda, K.; Dopkins, N.; Nagarkatti, P.; Nagarkatti, M. AhR Activation Leads to Attenuation of Murine Autoimmune Hepatitis: Single-Cell RNA-Seq Analysis Reveals Unique Immune Cell Phenotypes and Gene Expression Changes in the Liver. Front. Immunol. 2022, 13, 899609. [Google Scholar] [CrossRef]
- Buttner, F.A.; Winter, S.; Stuhler, V.; Rausch, S.; Hennenlotter, J.; Fussel, S.; Zastrow, S.; Meinhardt, M.; Toma, M.; Jeronimo, C.; et al. A novel molecular signature identifies mixed subtypes in renal cell carcinoma with poor prognosis and independent response to immunotherapy. Genome Med. 2022, 14, 105. [Google Scholar] [CrossRef]
- Lee, M.H.; Jarvinen, P.; Nisen, H.; Bruck, O.; Ilander, M.; Uski, I.; Theodoropoulos, J.; Kankainen, M.; Mirtti, T.; Mustjoki, S.; et al. T and NK cell abundance defines two distinct subgroups of renal cell carcinoma. Oncoimmunology 2022, 11, 1993042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, M.; Zhou, K.; Brown, J.; Tsao, T.; Cen, X.; Husman, T.; Bajpai, A.; Dunn, Z.S.; Yang, L. Engineering Induced Pluripotent Stem Cells for Cancer Immunotherapy. Cancers 2022, 14, 2266. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Y.; Xiao, W.; Tian, Z. Chimeric antigen receptor- and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy. Cell Mol. Immunol. 2021, 18, 2083–2100. [Google Scholar] [CrossRef]
- Schafer, R. Advanced cell therapeutics are changing the clinical landscape: Will mesenchymal stromal cells be a part of it? BMC Med. 2019, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Poli, A.; Cuapio, A.; Briquemont, B.; Iserentant, G.; Ollert, M.; Zimmer, J. Human CD56bright NK Cells: An Update. J. Immunol. 2016, 196, 2923–2931. [Google Scholar] [CrossRef]
- Kamizono, S.; Duncan, G.S.; Seidel, M.G.; Morimoto, A.; Hamada, K.; Grosveld, G.; Akashi, K.; Lind, E.F.; Haight, J.P.; Ohashi, P.S.; et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J. Exp. Med. 2009, 206, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Farahzadi, R.; Fathi, E.; Mesbah-Namin, S.A.; Zarghami, N. Zinc sulfate contributes to promote telomere length extension via increasing telomerase gene expression, telomerase activity and change in the TERT gene promoter CpG island methylation status of human adipose-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0188052. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Finlay, D.K. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol. 2019, 19, 282–290. [Google Scholar] [CrossRef]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Z. NK cell recognition of hematopoietic cells by SLAM-SAP families. Cell Mol. Immunol. 2019, 16, 452–459. [Google Scholar] [CrossRef]
- Tian, Z.; Gershwin, M.E.; Zhang, C. Regulatory NK cells in autoimmune disease. J. Autoimmun. 2012, 39, 206–215. [Google Scholar] [CrossRef]
- Fu, B.; Tian, Z.; Wei, H. Subsets of human natural killer cells and their regulatory effects. Immunology 2014, 141, 483–489. [Google Scholar] [CrossRef]
- Schuster, I.S.; Coudert, J.D.; Andoniou, C.E.; Degli-Esposti, M.A. “Natural Regulators”: NK Cells as Modulators of T Cell Immunity. Front. Immunol. 2016, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Montaldo, E.; Del Zotto, G.; Della Chiesa, M.; Mingari, M.C.; Moretta, A.; De Maria, A.; Moretta, L. Human NK cell receptors/markers: A tool to analyze NK cell development, subsets and function. Cytom. A 2013, 83, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Jiang, X.; Chen, Y.; Sojka, D.K.; Wei, H.; Gao, X.; Sun, R.; Yokoyama, W.M.; Tian, Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Investig. 2013, 123, 1444–1456. [Google Scholar] [CrossRef]
- Hammer, Q.; Romagnani, C. About Training and Memory: NK-Cell Adaptation to Viral Infections. Adv. Immunol. 2017, 133, 171–207. [Google Scholar] [CrossRef]
- O’Sullivan, T.E.; Sun, J.C.; Lanier, L.L. Natural Killer Cell Memory. Immunity 2015, 43, 634–645. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Z.; Peng, H. Immunological memory: ILC1s come into view. Cell Mol. Immunol. 2019, 16, 895–896. [Google Scholar] [CrossRef]
- Wang, X.; Peng, H.; Tian, Z. Innate lymphoid cell memory. Cell Mol. Immunol. 2019, 16, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tian, Z. NK cell subsets in autoimmune diseases. J. Autoimmun. 2017, 83, 22–30. [Google Scholar] [CrossRef]
- Turner, J.E.; Rickassel, C.; Healy, H.; Kassianos, A.J. Natural Killer Cells in Kidney Health and Disease. Front. Immunol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M. Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity. Immunity 2018, 48, 1104–1117. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Schlums, H.; Cichocki, F.; Tesi, B.; Theorell, J.; Beziat, V.; Holmes, T.D.; Han, H.; Chiang, S.C.; Foley, B.; Mattsson, K.; et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015, 42, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, G.; Antonangeli, F.; Bonanni, V.; Santoni, A. Dysregulation of Chemokine/Chemokine Receptor Axes and NK Cell Tissue Localization during Diseases. Front. Immunol. 2016, 7, 402. [Google Scholar] [CrossRef]
- Weng, W.K.; Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003, 21, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Varchetta, S.; Gibelli, N.; Oliviero, B.; Nardini, E.; Gennari, R.; Gatti, G.; Silva, L.S.; Villani, L.; Tagliabue, E.; Menard, S.; et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007, 67, 11991–11999. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Cantoni, C.; Pietra, G.; Mingari, M.C.; Moretta, L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur. J. Immunol. 2014, 44, 1582–1592. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, E.; Malarkannan, S. Tissue-Resident NK Cells: Development, Maturation, and Clinical Relevance. Cancers 2020, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.K.; Plougastel-Douglas, B.; Yang, L.; Pak-Wittel, M.A.; Artyomov, M.N.; Ivanova, Y.; Zhong, C.; Chase, J.M.; Rothman, P.B.; Yu, J.; et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 2014, 3, e01659. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, K.; Donadon, M.; Cimino, M.; Pontarini, E.; Tentorio, P.; Preti, M.; Hong, M.; Bertoletti, A.; Bicciato, S.; Invernizzi, P.; et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 2016, 66, 40–50. [Google Scholar] [CrossRef]
- Lugthart, G.; Melsen, J.E.; Vervat, C.; van Ostaijen-Ten Dam, M.M.; Corver, W.E.; Roelen, D.L.; van Bergen, J.; van Tol, M.J.; Lankester, A.C.; Schilham, M.W. Human Lymphoid Tissues Harbor a Distinct CD69+CXCR6+ NK Cell Population. J. Immunol. 2016, 197, 78–84. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.K.; Minnich, M.; Kragten, N.A.; Liao, Y.; Nota, B.; Seillet, C.; Zaid, A.; Man, K.; Preston, S.; Freestone, D.; et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016, 352, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Shin, J.H.; Haggadone, M.D.; Sunwoo, J.B. The aryl hydrocarbon receptor is required for the maintenance of liver-resident natural killer cells. J. Exp. Med. 2016, 213, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A. ILC1s in Tissue Inflammation and Infection. Front. Immunol. 2016, 7, 104. [Google Scholar] [CrossRef]
- Shreeve, N.; Depierreux, D.; Hawkes, D.; Traherne, J.A.; Sovio, U.; Huhn, O.; Jayaraman, J.; Horowitz, A.; Ghadially, H.; Perry, J.R.B.; et al. The CD94/NKG2A inhibitory receptor educates uterine NK cells to optimize pregnancy outcomes in humans and mice. Immunity 2021, 54, 1231–1244.e1234. [Google Scholar] [CrossRef]
- Wells, A.I.; Coyne, C.B. Uterine NK cell education: Learning the ropes in pregnancy. Immunity 2021, 54, 1102–1104. [Google Scholar] [CrossRef]
- Strunz, B.; Bister, J.; Jonsson, H.; Filipovic, I.; Crona-Guterstam, Y.; Kvedaraite, E.; Sleiers, N.; Dumitrescu, B.; Brannstrom, M.; Lentini, A.; et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci. Immunol. 2021, 6, eabb7800. [Google Scholar] [CrossRef]
- Von Woon, E.; Greer, O.; Shah, N.; Nikolaou, D.; Johnson, M.; Male, V. Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: A systematic review and meta-analysis. Hum. Reprod. Update 2022, 28, 548–582. [Google Scholar] [CrossRef]
- Whettlock, E.M.; Woon, E.V.; Cuff, A.O.; Browne, B.; Johnson, M.R.; Male, V. Dynamic Changes in Uterine NK Cell Subset Frequency and Function Over the Menstrual Cycle and Pregnancy. Front. Immunol. 2022, 13, 880438. [Google Scholar] [CrossRef]
- Woon, E.V.; Nikolaou, D.; MacLaran, K.; Norman-Taylor, J.; Bhagwat, P.; Cuff, A.O.; Johnson, M.R.; Male, V. Uterine NK cells underexpress KIR2DL1/S1 and LILRB1 in reproductive failure. Front. Immunol. 2022, 13, 1108163. [Google Scholar] [CrossRef]
- Brownlie, D.; Scharenberg, M.; Mold, J.E.; Hard, J.; Kekalainen, E.; Buggert, M.; Nguyen, S.; Wilson, J.N.; Al-Ameri, M.; Ljunggren, H.G.; et al. Expansions of adaptive-like NK cells with a tissue-resident phenotype in human lung and blood. Proc. Natl. Acad. Sci. USA 2021, 118, e2016580118. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, C.; Li, M.; Yu, H.; Zhao, D.; Xue, W.; Qin, L.; Peng, A. Depletion of NK cells attenuates paraquat-induced acute lung injury by manipulating macrophage polarization. Int. Immunopharmacol. 2020, 86, 106698. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, D.R.; Aminian, E.; Mallavia, B.; Liu, F.; Cleary, S.J.; Aguilar, O.A.; Wang, P.; Singer, J.P.; Hays, S.R.; Golden, J.A.; et al. Natural killer cells activated through NKG2D mediate lung ischemia-reperfusion injury. J. Clin. Investig. 2021, 131, e137047. [Google Scholar] [CrossRef]
- Tan, S.; Xu, Y.; Wang, Z.; Wang, T.; Du, X.; Song, X.; Guo, X.; Peng, J.; Zhang, J.; Liang, Y.; et al. Tim-3 Hampers Tumor Surveillance of Liver-Resident and Conventional NK Cells by Disrupting PI3K Signaling. Cancer Res. 2020, 80, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Stiglund, N.; Strand, K.; Cornillet, M.; Stal, P.; Thorell, A.; Zimmer, C.L.; Naslund, E.; Karlgren, S.; Nilsson, H.; Mellgren, G.; et al. Retained NK Cell Phenotype and Functionality in Non-alcoholic Fatty Liver Disease. Front. Immunol. 2019, 10, 1255. [Google Scholar] [CrossRef]
- Melhem, A.; Muhanna, N.; Bishara, A.; Alvarez, C.E.; Ilan, Y.; Bishara, T.; Horani, A.; Nassar, M.; Friedman, S.L.; Safadi, R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J. Hepatol. 2006, 45, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Radaeva, S.; Sun, R.; Jaruga, B.; Nguyen, V.T.; Tian, Z.; Gao, B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 2006, 130, 435–452. [Google Scholar] [CrossRef]
- Szabo, P.A.; Miron, M.; Farber, D.L. Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol. 2019, 4, eaas9673. [Google Scholar] [CrossRef]
- Yang, K.; Kallies, A. Tissue-specific differentiation of CD8(+) resident memory T cells. Trends Immunol. 2021, 42, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef]
- Bjorkstrom, N.K.; Riese, P.; Heuts, F.; Andersson, S.; Fauriat, C.; Ivarsson, M.A.; Bjorklund, A.T.; Flodstrom-Tullberg, M.; Michaelsson, J.; Rottenberg, M.E.; et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 2010, 116, 3853–3864. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Verges, S.; Milush, J.M.; Pandey, S.; York, V.A.; Arakawa-Hoyt, J.; Pircher, H.; Norris, P.J.; Nixon, D.F.; Lanier, L.L. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010, 116, 3865–3874. [Google Scholar] [CrossRef] [PubMed]
- Bjorkstrom, N.K.; Ljunggren, H.G.; Michaelsson, J. Emerging insights into natural killer cells in human peripheral tissues. Nat. Rev. Immunol. 2016, 16, 310–320. [Google Scholar] [CrossRef]
- Moffett, A.; Colucci, F. Uterine NK cells: Active regulators at the maternal-fetal interface. J. Clin. Investig. 2014, 124, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.A.; Zmuidzinas, A.; Manley, T.J.; Levine, H.; Smith, K.A.; Ritz, J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J. Exp. Med. 1990, 171, 1509–1526. [Google Scholar] [CrossRef]
- Kiso, Y.; McBey, B.A.; Mason, L.; Croy, B.A. Histological assessment of the mouse uterus from birth to puberty for the appearance of LGL-1+ natural killer cells. Biol. Reprod. 1992, 47, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Becknell, B.; Caligiuri, M.A. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 2005, 86, 209–239. [Google Scholar] [CrossRef]
- Kitaya, K.; Yamaguchi, T.; Honjo, H. Central role of interleukin-15 in postovulatory recruitment of peripheral blood CD16(-) natural killer cells into human endometrium. J. Clin. Endocrinol. Metab. 2005, 90, 2932–2940. [Google Scholar] [CrossRef]
- Oppenheim, D.E.; Roberts, S.J.; Clarke, S.L.; Filler, R.; Lewis, J.M.; Tigelaar, R.E.; Girardi, M.; Hayday, A.C. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 2005, 6, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Barchet, W.; Cella, M.; Colonna, M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J. Exp. Med. 2007, 204, 2561–2568. [Google Scholar] [CrossRef]
- Manaster, I.; Mizrahi, S.; Goldman-Wohl, D.; Sela, H.Y.; Stern-Ginossar, N.; Lankry, D.; Gruda, R.; Hurwitz, A.; Bdolah, Y.; Haimov-Kochman, R.; et al. Endometrial NK cells are special immature cells that await pregnancy. J. Immunol. 2008, 181, 1869–1876. [Google Scholar] [CrossRef]
- Yadi, H.; Burke, S.; Madeja, Z.; Hemberger, M.; Moffett, A.; Colucci, F. Unique receptor repertoire in mouse uterine NK cells. J. Immunol. 2008, 181, 6140–6147. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, T.V.; Craig, L.E.; Schloemann, S.R.; Riley, J.K. Murine endometrial and decidual NK1.1+ natural killer cells display a B220+CD11c+ cell surface phenotype. Biol. Reprod. 2009, 81, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, N.; Kekalainen, E.; Chen, P.; Kvedaraite, E.; Wilson, J.N.; Ivarsson, M.A.; Mjosberg, J.; Berglin, L.; Safholm, J.; Manson, M.L.; et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69(-)CD56(dim) cells. J. Allergy Clin. Immunol. 2017, 139, 1321–1330.e1324. [Google Scholar] [CrossRef] [PubMed]
- Mailliard, R.B.; Alber, S.M.; Shen, H.; Watkins, S.C.; Kirkwood, J.M.; Herberman, R.B.; Kalinski, P. IL-18-induced CD83+CCR7+ NK helper cells. J. Exp. Med. 2005, 202, 941–953. [Google Scholar] [CrossRef]
- Carrega, P.; Bonaccorsi, I.; Di Carlo, E.; Morandi, B.; Paul, P.; Rizzello, V.; Cipollone, G.; Navarra, G.; Mingari, M.C.; Moretta, L.; et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J. Immunol. 2014, 192, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Wang, S.; Huang, X.; Min, W.P.; Sun, H.; Liu, W.; Garcia, B.; Jevnikar, A.M. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J. Immunol. 2008, 181, 7489–7498. [Google Scholar] [CrossRef]
- Ascon, D.B.; Lopez-Briones, S.; Liu, M.; Ascon, M.; Savransky, V.; Colvin, R.B.; Soloski, M.J.; Rabb, H. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J. Immunol. 2006, 177, 3380–3387. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.S.; Kim, A.; Koo, S.; Cha, H.J.; Han, J.A.; Do, Y.; Kim, K.M.; Kwon, B.S.; Mittler, R.S.; et al. TLR2 signaling in tubular epithelial cells regulates NK cell recruitment in kidney ischemia-reperfusion injury. J. Immunol. 2013, 191, 2657–2664. [Google Scholar] [CrossRef]
- Victorino, F.; Sojka, D.K.; Brodsky, K.S.; McNamee, E.N.; Masterson, J.C.; Homann, D.; Yokoyama, W.M.; Eltzschig, H.K.; Clambey, E.T. Tissue-Resident NK Cells Mediate Ischemic Kidney Injury and Are Not Depleted by Anti-Asialo-GM1 Antibody. J. Immunol. 2015, 195, 4973–4985. [Google Scholar] [CrossRef]
- Chen, G.E.; Wu, H.; Ma, J.; Chadban, S.J.; Sharland, A. Toll-like receptor 4 engagement contributes to expression of NKG2D ligands by renal tubular epithelial cells. Nephrol. Dial. Transpl. 2011, 26, 3873–3881. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.S.; Kim, J.D.; Cha, H.J.; Kim, A.; Lee, S.K.; Lee, S.C.; Kwon, B.S.; Mittler, R.S.; Cho, H.R.; et al. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, E13–E22. [Google Scholar] [CrossRef]
- Law, B.M.P.; Wilkinson, R.; Wang, X.; Kildey, K.; Lindner, M.; Rist, M.J.; Beagley, K.; Healy, H.; Kassianos, A.J. Interferon-gamma production by tubulointerstitial human CD56(bright) natural killer cells contributes to renal fibrosis and chronic kidney disease progression. Kidney Int. 2017, 92, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zheng, L.; Wang, Y.; Wu, H.; Kairaitis, L.; Zhang, C.; Tay, Y.C.; Wang, Y.; Alexander, S.I.; Harris, D.C. NK cells do not mediate renal injury in murine adriamycin nephropathy. Kidney Int. 2006, 69, 1159–1165. [Google Scholar] [CrossRef]
- Wee, Y.M.; Go, H.; Choi, M.Y.; Jung, H.R.; Cho, Y.M.; Kim, Y.H.; Han, D.J.; Shin, S. Tissue-resident natural killer cells exacerbate tubulointerstitial fibrosis by activating transglutaminase 2 and syndecan-4 in a model of aristolochic acid-induced nephropathy. BMB Rep. 2019, 52, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Shek, K.; Wang, S.; Huang, X.; Lau, A.; Yin, Z.; Sun, H.; Liu, W.; Garcia, B.; Rittling, S.; et al. Osteopontin expressed in tubular epithelial cells regulates NK cell-mediated kidney ischemia reperfusion injury. J. Immunol. 2010, 185, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Schleypen, J.S.; Von Geldern, M.; Weiss, E.H.; Kotzias, N.; Rohrmann, K.; Schendel, D.J.; Falk, C.S.; Pohla, H. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int. J. Cancer 2003, 106, 905–912. [Google Scholar] [CrossRef]
- Schleypen, J.S.; Baur, N.; Kammerer, R.; Nelson, P.J.; Rohrmann, K.; Grone, E.F.; Hohenfellner, M.; Haferkamp, A.; Pohla, H.; Schendel, D.J.; et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin. Cancer Res. 2006, 12, 718–725. [Google Scholar] [CrossRef]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumor immunotherapy. Mol. Asp. Med. 2021, 80, 100870. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e1014. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Liang, Z.; Nong, F.; Zhao, J.; Wei, D.; Tang, Q.; Song, J.; Meng, L. Heterogeneity in NK Cell Subpopulations May Be Involved in Kidney Cancer Metastasis. J. Immunol. Res. 2022, 2022, 6378567. [Google Scholar] [CrossRef]

| Receptors Type | Surface Receptor in Human NK Cells |
|---|---|
| Activating Receptors | NKG2C NKG2D NKp30 NKp44 NKp46 NKp80 CD2 CD16 CD95L DNAM1(CD226) Activating KIR |
| Inhibitory Receptors | Inhibitory KIR TIGIT NKG2A NKG2B CD96 PD-1 KLRG-1 TIM-3 |
| Cytokine Receptors | IL-1R IL-2R IL12R IL18R IL21R IFN-AR |
| Death Receptors Ligands | Fas-L TRAIL |
| Homing Receptors and Adhesion molecules | CCR2 CCR5 CCR7 CXCR1 CXCR3 CXCR4 CXCR6 |
| Tissue | The Effect of NK Cells in Organs | Authors |
|---|---|---|
| Uterine | NK cells’ NKG2A pathway leads to a healthier pregnancy. | Shreeve et al. [65] |
| Uterine NK (uNK) cells take part in remodeling of spiral arteries. | Wells et al. [66] | |
| uNK cell differentiation driven by interleukin-15 occurred to counter the endometrial regeneration. | Strunz et al. [67] | |
| significantly increased uNK level in endometrium of women with RM and RIF may point to an underlying disturbance of the immune milieu culminating in implantation and/or placentation failure. | Von Woon et al. [68] | |
| Dynamic Changes in Uterine NK Cell Subset Frequency and Function Over the Menstrual Cycle and Pregnancy | Whettlock et al. [69] | |
| Uterine NK cells underexpress KIR2DL1/S1 and LILRB1 in reproductive failure | Woon et al. [70] | |
| Lung | Populations of CD56dimCD16+ NK cells with different receptors could be found in the lungs of patients undergoing surgery for suspected lung cancer. | Brownlie et al. [71] |
| Manipulated macrophage polarization by depletion of NK cells attenuates acute lung injury | Wu et al. [72] | |
| Depletion of NK cells or using NKG2D stress receptor blockade can alleviate acute lung injury. | Calabrese et al. [73] | |
| Liver | Tim-3 enhances hepatocellular carcinoma (HCC) growth by blocking natural killer cell function. | Tan et al. [74] |
| NASH have an increased expression of NKG2D on human NK cells | Stiglund et al. [75] | |
| NK cells killed of activated HSCs to anti-fibrotic | Melhem et al. [76] | |
| Natural killer cells reduce liver fibrosis by killing activated stellate cells | Radaeva et al. [77] |
| Renal Disease Type | Function of NK Cells in Disease | Authors |
|---|---|---|
| Ischemia–reperfusion injury (IRI) | NK cells cause tubular epithelial cell (TEC) apoptosis and contribute to renal IRI after being activated by osteopontin. | Zhang et al. [106] |
| NK cells can directly kill TECs in vitro, correlated with TEC expression of RAE-1 and NKG2D on NK cells. | Zhang et al. [97] | |
| Increased infiltration of NK1.1+ and CD4+NK1.1+ cells were observed 3 and 24 h after renal IRI. | Ascon et al. [98] | |
| TECs produced a high CXCR2 level that promoted neutrophil chemotaxis after binding CD137 from NK cells with CD137 ligand (CD137L) on TECs, and toll-like receptor (TLR) 2 ligands released from ischemic TECs induce NK cell recruitment. | Kim et al. [99] | |
| Depleting NK cells protects mice from kidney dysfunction during IRI, and tissue-resident NK cells promote AKI | Victorino et al. [100] | |
| TLR4 signaling affects the expression of the NKG2D ligands RAE-1 and MULT-1 on kidney cells to participate in the pathogenesis of renal IRI | Chen et al. [101] | |
| NK cells are essential in recruiting neutrophils during kidney IRI | Kim et al. [102] | |
| Renal fibrosis | CD56bright natural killer cells produce Interferon-γ (IFNγ), contributing to renal fibrosis and chronic kidney disease (CKD) progression | Law et al. [103] |
| Adriamycin nephropathy | NKs activating receptor NKG2D and its ligand RAE-1 are upregulated by AN | Zheng et al. [104] |
| Aristolochic acid-induced nephropathy (AAN) | Tissue-resident NK cells exacerbated tubulointerstitial fibrosis by activating transglutaminase 2 and syndecan-4 in the AAN model | Wee et al. [105] |
| Renal cell carcinoma | NK cells were found to express various inhibitory receptors (IRs) such as CD94/NKG2A receptor complex | Schleypen et al. [107] |
| The function of NK cells can be predicted by NK cell infiltration level and the expression of markers (CD16 and cytotoxins) in renal cell carcinoma | Schleypen et al. [108] | |
| Kidney Transplantation | NK cells can activate monocytes by secreting cytokines or directly cause renal microvascular inflammation, renal interstitial inflammation, renal tubular inflammation, etc. | Jasper Callemeyn et al. [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, K.; Zheng, Z.-R.; Meng, Y. Natural Killer Cells, as the Rising Point in Tissues, Are Forgotten in the Kidney. Biomolecules 2023, 13, 748. https://doi.org/10.3390/biom13050748
Ma K, Zheng Z-R, Meng Y. Natural Killer Cells, as the Rising Point in Tissues, Are Forgotten in the Kidney. Biomolecules. 2023; 13(5):748. https://doi.org/10.3390/biom13050748
Chicago/Turabian StyleMa, Ke, Zi-Run Zheng, and Yu Meng. 2023. "Natural Killer Cells, as the Rising Point in Tissues, Are Forgotten in the Kidney" Biomolecules 13, no. 5: 748. https://doi.org/10.3390/biom13050748
APA StyleMa, K., Zheng, Z.-R., & Meng, Y. (2023). Natural Killer Cells, as the Rising Point in Tissues, Are Forgotten in the Kidney. Biomolecules, 13(5), 748. https://doi.org/10.3390/biom13050748






