Enzymatic Modification of Pomace Olive Oil with Natural Antioxidants: Effect on Oxidative Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Immobilization of Lipase on Green Nanoparticles
2.2.2. Analysis of the Fatty Acid Composition of Pomace Olive Oil
2.2.3. Enzymatic Preparation of Modified Oils
Effect of Temperature and Enzyme Concentration
Reusability of the Nanobiocatalytic System
2.2.4. Characterization and Quantification of the Products
High-Performance Liquid Chromatography Analysis
Mass Spectrometry Analysis
2.2.5. Antioxidant Activity Tests
2.2.6. Oxidative Stability Tests
Determination of Conjugated Dienes
Determination of Thiobarbituric Acid Reactive Substances
Determination of Volatile Oxidative Products
Fluorescence Spectroscopy
2.2.7. Mayonnaise Preparation
2.2.8. Statistical Analysis
3. Results
3.1. Enzymatic Modification of Oil with Natural Antioxidants
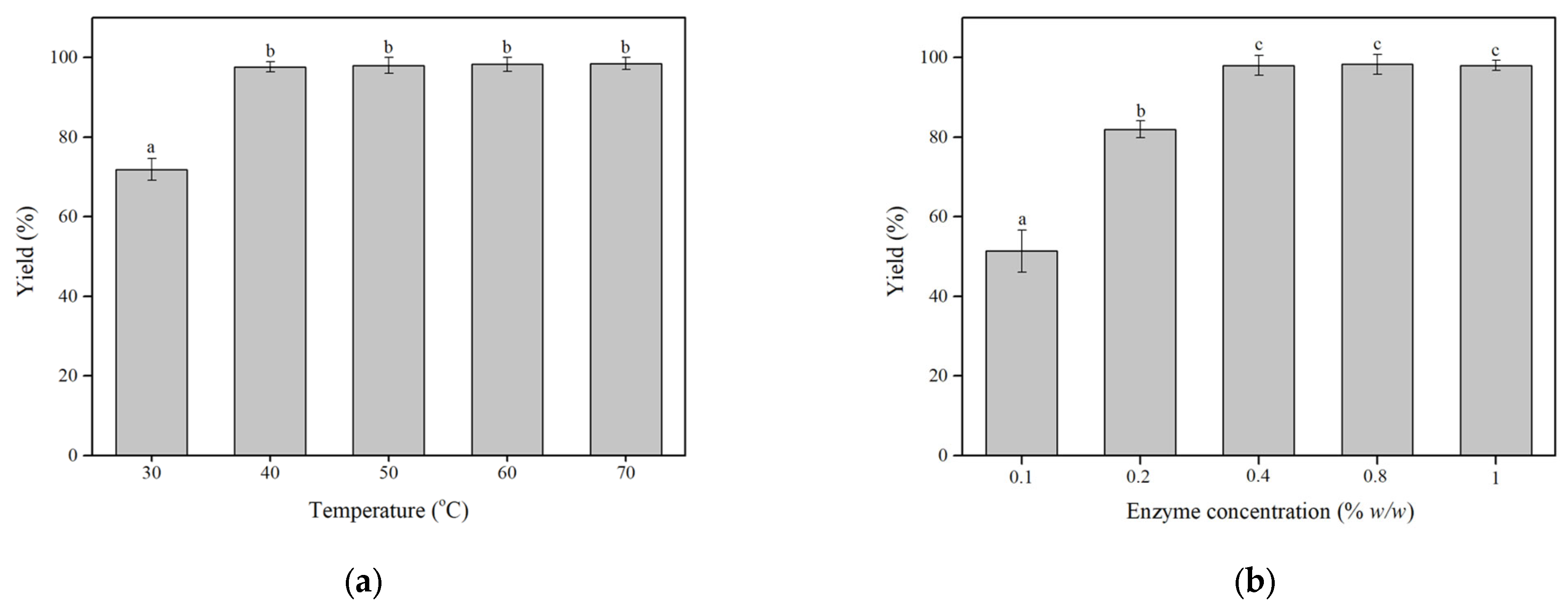
3.1.1. Optimization of the Enzymatic Modification of Oil with HTYR
3.1.2. Operational Stability of the Nanobiocatalytic System
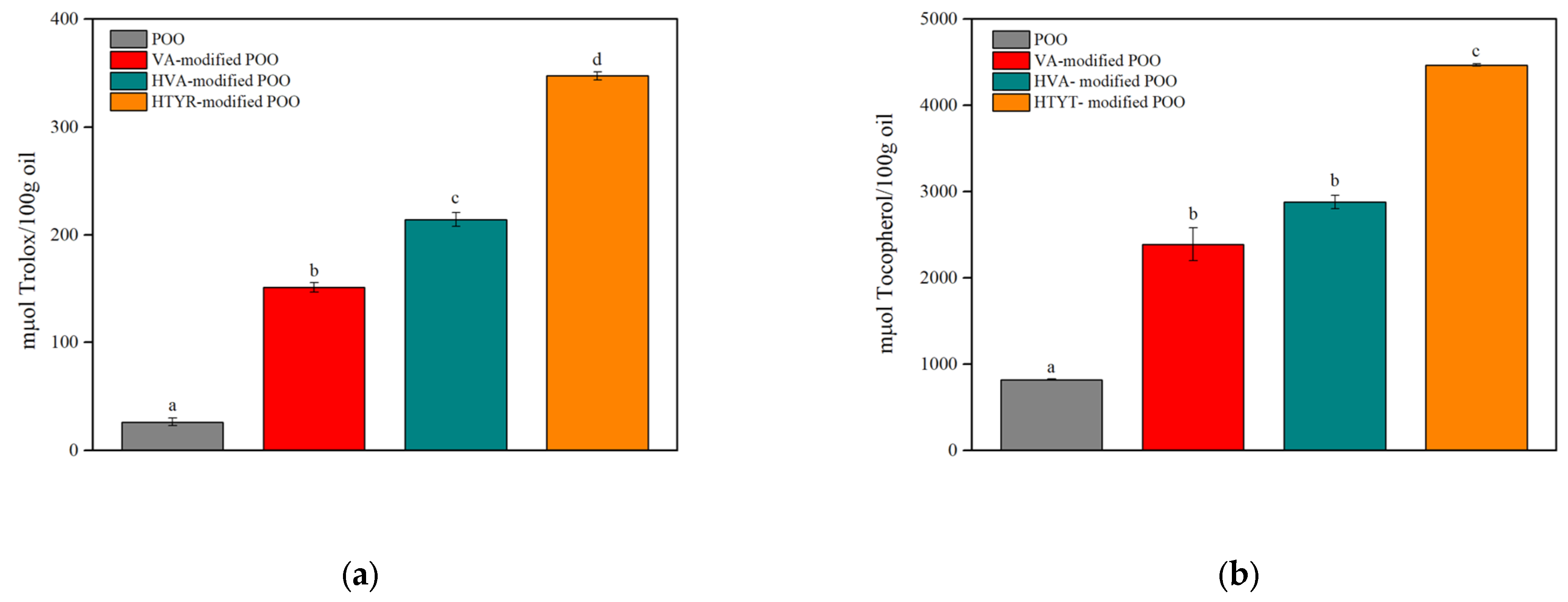
3.2. Antioxidant Evaluation of the Control and Modified Oils
3.3. Oxidative Stability of the Control and Modified Oils
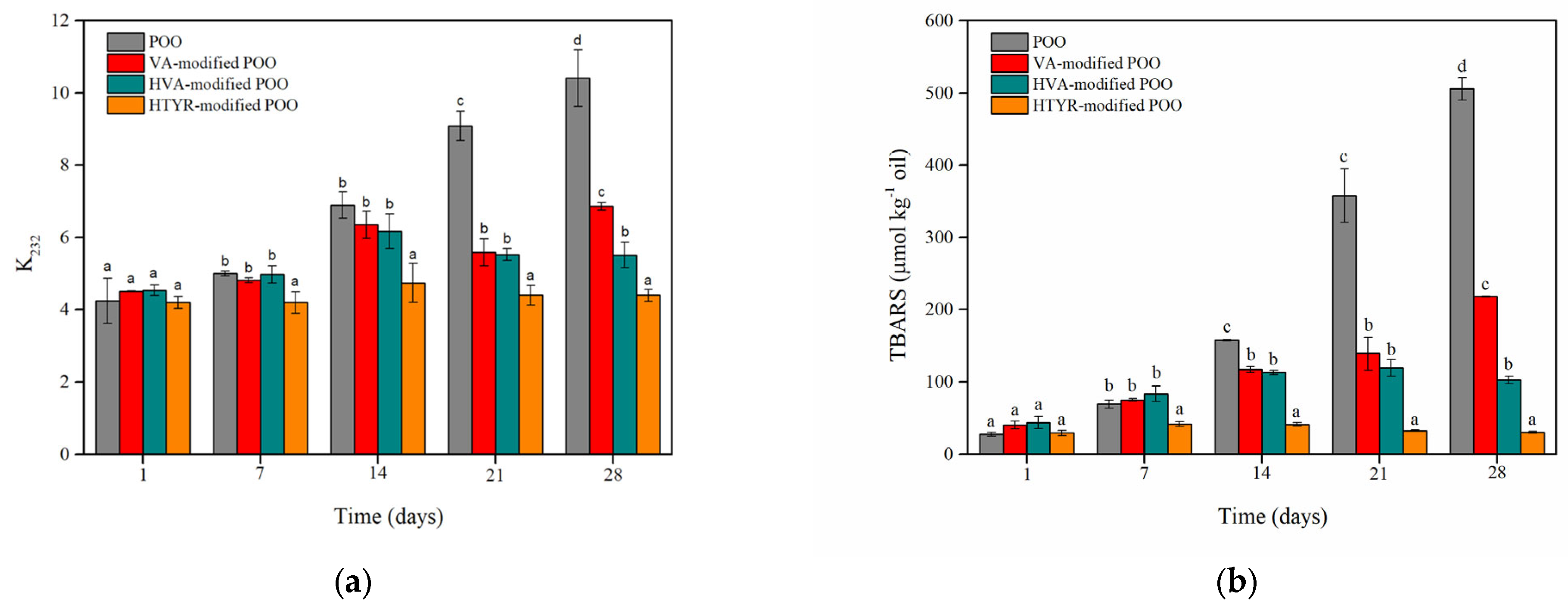
3.3.1. Evaluation of the Primary and Secondary Oxidation Products
3.3.2. Evaluation of Volatile Oxidative Products by SPME-GC/MS
3.3.3. Fluorescence Spectroscopy
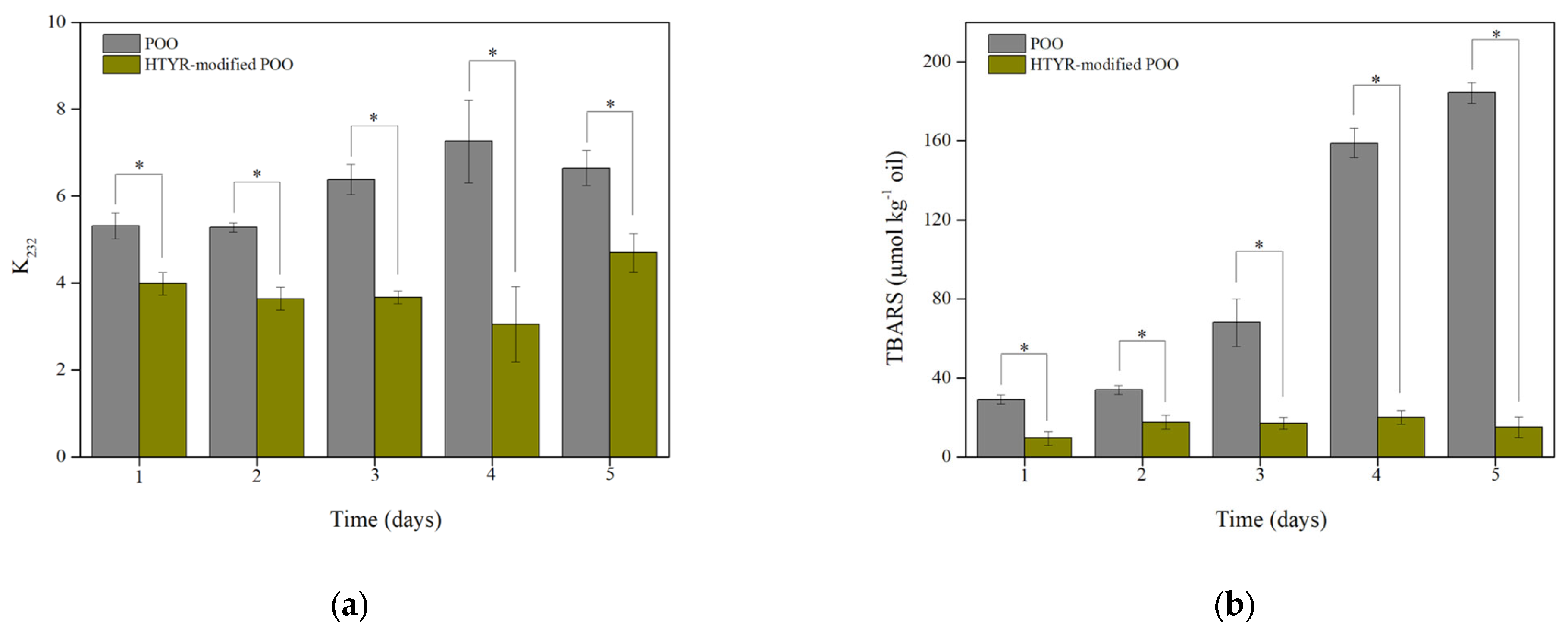
3.4. Oxidative Stability of Mayonnaise Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atallah, E.; Kwapinski, W.; Ahmad, M.N.; Leahy, J.J.; Al-Muhtaseb, A.H.; Zeaiter, J. Hydrothermal Carbonization of Olive Mill Wastewater: Liquid Phase Product Analysis. J. Environ. Chem. Eng. 2019, 7, 102833. [Google Scholar] [CrossRef]
- Bernini, R.; Carastro, I.; Santoni, F.; Clemente, M. Synthesis of Lipophilic Esters of Tyrosol, Homovanillyl Alcohol and Hydroxytyrosol. Antioxidants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzikonstantinou, A.V.; Giannakopoulou, A.; Spyrou, S.; Simos, Y.V.; Kontogianni, V.G.; Peschos, D.; Katapodis, P.; Polydera, A.C.; Stamatis, H. Production of Hydroxytyrosol Rich Extract from Olea Europaea Leaf with Enhanced Biological Activity Using Immobilized Enzyme Reactors. Environ. Sci. Pollut. Res. 2022, 29, 29624–29637. [Google Scholar] [CrossRef] [PubMed]
- Natalia, A.; Kim, S.J.; Kim, H.K. Antioxidant and Antibacterial Activity of Fatty Acid Vanillyl Ester Produced by Proteus Vulgaris K80 Lipase-Mediated Transesterification. J. Mol. Catal. B Enzym. 2016, 133, S475–S481. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Achmon, Y.; Fishman, A. The Antioxidant Hydroxytyrosol: Biotechnological Production Challenges and Opportunities. Appl. Microbiol. Biotechnol. 2015, 99, 1119–1130. [Google Scholar] [CrossRef]
- Liu, L.; Jin, C.; Zhang, Y. Lipophilic Phenolic Compounds (Lipo-PCs): Emerging Antioxidants Applied in Lipid Systems. RSC Adv. 2014, 4, 2879–2891. [Google Scholar] [CrossRef]
- González-Rámila, S.; Mateos, R.; García-Cordero, J.; Seguido, M.A.; Bravo-Clemente, L.; Sarriá, B. Olive Pomace Oil versus High Oleic Sunflower Oil and Sunflower Oil: A Comparative Study in Healthy and Cardiovascular Risk Humans. Foods 2022, 11, 2186. [Google Scholar] [CrossRef]
- Mateos, R.; Sarria, B.; Bravo, L. Nutritional and Other Health Properties of Olive Pomace Oil. Crit. Rev. Food Sci. Nutr. 2019, 60, 3506–3521. [Google Scholar] [CrossRef]
- Serra, A.; Conte, G.; Giovannetti, M.; Casarosa, L.; Agnolucci, M.; Ciucci, F.; Palla, M.; Bulleri, E.; Cappucci, A.; Servili, M.; et al. Olive Pomace in Diet Limits Lipid Peroxidation of Sausages from Cinta Senese Swine. Eur. J. Lipid Sci. Technol. 2018, 120, 1700236. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Agriopoulou, S.; Koidis, A.; Proestos, C.; Enshasy, H.A.E.; Varzakas, T. Recent Advances in Analytical Methods for the Detection of Olive Oil Oxidation Status during Storage along with Chemometrics, Authenticity and Fraud Studies. Biomolecules 2022, 12, 1180. [Google Scholar] [CrossRef]
- Arzola-Rodríguez, S.I.; Muñoz-Castellanos, L.N.; López-Camarillo, C.; Salas, E. Phenolipids, Amphipilic Phenolic Antioxidants with Modified Properties and Their Spectrum of Applications in Development: A Review. Biomolecules 2022, 12, 1897. [Google Scholar] [CrossRef]
- Xanthakis, E.; Theodosiou, E.; Magkouta, S.; Stamatis, H.; Loutrari, H.; Roussos, C.; Kolisis, F. Enzymatic Transformation of Flavonoids and Terpenoids: Structural and Functional Diversity of the Novel Derivatives. Pure Appl. Chem. 2010, 82, 1–16. [Google Scholar] [CrossRef]
- Zieniuk, B.; Groborz, K.; Wołoszynowska, M.; Ratusz, K.; Białecka-florjańczyk, E.; Fabiszewska, A. Enzymatic Synthesis of Lipophilic Esters of Phenolic Compounds, Evaluation of Their Antioxidant Activity and Effect on the Oxidative Stability of Selected Oils. Biomolecules 2021, 11, 314. [Google Scholar] [CrossRef]
- Fotiadou, R.; Chatzikonstantinou, A.V.; Hammami, M.A.; Chalmpes, N.; Moschovas, D.; Spyrou, K.; Polydera, A.C.; Avgeropoulos, A.; Gournis, D.; Stamatis, H. Green Synthesized Magnetic Nanoparticles as Effective Nanosupport for the Immobilization of Lipase: Application for the Synthesis of Lipophenols. Nanomaterials 2021, 11, 458. [Google Scholar] [CrossRef]
- Fotiadou, R.; Patila, M.; Hammami, M.A.; Enotiadis, A.; Moschovas, D.; Tsirka, K.; Spyrou, K.; Giannelis, E.P.; Avgeropoulos, A.; Paipetis, A.; et al. Development of Effective Lipase-Hybrid Nanoflowers Enriched with Carbon and Magnetic Nanomaterials for Biocatalytic Transformations. Nanomaterials 2019, 9, 808. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhu, S.; Bi, Y. Solvent-Free Enzymatic Synthesis of Feruloylated Structured Lipids by the Transesterification of Ethyl Ferulate with Castor Oil. Food Chem. 2014, 158, 292–295. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, M.; Shi, J.; Tang, H.; Deng, Q.; Huang, F.; Luo, D. Enzymatic Preparation of “Functional Oil” Rich in Feruloylated Structured Lipids with Solvent-Free Ultrasound Pretreatment. Food Chem. 2018, 248, 272–278. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EEC) No. 2568/91; On the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis; European Union: Brussels, Belgium, 1991. [Google Scholar]
- Ćorović, M.; Milivojević, A.; Simović, M.; Banjanac, K.; Pjanović, R.; Bezbradica, D. Enzymatically Derived Oil-Based L-Ascorbyl Esters: Synthesis, Antioxidant Properties and Controlled Release from Cosmetic Formulations. Sustain. Chem. Pharm. 2020, 15, 100231. [Google Scholar] [CrossRef]
- Milivojević, A.D.; Ćorović, M.M.; Simović, M.B.; Banjanac, K.M.; Blagojević, S.N.; Pjanović, R.V.; Bezbradica, D.I. Novel Approach for Flavonoid Esters Production: Statistically Optimized Enzymatic Synthesis Using Natural Oils and Application in Cosmetics. Ind. Eng. Chem. Res. 2019, 58, 3640–3649. [Google Scholar] [CrossRef]
- Harris, C.S.; Burt, A.J.; Saleem, A.; Le, P.M.; Martineau, L.C.; Haddad, P.S.; Bennett, S.A.L.; Arnason, J.T. A Single HPLC-PAD-APCI/MS Method for the Quantitative Comparison of Phenolic Compounds Found in Leaf, Stem, Root and Fruit Extracts of Vaccinium Angustifolium. Phytochem. Anal. 2007, 18, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tose, L.; Silva, S.; Barros, E.; Souza, L.; Pinto, F.; Palomino, D.; Freitas, J.; Thompson, C.; Vaz, B.; Lacerda, V., Jr.; et al. APCI(+)FT-ICR MS Analysis of Hydrocarbons Using Isooctane as Ionizing Reagent—A Comparison with HTGC-FID, GC×GC-MS and NMR. J. Braz. Chem. Soc. 2019, 30, 997–1009. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Bakan, K.S.; Erçaǧ, E.; Esin Çelik, S.; Baki, S.; Yildiz, L.; Karaman, Ş.; Apak, R. A Comprehensive Review of CUPRAC Methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Zeb, A.; Ullah, F. A Simple Spectrophotometric Method for the Determination of Thiobarbituric Acid Reactive Substances in Fried Fast Foods. J. Anal. Methods Chem. 2016, 2016, 9412767. [Google Scholar] [CrossRef] [Green Version]
- Beltrán, A.; Ramos, M.; Grané, N.; Martín, M.L.; Garrigós, M.C. Monitoring the Oxidation of Almond Oils by HS-SPME-GC-MS and ATR-FTIR: Application of Volatile Compounds Determination to Cultivar Authenticity. Food Chem. 2011, 126, 603–609. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of Secondary and Tertiary Volatile Compounds Resulting from the Lipid Oxidation of Rapeseed Oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef]
- Savaghebi, D.; Ghaderi-Ghahfarokhi, M.; Barzegar, M. Encapsulation of Sargassum Boveanum Algae Extract in Nano-Liposomes: Application in Functional Mayonnaise Production. Food Bioprocess Technol. 2021, 14, 1311–1325. [Google Scholar] [CrossRef]
- Lagunes-Galvez, L.; Cuvelier, M.-E.; Ordonnaud, C.; Berset, C. OXIDATIVE STABILITY OF SOME MAYONNAISE FORMULATIONS DURING STORAGE AND DAYLIGHT IRRADIATION. J. Food Lipids 2002, 9, 221–224. [Google Scholar] [CrossRef]
- Roby, M.H.; Allouche, A.; Dahdou, L.; De Castro, V.C.; Alves Da Silva, P.H.; Targino, B.N.; Huguet, M.; Paris, C.; Chrétien, F.; Guéant, R.M.; et al. Enzymatic Production of Bioactive Docosahexaenoic Acid Phenolic Ester. Food Chem. 2015, 171, 397–404. [Google Scholar] [CrossRef]
- Bouguerra Neji, S.; Bouaziz, M. Production of Biologically Active Hydroxytyrosol Rich Extract: Via Catalytic Conversion of Tyrosol. RSC Adv. 2022, 12, 2595–2602. [Google Scholar] [CrossRef]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.A.; Lindenblatt, C.T.; Masson, L.M.P.; Gomes, F.D.S.; Freitas-Silva, O.; Silva, J.P.L. Effect of oregano essential oil on oxidative stability of low-acid mayonnaise. IOSR J. Pharm. 2016, 11, 45–52. [Google Scholar]
- Chandrasekar, V.; Prasanna, D.B.; Regupathi, I. Effectiveness of Rutin and Its Lipophilic Ester in Improving Oxidative Stability of Sardine Oil Containing Trace Water. Int. J. Food Sci. Technol. 2018, 53, 541–548. [Google Scholar] [CrossRef]
- Koh, E.; Ryu, D.; Surh, J. Ratio of Malondialdehyde to Hydroperoxides and Color Change as an Index of Thermal Oxidation of Linoleic Acid and Linolenic Acid. J. Food. Process. Preserv. 2015, 39, 318–326. [Google Scholar] [CrossRef]
- Difonzo, G.; Pasqualone, A.; Silletti, R.; Cosmai, L.; Summo, C.; Paradiso, V.M.; Caponio, F. Use of Olive Leaf Extract to Reduce Lipid Oxidation of Baked Snacks. Food Res. Int. 2018, 108, 48–56. [Google Scholar] [CrossRef]
- Hao, S.; Zhu, L.; Sui, R.; Zuo, M.; Luo, N.; Shi, J.; Zhang, W.; He, X.; Chen, Z. Identification and Quantification of Vegetable Oil Adulteration with Waste Frying Oil by Laser-Induced Fluorescence Spectroscopy. OSA Contin. 2019, 2, 1148. [Google Scholar] [CrossRef]
- Kongbonga, Y.G.M.; Ghalila, H.; Onana, M.B.; Majdi, Y.; Lakhdar, Z.B.; Mezlini, H.; Sevestre-Ghalila, S. Characterization of Vegetable Oils by Fluorescence Spectroscopy. Food Nutr. Sci. 2011, 2, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, L.; Abdolmaleki, K.; Nayebzadeh, K.; Shahin, R. Effects of Tocopherol, Rosemary Essential Oil and Ferulago Angulata Extract on Oxidative Stability of Mayonnaise during Its Shelf Life: A Comparative Study. Food Chem. 2019, 285, 46–52. [Google Scholar] [CrossRef]
- Ozdemir, N.; Kantekin-Erdogan, M.N.; Tat, T.; Tekin, A. Effect of Black Cumin Oil on the Oxidative Stability and Sensory Characteristics of Mayonnaise. J. Food Sci. Technol. 2018, 55, 1562–1568. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotiadou, R.; Lefas, D.; Vougiouklaki, D.; Tsakni, A.; Houhoula, D.; Stamatis, H. Enzymatic Modification of Pomace Olive Oil with Natural Antioxidants: Effect on Oxidative Stability. Biomolecules 2023, 13, 1034. https://doi.org/10.3390/biom13071034
Fotiadou R, Lefas D, Vougiouklaki D, Tsakni A, Houhoula D, Stamatis H. Enzymatic Modification of Pomace Olive Oil with Natural Antioxidants: Effect on Oxidative Stability. Biomolecules. 2023; 13(7):1034. https://doi.org/10.3390/biom13071034
Chicago/Turabian StyleFotiadou, Renia, Dimitrios Lefas, Despina Vougiouklaki, Aliki Tsakni, Dimitra Houhoula, and Haralambos Stamatis. 2023. "Enzymatic Modification of Pomace Olive Oil with Natural Antioxidants: Effect on Oxidative Stability" Biomolecules 13, no. 7: 1034. https://doi.org/10.3390/biom13071034
APA StyleFotiadou, R., Lefas, D., Vougiouklaki, D., Tsakni, A., Houhoula, D., & Stamatis, H. (2023). Enzymatic Modification of Pomace Olive Oil with Natural Antioxidants: Effect on Oxidative Stability. Biomolecules, 13(7), 1034. https://doi.org/10.3390/biom13071034








_Stamatis.png)
