5-HT6 Receptors Sex-Dependently Modulate Hippocampal Synaptic Activity through GABA Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Pharmacology
2.3. Extracellular Electrophysiology
2.4. Data Analysis
3. Results
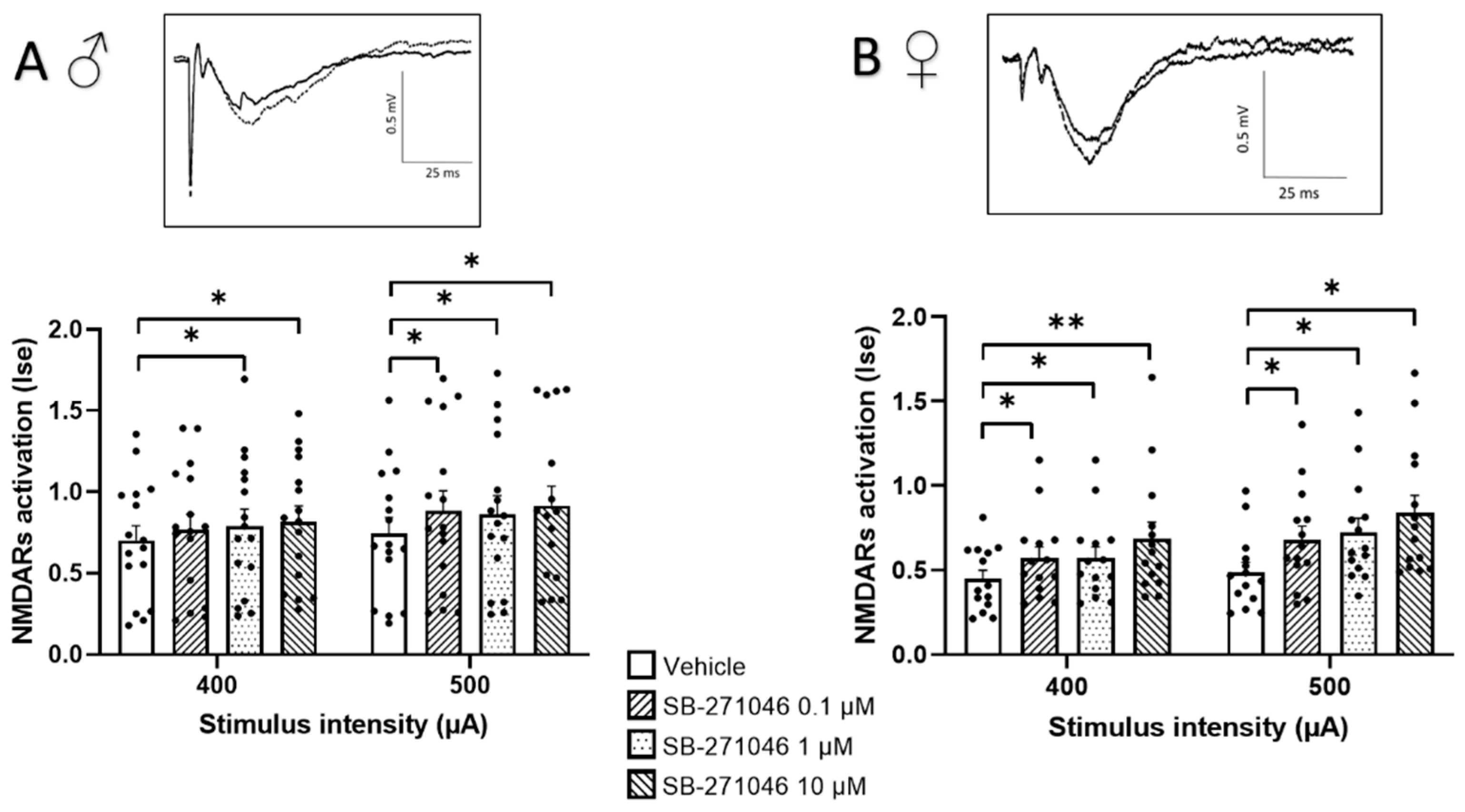
3.1. Effects of SB-271046 on NMDARs’ Activation
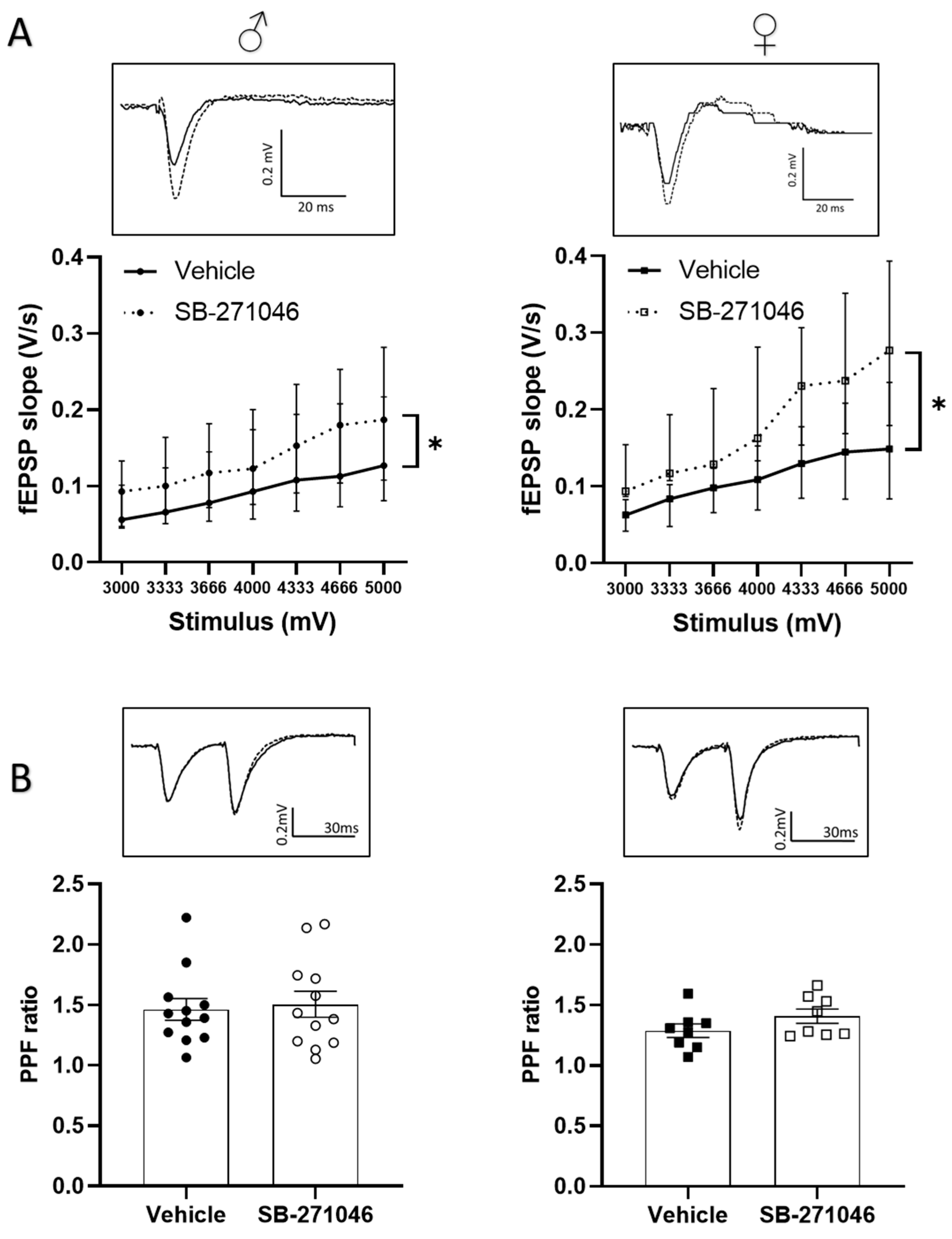
3.2. Effects of SB-271046 on Basal Synaptic Transmission
3.3. Effects of SB-271046 on Functional Plasticity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruat, M.; Traiffort, E.; Arrang, J.M.; Tardivellacombe, J.; Diaz, J.; Leurs, R.; Schwartz, J.C. A Novel Rat Serotonin (5-HT6) Receptor: Molecular Cloning, Localization and Stimulation of CAMP Accumulation. Biochem. Biophys. Res. Commun. 1993, 193, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Metcalf, M.A.; Khan, N.; Druck, T.; Huebner, K.; Lachowicz, J.E.; Meltzer, H.Y.; Sibley, D.R.; Roth, B.L.; Hamblin, M.W. Cloning, Characterization, and Chromosomal Localization of a Human 5-HT6 Serotonin Receptor. J. Neurochem. 1996, 66, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Woolley, M.L.; Marsden, C.A.; Fone, K.C.F. 5-Ht6 Receptors. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Bromidge, S.M.; Brown, A.M.; Clarke, S.E.; Dodgson, K.; Gager, T.; Grassam, H.L.; Jeffrey, P.M.; Joiner, G.F.; King, F.D.; Middlemiss, D.N.; et al. 5-Chloro-N-(4-Methoxy-3-Piperazin-1-Yl-Phenyl)-3-Methyl-2- Benzothiophenesulfonamide (SB-271046): A Potent, Selective, and Orally Bioavailable 5-HT6 Receptor Antagonist. J. Med. Chem. 1999, 42, 202–205. [Google Scholar] [CrossRef]
- Routledge, C.; Bromidge, S.M.; Moss, S.F.; Price, G.W.; Hirst, W.; Newman, H.; Riley, G.; Gager, T.; Stean, T.; Upton, N.; et al. Characterization of SB-271046: A Potent, Selective and Orally Active 5-HT6 Receptor Antagonist. Br. J. Pharmacol. 2000, 130, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Craigo, S.C.; Choudhary, M.S.; Uluer, A.; Monsma, F.J.; Shen, Y.; Meltzer, H.Y.; Sibley, D.R. Binding of Typical and Atypical Antipsychotic Agents to 5- Hydroxytryptamine-6 and 5-Hydroxytryptamine-7 Receptors. J. Pharmacol. Exp. Ther. 1994, 268, 1403–1410. [Google Scholar]
- Monsma, F.J.; Shen, Y.; Ward, R.P.; Hamblin, M.W.; Sibley, D.R. Cloning and Expression of a Novel Serotonin Receptor with High Affinity for Tricyclic Psychotropic Drugs. Mol. Pharmacol. 1993, 43, 320–327. [Google Scholar]
- Glatt, C.E.; Snowman, A.M.; Sibley, D.R.; Snyder, S.H. Clozapine: Selective Labeling of Sites Resembling 5HT6 Serotonin Receptors May Reflect Psychoactive Profile. Mol. Med. 1995, 1, 398–406. [Google Scholar] [CrossRef]
- Mørk, A.; Witten, L.M.; Arnt, J. Effect of Sertindole on Extracellular Dopamine, Acetylcholine, and Glutamate in the Medial Prefrontal Cortex of Conscious Rats: A Comparison with Risperidone and Exploration of Mechanisms Involved. Psychopharmacology 2009, 206, 39–49. [Google Scholar] [CrossRef]
- Correll, C.U.; Howes, O.D. Treatment-Resistant Schizophernia. J. Clin. Psychiatry 2021, 82, MY20096AH1C. [Google Scholar] [CrossRef]
- Shimizu, S.; Mizuguchi, Y.; Ohno, Y. Improving the Treatment of Schizophrenia: Role of 5-HT Receptors in Modulating Cognitive and Extrapyramidal Motor Functions. CNS Neurol. Disord. Drug Targets 2013, 12, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Jayarajan, P.; Shinde, A.; Mohammed, A.R.; Grandhi, V.R.; Benade, V.; Goyal, V.K.; Abraham, R.; Jasti, V.; Cummings, J. Progress in Investigational Agents Targeting Serotonin-6 Receptors for the Treatment of Brain Disorders. Biomolecules 2023, 13, 309. [Google Scholar] [CrossRef]
- Rosse, G.; Schaffhauser, H. 5-HT6 Receptor Antagonists as Potential Therapeutics for Cognitive Impairment. Curr. Top. Med. Chem. 2010, 10, 207–221. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, N.; Kruse, C. 5-HT6 Receptor Antagonists: Potential Efficacy for the Treatment of Cognitive Impairment in Schizophrenia. Curr. Pharm. Des. 2015, 21, 3739–3759. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Costa-Aze, V.; Quiedeville, A.; Boulouard, M.; Dauphin, F. 5-HT6 Receptor Blockade Differentially Affects Scopolamine-Induced Deficits of Working Memory, Recognition Memory and Aversive Learning in Mice. Psychopharmacology 2012, 222, 99–115. [Google Scholar] [CrossRef]
- Foley, A.G.; Murphy, K.J.; Hirst, W.D.; Gallagher, H.C.; Hagan, J.J.; Upton, N.; Walsh, F.S.; Regan, C.M. The 5-HT6 Receptor Antagonist SB-271046 Reverses Scopolamine-Disrupted Consolidation of a Passive Avoidance Task and Ameliorates Spatial Task Deficits in Aged Rats. Neuropsychopharmacology 2004, 29, 93–100. [Google Scholar] [CrossRef]
- Loiseau, F.; Dekeyne, A.; Millan, M.J. Pro-Cognitive Effects of 5-HT6 Receptor Antagonists in the Social Recognition Procedure in Rats: Implication of the Frontal Cortex. Psychopharmacology 2008, 196, 93–104. [Google Scholar] [CrossRef]
- Kendall, I.; Slotten, H.A.; Codony, X.; Burgueño, J.; Pauwels, P.J.; Vela, J.M.; Fone, K.C.F. E-6801, a 5-HT6 Receptor Agonist, Improves Recognition Memory by Combined Modulation of Cholinergic and Glutamatergic Neurotransmission in the Rat. Psychopharmacology 2011, 213, 413–430. [Google Scholar] [CrossRef]
- Da Silva Costa-Aze, V.; Dauphin, F.; Boulouard, M. Serotonin 5-HT6 Receptor Blockade Reverses the Age-Related Deficits of Recognition Memory and Working Memory in Mice. Behav. Brain Res. 2011, 222, 134–140. [Google Scholar] [CrossRef]
- King, M.V.; Marsden, C.A.; Fone, K.C.F. A Role for the 5-HT1A, 5-HT4 and 5-HT6 Receptors in Learning and Memory. Trends Pharmacol. Sci. 2008, 29, 482–492. [Google Scholar] [CrossRef]
- Marcos, B.; Chuang, T.T.; Gil-Bea, F.J.; Ramirez, M.J. Effects of 5-HT 6 Receptor Antagonism and Cholinesterase Inhibition in Models of Cognitive Impairment in the Rat. Br. J. Pharmacol. 2008, 155, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Quiedeville, A.; Boulouard, M.; Hamidouche, K.; Da Silva Costa-Aze, V.; Nee, G.; Rochais, C.; Dallemagne, P.; Fabis, F.; Freret, T.; Bouet, V. Chronic Activation of 5-HT4 Receptors or Blockade of 5-HT6 Receptors Improve Memory Performances. Behav. Brain Res. 2015, 293, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, D.A.; Peterson, S.; Pahua, A.; Posadas, R.; Hernandez, A.; Hefner, E.; Qi, D.; Vega, J. 5-HT6 Receptor Agonist EMD386088 Impairs Behavioral Flexibility and Working Memory. Behav. Brain Res. 2018, 349, 8–15. [Google Scholar] [CrossRef]
- Suárez-Santiago, J.E.; Roldán Roldán, G.; Picazo Picazo, O. The 5-HT6R Agonist E-6837 and the Antagonist SB-271046 Reverse the Psychotic-like Behaviors Induced by Ketamine. Behav. Pharmacol. 2022, 33, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, B.; Chen, C.; Li, C.; Zhang, Y. 5-HT6R Null Mutatrion Induces Synaptic and Cognitive Defects. Aging Cell 2021, 20, e13369. [Google Scholar] [CrossRef] [PubMed]
- Hirst, W.D.; Stean, T.O.; Rogers, D.C.; Sunter, D.; Pugh, P.; Moss, S.F.; Bromidge, S.M.; Riley, G.; Smith, D.R.; Bartlett, S.; et al. SB-399885 Is a Potent, Selective 5-HT6 Receptor Antagonist with Cognitive Enhancing Properties in Aged Rat Water Maze and Novel Object Recognition Models. Eur. J. Pharmacol. 2006, 553, 109–119. [Google Scholar] [CrossRef]
- Asselot, R.; Simon-O’Brien, E.; Lebourgeois, S.; Nee, G.; Delaunay, V.; Duchatelle, P.; Bouet, V.; Dauphin, F. Time-Dependent Impact of Glutamatergic Modulators on the Promnesiant Effect of 5-HT6R Blockade on Mice Recognition Memory. Pharmacol. Res. 2017, 118, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, L.P.; Dawson, L.A.; Hagan, J.J.; Heidbreder, C.A. 5-HT6 Receptor Antagonist SB-271046 Enhances Extracellular Levels of Monoamines in the Rat Medial Prefrontal Cortex. Synapse 2004, 51, 158–164. [Google Scholar] [CrossRef]
- Dawson, L.A.; Nguyen, H.Q.; Li, P. In Vivo Effects of the 5-HT6 Antagonist SB-271046 on Striatal and Frontal Cortex Extracellular Concentrations of Noradrenaline, Dopamine, 5-HT, Glutamate and Aspartate. Br. J. Pharmacol. 2000, 130, 23–26. [Google Scholar] [CrossRef]
- Dawson, L.A.; Nguyen, H.Q.; Li, P. The 5-HT6 Receptor Antagonist SB-271046 Selectively Enhances Excitatory Neurotransmission in the Rat Frontal Cortex and Hippocampus. Neuropsychopharmacology 2001, 25, 662–668. [Google Scholar] [CrossRef]
- Dawson, L.A.; Li, P. Effects of 5-HT6 Receptor Blockade on the Neurochemical Outcome of Antidepressant Treatment in the Frontal Cortex of the Rat. J. Neural Transm. 2003, 110, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Schechter, L.E.; Lin, Q.; Smith, D.L.; Zhang, G.; Shan, Q.; Platt, B.; Brandt, M.R.; Dawson, L.A.; Cole, D.; Bernotas, R.; et al. Neuropharmacological Profile of Novel and Selective 5-HT6 Receptor Agonists: WAY-181187 and WAY-208466. Neuropsychopharmacology 2008, 33, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Helboe, L.; Egebjerg, J.; de Jong, I.E.M. Distribution of Serotonin Receptor 5-HT6 MRNA in Rat Neuronal Subpopulations: A Double in Situ Hybridization Study. Neuroscience 2015, 310, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Chaumont-Dubel, S.; Dupuy, V.; Bockaert, J.; Bécamel, C.; Marin, P. The 5-HT6 Receptor Interactome: New Insight in Receptor Signaling and Its Impact on Brain Physiology and Pathologies. Neuropharmacology 2020, 172, 107839. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, V.; Prieur, M.; Pizzoccaro, A.; Margarido, C.; Valjent, E.; Bockaert, J.; Bouschet, T.; Marin, P.; Chaumont-Dubel, S. Spatiotemporal Dynamics of 5-HT6 Receptor Ciliary Localization during Mouse Brain Development. Neurobiol. Dis. 2023, 176, 105949. [Google Scholar] [CrossRef] [PubMed]
- Chaumont-Dubel, S.; Galant, S.; Prieur, M.; Bouschet, T.; Bockaert, J.; Marin, P. Impact of 5-HT6 Receptor Subcellular Localization on Its Signaling and Its Pathophysiological Roles. Cells 2023, 12, 426. [Google Scholar] [CrossRef]
- Morris, R.G.; Davis, S.; Butcher, S.P. Hippocampal Synaptic Plasticity and NMDA Receptors: A Role in Information Storage? Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 329, 187–204. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Collingridge, G.L. A Synaptic Model of Memory: LTP in the Hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Izquierdo, I.; Medina, J.H. Correlation between the Pharmacology of Long-Term Potentiation and the Pharmacology of Memory. Neurobiol. Learn. Mem. 1995, 63, 19–32. [Google Scholar] [CrossRef]
- Lisman, J.E.; McIntyre, C.C. Synaptic Plasticity: A Molecular Memory Switch. Curr. Biol. 2001, 11, 788–791. [Google Scholar] [CrossRef]
- Kim, S.J.; Linden, D.J. Ubiquitous Plasticity and Memory Storage. Neuron 2007, 56, 582–592. [Google Scholar] [CrossRef]
- West, P.J.; Marcy, V.R.; Marino, M.J.; Schaffhauser, H. Activation of the 5-HT6 Receptor Attenuates Long-Term Potentiation and Facilitates GABAergic Neurotransmission in Rat Hippocampus. Neuroscience 2009, 164, 692–701. [Google Scholar] [CrossRef]
- Ivan, S.; Daniela, O.; Jaroslava, B.D. Sex Differences Matter: Males and Females Are Equal but Not the Same. Physiol. Behav. 2023, 259, 114038. [Google Scholar] [CrossRef]
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex Differences in Schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef]
- Leger, M.; Neill, J.C. A Systematic Review Comparing Sex Differences in Cognitive Function in Schizophrenia and in Rodent Models for Schizophrenia, Implications for Improved Therapeutic Strategies. Neurosci. Biobehav. Rev. 2016, 68, 979–1000. [Google Scholar] [CrossRef] [PubMed]
- Mendrek, A.; Mancini-Marïe, A. Sex/Gender Differences in the Brain and Cognition in Schizophrenia. Neurosci. Biobehav. Rev. 2016, 67, 57–78. [Google Scholar] [CrossRef]
- Crawford, M.B.; DeLisi, L.E. Issues Related to Sex Differences in Antipsychotic Treatment. Curr. Opin. Psychiatry 2016, 29, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.; Werbeloff, N.; Caers, I.; Mandel, F.S.; Stauffer, V.; Ménard, F.; Kinon, B.J.; Kapur, S. Determinants of Antipsychotic Response in Schizophrenia. J. Clin. Psychiatry 2014, 75, e308–e316. [Google Scholar] [CrossRef]
- Morozova, M.; Burminskiy, D.; Rupchev, G.; Lepilkina, T.; Potanin, S.; Beniashvili, A.; Lavrovsky, Y.; Vostokova, N.; Ivaschenko, A. 5-HT6 Receptor Antagonist as an Adjunct Treatment Targeting Residual Symptoms in Patients with Schizophrenia: Unexpected Sex-Related Effects (Double-Blind Placebo-Controlled Trial). J. Clin. Psychopharmacol. 2017, 37, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.W.; Collingridge, G.L. The LTP Program: A Data Acquisition Program for on-Line Analysis of Long-Term Potentiation and Other Synaptic Events. J. Neurosci. Methods 2001, 108, 71–83. [Google Scholar] [CrossRef]
- Anderson, W.W.; Collingridge, G.L. Capabilities of the WinLTP Data Acquisition Program Extending beyond Basic LTP Experimental Functions. J. Neurosci. Methods 2007, 162, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.S.; Neumaier, J.F. 5-HT6 Receptors: A Novel Target for Cognitive Enhancement. Pharmacol. Ther. 2005, 108, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Doleviczényi, Z.; Vizi, E.S.; Gacsályi, I.; Pallagi, K.; Volk, B.; Hársing, L.G.; Halmos, G.; Lendvai, B.; Zelles, T. 5-HT6/7 Receptor Antagonists Facilitate Dopamine Release in the Cochlea via a GABAergic Disinhibitory Mechanism. Neurochem. Res. 2008, 33, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Creager, B.Y.R.; Dunwiddiet, T.; Lynch, G. Paired-Pulse and Frequency Facilitation in the Cai Region. Physiol. Soc. 1980, 299, 409–424. [Google Scholar]
- Wigström, H.; Gustafsson, B. Two Types of Synaptic Facilitation Recorded in Pyramidal Cells of in Vitro Hippocampal Slices from Guinea Pigs. Neurosci. Lett. 1981, 26, 73–78. [Google Scholar] [CrossRef]
- Gérard, C.; Martres, M.P.; Lefèvre, K.; Miquel, M.C.; Vergé, D.; Lanfumey, L.; Doucet, E.; Hamon, M.; El Mestikawy, S. Immune-Localization of Serotonin 5-HT6 Receptor-like Material in the Rat Central Nervous System. Brain Res. 1997, 746, 207–219. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J. SB-271046 (SmithKline Beecham). Curr. Opin. Investig. Drugs 2001, 2, 118–122. [Google Scholar]
- Rogers, D.C.; Hagan, J.J. 5-HT6 Receptor Antagonists Enhance Retention of a Water Maze Task in the Rat. Psychopharmacology 2001, 158, 114–119. [Google Scholar] [CrossRef]
- King, M.V.; Sleight, A.J.; Woolley, M.L.; Topham, I.A.; Marsden, C.A.; Fone, K.C.F. 5-HT6 Receptor Antagonists Reverse Delay-Dependent Deficits in Novel Object Discrimination by Enhancing Consolidation—An Effect Sensitive to NMDA Receptor Antagonism. Neuropharmacology 2004, 47, 195–204. [Google Scholar] [CrossRef]
- Tassone, A.; Madeo, G.; Sciamanna, G.; Pisani, A.; Bonsi, P. Electrophysiology of 5-HT6 Receptors; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 94, ISBN 9780123849762. [Google Scholar]
- Marrocco, J.; McEwen, B.S. Sex in the Brain: Hormones and Sex Differences. Dialogues Clin. Neurosci. 2016, 18, 373–383. [Google Scholar] [CrossRef]
- Bauer, G.R. Sex and Gender Multidimensionality in Epidemiologic Research. Am. J. Epidemiol. 2022, 192, 122–132. [Google Scholar] [CrossRef]
- Meoni, S.; Macerollo, A.; Moro, E. Sex Differences in Movement Disorders. Nat. Rev. Neurol. 2020, 16, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and Gender: Modifiers of Health, Disease, and Medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Zalewska, T.; Pawelec, P.; Ziabska, K.; Ziemka-Nalecz, M. Sexual Dimorphism in Neurodegenerative Diseases and in Brain Ischemia. Biomolecules 2023, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Pillerová, M.; Pastorek, M.; Borbélyová, V.; Riljak, V.; Frick, K.M.; Hodosy, J.; Tóthová, L. Sex Steroid Hormones in Depressive Disorders as a Basis for New Potential Treatment Strategies. Physiol. Res. 2022, 71, S187–S202. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. Men and Women Respond Differently to Antipsychotic Drugs. Neuropharmacology 2020, 163, 107631. [Google Scholar] [CrossRef]
- Bouet, V.; Percelay, S.; Leroux, E.; Diarra, B.; Léger, M.; Delcroix, N.; Andrieux, A.; Dollfus, S.; Freret, T.; Boulouard, M. A New 3-Hit Mouse Model of Schizophrenia Built on Genetic, Early and Late Factors. Schizophr. Res. 2021, 228, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Kekesi, G.; Petrovszki, Z.; Benedek, G.; Horvath, G. Sex-Specific Alterations in Behavioral and Cognitive Functions in a “Three Hit” Animal Model of Schizophrenia. Behav. Brain Res. 2015, 284, 85–93. [Google Scholar] [CrossRef]


| HFS | TBS | ||||
|---|---|---|---|---|---|
| STP | LTP | STP | LTP | ||
| Male | Vehicle | 180.6 ± 13.35% | 158.2 ± 8.45% | 160.5 ± 8.97% | 180.1 ± 17.67% |
| SB-271046 | 174.5 ± 8.56% | 157.6 ± 8.13% | 177.0 ± 6.91% | 181.2 ± 28.48% | |
| Female | Vehicle | 166.6 ± 6.50% | 148.0 ± 2.87% | 216.8 ± 5.62% | 151.1 ± 7.62% |
| SB-271046 | 181.7.5 ± 8.85% | 148.5 ± 4.44% | 211.7 ± 28.76% | 146.2 ± 4.48% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahogue, C.; Billard, J.-M.; Freret, T.; Bouet, V. 5-HT6 Receptors Sex-Dependently Modulate Hippocampal Synaptic Activity through GABA Inhibition. Biomolecules 2023, 13, 751. https://doi.org/10.3390/biom13050751
Lahogue C, Billard J-M, Freret T, Bouet V. 5-HT6 Receptors Sex-Dependently Modulate Hippocampal Synaptic Activity through GABA Inhibition. Biomolecules. 2023; 13(5):751. https://doi.org/10.3390/biom13050751
Chicago/Turabian StyleLahogue, Caroline, Jean-Marie Billard, Thomas Freret, and Valentine Bouet. 2023. "5-HT6 Receptors Sex-Dependently Modulate Hippocampal Synaptic Activity through GABA Inhibition" Biomolecules 13, no. 5: 751. https://doi.org/10.3390/biom13050751






