The Role of Trace Elements and Minerals in Osteoporosis: A Review of Epidemiological and Laboratory Findings
Abstract
:1. Introduction
2. Magnesium (Mg)
3. Selenium (Se)
4. Zinc (Zn)
5. Iron (Fe)
6. Copper (Cu)
7. Cobalt (Co)
8. Fluoride (F)
9. Strontium (Sr)
10. Silicon (Si)
11. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Akkawi, I.; Zmerly, H. Osteoporosis: Current Concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorentzon, M.; Cummings, S.R. Osteoporosis: The evolution of a diagnosis. J. Intern. Med. 2015, 277, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, G.; Boundin, E.; Wim Van Hum, E. A look behind the scenes: The risk and pathogenesis of primary osteoporosis. Nat. Rev. Rheumatol. 2015, 11, 462–474. [Google Scholar] [CrossRef]
- Colangelo, L.; Biamonte, F.; Pepe, J.; Cipriani, C.; Minisola, S. Understanding and managing secondary osteoporosis. Expert Rev. Endocrinol. Metab. 2019, 14, 111–122. [Google Scholar] [CrossRef]
- Ebeling, P.R.; Nguyen, H.H.; Aleksova, J.; Vincent, A.J.; Wong, P.; Milat, F. Secondary osteoporosis. Endocrine Rev. 2022, 43, 240–313. [Google Scholar] [CrossRef]
- Marcucci, G.; Brandi, M.L. Rare causes of osteoporosis. Clin. Cases Miner. Bone Metab. 2015, 12, 151–156. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Zhang, J.; Dennison, E.; Prieto-Alhambra, D. Osteoporosis epidemiology using international cohorts. Curr. Opin. Rheumatol. 2020, 32, 387–393. [Google Scholar] [CrossRef] [PubMed]
- A Clynes, M.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Fassio, A.; Gatti, D.; Viapiana, O.; Benini, C.; Danila, M.I.; Saag, K.G.; Rossini, M. Osteoporosis in 10 years time: A glimpse into the future of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221083541. [Google Scholar] [CrossRef]
- Liang, B.; Burley, G.; Lin, S.; Shi, Y.-C. Osteoporosis pathogenesis and treatment: Existing and emerging avenues. Cell. Mol. Biol. Lett. 2022, 27, 72. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Hampson, G. The pathogenesis, diagnosis, investigation and management of osteoporosis. J. Clin. Pathol. 2011, 64, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Pouresmaeili, F.; Dehghan, B.K.; Kamarehei, M.; Meng, G.Y. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Guo, Y.; Yang, Y.; Fu, D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther. 2022, 237, 108168. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Khosla, S. The role of sex steroids in the pathogenesis of osteoporosis. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 8th ed.; Clifford, J., Rosen, M.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 367–375. [Google Scholar]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef]
- Black, J.D.; Tadros, B.J. Bone structure: From cortical to calcium. Orthop. Trauma 2020, 34, 113–119. [Google Scholar] [CrossRef]
- Song, L. Calcium and Bone Metabolism Indices. Adv. Clin. Chem. 2017, 82, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, G.; Dermauw, V.; Bouillon, R. Vitamin D signaling in calcium and bone homeostasis: A delicate balance. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016, 4, 16041. [Google Scholar] [CrossRef] [Green Version]
- Shlisky, J.; Mandlik, R.; Askari, S.; Abrams, S.; Belizan, J.M.; Bourassa, M.W.; Cormick, G.; Driller-Colangelo, A.; Gomes, F.; Khadilkar, A.; et al. Calcium deficiency worldwide: Prevalence of inadequate intakes and associated health outcomes. Ann. N. Y. Acad. Sci. 2022, 1512, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Tai, V.; Leung, W.; Grey, A.; Reid, I.; Bolland, M.J. Calcium intake and bone mineral density: Systematic review and meta-analysis. BMJ 2015, 351, h4183. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Do Only Calcium and Vitamin D Matter? Micronutrients in the Diet of Inflammatory Bowel Diseases Patients and the Risk of Osteoporosis. Nutrients 2021, 13, 525. [Google Scholar] [CrossRef]
- Stazi, A.V. Micronutrient deficiencies in osteoporosis. Minerva Med. 2013, 104, 455–470. [Google Scholar] [PubMed]
- Feng, W.; Wang, X.; Huang, D.; Lu, A. Role of diet in osteoporosis incidence: Umbrella review of meta-analyses of prospective observational studies. Crit. Rev. Food Sci. Nutr. 2021, 1–10. [Google Scholar] [CrossRef]
- Aaseth, J.O.; Alexander, J. Postoperative Osteoporosis in Subjects with Morbid Obesity Undergoing Bariatric Surgery with Gastric Bypass or Sleeve Gastrectomy. Nutrients 2023, 15, 1302. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Mao, X.; Ling, J.; He, Q.; Quan, J. Low Serum Levels of Zinc, Copper, and Iron as Risk Factors for Osteoporosis: A Meta-analysis. Biol. Trace Elem. Res. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef]
- Dermience, M.; Lognay, G.; Mathieu, F.; Goyens, P. Effects of thirty elements on bone metabolism. J. Trace Elem. Med. Biol. 2015, 32, 86–106. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An update on magnesium and bone health. Biometals 2021, 34, 715–736. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Mao, X.; Ling, J.; He, Q.; Quan, J.; Jiang, H. Association Between Serum Level of Magnesium and Postmenopausal Osteoporosis: A Meta-analysis. Biol. Trace Elem. Res. 2014, 159, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Yu, D.; Ji, J.; Wang, N.; Yu, S.; Yu, B. The Association Between the Concentration of Serum Magnesium and Postmenopausal Osteoporosis. Front. Med. 2020, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, I.; van Delft, M.; Versloot, P.; van Loon, L.J.; de Groot, L.C. Impact of magnesium on bone health in older adults: A systematic review and meta-analysis. Bone 2022, 154, 116233. [Google Scholar] [CrossRef]
- Wang, J.; Xing, F.; Sheng, N.; Xiang, Z. Associations of the Dietary Magnesium Intake and Magnesium Depletion Score with Osteoporosis among American Adults: Data From the National Health and Nutrition Examination Survey. Front. Nutr. 2022, 9, 883264. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Solmi, M.; Noale, M.; Vaona, A.; Demurtas, J.; Maggi, S. Dietary magnesium intake and fracture risk: Data from a large prospective study. Br. J. Nutr. 2017, 117, 1570–1576. [Google Scholar] [CrossRef] [Green Version]
- Aydın, H.; Deyneli, O.; Yavuz, D.; Gözü, H.; Mutlu, N.; Kaygusuz, I.; Akalın, S.; Kaygusuz, I. Short-Term Oral Magnesium Supplementation Suppresses Bone Turnover in Postmenopausal Osteoporotic Women. Biol. Trace Elem. Res. 2010, 133, 136–143. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Laukkanen, J.A. Low serum magnesium levels are associated with increased risk of fractures: A long-term prospective cohort study. Eur. J. Epidemiol. 2017, 32, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Qi, T.; Weng, J.; Yu, F.; Zhang, W.; Li, G.; Qin, H.; Tan, Z.; Zeng, H. Insights into the Role of Magnesium Ions in Affecting Osteogenic Differentiation of Mesenchymal Stem Cells. Biol. Trace Elem. Res. 2021, 199, 559–567. [Google Scholar] [CrossRef]
- Xu, J.; Hu, P.; Zhang, X.; Chen, J.; Wang, J.; Zhang, J.; Chen, Z.; Yu, M.K.; Chung, Y.W.; Wang, Y.; et al. Magnesium implantation or supplementation ameliorates bone disorder in CFTR-mutant mice through an ATF4-dependent Wnt/β-catenin signaling. Bioact. Mater. 2021, 8, 95–108. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, L.; Liu, C.; Yuan, Y.; Lin, X.; Tan, L.; Chen, S.; Yang, K.; Mei, X. Effect of implantation of biodegradable magnesium alloy on BMP-2 expression in bone of ovariectomized osteoporosis rats. Mater. Sci. Eng. C 2013, 33, 4470–4474. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.; Stocchero, M.; Andersson, M.; Karlsson, J.; He, W.; Lilin, T.; Wennerberg, A.; Jimbo, R. The effect of magnesium on early osseointegration in osteoporotic bone: A histological and gene expression investigation. Osteoporos. Int. 2017, 28, 2195–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Geng, Z.; Huang, Y.; Jia, Z.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y.; Zhu, S.; Yang, X.; et al. Unraveling the osteogenesis of magnesium by the activity of osteoblasts in vitro. J. Mater. Chem. B 2018, 6, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.-Y.; Feng, Y.-F.; Ma, Z.-S.; Ma, T.-C.; Zhang, Y.; Li, X.; Wang, L.; Lei, W. Magnesium Ions Promote the Biological Behaviour of Rat Calvarial Osteoblasts by Activating the PI3K/Akt Signalling Pathway. Biol. Trace Elem. Res. 2017, 179, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tocados, J.M.; Herencia, C.; Martínez-Moreno, J.M.; de Oca, A.M.; Rodríguez-Ortiz, M.E.; Vergara, N.; Blanco, A.; Steppan, S.; Almadén, Y.; Rodríguez, M.; et al. Magnesium Chloride promotes Osteogenesis through Notch signaling activation and expansion of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 7839. [Google Scholar] [CrossRef] [Green Version]
- Leidi, M.; Dellera, F.; Mariotti, M.; Banfi, G.; Crapanzano, C.; Albisetti, W.; Maier, J.A. Nitric oxide mediates low magnesium inhibition of osteoblast-like cell proliferation. J. Nutr. Biochem. 2011, 23, 1224–1229. [Google Scholar] [CrossRef]
- Kim, K.-J.; Choi, S.; Cho, Y.S.; Yang, S.-J.; Cho, Y.-S.; Kim, K.K. Magnesium ions enhance infiltration of osteoblasts in scaffolds via increasing cell motility. J. Mater. Sci. Mater. Med. 2017, 28, 96. [Google Scholar] [CrossRef]
- Choi, S.; Kim, K.-J.; Cheon, S.; Kim, E.-M.; Kim, Y.-A.; Park, C.; Kim, K.K. Biochemical activity of magnesium ions on human osteoblast migration. Biochem. Biophys. Res. Commun. 2020, 531, 588–594. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Liu, B.; Tian, Y.; Ma, W. Effect of magnesium ion on human osteoblast activity. Braz. J. Med. Biol. Res. 2016, 49, e5257. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and re-generation: From mechanism to application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Leidi, M.; Dellera, F.; Mariotti, M.; Maier, J.A.M. High magnesium inhibits human osteoblast differentiation in vitro. Magnes. Res. 2011, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-C.; Pringa, E.; Chou, L. Effect of magnesium on the osteogenesis of normal human osteoblasts. Magnes. Res. 2017, 30, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Duan, Y. Magnesium Lithospermate B Protects against Lipopolysaccharide-Induced Bone Loss by Inhibiting RANKL/RANK Pathway. Front. Pharmacol. 2018, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.; Qu, X.; Li, H.; Yang, K.; Wan, P.; Tan, L.; Ouyang, Z.; Liu, X.; Tian, B.; Xiao, F.; et al. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials 2014, 35, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Belluci, M.M.; de Molon, R.S.; Rossa, C., Jr.; Tetradis, S.; Giro, G.; Cerri, P.S.; Marcantonio, E., Jr.; Orrico, S.R.P. Severe magnesium deficiency compromises systemic bone mineral density and aggravates inflammatory bone resorption. J. Nutr. Biochem. 2020, 77, 108301. [Google Scholar] [CrossRef]
- Rude, R.; Gruber, H.; Wei, L.; Frausto, A.; Mills, B. Magnesium Deficiency: Effect on Bone and Mineral Metabolism in the Mouse. Calcif. Tissue Int. 2003, 72, 32–41. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone 2005, 37, 211–219. [Google Scholar] [CrossRef]
- Rude, R.K.; E Gruber, H.; Wei, L.Y.; Frausto, A. Immunolocalization of RANKL is Increased and OPG Decreased During Dietary Magnesium Deficiency in the Rat. Nutr. Metab. 2005, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Belluci, M.M.; Schoenmaker, T.; Rossa-Junior, C.; Orrico, S.R.; de Vries, T.J.; Everts, V. Magnesium deficiency results in an increased formation of osteoclasts. J. Nutr. Biochem. 2013, 24, 1488–1498. [Google Scholar] [CrossRef]
- Gruber, H.E.; Rude, R.K. Alterations in osteoclast morphology following osteoprotegerin administration in the magnesium-deficient mouse. Biotech. Histochem. 2003, 78, 231–236. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Luthringer, B.J.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, J.; Xiao, J. Selenoproteins and selenium status in bone physiology and pathology. Biochim. Biophys. Acta 2014, 1840, 3246–3256. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-C.; Kwon, Y.; Lee, Y.; Kim, D.K.; Jang, Y.; Lee, S. Low selenium levels are associated with decreased bone mineral densities. J. Trace Elem. Med. Biol. 2020, 61, 126534. [Google Scholar] [CrossRef]
- Grili, P.P.d.F.; Vidigal, C.V.; da Cruz, G.F.; Albergaria, B.H.; Marques-Rocha, J.L.; Pereira, T.S.S.; Guandalini, V.R. Dietary consumption of selenium inversely associated with osteoporosis in postmenopausal women. Front. Nutr. 2022, 9, 997414. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wang, C.-K.; Yang, A.-M.; Lu, C.-S.; Lin, C.-Y. Selenium status is independently related to bone mineral density, FRAX score, and bone fracture history: NHANES, 2013 to 2014. Bone 2021, 143, 115631. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, D.; Li, J.; Long, H.; Wu, J.; Wu, Z.; He, H.; Wang, H.; Yang, T.; Wang, Y. Association between dietary selenium intake and the prevalence of osteoporosis: A cross-sectional study. BMC Musculoskelet. Disord. 2019, 20, 585. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.J.; Gregoire, B.R.; Zeng, H. Selenium Deficiency Decreases Antioxidative Capacity and Is Detrimental to Bone Microarchitecture in Mice. J. Nutr. 2012, 142, 1526–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, R.; Jakob, F. Selenium deficiency as a putative risk factor for osteoporosis. Int. Congr. Ser. 2007, 1297, 158–164. [Google Scholar] [CrossRef]
- Pietschmann, N.; Rijntjes, E.; Hoeg, A.; Stoedter, M.; Schweizer, U.; Seemann, P.; Schomburg, L. Selenoprotein P is the essential selenium transporter for bones. Metallomics 2014, 6, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, K.; Kim, J.M.; Kim, M.Y.; Kim, J.-R.; Lee, H.-W.; Chung, Y.W.; Shin, H.-I.; Kim, T.; Park, E.-S.; et al. Selenoprotein W ensures physiological bone remodeling by preventing hyperactivity of osteoclasts. Nat. Commun. 2021, 12, 2258. [Google Scholar] [CrossRef]
- Gilbert, A.K.; Newton, T.D.; Hettiaratchi, M.H.; Pluth, M.D. Reactive sulfur and selenium species in the regulation of bone homeostasis. Free. Radic. Biol. Med. 2022, 190, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Lee, N.-H.; Patel, K.D.; Jang, T.-S.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. The Effect of Selenium Nanoparticles on the Osteogenic Differentiation of MC3T3-E1 Cells. Nanomaterials 2021, 11, 557. [Google Scholar] [CrossRef]
- Sun, J.Y.; Hou, Y.J.; Fu, X.Y.; Fu, X.T.; Ma, J.K.; Yang, M.F.; Sun, B.L.; Fan, C.D.; Oh, J. Selenium-Containing Protein From Selenium-Enriched Spirulina platensis Attenuates Cisplatin-Induced Apoptosis in MC3T3-E1 Mouse Preosteoblast by Inhibiting Mitochondrial Dysfunction and ROS-Mediated Oxidative Damage. Front. Physiol. 2019, 9, 1907. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lee, S.-Y.; Park, K.-C.; Park, S.-H.; Chung, J.; Lee, S. The Effects of Selenium on Bone Health: From Element to Therapeutics. Molecules 2022, 27, 392. [Google Scholar] [CrossRef]
- Sharma, A.R.; Sharma, G.; Lee, Y.H.; Chakraborty, C.; Lee, S.S.; Seo, E.M. Sodium Selenite Promotes Osteoblast Differ-entiation via The WNT/ß-Catenin Signaling Pathway. Cell J. 2022, 24, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bian, W.; Liu, S.; Huang, K. Selenium Protects Bone Marrow Stromal Cells Against Hydrogen Peroxide-Induced Inhibition of Osteoblastic Differentiation by Suppressing Oxidative Stress and ERK Signaling Pathway. Biol. Trace Elem. Res. 2012, 150, 441–450. [Google Scholar] [CrossRef]
- Fatima, S.; Alfrayh, R.; Alrashed, M.; Alsobaie, S.; Ahmad, R.; Mahmood, A. Selenium Nanoparticles by Moderating Oxidative Stress Promote Differentiation of Mesenchymal Stem Cells to Osteoblasts. Int. J. Nanomed. 2021, 16, 331–343. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, J.; Liu, Y.; Yu, Q.; Liu, Y.; Deng, N.; Liu, J. Functional selenium nanoparticles enhanced stem cell osteo-blastic differentiation through BMP signaling pathways. Adv. Funct. Mater. 2014, 24, 6872–6883. [Google Scholar] [CrossRef]
- Poleboina, S.; Sheth, V.G.; Sharma, N.; Sihota, P.; Kumar, N.; Tikoo, K. Selenium nanoparticles stimulate osteoblast differentiation via BMP-2/MAPKs/β-catenin pathway in diabetic osteoporosis. Nanomedicine 2022, 17, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Lin, H.; Li, H.; Zou, B.; Xie, B.; Yu, Y.; He, L.; Chen, T. Chiral Selenium Nanotherapeutics Regulates Selenoproteins to Attenuate Glucocorticoid-Induced Osteoporosis. Adv. Funct. Mater. 2023, 33, 2212970. [Google Scholar] [CrossRef]
- Yazıcı, T.; Koçer, G.; Nazıroğlu, M.; Övey, I.S.; Öz, A. Zoledronic Acid, Bevacizumab and Dexamethasone-Induced Apoptosis, Mitochondrial Oxidative Stress, and Calcium Signaling Are Decreased in Human Osteoblast-Like Cell Line by Selenium Treatment. Biol. Trace Elem. Res. 2018, 184, 358–368. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Gu, C.; Liu, H.; Hu, X.; Deng, L.; He, W.; Xu, Y.; Zhu, X.; Yang, H.; et al. Selenium-modified bone cement promotes osteoporotic bone defect repair in ovariectomized rats by restoring GPx1-mediated mitochondrial antioxidant functions. Regen. Biomater. 2023, 10, bad011. [Google Scholar] [CrossRef]
- Li, T.-L.; Tao, Z.-S.; Wu, X.-J.; Yang, M.; Xu, H.-G. Selenium-modified calcium phosphate cement can accelerate bone regeneration of osteoporotic bone defect. J. Bone Miner. Metab. 2021, 39, 934–943. [Google Scholar] [CrossRef]
- Huang, Y.; Jia, Z.; Xu, Y.; Qin, M.; Feng, S. Selenium protects against LPS-induced MC3T3-E1 cells apoptosis through modulation of microRNA-155 and PI3K/Akt signaling pathways. Genet. Mol. Biol. 2020, 43, e20190153. [Google Scholar] [CrossRef]
- Moon, H.-J.; Ko, W.-K.; Han, S.W.; Kim, D.-S.; Hwang, Y.-S.; Park, H.-K.; Kwon, I.K. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem. Biophys. Res. Commun. 2012, 418, 247–253. [Google Scholar] [CrossRef]
- Chung, Y.W.; Kim, T.S.; Lee, S.Y.; Lee, S.H.; Choi, Y.; Kim, N.; Min, B.-M.; Jeong, D.-W.; Kim, I.Y. Selenite-induced apoptosis of osteoclasts mediated by the mitochondrial pathway. Toxicol. Lett. 2006, 160, 143–150. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, X.; Feng, Y.; Zheng, L.; Jian, J. Selenium donors inhibits osteoclastogenesis through inhibiting IL-6 and plays a pivotal role in bone metastasis from breast cancer. Toxicol. Res. 2020, 9, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Sosnoski, D.M.; Gandhi, U.H.; Novinger, L.J.; Prabhu, K.S.; Mastro, A.M. Selenium modifies the osteoblast inflammatory stress response to bone metastatic breast cancer. Carcinogenesis 2009, 30, 1941–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Yan, G.; Guan, M. Zinc Homeostasis in Bone: Zinc Transporters and Bone Diseases. Int. J. Mol. Sci. 2020, 21, 1236. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, M.N.; Akdas, S.; Yazihan, N. Is Zinc an Important Trace Element on Bone-Related Diseases and Complications? A Meta-analysis and Systematic Review from Serum Level, Dietary Intake, and Supplementation Aspects. Biol. Trace Elem. Res. 2021, 199, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Arikan, D.C.; Coskun, A.; Ozer, A.; Kilinc, M.; Atalay, F.; Arikan, T. Plasma Selenium, Zinc, Copper and Lipid Levels in Postmenopausal Turkish Women and Their Relation with Osteoporosis. Biol. Trace Elem. Res. 2011, 144, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, M.; Foldes, J.; Steinberg, R.; Menczel, J. Zinc excretion in osteoporotic women. J. Bone Miner. Res. 1990, 5, 251–257. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Ran, X.; Cui, J.; Wei, F.; Yi, G.; Chen, W.; Luo, X.; Chen, Z. The Role of Zinc in Bone Mesenchymal Stem Cell Differentiation. Cell Reprogram. 2022, 24, 80–94. [Google Scholar] [CrossRef]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Yusa, K.; Yamamoto, O.; Iino, M.; Takano, H.; Fukuda, M.; Qiao, Z.; Sugiyama, T. Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering. Arch. Oral Biol. 2016, 71, 162–169. [Google Scholar] [CrossRef]
- Alcantara, E.H.; Lomeda, R.-A.R.; Feldmann, J.; Nixon, G.F.; Beattie, J.H.; Kwun, I.-S. Zinc deprivation inhibits extracellular matrix calcification through decreased synthesis of matrix proteins in osteoblasts. Mol. Nutr. Food Res. 2011, 55, 1552–1560. [Google Scholar] [CrossRef]
- Cerovic, A.; Miletic, I.; Sobajic, S.; Blagojevic, D.; Radusinovic, M.; El-Sohemy, A. Effects of zinc on the mineralization of bone nodules from human osteoblast-like cells. Biol. Trace Elem. Res. 2007, 116, 61–71. [Google Scholar] [CrossRef]
- Hesse, E.; Kiviranta, R.; Wu, M.; Saito, H.; Yamana, K.; Correa, D.; Atfi, A.; Baron, R. Zinc finger protein 521, a new player in bone formation. Ann. N. Y. Acad. Sci. 2010, 1192, 32–37. [Google Scholar] [CrossRef]
- Liang, D.; Yang, M.; Guo, B.; Cao, J.; Yang, L.; Guo, X. Zinc Upregulates the Expression of Osteoprotegerin in Mouse Osteoblasts MC3T3-E1 Through PKC/MAPK Pathways. Biol. Trace Elem. Res. 2012, 146, 340–348. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, Y.; Yoon, D.S.; Lee, K.-M.; Kim, D.; Lee, J.W. Zinc Promotes Osteoblast Differentiation in Human Mesenchymal Stem Cells Via Activation of the cAMP-PKA-CREB Signaling Pathway. Stem Cells Dev. 2018, 27, 1125–1135. [Google Scholar] [CrossRef]
- Guo, B.; Yang, M.; Liang, D.; Yang, L.; Cao, J.; Zhang, L. Cell apoptosis induced by zinc deficiency in osteoblastic MC3T3-E1 cells via a mitochondrial-mediated pathway. Mol. Cell Biochem. 2011, 361, 209–216. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, J.; Chen, Y.; Li, Z.; Sun, Y.; Fan, L.; Wang, M.; Peng, C. Zinc deficiency decreases bone mineral density of rat by cal-modulin-induced change in calcium metabolism. bioRxiv 2020. [Google Scholar] [CrossRef]
- Suzuki, T.; Kajita, Y.; Katsumata, S.-I.; Matsuzaki, H.; Suzuki, K. Zinc Deficiency Increases Serum Concentrations of Parathyroid Hormone through a Decrease in Serum Calcium and Induces Bone Fragility in Rats. J. Nutr. Sci. Vitaminol. 2015, 61, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Kwun, I.-S.; Cho, Y.-E.; Lomeda, R.-A.R.; Shin, H.-I.; Choi, J.-Y.; Kang, Y.-H.; Beattie, J.H. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone 2010, 46, 732–741. [Google Scholar] [CrossRef]
- Hie, M.; Iitsuka, N.; Otsuka, T.; Nakanishi, A.; Tsukamoto, I. Zinc deficiency decreases osteoblasts and osteoclasts associated with the reduced expression of Runx2 and RANK. Bone 2011, 49, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, H.; Jia, S. Zinc Enhances Bone Metabolism in Ovariectomized Rats and Exerts Anabolic Osteoblastic/Adipocytic Marrow Effects Ex Vivo. Biol. Trace Elem. Res. 2015, 163, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Weitzmann, M.N. Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol. Cell Biochem. 2011, 355, 179–186. [Google Scholar] [CrossRef]
- Hadley, K.B.; Newman, S.M.; Hunt, J.R. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J. Nutr. Biochem. 2010, 21, 297–303. [Google Scholar] [CrossRef]
- Park, J.-H.; A Park, S.; Kang, Y.-H.; Hwa, S.M.; Koh, E.-B.; Hwang, S.-C.; Oh, S.H.; Byun, J.-H. Zinc Sulfate Stimulates Osteogenic Phenotypes in Periosteum-Derived Cells and Co-Cultures of Periosteum-Derived Cells and THP-1 Cells. Life 2021, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Uchiyama, S. Receptor activator of NF-kappaB ligand-stimulated osteoclastogenesis in mouse marrow cul-ture is suppressed by zinc in vitro. Int. J. Mol. Med. 2004, 14, 81–85. [Google Scholar]
- Park, K.H.; Park, B.; Yoon, D.S.; Kwon, S.-H.; Shin, D.M.; Lee, J.W.; Lee, H.G.; Shim, J.-H.; Park, J.H.; Lee, J.M. Zinc inhibits osteoclast differentiation by suppression of Ca2+-Calcineurin-NFATc1 signaling pathway. Cell Commun. Signal. 2013, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.C.; Bortolin, R.H.; Freire-Neto, F.P.; Souza, K.S.; Bezerra, J.F.; Ururahy, M.A.; Ramos, A.M.; Himelfarb, S.T.; Abreu, B.J.; Didone, T.V.; et al. Zinc supplementation reduces RANKL/OPG ratio and prevents bone architecture alterations in ovariectomized and type 1 diabetic rats. Nutr. Res. 2017, 40, 48–56. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Z.; Luo, H.; Li, Z.; Yuan, Z.; Tang, J.; Lin, L.; Du, Z.; Zhou, J.-R. Effects of a calcium/vitamin D/Zinc combination on anti-osteoporosis in ovariectomized rats. J. Trace Elem. Med. Biol. 2023, 77, 127138. [Google Scholar] [CrossRef]
- Iitsuka, N.; Hie, M.; Tsukamoto, I. Zinc supplementation inhibits the increase in osteoclastogenesis and decrease in osteoblastogenesis in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2013, 714, 41–47. [Google Scholar] [CrossRef]
- Yu, J.; Xu, L.; Li, K.; Xie, N.; Xi, Y.; Wang, Y.; Zheng, X.; Chen, X.; Wang, M.; Ye, X. Zinc-modified Calcium Silicate Coatings Promote Osteogenic Differentiation through TGF-β/Smad Pathway and Osseointegration in Osteopenic Rabbits. Sci. Rep. 2017, 7, 3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Dong, W.-J.; He, F.-M.; Wang, X.-X.; Zhao, S.-F.; Yang, G.-L. Osteoblast response to porous titanium surfaces coated with zinc-substituted hydroxyapatite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 313–318. [Google Scholar] [CrossRef]
- Luo, X.; Barbieri, D.; Davison, N.; Yan, Y.; de Bruijn, J.D.; Yuan, H. Zinc in calcium phosphate mediates bone induction: In vitro and in vivo model. Acta Biomater. 2014, 10, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.H.; Alves, M.M.; Cebotarenco, M.; Ribeiro, I.A.; Grenho, L.; Gomes, P.S.; Carmezim, M.J.; Santos, C.F. Citrate zinc hydroxyapatite nanorods with enhanced cytocompatibility and osteogenesis for bone regeneration. Mater. Sci. Eng. C 2020, 115, 111147. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, M.; Liu, L.; Yan, G.; Yan, H.; Feng, J.; Li, Z.; Li, D.; Sun, H.; Yang, B. Osteogenic potential of Zn2+-passivated carbon dots for bone regeneration in vivo. Biomater. Sci. 2019, 7, 5414–5423. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Yamamoto, O.; Takano, H.; Fukuda, M.; Iino, M. Zinc-modified titanium surface enhances osteoblast differentiation of dental pulp stem cells in vitro. Sci. Rep. 2016, 6, 29462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh, E.; Paragh, G.; Jeney, V. Influence of Iron on Bone Homeostasis. Pharmaceuticals 2018, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Che, J.; Yang, J.; Zhao, B.; Zhang, G.; Wang, L.; Peng, S.; Shang, P. Effect of Abnormal Iron Metabolism on Osteoporosis. Biol. Trace Elem. Res. 2020, 195, 353–365. [Google Scholar] [CrossRef]
- Valenti, L.; Varenna, M.; Fracanzani, A.L.; Rossi, V.; Fargion, S.; Sinigaglia, L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos. Int. 2009, 20, 549–555. [Google Scholar] [CrossRef]
- Rossi, F.; Perrotta, S.; Bellini, G.; Luongo, L.; Tortora, C.; Siniscalco, D.; Francese, M.; Torella, M.; Nobili, B.; Di Marzo, V.; et al. Iron overload causes osteoporosis in thalassemia major patients through interaction with transient receptor potential vanilloid type 1 (TRPV1) channels. Haematologica 2014, 99, 1876–1884. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Men, P.; Kenner, G.H.; Miller, S.C. Age-associated Iron Accumulation in Bone: Implications for Postmenopausal Osteoporosis and a New Target for Prevention and Treatment by Chelation. Biometals 2006, 19, 245–251. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, H.; Yao, G.; Qiao, P.; Li, L.; Wu, S. Therapeutic potential of iron chelators on osteoporosis and their cellular mechanisms. Biomed. Pharmacother. 2021, 137, 111380. [Google Scholar] [CrossRef]
- Toxqui, L.; Vaquero, M.P. Chronic Iron Deficiency as an Emerging Risk Factor for Osteoporosis: A Hypothesis. Nutrients 2015, 7, 2324–2344. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.-L.; Chen, L.-R.; Tsao, H.-M.; Chen, K.-H. Iron Deficiency Anemia as a Risk Factor for Osteoporosis in Taiwan: A Nationwide Population-Based Study. Nutrients 2017, 9, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.-Y.; Zhao, L.-P.; He, Y.-F.; Li, G.-F.; Gao, C.; Li, K.; Xu, Y.-J. A Comparison of the Biological Activities of Human Osteoblast hFOB1.19 Between Iron Excess and Iron Deficiency. Biol. Trace Elem. Res. 2012, 150, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Liu, Z.; Zhong, Y.; Huang, J.; Chen, B.; Wang, H.; Xu, Y. Iron deficiency anemia’s effect on bone formation in zebrafish mutant. Biochem. Biophys. Res. Commun. 2016, 475, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jian, J.; Abramson, S.; Huang, X. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J. Bone Miner. Res. 2011, 26, 1188–1196. [Google Scholar] [CrossRef]
- Edwards, D.F.; Miller, C.J., III; Quintana-Martinez, A.; Wright, C.S.; Prideaux, M.; Atkins, G.J.; Thompson, W.R.; Clinkenbeard, E.L. Differential Iron Requirements for Osteoblast and Adipocyte Differentiation. JBMR Plus 2021, 5, e10529. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Katsumata, R.; Matsumoto, N.; Inoue, H.; Takahashi, N.; Uehara, M. Iron deficiency decreases renal 25-hydroxyvitamin D3-1α-hydroxylase activity and bone formation in rats. BMC Nutr. 2016, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, K.; Hagiwara, H. Excess iron inhibits osteoblast metabolism. Toxicol. Lett. 2009, 191, 211–215. [Google Scholar] [CrossRef]
- Che, J.; Lv, H.; Yang, J.; Zhao, B.; Zhou, S.; Yu, T.; Shang, P. Iron overload induces apoptosis of osteoblast cells via eliciting ER stress-mediated mitochondrial dysfunction and p-eIF2α/ATF4/CHOP pathway in vitro. Cell Signal. 2021, 84, 110024. [Google Scholar] [CrossRef]
- Xia, D.; Wu, J.; Xing, M.; Wang, Y.; Zhang, H.; Xia, Y.; Zhou, P.; Xu, S. Iron overload threatens the growth of osteoblast cells via inhibiting the PI3K/AKT/FOXO3a/DUSP14 signaling pathway. J. Cell Physiol. 2019, 234, 15668–15677. [Google Scholar] [CrossRef]
- Tian, Q.; Qin, B.; Gu, Y.; Zhou, L.; Chen, S.; Zhang, S.; Zhang, S.; Han, Q.; Liu, Y.; Wu, X. ROS-Mediated Necroptosis Is Involved in Iron Overload-Induced Osteoblastic Cell Death. Oxidative Med. Cell Longev. 2020, 2020, 1295382. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, H.; Qi, G.; Jiang, C.; Chen, K.; Yan, Z. Iron overload-induced ferroptosis of osteoblasts inhibits osteogenesis and promotes osteoporosis: An in vitro and in vivo study. IUBMB Life 2022, 74, 1052–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, A.; Li, G.; Zhai, Q.; Huang, Z.; Wang, X.; Cao, Z.; Liu, L.; Liu, G.; Chen, B.; et al. Osteoporotic bone loss from excess iron accumulation is driven by NOX4-triggered ferroptosis in osteoblasts. Free. Radic. Biol. Med. 2023, 198, 123–136. [Google Scholar] [CrossRef]
- Xu, W.; Yu, R.; Zhu, X.; Li, Z.; Jia, J.; Li, D.; Chen, Y.; Zhang, X. Iron-Chelating Agent Can Maintain Bone Homeostasis Disrupted by Iron Overload by Upregulating Wnt/Beta-Catenin Signaling. BioMed Res. Int. 2020, 2020, 8256261. [Google Scholar] [CrossRef]
- Luo, C.; Xu, W.; Tang, X.; Liu, X.; Cheng, Y.; Wu, Y.; Xie, Z.; Wu, X.; He, X.; Wang, Q.; et al. Canonical Wnt signaling works downstream of iron overload to prevent ferroptosis from damaging osteoblast differentiation. Free. Radic. Biol. Med. 2022, 188, 337–350. [Google Scholar] [CrossRef]
- Baschant, U.; Rauner, M.; Balaian, E.; Weidner, H.; Roetto, A.; Platzbecker, U.; Hofbauer, L.C. Wnt5a is a key target for the pro-osteogenic effects of iron chelation on osteoblast progenitors. Haematologica 2016, 101, 1499–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Li, X.; Zhu, Z.; Wang, H.; Bai, X. Iron Overload Induces Apoptosis and Cytoprotective Autophagy Regulated by ROS Generation in Mc3t3-E1 Cells. Biol. Trace Elem. Res. 2021, 199, 3781–3792. [Google Scholar] [CrossRef]
- Wu, J.; Wang, A.; Wang, X.; Li, G.; Jia, P.; Shen, G.; Chen, B.; Yuan, Y.; Zhang, H.; Yang, F.; et al. Rapamycin improves bone mass in high-turnover osteoporosis with iron accumulation through positive effects on osteogenesis and angiogenesis. Bone 2019, 121, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takai, C.; Kazama, J.J.; Wakamatsu, A.; Hasegawa, E.; Kobayashi, D.; Kondo, N.; Nakatsue, T.; Abe, A.; Ito, S.; et al. Serum hepcidin level, iron metabolism and osteoporosis in patients with rheumatoid arthritis. Sci. Rep. 2020, 10, 9882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, S.; Wang, L.; Shan, B.C.; Zhang, H.; Yang, F.; Zhou, Z.Q.; Wang, X.; Yuan, Y.; Xu, Y. Hepcidin is an endogenous protective factor for osteoporosis by reducing iron levels. J. Mol. Endocrinol. 2018, 60, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Chen, B.; Yan, Y.; Zhu, G.-X. Hepcidin protects against iron overload-induced inhibition of bone formation in zebrafish. Fish Physiol. Biochem. 2018, 45, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, G.-F.; Shen, Y.; Huang, X.; Xu, Y.-J. Reducing iron accumulation: A potential approach for the prevention and treatment of postmenopausal osteoporosis. Exp. Ther. Med. 2015, 10, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Beibei, F.; Guangsi, S.; Yu, J.; Wen, Z.; Xi, H.; Youjia, X. Iron overload increases osteoclastogenesis and aggravates the effects of ovariectomy on bone mass. J. Endocrinol. 2015, 226, 121–134. [Google Scholar] [CrossRef]
- Jia, P.; Xu, Y.J.; Zhang, Z.L.; Li, K.; Li, B.; Zhang, W.; Yang, H. Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. J. Orthop. Res. 2012, 30, 1843–1852. [Google Scholar] [CrossRef]
- Yang, J.; Dong, D.; Luo, X.; Zhou, J.; Shang, P.; Zhang, H. Iron Overload-Induced Osteocyte Apoptosis Stimulates Osteoclast Differentiation Through Increasing Osteocytic RANKL Production In Vitro. Calcif. Tissue Int. 2020, 107, 499–509. [Google Scholar] [CrossRef]
- Wang, X.; Chen, B.; Sun, J.; Jiang, Y.; Zhang, H.; Zhang, P.; Fei, B.; Xu, Y. Iron-induced oxidative stress stimulates osteoclast differentiation via NF-κB signaling pathway in mouse model. Metabolism 2018, 83, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Lorenz, S.; Dolder, S.; Hofstetter, W. Extracellular Iron is a Modulator of the Differentiation of Osteoclast Lineage Cells. Calcif. Tissue Int. 2016, 98, 275–283. [Google Scholar] [CrossRef]
- Das, B.K.; Wang, L.; Fujiwara, T.; Zhou, J.; Aykin-Burns, N.; Krager, K.J.; Lan, R.; Mackintosh, S.G.; Edmondson, R.; Jennings, M.L.; et al. Transferrin receptor 1-mediated iron uptake regulates bone mass in mice via osteoclast mitochondria and cytoskeleton. eLife 2022, 11, e73539. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, A.; Shen, G.; Wang, X.; Liu, G.; Yang, F.; Chen, B.; Wang, M.; Xu, Y. Hepcidin-induced reduction in iron content and PGC-1β expression negatively regulates osteoclast differentiation to play a protective role in postmenopausal osteoporosis. Aging 2021, 13, 11296–11314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-Y.; Di, D.-H.; Wang, B.; Huang, X.; Xu, Y.-J. Effects of Mouse Hepcidin 1 Treatment on Osteoclast Differentiation and Intracellular Iron Concentration. Inflammation 2015, 38, 718–727. [Google Scholar] [CrossRef]
- Fan, Y.; Ni, S.; Zhang, H. Associations of Copper Intake with Bone Mineral Density and Osteoporosis in Adults: Data from the National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2022, 200, 2062–2068. [Google Scholar] [CrossRef]
- Qu, X.; He, Z.; Qiao, H.; Zhai, Z.; Mao, Z.; Yu, Z.; Dai, K. Serum copper levels are associated with bone mineral density and total fracture. J. Orthop. Transl. 2018, 14, 34–44. [Google Scholar] [CrossRef]
- Chenbhanich, J.; Thongprayoon, C.; Atsawarungruangkit, A.; Phupitakphol, T.; Cheungpasitporn, W. Osteoporosis and bone mineral density in patients with Wilson’s disease: A systematic review and meta-analysis. Osteoporos. Int. 2018, 29, 315–322. [Google Scholar] [CrossRef]
- Bane, T.; Siegel, L.; Bertels, J.; Ratz, K.; Rubessa, M.; Wheeler, M. The effect of copper on the differentiation of adi-pose-derived stem cells into osteoblasts. Reprod. Fertil. Dev. 2019, 31, 229–230. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Ríos, S.; González, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef]
- Vimalraj, S.; Rajalakshmi, S.; Preeth, D.R.; Kumar, S.V.; Deepak, T.; Gopinath, V.; Murugan, K.; Chatterjee, S. Mixed-ligand copper(II) complex of quercetin regulate osteogenesis and angiogenesis. Mater. Sci. Eng. C 2018, 83, 187–194. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, S.; Qi, X.; Chen, X.; Zhang, W.; Yang, K.; Zhong, H. Osteogenesis stimulation by copper-containing 316L stainless steel via activation of akt cell signaling pathway and Runx2 upregulation. J. Mater. Sci. Technol. 2019, 35, 2727–2733. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wang, X.; Wang, Y.; Hang, R.; Huang, X.; Tang, B.; Chu, P.K. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C 2018, 82, 110–120. [Google Scholar] [CrossRef]
- Wang, L.-J.; Ni, X.-H.; Zhang, F.; Peng, Z.; Yu, F.-X.; Zhang, L.-B.; Li, B.; Jiao, Y.; Li, Y.-K.; Yang, B.; et al. Osteoblast Response to Copper-Doped Microporous Coatings on Titanium for Improved Bone Integration. Nanoscale Res. Lett. 2021, 16, 146. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, C.; Wu, M.; Tao, B. Fabrication of copper ions-substituted hydroxyapatite coating on titanium substrates for antibacterial and osteogenic applications. Mater. Lett. 2022, 307, 131072. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Chen, X.; Zhu, Y. The role of TGF-β1/Smad3 signaling pathway and oxidative stress in the inhibition of osteoblast mineralization by copper chloride. Environ. Toxicol. Pharmacol. 2021, 84, 103613. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Chen, X.; Li-Ling, J.; Xie, H.-Q. Inhibition of osteogenic differentiation of mesenchymal stem cells by copper supplementation. Cell Prolif. 2014, 47, 81–90. [Google Scholar] [CrossRef]
- Li, B.-B.; Yu, S.-F. In vitro study of the effects of copper ion on osteoclastic resorption in various dental mineralized tissues. Zhonghua Kou Qiang Yi Xue Za Zhi = Chin. J. Stomatol. 2007, 42, 110–113. [Google Scholar]
- Bernhardt, A.; Schamel, M.; Gbureck, U.; Gelinsky, M. Osteoclastic differentiation and resorption is modulated by bioactive metal ions Co2+, Cu2+ and Cr3+ incorporated into calcium phosphate bone cements. PLoS ONE 2017, 12, e0182109. [Google Scholar] [CrossRef] [Green Version]
- Rico, H.; Roca-Botran, C.; Hernández, E.R.; Seco, C.; Paez, E.; Valencia, M.J.; Villa, L.F. The effect of supplemental copper on osteopenia induced by ovariectomy in rats. Menopause 2000, 7, 413–416. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, X.; Yang, C.; Hosseinkhani, S.; Zhang, C.; Luo, K.; Tang, K.; Yang, K.; Lin, J. Copper modified cobalt-chromium particles for attenuating wear particle induced-inflammation and osteoclastogenesis. Biomater. Adv. 2023, 147, 213315. [Google Scholar] [CrossRef]
- Xu, X.; Zhuo, J.; Xu, Y.; Luo, K.; Chen, C.; Zhong, Q. Copper-doped Titanium Alloy Inhibited RANKL-induced Osteo-clasts Differentiation in Vitro. J. Oral Sci. Res. 2021, 37, 371. [Google Scholar]
- Bernhardt, A.; Bacova, J.; Gbureck, U.; Gelinsky, M. Influence of Cu2+ on Osteoclast Formation and Activity In Vitro. Int. J. Mol. Sci. 2021, 22, 2451. [Google Scholar] [CrossRef]
- Skalny, A.V.; Zaitseva, I.P.; Gluhcheva, Y.G.; Skalny, A.A.; Achkasov, E.E.; Skalnaya, M.G.; Tinkov, A.A. Cobalt in athletes: Hypoxia and doping—New crossroads. J. Appl. Biomed. 2019, 17, 28. [Google Scholar] [CrossRef] [Green Version]
- Aherwar, A.; Singh, A.K.; Patnaik, A. Cobalt Based Alloy: A Better Choice Biomaterial for Hip Implants. Trends Biomater. Artif. Organs 2016, 30, 50–55. [Google Scholar]
- Ignjatovic, N.; Ajduković, Z.; Savić, V.; Najman, S.; Mihailović, D.; Vasiljević, P.; Stojanović, Z.S.; Uskoković, V.; Uskokovic, D. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J. Mater. Sci. Mater. Med. 2013, 24, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Patntirapong, S.; Habibovic, P.; Hauschka, P.V. Effects of soluble cobalt and cobalt incorporated into calcium phosphate layers on osteoclast differentiation and activation. Biomaterials 2009, 30, 548–555. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Zhou, X.; Zhang, L.; Mi, J.; Shan, Z.; Huang, B.; Chen, Z.; Chen, Z. Modulating the cobalt dose range to manipulate multisystem cooperation in bone environment: A strategy to resolve the controversies about cobalt use for orthopedic applications. Theranostics 2020, 10, 1074–1089. [Google Scholar] [CrossRef]
- Pu, Y.; Sun, H.; Liu, J.; Amantai, D.; Yao, W.; Han, X.; He, H. Cobalt Chloride Promotes Osteogenesis of Rat Bone Marrow Mesenchymal Stem Cells In Vitro and In Vivo. Indian J. Pharm Sci. 2023, 85, 1–10. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Y.; Deng, Y. Dual therapeutic cobalt-incorporated bioceramics accelerate bone tissue regeneration. Mater. Sci. Eng. C 2019, 99, 770–782. [Google Scholar] [CrossRef]
- Kim, H.-H.; Lee, S.E.; Chung, W.J.; Choi, Y.; Kwack, K.; Kim, S.W.; Kim, M.S.; Park, H.; Lee, Z.H. Stabilization of hypoxia-inducible factor-1alpha is involved in the hypoxic stimuli-induced expression of vascular endothelial growth factor in osteoblastic cells. Cytokine 2002, 17, 14–27. [Google Scholar] [CrossRef]
- Khosrowshahi, A.K.; Khoshfetrat, A.B.; Khosrowshahi, Y.B.; Maleki-Ghaleh, H. Cobalt content modulates characteris-tics and osteogenic properties of cobalt-containing hydroxyapatite in in-vitro milieu. Mater. Today Commun. 2021, 27, 102392. [Google Scholar] [CrossRef]
- Li, C.-T.; Liu, J.-X.; Yu, B.; Liu, R.; Dong, C.; Li, S.-J. Notch signaling represses hypoxia-inducible factor-1α-induced activation of Wnt/β-catenin signaling in osteoblasts under cobalt-mimicked hypoxia. Mol. Med. Rep. 2016, 14, 689–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osathanon, T.; Vivatbutsiri, P.; Sukarawan, W.; Sriarj, W.; Pavasant, P.; Sooampon, S. Cobalt chloride supplementation induces stem-cell marker expression and inhibits osteoblastic differentiation in human periodontal ligament cells. Arch. Oral Biol. 2015, 60, 29–36. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Q.; Yang, X.; Yu, X.; Yu, D.; Zhao, W. Effects of cobalt chloride on the stem cell marker expression and osteogenic differentiation of stem cells from human exfoliated deciduous teeth. Cell Stress Chaperones 2019, 24, 527–538. [Google Scholar] [CrossRef]
- Drynda, S.; Drynda, A.; Feuerstein, B.; Kekow, J.; Lohmann, C.H.; Bertrand, J. The effects of cobalt and chromium ions on transforming growth factor-beta patterns and mineralization in human osteoblast-like MG63 and SaOs-2 cells. J. Biomed. Mater. Res. Part A 2018, 106, 2105–2115. [Google Scholar] [CrossRef]
- Fleury, C.; Petit, A.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity, and oxidative stress. Biomaterials 2006, 27, 3351–3360. [Google Scholar] [CrossRef]
- Kanaji, A.; Orhue, V.; Caicedo, M.S.; Virdi, A.S.; Sumner, D.R.; Hallab, N.J.; Yoshiaki, T.; Sena, K. Cytotoxic effects of cobalt and nickel ions on osteocytes in vitro. J. Orthop. Surg. Res. 2014, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Drynda, A.; Drynda, S.; Kekow, J.; Lohmann, C.H.; Bertrand, J. Differential Effect of Cobalt and Chromium Ions as Well as CoCr Particles on the Expression of Osteogenic Markers and Osteoblast Function. Int. J. Mol. Sci. 2018, 19, 3034. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, E.M.; Floyd, H.; Addison, O.; Zhang, Z.J.; Oppenheimer, P.G.; Grover, L.M. Influence of Cobalt Ions on Collagen Gel Formation and Their Interaction with Osteoblasts. ACS Omega 2018, 3, 10129–10138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuen, J.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated β-tricalcium phosphate. Biomaterials 2015, 61, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Queally, J.; Devitt, B.; Butler, J.; Malizia, A.; Murray, D.; Doran, P.; O’Byrne, J. Cobalt ions induce chemokine secretion in primary human osteoblasts. J. Orthop. Res. 2009, 27, 855–864. [Google Scholar] [CrossRef]
- Anissian, L.; Stark, A.; Dahlstrand, H.; Granberg, B.; Good, V.; Bucht, E. Cobalt ions influence proliferation and function of human osteoblast-like cells. Acta Orthop. 2002, 73, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Jonitz-Heincke, A.; Sellin, M.-L.; Seyfarth, A.; Peters, K.; Mueller-Hilke, B.; Fiedler, T.; Bader, R.; Klinder, A. Analysis of Cellular Activity and Induction of Inflammation in Response to Short-Term Exposure to Cobalt and Chromium Ions in Mature Human Osteoblasts. Materials 2019, 12, 2771. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, W.P.; Bulstra, S.K.; van Raay, J.J.; van Leeuwen, B.M.; Kuijer, R. Cobalt and chromium ions reduce human osteoblast-like cell activity in vitro, reduce the OPG to RANKL ratio, and induce oxidative stress. J. Orthop. Res. 2012, 30, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Andrews, R.E.; Shah, K.M.; Wilkinson, J.M.; Gartland, A. Effects of cobalt and chromium ions at clinically equivalent concentrations after metal-on-metal hip replacement on human osteoblasts and osteoclasts: Implications for skeletal health. Bone 2011, 49, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-K.; Ye, J.; Han, Q.-L.; Tao, R.; Liu, F.; Wang, W. Toxicity and Bioactivity of Cobalt Nanoparticles on the Monocytes. Orthop. Surg. 2015, 7, 168–173. [Google Scholar] [CrossRef]
- Yashima, Y.; Okamoto, K.; Sakai, E.; Iwatake, M.; Fukuma, Y.; Nishishita, K.; Tsukuba, T. Cobalt protoporphyrin represses osteoclastogenesis through blocking multiple signaling pathways. Biometals 2015, 28, 725–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaseth, J.; Shimshi, M.; Gabrilove, J.L.; Birketvedt, G.S. Fluoride: A toxic or therapeutic agent in the treatment of osteo-porosis? J. Trace Elem. Exp. Med. 2004, 17, 83–92. [Google Scholar] [CrossRef]
- Vestergaard, P.; Jorgensen, N.R.; Schwarz, P.; Mosekilde, L. Effects of treatment with fluoride on bone mineral density and fracture risk—A meta-analysis. Osteoporos. Int. 2008, 19, 257–268. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Hung, M.-C.; Chang, S.-F.; Tsuang, F.-Y.; Chang, J.Z.-C.; Sun, J.-S. Efficacy and Safety of Postmenopausal Osteoporosis Treatments: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 3043. [Google Scholar] [CrossRef]
- Sowers, M.; Whitford, G.M.; Clark, M.K.; Jannausch, M.L. Elevated Serum Fluoride Concentrations in Women Are Not Related to Fractures and Bone Mineral Density. J. Nutr. 2005, 135, 2247–2252. [Google Scholar] [CrossRef] [Green Version]
- Gazzano, E.; Bergandi, L.; Riganti, C.; Aldieri, E.; Doublier, S.; Costamagna, C.; Bosia, A.; Ghigo, D. Fluoride Effects: The Two Faces of Janus. Curr. Med. Chem. 2010, 17, 2431–2441. [Google Scholar] [CrossRef]
- Lee, N.; Kang, S.; Lee, W.; Hwang, S.-S. The Association between Community Water Fluoridation and Bone Diseases: A Natural Experiment in Cheongju, Korea. Int. J. Environ. Res. Public Health 2020, 17, 9170. [Google Scholar] [CrossRef]
- Yin, X.-H.; Huang, G.-L.; Lin, D.-R.; Wan, C.-C.; Wang, Y.-D.; Song, J.-K.; Xu, P. Exposure to Fluoride in Drinking Water and Hip Fracture Risk: A Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0126488. [Google Scholar] [CrossRef]
- Levy, S.; Warren, J.; Phipps, K.; Letuchy, E.; Broffitt, B.; Eichenberger-Gilmore, J.; Burns, T.; Kavand, G.; Janz, K.; Torner, J.; et al. Effects of Life-long Fluoride Intake on Bone Measures of Adolescents: A prospective cohort study. J. Dent. Res. 2014, 93, 353–359. [Google Scholar] [CrossRef]
- Helte, E.; Vargas, C.D.; Kippler, M.; Wolk, A.; Michaëlsson, K.; Åkesson, A. Fluoride in Drinking Water, Diet, and Urine in Relation to Bone Mineral Density and Fracture Incidence in Postmenopausal Women. Environ. Health Perspect. 2021, 129, 47005. [Google Scholar] [CrossRef]
- Näsman, P.; Ekstrand, J.; Granath, F.; Ekbom, A.; Fored, C. Estimated Drinking Water Fluoride Exposure and Risk of Hip Fracture: A cohort study. J. Dent. Res. 2013, 92, 1029–1034. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, P.K.; Sood, S.; Singh, R.; Gupta, A.; Rastogi, A. Distribution of Fluoride in Plasma, Brain, and Bones and Associated Oxidative Damage After Induced Chronic Fluorosis in Wistar Rats. Biol. Trace Elem. Res. 2021, 200, 1710–1721. [Google Scholar] [CrossRef]
- Rezaee, T.; Bouxsein, M.L.; Karim, L. Increasing fluoride content deteriorates rat bone mechanical properties. Bone 2020, 136, 115369. [Google Scholar] [CrossRef]
- Qi, X.L. Effect of Fluoride on Signal Transduction Pathways. In Coal-Burning Type of Endemic Fluorosis: Pathophysiology and Clinical Treatments; Springer: Berlin/Heidelberg, Germany, 2021; pp. 225–249. [Google Scholar]
- Wang, J.; Yang, J.; Cheng, X.; Yin, F.; Zhao, Y.; Zhu, Y.; Yan, Z.; Khodaei, F.; Ommati, M.M.; Manthari, R.K.; et al. Influence of Calcium Supplementation against Fluoride-Mediated Osteoblast Impairment in Vitro: Involvement of the Canonical Wnt/β-Catenin Signaling Pathway. J. Agric. Food Chem. 2019, 67, 10285–10295. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, Y.; Yang, Y.; Liu, Y.; Guo, N.; Wang, L.; Huang, W.; Wu, L.; Sun, D.; Gu, W. β-catenin mediates fluoride-induced aberrant osteoblasts activity and osteogenesis. Environ. Pollut. 2020, 265 Pt A, 114734. [Google Scholar] [CrossRef]
- Pan, L.; Shi, X.; Liu, S.; Guo, X.; Zhao, M.; Cai, R.; Sun, G. Fluoride promotes osteoblastic differentiation through canonical Wnt/β-catenin signaling pathway. Toxicol. Lett. 2014, 225, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-Y.; Ren, L.-Q.; Li, X.-N.; Wu, N.; Li, G.-S.; Liu, Q.-Y.; Xu, H. Effect of Fluoride on Insulin Level of Rats and Insulin Receptor Expression in the MC3T3-E1 Cells. Biol. Trace Elem. Res. 2012, 150, 297–305. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, H.; Yu, X.; Wang, Y.; Yang, C.; Xu, H. Analysis of the Role of Insulin Signaling in Bone Turnover Induced by Fluoride. Biol. Trace Elem. Res. 2016, 171, 380–390. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Gao, Y.; Yuan, M.; Manthari, R.K.; Wang, J.; Wang, J. TGF-β1 acts as mediator in fluoride-induced autophagy in the mouse osteoblast cells. Food Chem. Toxicol. 2018, 115, 26–33. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Y.; Zhao, Y.; Manthari, R.K.; Wang, J. The Effects of Fluoride on the Gap-Junctional Intercellular Communication of Rats’ Osteoblast. Biol. Trace Elem. Res. 2020, 193, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Fookes, F.A.; Mengatto, L.N.; Rigalli, A.; Luna, J.A. Controlled fluoride release for osteoporosis treatment using orally administered chitosan hydrogels. J. Drug Deliv. Sci. Technol. 2019, 51, 268–275. [Google Scholar] [CrossRef]
- Zhu, Q.X.; Nie, Q.Y.; Liu, L.; Xu, Y.L.; Liu, J.X. Synthesis of fluoride-releasing strontium-substituted porous apatite mi-crospheres for bone osteoporosis treatment. Ceramics Int. 2023, 49, 14666–14672. [Google Scholar] [CrossRef]
- Gentleman, E.; Stevens, M.; Hill, R.; Brauer, D. Surface properties and ion release from fluoride-containing bioactive glasses promote osteoblast differentiation and mineralization in vitro. Acta Biomater. 2013, 9, 5771–5779. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Rawlinson, S.C.; Hill, R.G.; Fortune, F. Fluoride incorporation in high phosphate containing bioactive glasses and in vitro osteogenic, angiogenic and antibacterial effects. Dent. Mater. 2016, 32, e221–e237. [Google Scholar] [CrossRef]
- Lu, P.; Li, X.; Ruan, L.; Xu, H.; Liu, Q. Effect of siRNA PERK on Fluoride-Induced Osteoblastic Differentiation in OS732 Cells. Biol. Trace Elem. Res. 2014, 159, 434–439. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Shi, H.-Y.; Li, X.-N.; Lv, P.; Li, G.-S.; Liu, Q.-Y.; Xu, H. Role of Endoplasmic Reticulum Stress in Aberrant Activation of Fluoride-Treated Osteoblasts. Biol. Trace Elem. Res. 2013, 154, 448–456. [Google Scholar] [CrossRef]
- Li, X.-N.; Lv, P.; Sun, Z.; Li, G.-S.; Xu, H. Role of Unfolded Protein Response in Affecting Osteoblast Differentiation Induced by Fluoride. Biol. Trace Elem. Res. 2014, 158, 113–121. [Google Scholar] [CrossRef]
- Li, X.; Meng, L.; Wang, F.; Hu, X.; Yu, Y. Sodium fluoride induces apoptosis and autophagy via the endoplasmic reticulum stress pathway in MC3T3-E1 osteoblastic cells. Mol. Cell. Biochem. 2019, 454, 77–85. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Gu, H.; Zhang, K.; Ma, L. Fluorosis Induces Endoplasmic Reticulum Stress and Apoptosis in Osteoblasts In Vivo. Biol. Trace Elem. Res. 2015, 164, 64–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, F.; Wang, Z.; Xu, B.; Zhang, T.; Wang, Q.; Lin, Q. Fluoride Exposure Provokes Mitochondria-Mediated Apoptosis and Increases Mitophagy in Osteocytes via Increasing ROS Production. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bian, S.; Wang, J.; Wang, J. Effects of fluoride and chitosan on the gene expressions of bone morphogenic protein 2 and collagen type-1 alpha 1 chain in the mouse femur. Fluoride 2016, 49, 47. [Google Scholar]
- Gu, X.; Wang, Z.; Gao, J.; Han, D.; Zhang, L.; Chen, P.; Luo, G.; Han, B. SIRT1 suppresses p53-dependent apoptosis by modulation of p21 in osteoblast-like MC3T3-E1 cells exposed to fluoride. Toxicol. Vitr. 2019, 57, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Han, D.; Chen, W.; Zhang, L.; Lin, Q.; Gao, J.; Fanning, S.; Han, B. SIRT1-mediated FoxOs pathways protect against apoptosis by promoting autophagy in osteoblast-like MC3T3-E1 cells exposed to sodium fluoride. Oncotarget 2016, 7, 65218–65230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.-Q.; Yu, Y.-J.; Xu, L.-N.; Ming, P.-P.; Shao, S.-Y.; Qiu, J. Regulation of osteoblast behaviors via cross-talk between Hippo/YAP and MAPK signaling pathway under fluoride exposure. J. Mol. Med. 2019, 97, 1003–1017. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Heuvel, E.G.H.M.v.D.; Castelein, S.; Buisman, J.K.; Bronckers, A.L.J.J.; Bakker, A.D.; Klein-Nulend, J. Fluoride inhibits the response of bone cells to mechanical loading. Odontology 2011, 99, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Duan, X.-Q.; Zhao, Z.-T.; Zhang, X.-Y.; Wang, H.; Liu, D.-W.; Li, G.-S.; Jing, L. Fluoride Affects Calcium Homeostasis by Regulating Parathyroid Hormone, PTH-Related Peptide, and Calcium-Sensing Receptor Expression. Biol. Trace Elem. Res. 2015, 165, 159–166. [Google Scholar] [CrossRef]
- Duan, X.-Q.; Zhao, Z.-T.; Zhang, X.-Y.; Wang, Y.; Wang, H.; Liu, D.-W.; Li, G.-S.; Jing, L. Fluoride Affects Calcium Homeostasis and Osteogenic Transcription Factor Expressions Through L-type Calcium Channels in Osteoblast Cell Line. Biol. Trace Elem. Res. 2014, 162, 219–226. [Google Scholar] [CrossRef]
- Wu, S.; Yan, W.; Qiu, B.; Liao, Y.; Gu, J.; Wei, S.; Zhang, A.; Pan, X. Aberrant methylation-induced dysfunction of p16 is associated with osteoblast activation caused by fluoride. Environ. Toxicol. 2019, 34, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Ming, J.; Wu, S.; You, T.; Wang, X.; Yu, C.; Luo, P.; Zhang, A.; Pan, X. Histone Deacetylation in the Promoter of p16 Is Involved in Fluoride-Induced Human Osteoblast Activation via the Inhibition of Sp1 Binding. Biol. Trace Elem. Res. 2019, 188, 373–383. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Xu, K.; Zhang, L.; Zhang, Y.; He, T.; Sun, R.; Huang, H.; Zhu, J.; Zhang, Y.; et al. Association between low-to-moderate fluoride exposure and bone mineral density in Chinese adults: Non-negligible role of RUNX2 promoter methylation. Ecotoxicol. Environ. Saf. 2020, 203, 111031. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, G.; Liu, L.; Ren, L.; Xi, Y.; Zhu, J.; Huang, H.; Li, Z.; Li, Y.; Cheng, X.; et al. Fluoride exposure and CALCA methylation is associated with the bone mineral density of Chinese women. Chemosphere 2020, 253, 126616. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, N.; Ding, Y.; Zhang, M.-L.; Li, M.; Zhong, J.-J.; Feng, S.-M. Effects of fluoride on the proliferation and activation of osteoblasts by regulating methylation of the DNA repair genes MGMT and MLH1. Regen. Ther. 2022, 19, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, Y.; Zhong, N.; Angwa, L.M.; Pei, J. The dose-time effects of fluoride on the expression and DNA methylation level of the promoter region of BMP-2 and BMP-7 in rats. Environ. Toxicol. Pharmacol. 2020, 75, 103331. [Google Scholar] [CrossRef] [PubMed]
- Daiwile, A.; Tarale, P.; Sivanesan, S.; Naoghare, P.K.; Bafana, A.; Parmar, D.; Kannan, K. Role of fluoride induced epigenetic alterations in the development of skeletal fluorosis. Ecotoxicol. Environ. Saf. 2018, 169, 410–417. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhao, Z.; Xu, H. Preliminary Analysis of MicroRNAs Expression Profiling in MC3T3-E1 Cells Exposed to Fluoride. Biol. Trace Elem. Res. 2017, 176, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Qin, Y.; Luo, K.; Han, X.; Yu, C.; Zhang, A.; Pan, X. miR -486-3p regulates CyclinD1 and promotes fluoride-induced osteoblast proliferation and activation. Environ. Toxicol. 2021, 36, 1817–1828. [Google Scholar] [CrossRef]
- Jiang, N.; Xu, W.; Zhang, Z.; Jin, H.; Yang, Y.; Zhang, J.; Xu, H. Role of TGF-β1 in Fluoride-Treated Osteoblasts at Different Stages. Biol. Trace Elem. Res. 2021, 200, 740–748. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, N.; Yu, H.; Yu, X.; Guo, F.; Zhao, Z.; Xu, H. Requirement of TGFβ Signaling for Effect of Fluoride on Osteoblastic Differentiation. Biol. Trace Elem. Res. 2019, 187, 492–498. [Google Scholar] [CrossRef]
- Luo, K.; Qin, Y.; Ouyang, T.; Wang, X.; Zhang, A.; Luo, P.; Pan, X. let-7c-5p regulates CyclinD1 in fluoride-mediated osteoblast proliferation and activation. Toxicol. Sci. 2021, 182, 275–287. [Google Scholar] [CrossRef]
- Guo, N.; Yu, Y.; Chu, Y.; Lou, Q.; Huang, W.; Wu, L.; Fan, C.; Su, M.; Zhang, M.; Yin, F.; et al. miR-21-5p and canonical Wnt signaling pathway promote osteoblast function through a feed-forward loop induced by fluoride. Toxicology 2021, 466, 153079. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, Y.; Wang, H.; Darko, G.M.; Sun, D.; Gao, Y. Identification of miR-200c-3p as a major regulator of SaoS2 cells activation induced by fluoride. Chemosphere 2018, 199, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Bondu, J.D.; Seshadri, M.S.; Selvakumar, R.; Fleming, J.J. Effects of Fluoride on Bone in an Animal Model of Vitamin D Deficiency. Indian J. Clin. Biochem. 2017, 34, 60–67. [Google Scholar] [CrossRef]
- Yu, J.; Gao, Y.; Sun, D. Effect of Fluoride and Low versus High Levels of Dietary Calcium on mRNA Expression of Osteoprotegerin and Osteoprotegerin Ligand in the Bone of Rats. Biol. Trace Elem. Res. 2013, 152, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Dede, S.; Taşpinar, M.; Yüksek, V.; Çetin, S.; Usta, A. The Effects of Vitamin D Application on NaF-Induced Cytotoxicity in Osteoblast Cells (hFOB 1.19). Biol. Trace Elem. Res. 2023, 201, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Li, B.; Gao, Y.; Wei, Y.; Zhou, L.; Yao, H.; Wang, J.; Sun, D. Fluoride decreased osteoclastic bone resorption through the inhibition of NFATc1 gene expression. Environ. Toxicol. 2014, 29, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ma, Y.; Zhong, N.; Pei, J. The Inverted U-Curve Association of Fluoride and Osteoclast Formation in Mice. Biol. Trace Elem. Res. 2019, 191, 419–425. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, N.; Yu, X.; Zhao, Z.; Zhang, X.; Xu, H. The role of TGFβ receptor 1-smad3 signaling in regulating the osteoclastic mode affected by fluoride. Toxicology 2018, 393, 73–82. [Google Scholar] [CrossRef]
- Oka, S.; Li, X.; Taguchi, C.; Wang, C.; Tewari, N.; Arikawa, K.; Liu, Y.; Bhawal, U.K. Treatment with 50 μM Sodi-um Fluoride Suppresses Aging-Induced Alveolar Bone Resorption in Mice. J. Hard Tissue Biol. 2021, 30, 225–230. [Google Scholar] [CrossRef]
- Bhawal, U.K.; Lee, H.-J.; Arikawa, K.; Shimosaka, M.; Suzuki, M.; Toyama, T.; Sato, T.; Kawamata, R.; Taguchi, C.; Hamada, N.; et al. Micromolar sodium fluoride mediates anti-osteoclastogenesis in Porphyromonas gingivalis-induced alveolar bone loss. Int. J. Oral Sci. 2015, 7, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Junrui, P.; Bingyun, L.; Yanhui, G.; Xu, J.; Darko, G.M.; Dianjun, S. Relationship between fluoride exposure and osteoclast markers during RANKL-induced osteoclast differentiation. Environ. Toxicol. Pharmacol. 2016, 46, 241–245. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, F.; Xu, W.; Zhang, Z.; Jin, H.; Shi, L.; Zhang, X.; Gao, J.; Xu, H. Effect of fluoride on osteocyte-driven osteoclastic differentiation. Toxicology 2020, 436, 152429. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-G.; Kang, L.; Wu, G. Fluorosis increases the risk of postmenopausal osteoporosis by stimulating interferon γ. Biochem. Biophys. Res. Commun. 2016, 479, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.; Yazdi, A.R.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Oden, A.; McCloskey, E.V. A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX®. Osteoporos. Int. 2011, 22, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Koukou, O.I.; Pappas, L.D.; Chloropoulou, P.; Kouroupi, M.A.; Koukos, K.I.; Karpathiou, G.; Galanos, A.A.; Drosos, G.I.; Magnisalis, E.; Giatromanolaki, A.N.; et al. The Effect of Strontium Ranelate on Fracture Healing: An Animal Study. BioMed Res. Int. 2020, 2020, 1085324. [Google Scholar] [CrossRef]
- Scaglione, M.; Fabbri, L.; Casella, F.; Guido, G. Strontium ranelate as an adjuvant for fracture healing: Clinical, radiological, and ultrasound findings in a randomized controlled study on wrist fractures. Osteoporos. Int. 2016, 27, 211–218. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Kaufman, J.-M.; Goemaere, S.; Devogelaer, J.P.; Benhamou, C.L.; Felsenberg, D.; Diaz-Curiel, M.; Brandi, M.-L.; Badurski, J.; Wark, J.; et al. Maintenance of antifracture efficacy over 10 years with strontium ranelate in postmenopausal osteoporosis. Osteoporos. Int. 2012, 23, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Morabito, N.; Catalano, A.; Gaudio, A.; Morini, E.; Bruno, L.M.; Basile, G.; Tsiantouli, E.; Bellone, F.; Agostino, R.M.; Piraino, B.; et al. Effects of strontium ranelate on bone mass and bone turnover in women with thalassemia major-related osteoporosis. J. Bone Miner. Metab. 2016, 34, 540–546. [Google Scholar] [CrossRef]
- Ali, M.; Berencsi, K.; Marinier, K.; Deltour, N.; Perez-Guthann, S.; Pedersen, L.; Rijnbeek, P.; Lapi, F.; Simonetti, M.; Reyes, C.; et al. Comparative cardiovascular safety of strontium ranelate and bisphosphonates: A multi-database study in 5 EU countries by the EU-ADR Alliance. Osteoporos. Int. 2020, 31, 2425–2438. [Google Scholar] [CrossRef]
- Lu, W.; Zhou, Y.; Yang, H.; Cheng, Z.; He, F. Efficacy of strontium supplementation on implant osseointegration under osteoporotic conditions: A systematic review. J. Prosthet. Dent. 2022, 128, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, Y.; Gu, Y.; Qiao, S.; Zhang, X.; Lai, H. Effect of titanium implants with strontium incorporation on bone apposition in animal models: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 15563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bizelli-Silveira, C.; Abildtrup, L.A.; Spin-Neto, R.; Foss, M.; Søballe, K.; Kraft, D.C.E. Strontium enhances proliferation and osteogenic behavior of bone marrow stromal cells of mesenchymal and ectomesenchymal origins in vitro. Clin. Exp. Dent. Res. 2019, 5, 541–550. [Google Scholar] [CrossRef]
- Nardone, V.; Zonefrati, R.; Mavilia, C.; Romagnoli, C.; Ciuffi, S.; Fabbri, S.; Palmini, G.; Galli, G.; Tanini, A.; Brandi, M.L. In Vitro Effects of Strontium on Proliferation and Osteoinduction of Human Preadipocytes. Stem Cells Int. 2015, 2015, 871863. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yue, J.; Liu, Y.; Wu, J.; Guan, M.; Chen, D.; Pan, H.; Zhao, X.; Lu, W.W. Strontium regulates stem cell fate during osteogenic differentiation through asymmetric cell division. Acta Biomater. 2021, 119, 432–443. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhu, S.; Luo, E.; Feng, G.; Chen, Q.; Hu, J. Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 418, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.M.; Nani, E.P.; Teixeira, L.N.; Peruzzo, D.C.; Joly, J.C.; Napimoga, M.H.; Martinez, E.F. Strontium ranelate increases osteoblast activity. Tissue Cell 2016, 48, 183–188. [Google Scholar] [CrossRef]
- Takaoka, S.; Yamaguchi, T.; Yano, S.; Yamauchi, M.; Sugimoto, T. The Calcium-sensing Receptor (CaR) is Involved in Strontium Ranelate-induced Osteoblast Differentiation and Mineralization. Horm. Metab. Res. 2010, 42, 627–631. [Google Scholar] [CrossRef]
- Ren, W.H.; Xin, S.; Yang, K.; Yu, Y.B.; Li, S.M.; Zheng, J.J.; Huang, K.; Zeng, R.C.; Yang, X.X.; Gao, L.; et al. Strontium-Doped Hydrox-yapatite Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in Osteoporotic Rats through the CaSR-JAK2/STAT3 Signaling Pathway. Adv. Nanobiomed Res. 2022, 2, 2200018. [Google Scholar] [CrossRef]
- Rybchyn, M.S.; Slater, M.; Conigrave, A.D.; Mason, R.S. An Akt-dependent Increase in Canonical Wnt Signaling and a Decrease in Sclerostin Protein Levels Are Involved in Strontium Ranelate-induced Osteogenic Effects in Human Osteoblasts. J. Biol. Chem. 2011, 286, 23771–23779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Yu, Z.; Chang, H.; Wang, Y.; Xiang, H.; Zhang, X.; Yu, B. Strontium-containing α-calcium sulfate hemihydrate promotes bone repair via the TGF-β/Smad signaling pathway. Mol. Med. Rep. 2019, 20, 3555–3564. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Yan, C.; Zhou, L.; Fan, X. Changes in BMP-2 expression and mechanical properties during treatment of rats with osteoporotic hindlimb fracture with strontium ranelate. J. Musculoskelet. Neuronal Interact. 2020, 20, 136–141. [Google Scholar]
- Cheng, Y.; Huang, L.; Wang, Y.; Huo, Q.; Shao, Y.; Bao, H.; Li, Z.; Liu, Y.; Li, X. Strontium promotes osteogenic differentiation by activating autophagy via the the AMPK/mTOR signaling pathway in MC3T3-E1 cells. Int. J. Mol. Med. 2019, 44, 652–660. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cui, J.; Cheng, L.; Lin, K. Enhancement of osteoporotic bone regeneration by strontium-substituted 45S5 bioglass via time-dependent modulation of autophagy and the Akt/mTOR signaling pathway. J. Mater. Chem. B 2021, 9, 3489–3501. [Google Scholar] [CrossRef]
- Kruppke, B.; Heinemann, C.; Wagner, A.-S.; Farack, J.; Wenisch, S.; Wiesmann, H.-P.; Hanke, T. Strontium ions promote in vitro human bone marrow stromal cell proliferation and differentiation in calcium-lacking media. Dev. Growth Differ. 2019, 61, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Long, Q.; Miron, R.J.; Yin, C.; Wei, Y.; Zhang, Y.; Wu, M. Setd2 is associated with strontium-induced bone regeneration. Acta Biomater. 2017, 53, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chen, X.; Xiong, Y.; Chen, J.; Li, J.; Li, D.; Zhou, G.; Zou, Y.; Liu, T. Strontium gluconate potently promotes osteoblast development and restores bone formation in glucocorticoid-induced osteoporosis rats. Biochem. Biophys. Res. Commun. 2021, 554, 33–40. [Google Scholar] [CrossRef]
- Aimaiti, A.; Wahafu, T.; Keremu, A.; Yicheng, L.; Li, C. Strontium Ameliorates Glucocorticoid Inhibition of Osteogenesis Via the ERK Signaling Pathway. Biol. Trace Elem. Res. 2020, 197, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, G.; Luk, K.D.K.; Cheung, K.; Li, Z.; Lam, W.M.; Zhou, Z.; Lu, W.W. Strontium Promotes Osteogenic Differentiation of Mesenchymal Stem Cells Through the Ras/MAPK Signaling Pathway. Cell. Physiol. Biochem. 2009, 23, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-H.; Kang, M.S.; Kim, T.-H.; Yoon, D.S.; Mandakhbayar, N.; Bin Jo, S.; Kim, H.S.; Knowles, J.C.; Lee, J.-H.; Kim, H.-W. Dual actions of osteoclastic-inhibition and osteogenic-stimulation through strontium-releasing bioactive nanoscale cement imply biomaterial-enabled osteoporosis therapy. Biomaterials 2021, 276, 121025. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, R.; Xing, Z.; Shang, T.; Yang, X.; Zhu, X.; Zhang, X. Strontium combined with bioceramics for osteoporot-ic bone repair: Oral intake or as a dopant? Appl. Mater. Today 2021, 22, 100927. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Tsigkou, O.; Li, S.; Porter, A.E.; Jones, J.R. Human mesenchymal stem cells differentiate into an osteogenic lineage in presence of strontium containing bioactive glass nanoparticles. Acta Biomater. 2019, 90, 373–392. [Google Scholar] [CrossRef]
- Verberckmoes, S.C.; De Broe, M.E.; D’Haese, P.C. Dose-dependent effects of strontium on osteoblast function and mineralization. Kidney Int. 2003, 64, 534–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimaiti, A.; Maimaitiyiming, A.; Boyong, X.; Aji, K.; Li, C.; Cui, L. Low-dose strontium stimulates osteogenesis but high-dose doses cause apoptosis in human adipose-derived stem cells via regulation of the ERK1/2 signaling pathway. Stem Cell Res. Ther. 2017, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-L.; Zaidi, S.; Peng, Y.; Zhou, H.; Moonga, B.S.; Blesius, A.; Dupin-Roger, I.; Zaidi, M.; Sun, L. Induction of a program gene expression during osteoblast differentiation with strontium ranelate. Biochem. Biophys. Res. Commun. 2007, 355, 307–311. [Google Scholar] [CrossRef]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, X.S.; Huang, S.; Li, Z.; Pan, H.; Zhen, W.; Luk, K.D.; Guo, X.E.; Lu, W.W. The cross-talk between osteoclasts and osteoblasts in response to strontium treatment: Involvement of osteoprotegerin. Bone 2011, 49, 1290–1298. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, X.; Tao, Y.; Ping, Z.; Wang, L.; Shi, J.; Wu, X.; Zhang, W.; Yang, H.; Nie, Z.; et al. Strontium inhibits titanium particle-induced osteoclast activation and chronic inflammation via suppression of NF-κB pathway. Sci. Rep. 2016, 6, 36251. [Google Scholar] [CrossRef] [PubMed]
- Caudrillier, A.; Hurtel-Lemaire, A.-S.; Wattel, A.; Cournarie, F.; Godin, C.; Petit, L.; Petit, J.-P.; Terwilliger, E.; Kamel, S.; Brown, E.M.; et al. Strontium Ranelate Decreases Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclastic Differentiation In Vitro: Involvement of the Calcium-Sensing Receptor. Mol. Pharmacol. 2010, 78, 569–576. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.; Zhong, X.; Cai, Z.; Ning, Z.; Hou, T.; Xiong, L.; Feng, Y.; Leung, F.; Lu, W.W.; et al. Strontium inhibits osteoclastogenesis by enhancing LRP6 and β-catenin-mediated OPG targeted by miR-181d-5p. J. Cell Commun. Signal. 2019, 13, 85–97. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on the possible nutritional importance of silicon. J. Trace Elem. Med. Biol. 2014, 28, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Bonazza, V.; Labanca, M.; Lonati, C.; Rezzani, R. A review of the effects of dietary silicon intake on bone homeostasis and regeneration. J. Nutr. Health Aging 2014, 18, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R.; Tucker, K.L.; Qiao, N.; Cupples, L.A.; Kiel, D.P.; Powell, J.J. Dietary Silicon Intake Is Positively Associated With Bone Mineral Density in Men and Premenopausal Women of the Framingham Offspring Cohort. J. Bone Miner. Res. 2003, 19, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, H.M.; Hardcastle, A.C.; Jugdaohsingh, R.; Fraser, W.D.; Reid, D.M.; Powell, J.J. Dietary silicon interacts with oestrogen to influence bone health: Evidence from the Aberdeen Prospective Osteoporosis Screening Study. Bone 2012, 50, 681–687. [Google Scholar] [CrossRef]
- Vigna, L.; De Liso, F.; Tomaino, L.; Cighetti, G.; Paroni, R.; Gestro, M.; Ingenito, M.R.; Napolitano, F.; Bamonti, F. Osteoporosis pre-vention in postmenopausal female workers: Beneficial effects of silicon dietary supplementation on oxidative status. A pilot study. Prog. Nutr. 2019, 21, 1052–1062. [Google Scholar]
- Yıldızgören, M.T.; Öziş, T.N.; Baki, A.E.; Tutkun, E.; Yılmaz, H.; Tiftik, T.; Ekiz, T.; Özgirgin, N. Evaluation of bone mineral density and 25-hydroxyvitamin D levels in subjects with silica exposure. Environ. Health Prev. Med. 2016, 21, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Hui, Z.; Dingjie, X.; Yuan, Y.; Zhongqiu, W.; Na, M.; Mingjian, B.; Yu, G.; Guangyuan, L.; Xu, H.; Shifeng, L.; et al. Silicosis decreases bone mineral density in rats. Toxicol. Appl. Pharmacol. 2018, 348, 117–122. [Google Scholar] [CrossRef]
- Li, Z.; Karp, H.; Zerlin, A.; Lee, T.Y.A.; Carpenter, C.; Heber, D. Absorption of silicon from artesian aquifer water and its impact on bone health in postmenopausal women: A 12 week pilot study. Nutr. J. 2010, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jugdaohsingh, R.; Watson, A.I.E.; Bhattacharya, P.; van Lenthe, H.; Powell, J.J. Positive association between serum silicon levels and bone mineral density in female rats following oral silicon supplementation with monomethylsilanetriol. Osteoporos. Int. 2015, 26, 1405–1415. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Bae, Y.-J.; Choi, M.-K.; Chung, Y.-S. Silicon Supplementation Improves the Bone Mineral Density of Calcium-Deficient Ovariectomized Rats by Reducing Bone Resorption. Biol. Trace Elem. Res. 2009, 128, 239–247. [Google Scholar] [CrossRef]
- Bae, Y.-J.; Kim, J.-Y.; Choi, M.-K.; Chung, Y.-S.; Kim, M.-H. Short-term Administration of Water-soluble Silicon Improves Mineral Density of the Femur and Tibia in Ovariectomized Rats. Biol. Trace Elem. Res. 2008, 124, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Ha, S.W.; Vikulina, T.; Roser-Page, S.; Lee, J.K.; Beck, G.R., Jr. Bioactive silica nanoparticles reverse age-associated bone loss in mice. Nanomedicine 2015, 11, 959–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Chen, S.; Shi, X.; Osaka, A.; Gao, H.; Hanagata, N. Facile synthesis, microstructure and BMP-2 delivery of novel silica hollow flowers for enhanced osteoblast differentiation. Chem. Eng. J. 2014, 246, 1–9. [Google Scholar] [CrossRef]
- Dong, M.; Jiao, G.; Liu, H.; Wu, W.; Li, S.; Wang, Q.; Xu, D.; Li, X.; Liu, H.; Chen, Y. Biological Silicon Stimulates Collagen Type 1 and Osteocalcin Synthesis in Human Osteoblast-Like Cells Through the BMP-2/Smad/RUNX2 Signaling Pathway. Biol. Trace Elem. Res. 2016, 173, 306–315. [Google Scholar] [CrossRef]
- Kim, E.-J.; Bu, S.Y.; Sung, M.-K.; Choi, M.-K. Effects of Silicon on Osteoblast Activity and Bone Mineralization of MC3T3-E1 Cells. Biol. Trace Elem. Res. 2013, 152, 105–112. [Google Scholar] [CrossRef]
- Uribe, P.; Johansson, A.; Jugdaohsingh, R.; Powell, J.J.; Magnusson, C.; Davila, M.; Westerlund, A.; Ransjö, M. Soluble silica stimulates osteogenic differentiation and gap junction communication in human dental follicle cells. Sci. Rep. 2020, 10, 9923. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, H.; Qiao, Y.; Zhang, Z.; Sun, J. Enhanced Performance of Osteoblasts by Silicon Incorporated Porous TiO2 Coating. J. Mater. Sci. Technol. 2012, 28, 109–117. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Zhou, H.; Jiao, G.; Dong, M.; Chi, H.; Wang, H.; Wu, W.; Liu, H.; Ren, S.; Kong, M.; Li, C.; et al. Orthosilicic Acid Accelerates Bone Formation in Human Osteoblast-Like Cells Through the PI3K–Akt–mTOR Pathway. Biol. Trace Elem. Res. 2019, 190, 327–335. [Google Scholar] [CrossRef]
- Gu, G.; Hou, D.; Jiao, G.; Wu, W.; Zhou, H.; Wang, H.; Chen, Y. Ortho-silicic Acid Plays a Protective Role in Glucocorticoid-Induced Osteoporosis via the Akt/Bad Signal Pathway In Vitro and In Vivo. Biol. Trace Elem. Res. 2023, 201, 843–855. [Google Scholar] [CrossRef]
- Jiao, K.; Niu, L.-N.; Li, Q.-H.; Chen, F.-M.; Zhao, W.; Li, J.-J.; Chen, J.-H.; Cutler, C.W.; Pashley, D.H.; Tay, F.R. Biphasic silica/apatite co-mineralized collagen scaffolds stimulate osteogenesis and inhibit RANKL-mediated osteoclastogenesis. Acta Biomater. 2015, 19, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Shie, M.-Y.; Ding, S.-J.; Chang, H.-C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-W.; Weitzmann, M.N.; Beck, G.R., Jr. Bioactive Silica Nanoparticles Promote Osteoblast Differentiation through Stimulation of Autophagy and Direct Association with LC3 and p62. ACS Nano 2014, 8, 5898–5910. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Moussa, F.M.; Mankoci, S.; Ustriyana, P.; Zhang, N.; Abdelmagid, S.; Molenda, J.; Murphy, W.L.; Safadi, F.F.; Sahai, N. Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation. Acta Biomater. 2016, 39, 192–202. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Reis, S.; Castro, A.; Fernandes, M.H. Bone Anabolic Effects of Soluble Si: In Vitro Studies with Human Mesenchymal Stem Cells and CD14+ Osteoclast Precursors. Stem Cells Int. 2016, 2016, 5653275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mladenović; Johansson, A.; Willman, B.; Shahabi, K.; Björn, E.; Ransjö, M. Soluble silica inhibits osteoclast formation and bone resorption in vitro. Acta Biomater. 2014, 10, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.R., Jr.; Ha, S.-W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.-K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Wang, F.; You, Y.; Wu, W.; Chi, H.; Jiao, G.; Zhang, L.; Zhou, H.; Wang, H.; Chen, Y. Ortho-silicic Acid Inhibits RANKL-Induced Osteoclastogenesis and Reverses Ovariectomy-Induced Bone Loss In Vivo. Biol. Trace Elem. Res. 2021, 199, 1864–1876. [Google Scholar] [CrossRef]
- Wiens, M.; Wang, X.; Schröder, H.C.; Kolb, U.; Schloßmacher, U.; Ushijima, H.; Müller, W.E. The role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cells. Biomaterials 2010, 31, 7716–7725. [Google Scholar] [CrossRef]
- Botelho, C.M.; Brooks, R.A.; Spence, G.; McFarlane, I.; Lopes, M.A.; Best, S.M.; Santos, J.D.; Rushton, N.; Bonfield, W. Differentiation of mononuclear precursors into osteoclasts on the surface of Si-substituted hydroxyapatite. J. Biomed. Mater. Res. Part A 2006, 78, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Wawer, A.A.; Gillings, R.; Jennings, A.; Myint, P.K. Iron status in the elderly. Mech. Ageing Dev. 2014, 136-137, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.P.; Ho, E. Zinc and its role in age-related inflammation and immune dysfunction. Mol. Nutr. Food Res. 2012, 56, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C. Trace elements and ageing, a genomic perspective using selenium as an example. J. Trace Elem. Med. Biol. 2011, 25, S11–S16. [Google Scholar] [CrossRef]
- Avinash, S.S.; Sreekantha; Goud, B.M. Magnesium Metabolism in Menopause. In Nutrition and Diet in Menopause; Humana Press: Totowa, NJ, USA, 2013; pp. 213–223. [Google Scholar]
- Bureau, I.; Anderson, R.A.; Arnaud, J.; Raysiguier, Y.; Favier, A.E.; Roussel, A.-M. Trace mineral status in post menopausal women: Impact of hormonal replacement therapy. J. Trace Elem. Med. Biol. 2002, 16, 9–13. [Google Scholar] [CrossRef]
- Karita, K.; Yamanouchi, Y.; Takano, T.; Oku, J.; Kisaki, T.; Yano, E. Associations of blood selenium and serum lipid levels in Japanese premenopausal and postmenopausal women. Menopause 2008, 15, 119–124. [Google Scholar] [CrossRef]
- Kim, C.; Nan, B.; Kong, S.; Harlow, S. Changes in Iron Measures over Menopause and Associations with Insulin Resistance. J. Women’s Health 2012, 21, 872–877. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.; Chu, A.; Petocz, P.; Samman, S. Zinc transporter gene expression and glycemic control in post-menopausal women with Type 2 diabetes mellitus. J. Trace Elem. Med. Biol. 2014, 28, 448–452. [Google Scholar] [CrossRef]
- Di Gioacchino, M.; Forcucci, R.; Tiboni, G.M.; Kouri, S.; Di Gioacchino, F.; Boscolo, P. The influence of menopause and habitual smoking upon serum zinc, serum copper and the cardiovascular and immune parameters of women. Int. J. Immunopathol. Pharmacol. 2000, 13, 91–97. [Google Scholar]
- Zhou, X.; Smith, A.M.; Failla, M.L.; Hill, K.E.; Yu, Z. Estrogen status alters tissue distribution and metabolism of selenium in female rats. J. Nutr. Biochem. 2012, 23, 532–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Zhang, S.; Wang, L.; Li, J.; Qu, G.; He, J.; Rong, H.; Ji, H.; Liu, S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 2012, 511, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, J.-H.; Hong, S.H.; Lee, J.Y.; Cherny, R.A.; Bush, A.I.; Palmiter, R.D.; Koh, J.-Y. Estrogen Decreases Zinc Transporter 3 Expression and Synaptic Vesicle Zinc Levels in Mouse Brain. J. Biol. Chem. 2004, 279, 8602–8607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zofková, I.; Nemcikova, P.; Matucha, P. Trace elements and bone health. Clin. Chem. Lab. Med. 2013, 51, 1555–1561. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Milne, D.B. A moderately high intake compared to a low intake of zinc depresses magnesium balance and alters indices of bone turnover in postmenopausal women. Eur. J. Clin. Nutr. 2004, 58, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Ciosek, Ż.; Kot, K.; Rotter, I. Iron, Zinc, Copper, Cadmium, Mercury, and Bone Tissue. Int. J. Environ. Res. Public Health 2023, 20, 2197. [Google Scholar] [CrossRef]
- Alagumuthu, G.; Singh, A.R. Impact of aluminum and selenium on fluoride level in serum and bones and on bone structure. Toxicol. Environ. Chem. 2009, 91, 363–373. [Google Scholar] [CrossRef]
- Yu, R.-A.; Xia, T.; Wang, A.-G.; Chen, X.-M. Effects of selenium and zinc on renal oxidative stress and apoptosis induced by fluoride in rats. Biomed. Environ. Sci. 2006, 19, 439–444. [Google Scholar]
- Doguer, C.; Ha, J.; Collins, J.F. Intersection of Iron and Copper Metabolism in the Mammalian Intestine and Liver. Compr. Physiol. 2018, 8, 1433–1461. [Google Scholar] [CrossRef]
- Skalny, A.A.; Tinkov, A.A.; Medvedeva, Y.S.; Alchinova, I.B.; Karganov, M.Y.; Skalny, A.V.; Nikonorov, A.A. Effect of short-term zinc supplementation on zinc and selenium tissue distribution and serum antioxidant enzymes. Acta Sci. Pol. Technol. Aliment. 2015, 14, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Gluhcheva, Y.; Pavlova, E.; Petrova, E.; Tinkov, A.A.; Ajsuvakova, O.P.; Skalnaya, M.G.; Vladov, I.; Skalny, A.V. The Impact of Perinatal Cobalt Chloride Exposure on Extramedullary Erythropoiesis, Tissue Iron Levels, and Transferrin Receptor Expression in Mice. Biol. Trace Elem. Res. 2020, 194, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Lertsuwan, K.; Wongdee, K.; Teerapornpuntakit, J.; Charoenphandhu, N. Intestinal calcium transport and its regulation in thalassemia: Interaction between calcium and iron metabolism. J. Physiol. Sci. 2018, 68, 221–232. [Google Scholar] [CrossRef] [PubMed]
- O’dell, B.L.; Browning, J.D. Impaired Calcium Entry into Cells Is Associated with Pathological Signs of Zinc Deficiency. Adv. Nutr. Int. Rev. J. 2013, 4, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paunier, L. Effect of magnesium on phosphorus and calcium metabolism. Mon. Kinderheilkd. 1992, 140, 17–20. [Google Scholar]
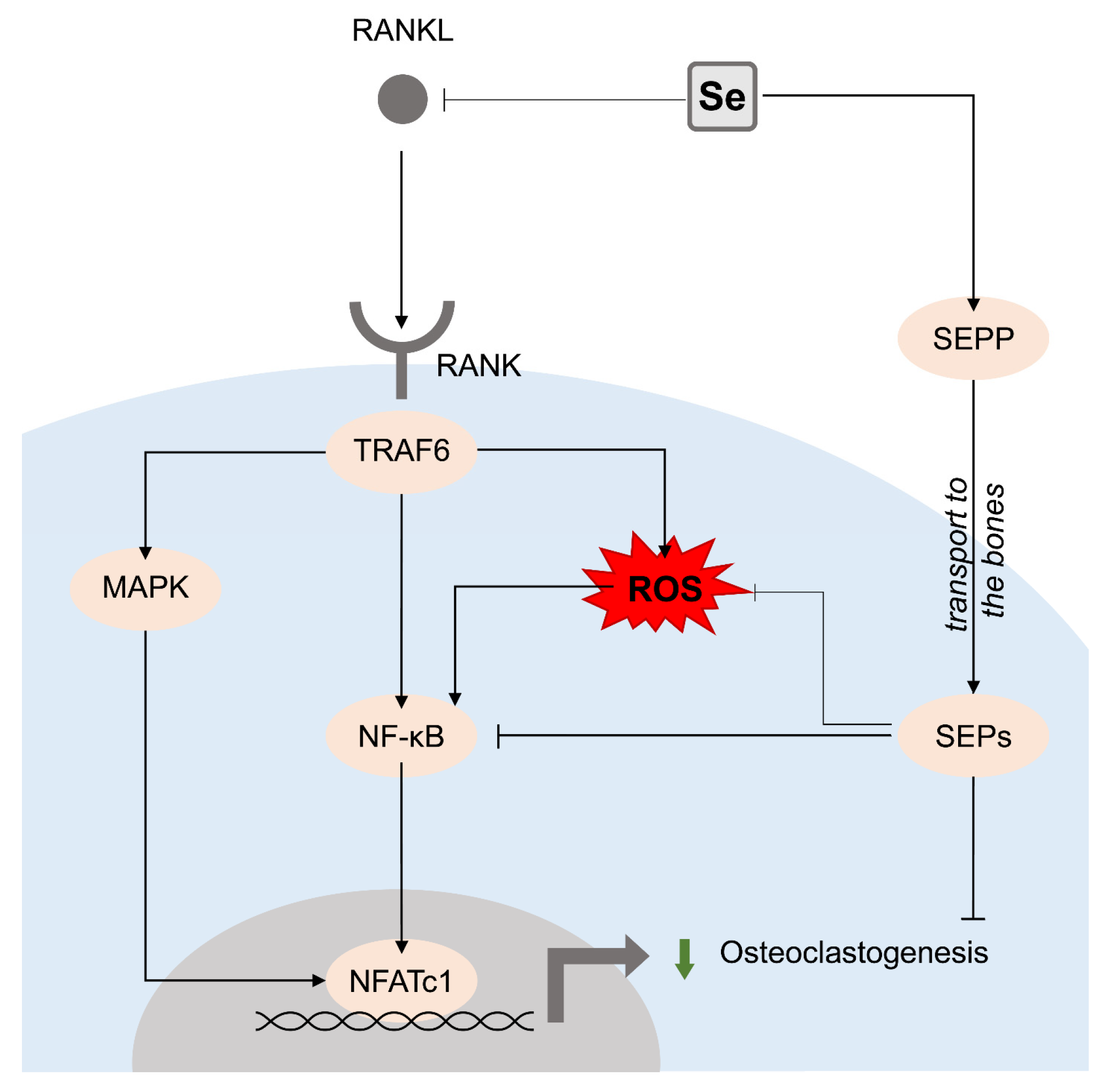

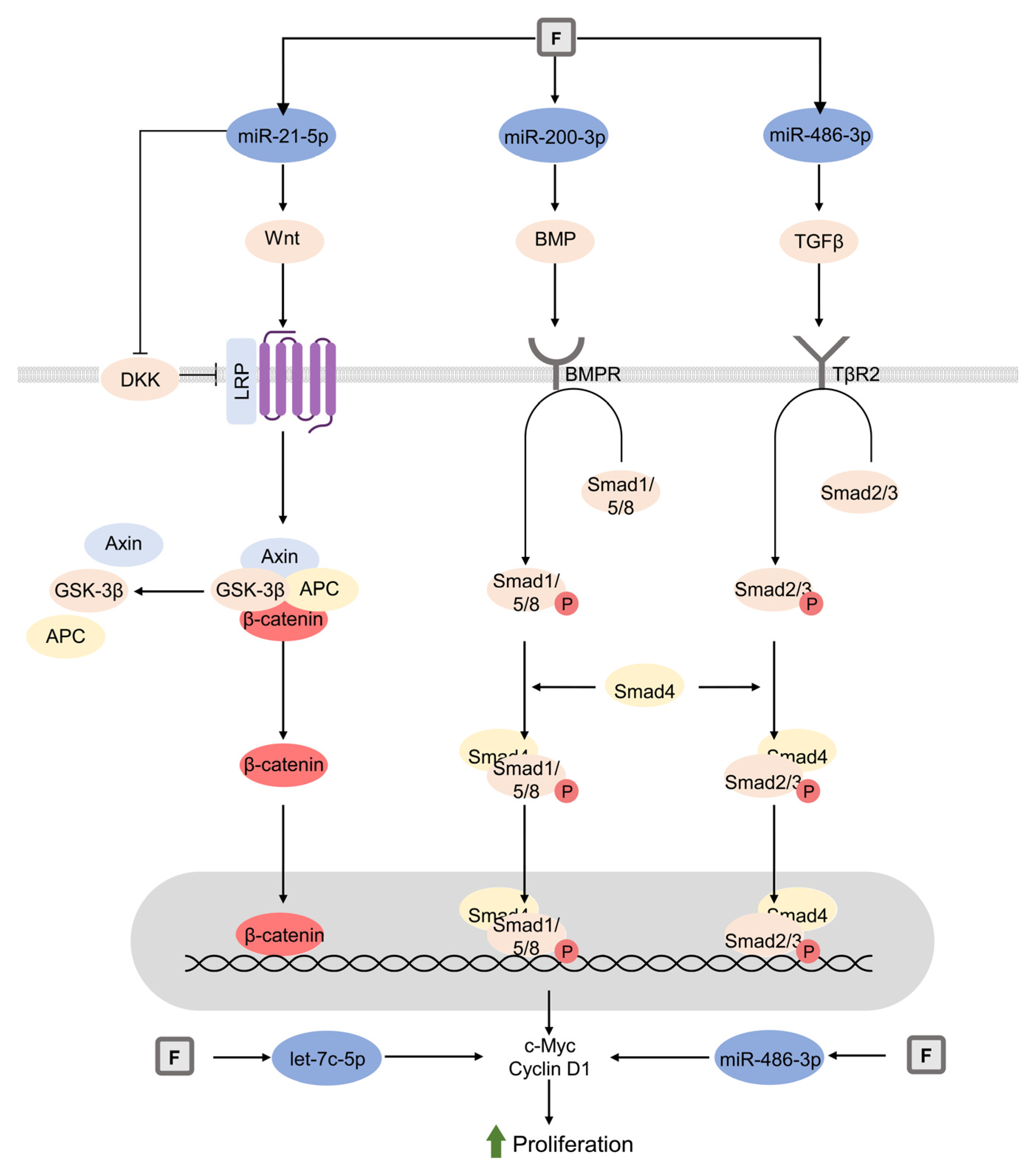
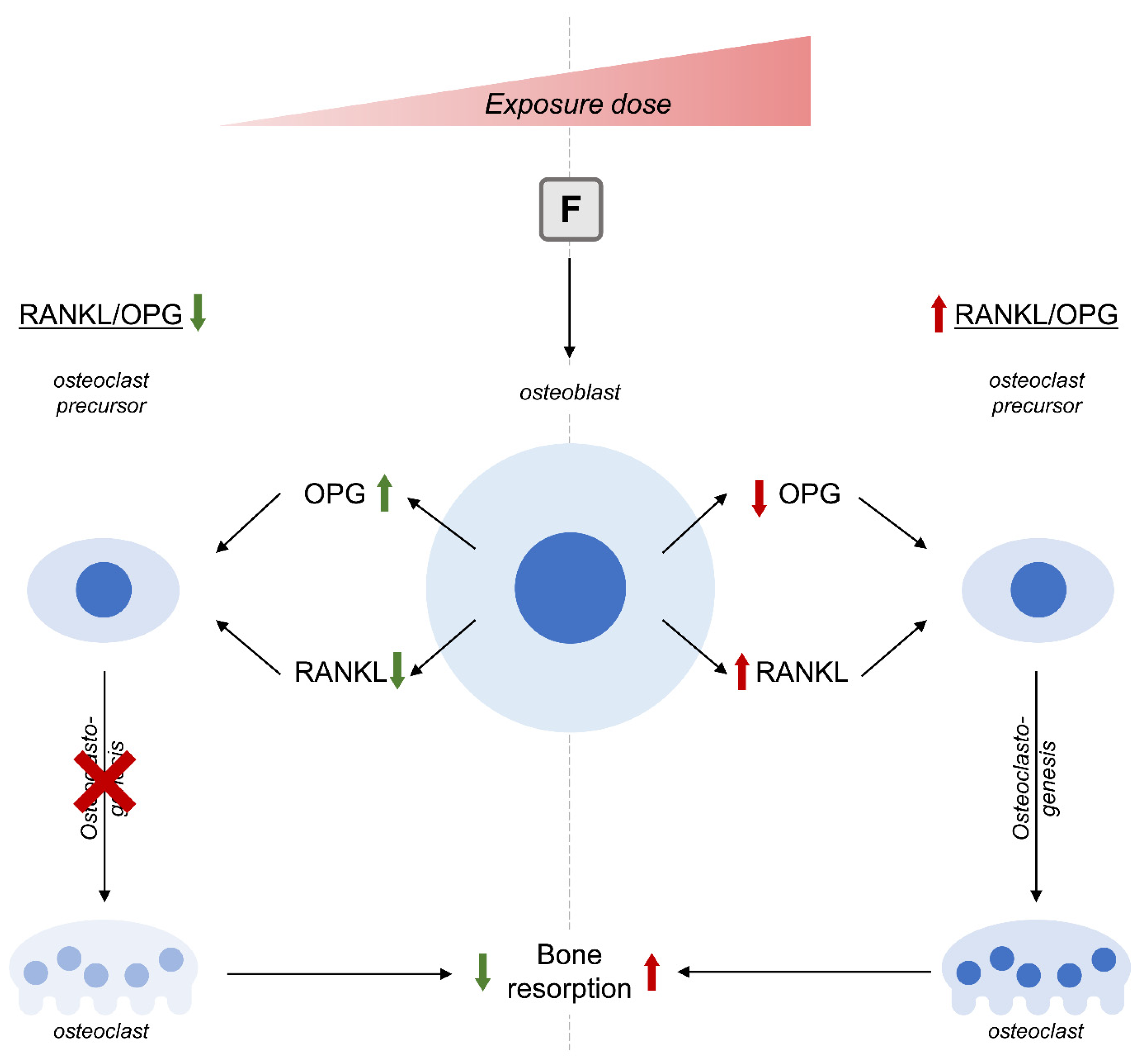
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalny, A.V.; Aschner, M.; Silina, E.V.; Stupin, V.A.; Zaitsev, O.N.; Sotnikova, T.I.; Tazina, S.I.; Zhang, F.; Guo, X.; Tinkov, A.A. The Role of Trace Elements and Minerals in Osteoporosis: A Review of Epidemiological and Laboratory Findings. Biomolecules 2023, 13, 1006. https://doi.org/10.3390/biom13061006
Skalny AV, Aschner M, Silina EV, Stupin VA, Zaitsev ON, Sotnikova TI, Tazina SI, Zhang F, Guo X, Tinkov AA. The Role of Trace Elements and Minerals in Osteoporosis: A Review of Epidemiological and Laboratory Findings. Biomolecules. 2023; 13(6):1006. https://doi.org/10.3390/biom13061006
Chicago/Turabian StyleSkalny, Anatoly V., Michael Aschner, Ekaterina V. Silina, Victor A. Stupin, Oleg N. Zaitsev, Tatiana I. Sotnikova, Serafima Ia. Tazina, Feng Zhang, Xiong Guo, and Alexey A. Tinkov. 2023. "The Role of Trace Elements and Minerals in Osteoporosis: A Review of Epidemiological and Laboratory Findings" Biomolecules 13, no. 6: 1006. https://doi.org/10.3390/biom13061006
APA StyleSkalny, A. V., Aschner, M., Silina, E. V., Stupin, V. A., Zaitsev, O. N., Sotnikova, T. I., Tazina, S. I., Zhang, F., Guo, X., & Tinkov, A. A. (2023). The Role of Trace Elements and Minerals in Osteoporosis: A Review of Epidemiological and Laboratory Findings. Biomolecules, 13(6), 1006. https://doi.org/10.3390/biom13061006





