RNA-Seq Analysis of Aboveground and Underground Parts of Biomass Sorghum Was Performed to Evaluate Its Suitability for Environmental Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Distribution of Cd Content at Subcellular Level in Biomass Sorghum
2.3. Determination of Oxidase Activity and MDA Content
2.4. RNA Extraction and Transcriptome Sequencing
2.5. qRT-PCR Validation of Differentially Expressed Genes (DEGs)
2.6. Data Analysis
3. Results
3.1. The Accumulation of Cd in Different Tissues and the Subcellular Localization of Cd
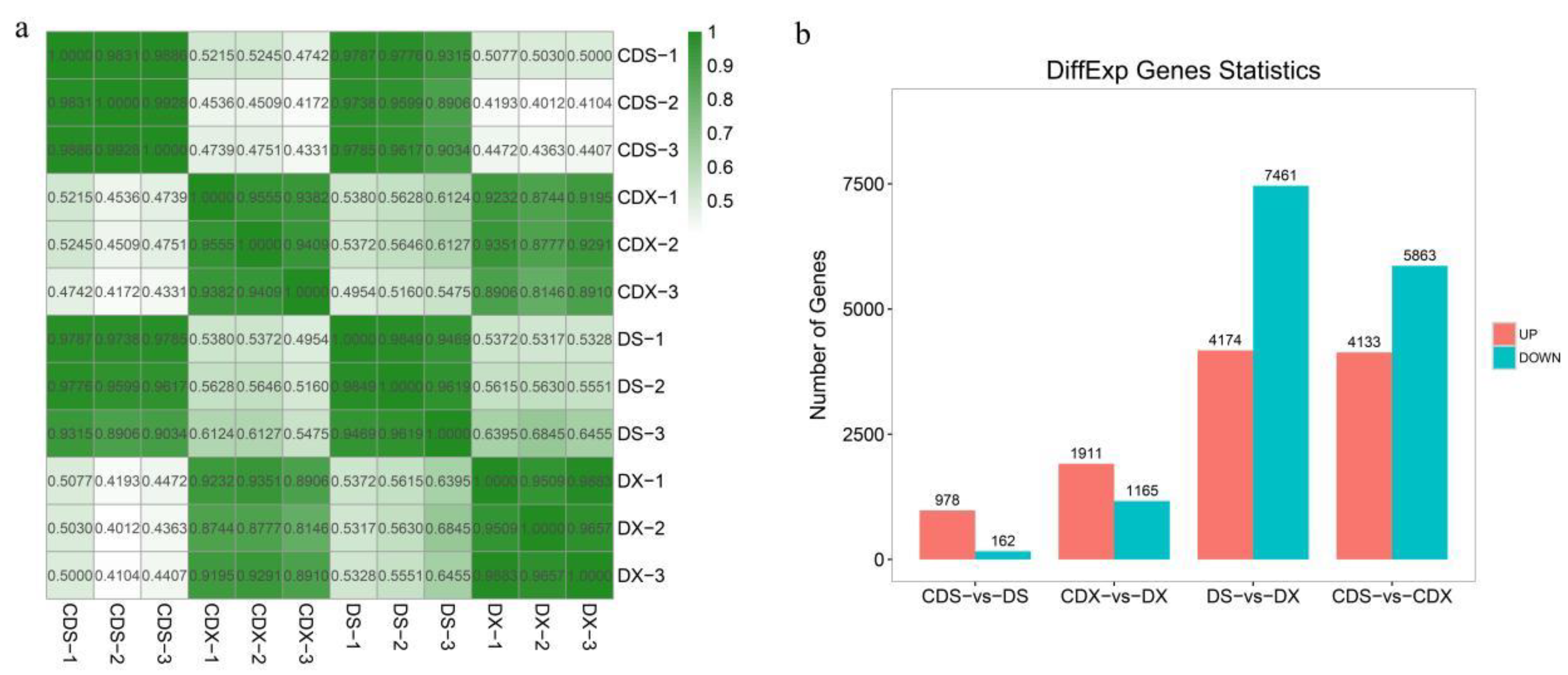
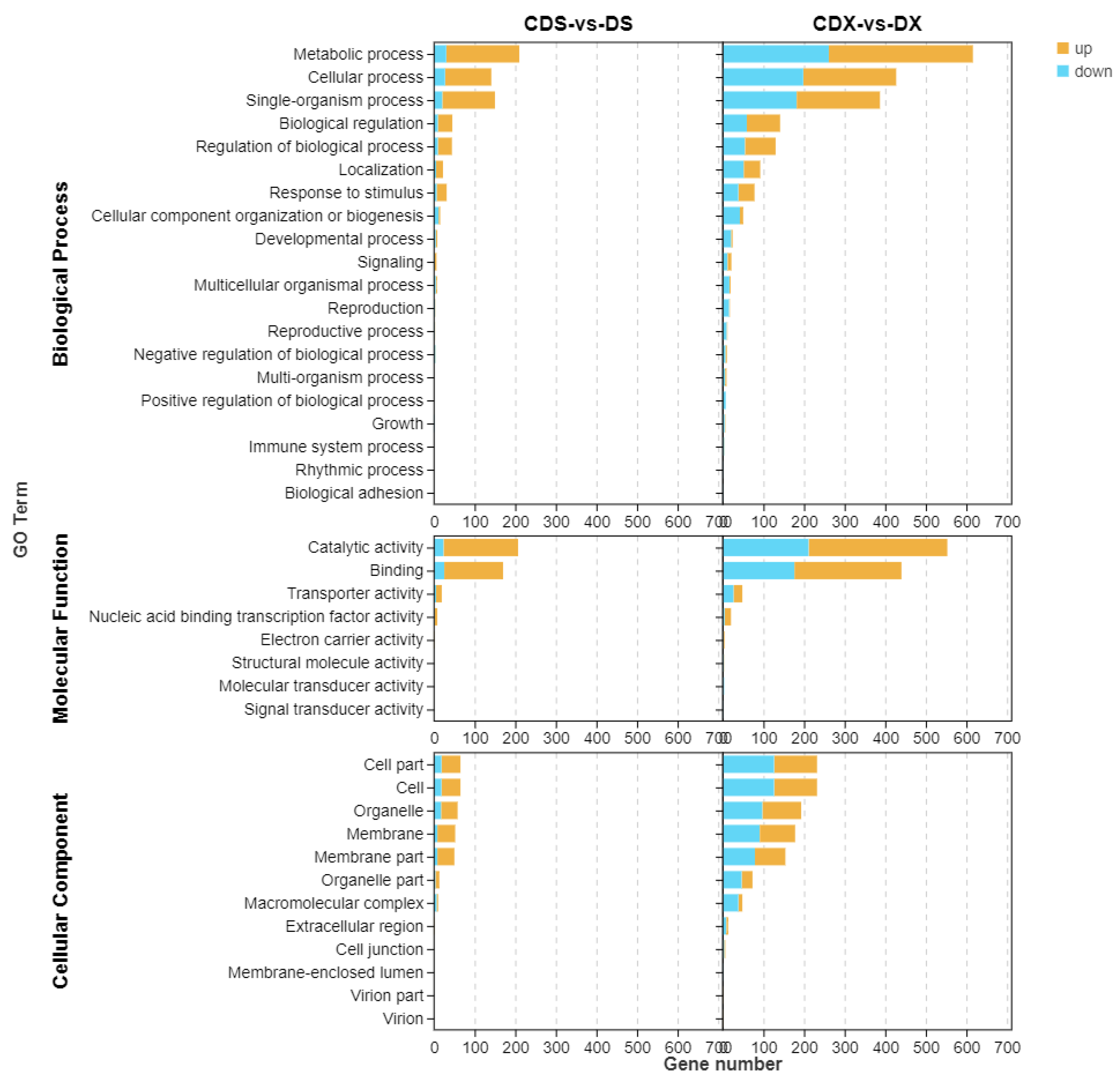
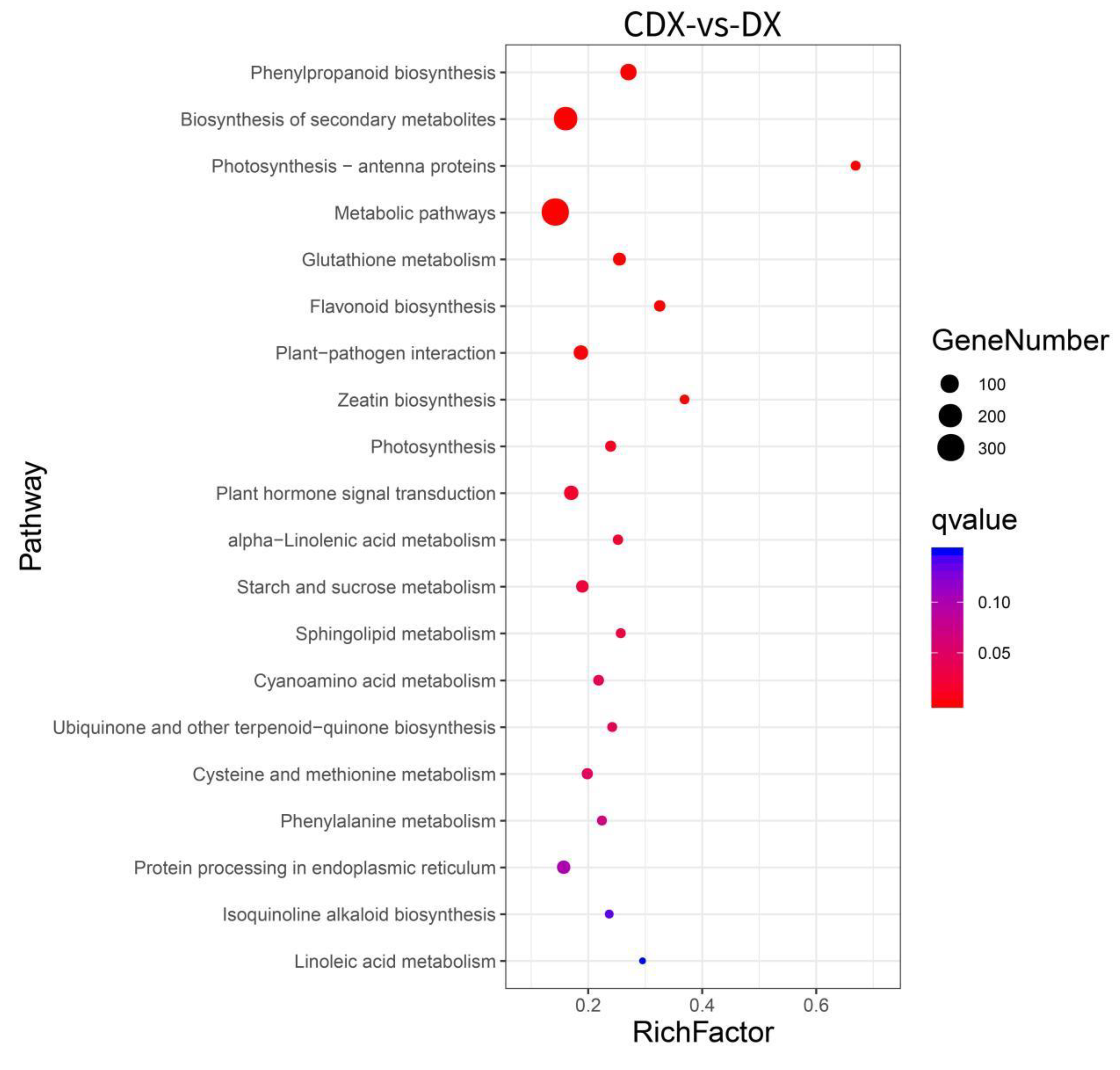
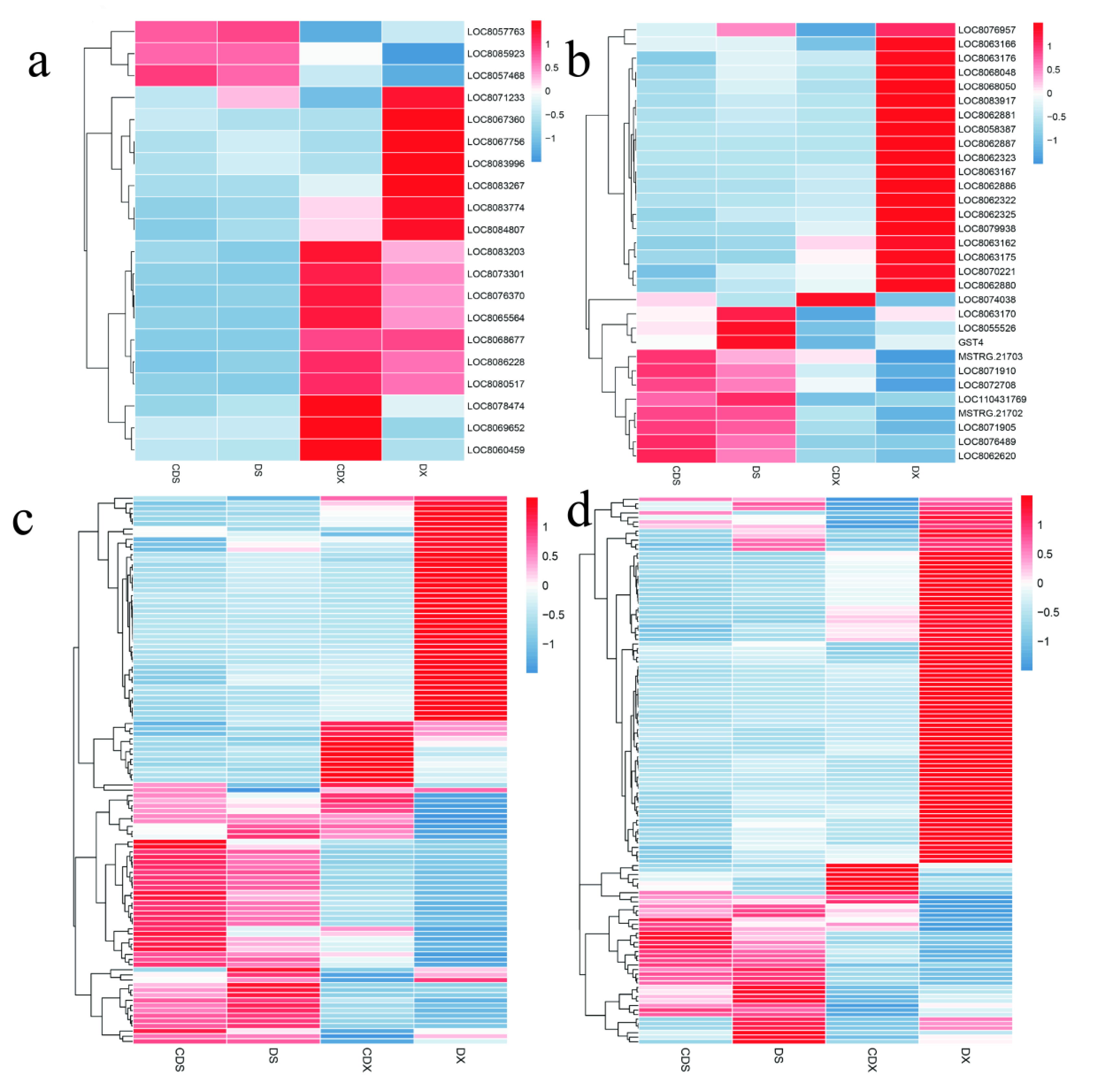
3.2. Transcriptome Profiling and Functional Analysis of Differentially Expressed Genes
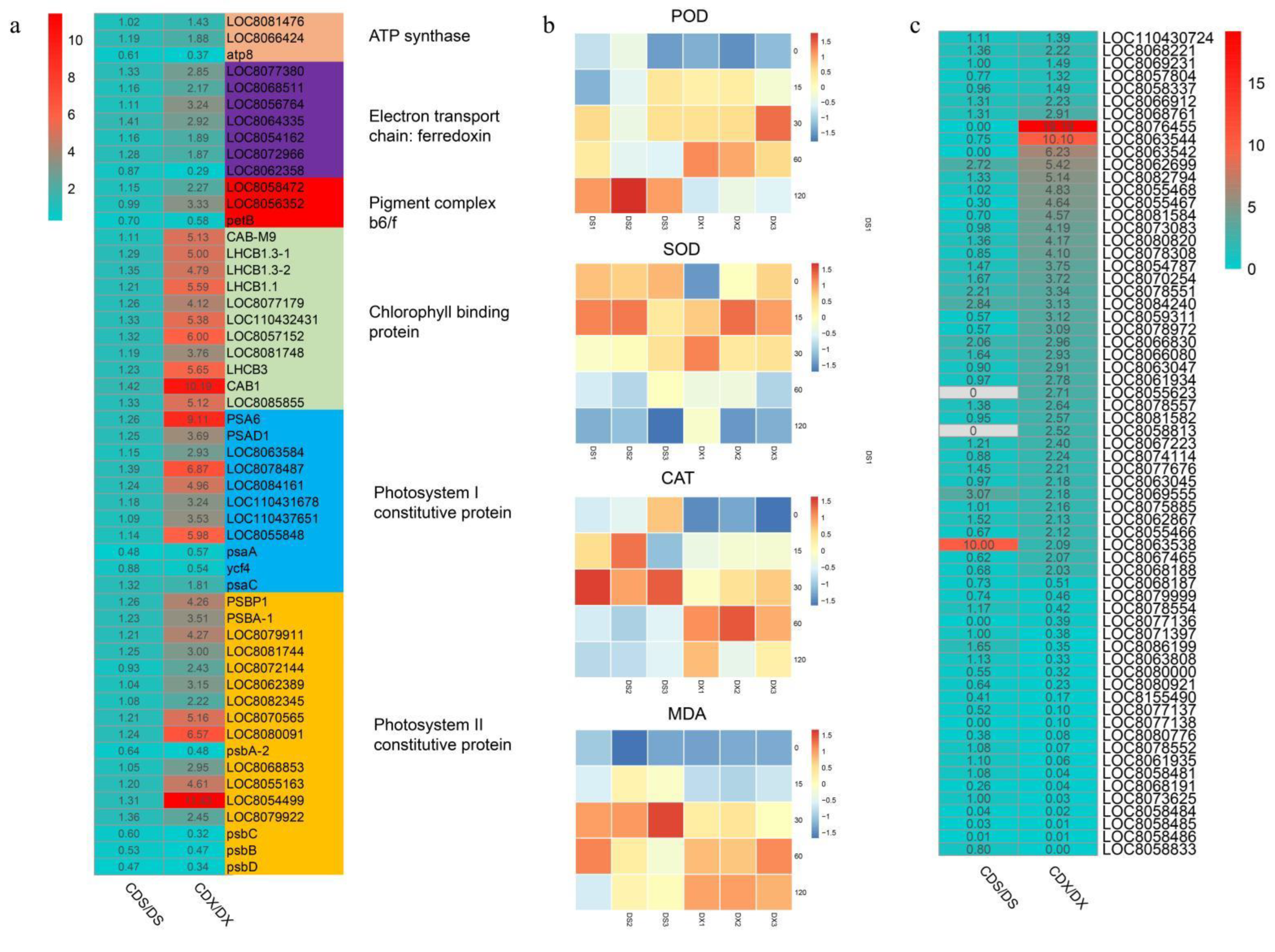
3.3. Gene Expression Profile Related to Photosynthesis and Oxidative Stress
3.4. Dynamic Changes in Genes Related to Metalloproteinase, Transporter, Chelator, and Glutathione
3.5. Expression Profile of Hormone-Related Genes in Response to Heavy Metal Stress
3.6. Identification of Transcriptional Regulators Related to Cd Stress
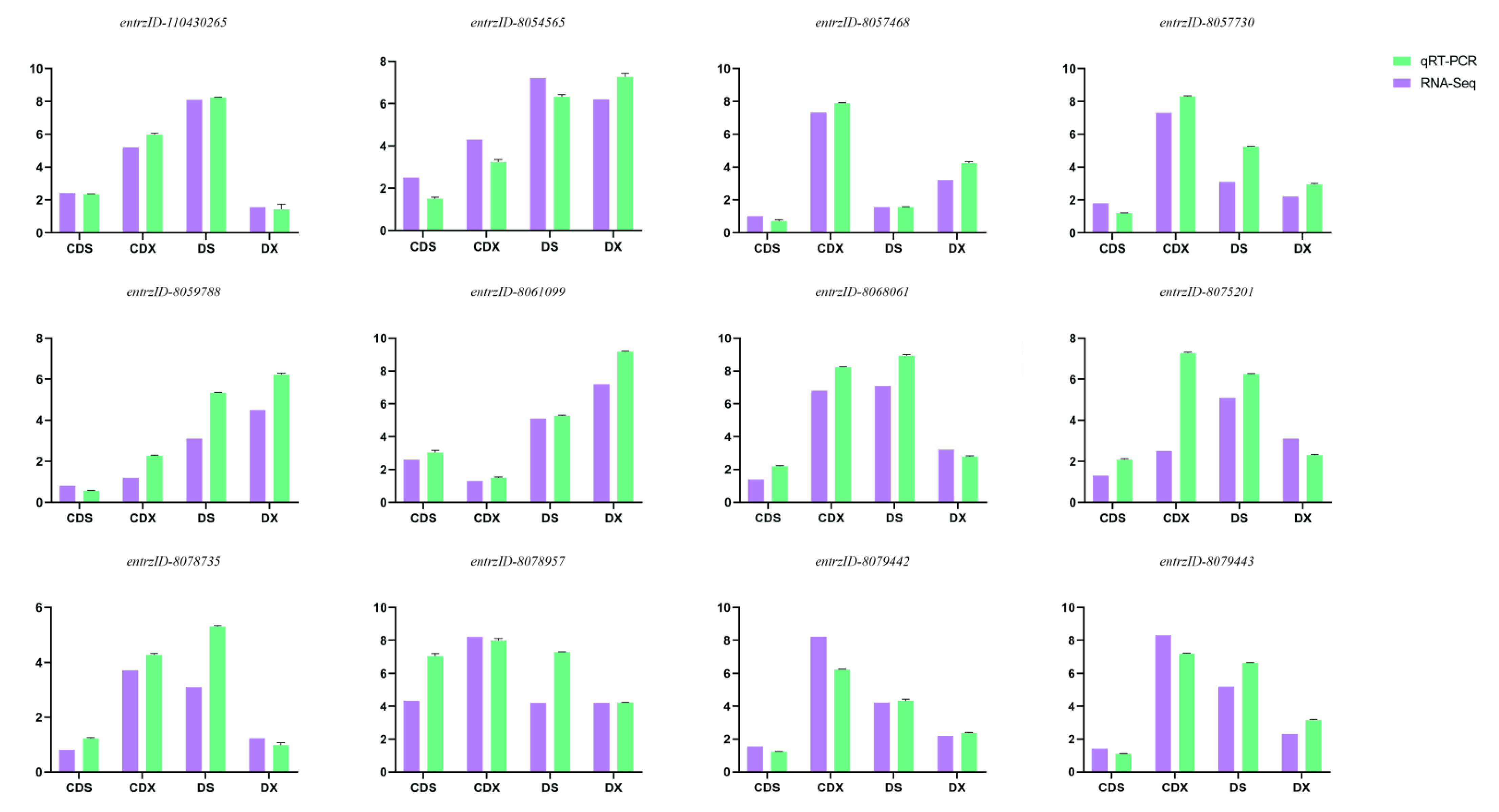
3.7. qRT-PCR Validation of DEGs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ikenaka, Y.; Nakayama, S.M.; Muzandu, K.; Choongo, K.; Teraoka, H.; Mizuno, N.; Ishizuka, M. Heavy metal contamination of soil and sediment in Zambia. Afr. J. Environ. Sci. Technol. 2010, 4, 729–739. [Google Scholar]
- Zhang, L.; Wong, M.H. Environmental mercury contamination in China: Sources and impacts. Environ. Int. 2007, 33, 108–121. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J.; Hamon, R.; McLaren, R.; Speir, T.; Rogers, S. A bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand. Soil Res. 2000, 38, 1037–1086. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M.; Abd El-Mageed, T.A.; Howladar, S.M.; Abdelhamid, M.T. Alleviation of cadmium toxicity in common bean (Phaseolus vulgaris L.) plants by the exogenous application of salicylic acid. J. Hortic. Sci. Biotechnol. 2015, 90, 83–91. [Google Scholar]
- Ali, E.F.; Aljarani, A.M.; Mohammed, F.A.; Desoky, E.-S.M.; Mohamed, I.A.A.; Rady, M.M.; El-Sharnouby, M.; Tammam, S.A.; Hassan, F.A.S.; Shaaban, A. Exploring the potential enhancing effects of trans-zeatin and silymarin on the productivity and antioxidant defense capacity of cadmium-stressed wheat. Biology 2022, 11, 1173. [Google Scholar] [CrossRef]
- Straif, K.; Benbrahim-Tallaa, L.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Guha, N.; Freeman, C.; Galichet, L. A review of human carcinogens—Part C: Metals, arsenic, dusts, and fibres. Lancet Oncol. 2009, 10, 453–454. [Google Scholar] [CrossRef]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S. A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J. Energy Environ. 2005, 6, 18. [Google Scholar]
- Pollard, A.J.; Powell, K.D.; Harper, F.A.; Smith, J.A.C. The genetic basis of metal hyperaccumulation in plants. Crit. Rev. Plant Sci. 2002, 21, 539–566. [Google Scholar] [CrossRef]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Sandalio, L.; Dalurzo, H.; Gomez, M.; Romero-Puertas, M.; Del Rio, L. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Sparkes, I.; Hawes, C.; Luis, A.; Sandalio, L.M. Peroxisome dynamics in Arabidopsis plants under oxidative stress induced by cadmium. Free. Radic. Biol. Med. 2009, 47, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mechanisms of adaptation of plants to acid soils. Plant Soil 1991, 134, 1–20. [Google Scholar] [CrossRef]
- Jaffré, T.; Brooks, R.; Lee, J.; Reeves, R. Sebertia acuminata: A hyperaccumulator of nickel from New Caledonia. Science 1976, 193, 579–580. [Google Scholar] [CrossRef]
- Haferburg, G.; Kothe, E. Microbes and metals: Interactions in the environment. J. Basic Microbiol. 2007, 47, 453–467. [Google Scholar] [CrossRef]
- Küpper, H.; Lombi, E.; Zhao, F.-J.; McGrath, S.P. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 2000, 212, 75–84. [Google Scholar] [CrossRef]
- Kim, D.Y.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50, 207–218. [Google Scholar] [CrossRef]
- Krämer, U.; Talke, I.N.; Hanikenne, M. Transition metal transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef]
- Rogers, E.E.; Eide, D.J.; Guerinot, M.L. Altered selectivity in an Arabidopsis metal transporter. Proc. Natl. Acad. Sci. USA 2000, 97, 12356–12360. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef]
- Wolf, A.E.; Dietz, K.-J.; Schröder, P. Degradation of glutathione S-conjugates by a carboxypeptidase in the plant vacuole. FEBS Lett. 1996, 384, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Heiss, S.; Wachter, A.; Bogs, J.; Cobbett, C.; Rausch, T. Phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. J. Exp. Bot. 2003, 54, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Masood, A.; Khan, M.I.R.; Fatma, M.; Asgher, M.; Per, T.S.; Khan, N.A. Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol. Biochem. 2016, 104, 1–10. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Gupta, D.K.; Archilla, A.; Romero-Puertas, M.C.; Luis, A. Reactive oxygen species and nitric oxide in plants under cadmium stress: From toxicity to signaling. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–215. [Google Scholar]
- Garnier, L.; Simon-Plas, F.; Thuleau, P.; Agnel, J.P.; Blein, J.P.; Ranjeva, R.; Montillet, J.L. Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ. 2006, 29, 1956–1969. [Google Scholar] [CrossRef]
- Remans, T.; Opdenakker, K.; Smeets, K.; Mathijsen, D.; Vangronsveld, J.; Cuypers, A. Metal-specific and NADPH oxidase dependent changes in lipoxygenase and NADPH oxidase gene expression in Arabidopsis thaliana exposed to cadmium or excess copper. Funct. Plant Biol. 2010, 37, 532–544. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Monde, R.-A.; Last, R.L. Superoxide dismutase in Arabidopsis: An eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar] [CrossRef]
- Costa, A.; Drago, I.; Behera, S.; Zottini, M.; Pizzo, P.; Schroeder, J.I.; Pozzan, T.; Schiavo, F.L. H2O2 in plant peroxisomes: An in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J. 2010, 62, 760–772. [Google Scholar] [CrossRef]
- Wang, X.K. The Experiment Principle and Technology in Plant Physiology Biochemistry; Higher Education Press: Beijing, China, 2015; p. 306. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. Plant Signal. Mol. 2019, 157–168. [Google Scholar] [CrossRef]
- Dicko, M.H.; Gruppen, H.; Barro, C.; Traoré, A.S.; van Berkel, W.J.; Voragen, A.G. Impact of phenolic compounds and related enzymes in sorghum varieties for resistance and susceptibility to biotic and abiotic stresses. J. Chem. Ecol. 2005, 31, 2671–2688. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia Obovata under Cd and Zn stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Štork, F.; Bačkor, M. Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 2009, 320, 231–242. [Google Scholar] [CrossRef]
- Fernandez, M.T.; Mira, M.L.; Florencio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef]
- Malešev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Cao, F.; Cheng, W.; Zhang, G.; Wu, F. Modulation of exogenous glutathione in phytochelatins and photosynthetic performance against Cd stress in the two rice genotypes differing in Cd tolerance. Biol. Trace Elem. Res. 2011, 143, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, X.; Gao, K.; Ge, Y.; Cheng, W. Non-protein thiols and glutathione S-transferase alleviate Cd stress and reduce root-to-shoot translocation of Cd in rice. J. Plant Nutr. Soil Sci. 2013, 176, 626–633. [Google Scholar] [CrossRef]
- Wójcik, M.; Tukiendorf, A. Glutathione in adaptation of Arabidopsis thaliana to cadmium stress. Biol. Plant. 2011, 55, 125–132. [Google Scholar] [CrossRef]
- Sheng, Y.; Yan, X.; Huang, Y.; Han, Y.; Zhang, C.; Ren, Y.; Fan, T.; Xiao, F.; Liu, Y.; Cao, S. The WRKY transcription factor, WRKY13, activates PDR8 expression to positively regulate cadmium tolerance in Arabidopsis. Plant Cell Environ. 2019, 42, 891–903. [Google Scholar] [CrossRef]
- Gonçalves, J.F.; Fiorenza, A.M.; Spanevello, R.M.; Mazzanti, C.M.; Bochi, G.V.; Antes, F.G.; Stefanello, N.; Rubin, M.A.; Dressler, V.L.; Morsch, V.M. N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem.-Biol. Interact. 2010, 186, 53–60. [Google Scholar] [CrossRef]
- Agarwal, P.; Mitra, M.; Banerjee, S.; Roy, S. MYB4 transcription factor, a member of R2R3-subfamily of MYB domain protein, regulates cadmium tolerance via enhanced protection against oxidative damage and increases expression of PCS1 and MT1C in Arabidopsis. Plant Sci. 2020, 297, 110501. [Google Scholar] [CrossRef]
- Karanja, B.K.; Xu, L.; Wang, Y.; Tang, M.; Muleke, E.M.; Dong, J.; Liu, L. Genome-wide characterization of the AP2/ERF gene family in radish (Raphanus sativus L.): Unveiling evolution and patterns in response to abiotic stresses. Gene 2019, 718, 144048. [Google Scholar] [CrossRef]
- Anuradha, S.; Rao, S. The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil Environ. 2007, 53, 465. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef]
- Hayat, S.; Ali, B.; Hasan, S.A.; Ahmad, A. Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ. Exp. Bot. 2007, 60, 33–41. [Google Scholar] [CrossRef]
- Hayat, S.; Alyemeni, M.N.; Hasan, S.A. Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J. Biol. Sci. 2012, 19, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hasan, S.A.; Hayat, Q.; Ahmad, A. Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma 2010, 239, 3–14. [Google Scholar] [CrossRef] [PubMed]

| Cd Concentration | Cd Content (mg kg−1 DW) | Percentage (%) | BCF | TF | |||||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Stems | Roots | Leaves | Stems | Roots | ||||
| 15 μmol L−1 Cd | A1 | 0.962 ± 0.24 a | 0.583 ± 0.12 b | 1.790 ± 0.14 c | 62.3 | 71.3 | 73.1 | 0.318 a | 0.703 a |
| A2 | 0.580 ± 0.15 a | 0.165 ± 0.05 d | 0.540 ± 0.12 d | 32.5 | 21.5 | 24.8 | |||
| A3 | 0.077 ± 0.03 a | 0.058 ± 0.02 b | 0.052 ± 0.02 b | 5.2 | 7.2 | 2.1 | |||
| Total | 1.78 ± 0.21 a | 0.84 ± 0.12 b | 2.33 ± 0.08 c | ||||||
| 30 μmol L−1 Cd | A1 | 1.197 ± 0.54 a | 0.753 ± 0.25 b | 3.090 ± 0.24 c | 52.3 | 66.1 | 75.7 | 0.135 b | 0.659 b |
| A2 | 0.958 ± 0.25 a | 0.333 ± 0.09 b | 0.819 ± 0.18 a | 41.1 | 25.7 | 21.4 | |||
| A3 | 0.127 ± 0.04 a | 0.105 ± 0.05 a | 0.119 ± 0.04 a | 6.6 | 8.2 | 2.9 | |||
| Total | 2.25 ± 0.24 a | 1.31 ± 0.09 b | 3.95 ± 0.18 c | ||||||
| 60 μmol L−1 Cd | A1 | 1.503 ± 0.85 a | 1.191 ± 0.29 a | 4.176 ± 0.45 b | 45.8 | 64.2 | 66.7 | 0.089 c | 0.501 c |
| A2 | 1.490 ± 0.64 a | 0.517 ± 0.12 b | 1.461 ± 0.21 a | 44.3 | 27.9 | 23.3 | |||
| A3 | 0.292 ± 0.08 a | 0.148 ± 0.08 b | 0.622 ± 0.09 c | 9.9 | 7.9 | 10.0 | |||
| Total | 3.43 ± 0.13 a | 1.95 ± 0.08 b | 6.33 ± 0.15 c | ||||||
| 120 μmol L−1 Cd | A1 | 1.755 ± 0.69 a | 1.469 ± 0.32 a | 5.908 ± 0.49 a | 43.6 | 59.2 | 63.7 | 0.052 d | 0.461 d |
| A2 | 2.780 ± 0.85 a | 0.780 ± 0.21 a | 1.933 ± 0.34 a | 46.2 | 31.4 | 25.1 | |||
| A3 | 0.402 ± 0.14 a | 0.203 ± 0.12 a | 0.869 ± 0.12 a | 10.2 | 9.4 | 11.2 | |||
| Total | 5.04 ± 0.29 a | 2.01 ± 0.14 a | 9.48 ± 0.17 a | ||||||
| DEGs ID | Description | Primer (5′-3′) |
|---|---|---|
| entrzID_8061099 | ABCB1 | F:CGGGAACACCTTCTAACT R:ACACCAACTCGTTAGGCT |
| entrzID_8068061 | ABCB2 | F:ATTTGGAGAAGGGGATTG R:TCTTCAAACTCTCCTCGC |
| entrzID_8060240 | CALM | F:CCTGTAGTCCTCCAACCTG R:AAGTTGCTGGAGGTGCTG |
| entrzID_8057730 | WRKY33 | F:AAGTTGCTGGAGGTGCTG R:AAGTTGCTGGAGGTGCTG |
| entrzID_8081117 | PYL | F:GCTGGATTCTGGAAGGTG R:CATTTTTCTCTACCCCATTC |
| entrzID_8054565 | PP2C | F:CCTCTGACAACACTTCCAAC R:ATTACTGGACTCTGGGAACA |
| entrzID_110431683 | SNRK2 | F:TCTAAACCCCTTGAACCTG R:TACCTGAACAAGGCACTACA |
| entrzID_8068938 | EIN3 | F:ATTGCGATACTCGGGTTG R:CGACTCTCCCTGTTGTTGA |
| entrzID_8059234 | ERF1 | F:GGAGAAGGCGATAGCAAC R:GCTCAAGTCCCTTCTCAA |
| entrzID_8075201 | ABF | F:CCAAGCGATGTCATAAAC R:GGCACAATCTTCACCTATG |
| entrzID_8078735 | IAA | F:TAGTCGGGTCAAAGGATAG R:CCTTTGACCCGACTACTGAC |
| entrzID_8059110 | GH3 | F:GGTGGAAGAGGAGTTTGGTG R:GAGGGTCGGTATGACGGAA |
| entrzID_8068884 | ARR-A | F:CGGGAACACCTTCTAACT R:ACACCAACTCGTTAGGCT |
| entrzID_110430265 | GID1 | F:CGGGAACACCTTCTAACT R:ACACCAACTCGTTAGGCT |
| entrzID_8084151 | PIF4 | F:CGGGAACACCTTCTAACT R:ACACCAACTCGTTAGGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Ling, D.; He, Q.; Wang, P.; Zhu, J. RNA-Seq Analysis of Aboveground and Underground Parts of Biomass Sorghum Was Performed to Evaluate Its Suitability for Environmental Remediation. Biomolecules 2023, 13, 925. https://doi.org/10.3390/biom13060925
Zhou T, Ling D, He Q, Wang P, Zhu J. RNA-Seq Analysis of Aboveground and Underground Parts of Biomass Sorghum Was Performed to Evaluate Its Suitability for Environmental Remediation. Biomolecules. 2023; 13(6):925. https://doi.org/10.3390/biom13060925
Chicago/Turabian StyleZhou, Tao, Dingxun Ling, Qihao He, Ping Wang, and Jian Zhu. 2023. "RNA-Seq Analysis of Aboveground and Underground Parts of Biomass Sorghum Was Performed to Evaluate Its Suitability for Environmental Remediation" Biomolecules 13, no. 6: 925. https://doi.org/10.3390/biom13060925
APA StyleZhou, T., Ling, D., He, Q., Wang, P., & Zhu, J. (2023). RNA-Seq Analysis of Aboveground and Underground Parts of Biomass Sorghum Was Performed to Evaluate Its Suitability for Environmental Remediation. Biomolecules, 13(6), 925. https://doi.org/10.3390/biom13060925






