Pharmacological Potential of Betulin as a Multitarget Compound
Abstract
:1. Introduction
2. Natural Sources of Betulin
Methods of Isolation of Betulin
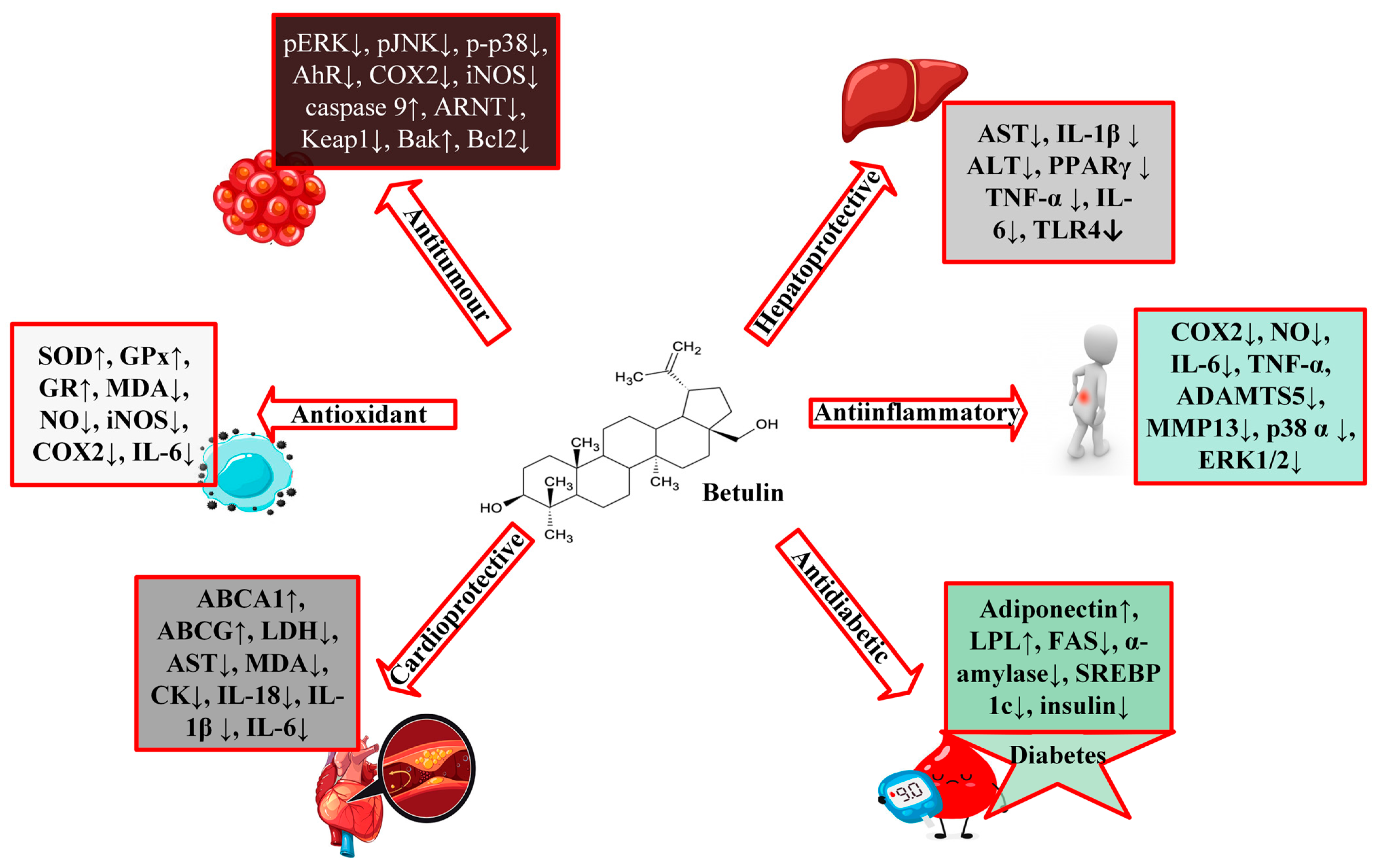
3. Pharmacological Activities of Betulin
3.1. Protective Effects of Betulin on Cardiovascular Diseases
3.2. Protective Effects of Betulin on Diabetes
3.3. Protective Effects of Betulin on Cancer
| Experimental Model | Dose/Concentration | Pharmacological Indicator | Molecular Mechanism | References | |
|---|---|---|---|---|---|
| Gastric SGC7901 cells | - | IC50 13 µg/mL | ROS ↑ Caspase 3 ↑ cleaved PARP ↑ Smac ↑ cytochrome c ↑ Bax ↑ Bak ↑ Bcl-2 ↓ XIAP ↓ Caspase 9 ↑ Bcl-xL * c-IAP1 * c-IAP2 * | Mitochondrial pathway | [68] |
| Human hepatoma HeLa cells | - | IC50 10 µg/mL | caspase9 ↑ caspase3/7 ↑ caspase 8 * cytochrome c ↑ Smac ↑ Bax ↑ Bak ↑ | Mitochondria pathway | [69] |
| Human lung adenocarcinomaA549 cells | - | 20 µM | enoyl-CoA hydratase ↓ PCBP 1 ↓ isoform 1 of 3-hydroxyacyl-CoA dehydrogenase type 2 ↓ malate dehydrogenase ↑ HSP 90-alpha 2 ↓ aconitate hydratase ↑ arginine/serine-rich splicing factor 1 ↑ | None | [65] |
| HepG2 cells | - | 10 µg/mL | Caspase 3 ↑ Caspase 9 ↑ | None | [80] |
| Murine CT26 human HCT116 | BALB/c mice injected intravenously with CT26 cells | 0–8 μM 5 and 10 mg/kg for 14 days | Bcl-2 ↓ CyclinD1/CDK4 ↓ Bax ↑ cleaved caspase-3, -9, and -PARP ↑ LC3-II ↑ beclin ↑ p-ERK ↓ p-p38 ↓ Bcl-xL ↓ p-JNK ↓ | AMPK activation Blockage of the MAPK signaling pathway Inhibition of Pi3k/Akt/mTOR signaling pathway | [72] |
| - | Female rats DMBA (25 mg/kg b.wt. s.c injection) | 20 mg/kg/b.wt. in corn oil (1 mL) | TBARS ↓ LOOH ↓ CAT ↑ SOD ↑ GPx ↑ Vit C ↑ Vit E ↑ GSH ↑ AhR ↓ ARnT ↓ CYP1A1 ↓ Keap1 ↓ HO-1 ↑ | Inhibition of MAPK proteins Activation of AhR/Nrf2 signaling axis | [81] |
| Human renal carcinoma cells (RCC4) | - | 10 and 25 μM | cleaved caspase3/7 ↑ cleaved caspase 8 ↑ cleaved PARP ↑ TRAIL R1/DR4 and R2/DR5 ↑ TNFR1 ↑ Bax ↑ XIAP ↓ PUMA ↑ Bcl-2 ↓ cleaved caspase 9 ↑ | Activated mitochondrial apoptotic signaling and inhibited NFκB pathway | [76] |
| Non-small lung cancer cells (H460) | - | 11 and 30 μM | p53 ↓ Bcl-2L1 ↓ MMP2/9 ↓ BAK ↑ BAX ↑ caspase 3 ↑ caspase 6 ↑ caspase 9 ↑ caspase 8 ↓ HRK ↑ VEGF ↓ COX2 ↓ osteopontin ↓ | Mitochondrial intrinsic pathway | [27] |
| Human colon cancer cells (HCT116 and HT29) | - | 10 μg/mL | cleaved caspase 9 ↑ cleaved caspase 3 ↑ cytochrome c ↑ Bim ↑ | Induction of NOXA | [82] |
| Renal cell carcinoma (786-O and Caki-2) | - | 5 μM | p-S6 ↓ p-4EBP1 ↓ PKM2 ↓ HK2 ↓ | Modulation of mTOR signaling pathway | [83] |
| Human osteosarcoma cell (HOS and MG-63) | - | 0–20 μM | cleaved caspase 3 ↑ cleaved PARP ↑ p-mTOR ↓ cleaved caspase 9 ↑ p-4E-BP1 ↓ LC3-II ↑ cleaved caspase 8 ↑ p-Akt ↑ | Inhibition of mTOR signaling Activating autophagy | [84] |
| - | Male Wistar Rat (DMH 20 mg/kg b.wt. s.c.) | 20 mg/kg b.wt for 16 weeks | GSH ↑ GPx ↑ SOD ↑ CAT ↑ IL-1β ↓ CYP450 ↓ CYT-b5 ↓ GST ↑ GR ↑ COX-2 ↓ iNOS ↓ TNF-α ↓ PCNA ↓ cyclin D1 ↓ IL-6 ↓ | None | [85] |
| human ovarian carcinoma cells (OVCAR-3) | - | 0–120 μM | Cyclin-D1 ↓ Bad ↑ Bax ↑ Bcl-2 ↓ Bcl-xL ↓ Cyclin-B1 ↑ Cyclin-E1 ↑ | modulating mTOR/Pi3k/Akt signaling pathway | [86] |
3.4. Protective Effects of Betulin on Liver Diseases
3.5. Protective Effects of Betulin on Inflammation
3.5.1. Preclinical Evidence
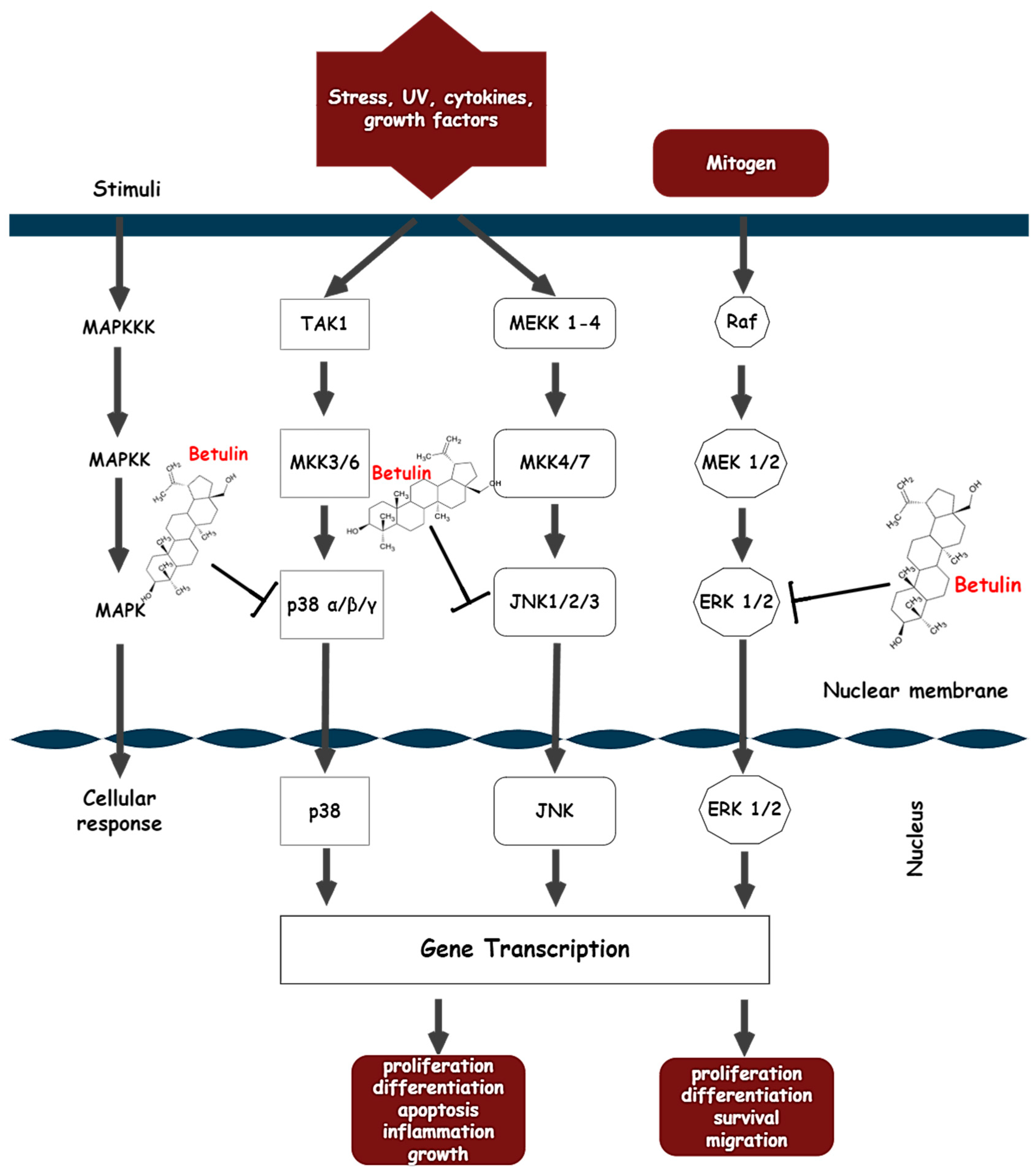
3.5.2. Molecular Mechanisms: Multiple Targets of Betulin
- Modulation of inflammatory cytokines
- Inhibition of reactive oxygen species (ROS) production
- Action on the Nrf2 signaling pathway
- Action on the NFκB signaling pathway
- Action on the MAPK signaling pathway
4. Clinical Evidence of Betulin
5. Betulin Derivatives
6. Pharmacokinetics of Betulin
7. Limitations and Future Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef] [Green Version]
- Šiman, P.; Filipová, A.; Tichá, A.; Niang, M.; Bezrouk, A.; Havelek, R. Effective method of purification of betulin from birch bark: The importance of its purity for scientific and medicinal use. PLoS ONE 2016, 11, e0154933. [Google Scholar] [CrossRef] [Green Version]
- Laavola, M.; Haavikko, R.; Hämäläinen, M.; Leppänen, T.; Nieminen, R.; Alakurtti, S.; Moreira, V.M.; Yli-Kauhaluoma, J.; Moilanen, E. Betulin Derivatives Effectively Suppress Inflammation in vitro and in vivo. J. Nat. Prod. 2016, 79, 274–280. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Z.; Liu, W.; Han, X.; Zhao, M. Betulin attenuates lung and liver injuries in sepsis. Int. Immunopharmacol. 2016, 30, 50–56. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Hayek, E.W.H.; Jordis, U.; Moche, W.; Sauter, F. A bicentennial of betulin. Phytochemistry 1989, 28, 2229–2242. [Google Scholar] [CrossRef]
- Tolstikov, G.A.; Flekhter, O.B.; Shultz, E.E.; Baltina, L.A.; Tolstikov, A.G. Betulin and Its Derivatives. Chemistry and Biological Activity. Chem. Sustain. Dev. 2005, 13, 1–29. [Google Scholar]
- Yamashita, K.; Lu, H.; Lu, J.; Chen, G.; Yokoyama, T.; Sagara, Y.; Manabe, M.; Kodama, H. Effect of three triterpenoids, lupeol, betulin, and betulinic acid on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta 2002, 325, 91–96. [Google Scholar] [CrossRef]
- Yang, X.; Peng, Q.; Liu, Q.; Hu, J.; Tang, Z.; Cui, L.; Lin, Z.; Xu, B.; Lu, K.; Yang, F.; et al. Antioxidant activity against h2o2-induced cytotoxicity of the ethanol extract and compounds from pyrola decorate leaves. Pharm. Biol. 2017, 55, 1843–1848. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.R.; Rajput, N.; Panchal, H.S.; Dalwadi, H.B. Quantification of Lupeol and Betulin in Ougenia dalbergioides Bark by Column Chromatography and TLC. J. Pharm. Sci. Biosci. Res. 2017, 7, 114–120. [Google Scholar]
- Barakat, K.; Saleh, M. Bioactive Betulin produced by marine Paecilomyces WE3-F. J. Appl. Pharm. Sci. 2016, 6, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Drag, M.; Surowiak, P.; Malgorzata, D.Z.; Dietel, M.; Lage, H.; Oleksyszyn, J. Comparision of the Cytotoxic Effects of Birch Bark Extract, Betulin and Betulinic Acid Towards Human Gastric Carcinoma and Pancreatic Carcinoma Drug-sensitive and Drug-Resistant Cell Lines. Molecules 2009, 14, 1639–1651. [Google Scholar] [CrossRef]
- De Moraes, W.F.; Galdino, P.M.; Nascimento, M.V.M.; Vanderlinde, F.A.; Bara, M.T.F.; Costa, E.A.; De Paula, J.R. Triterpenes involved in the anti-inflammatory effect of ethanolic extract of Pterodon emarginatus Vogel stem bark. J. Nat. Med. 2012, 66, 202–207. [Google Scholar] [CrossRef]
- Co, M.; Koskela, P.; Eklund-Åkergren, P.; Srinivas, K.; King, J.W.; Sjöberg, P.J.R.; Turner, C. Pressurized liquid extraction of betulin and antioxidants from birch bark. Green Chem. 2009, 11, 668–674. [Google Scholar] [CrossRef]
- Amol Singh, P.; Brindavanam, N.B.; Kimothi, G.P.; Verma, R.; Aeri, V. A Validated HPLC method for the determination of betulin in the stem bark of Tectona grandis Linn. Int. J. Pharm. Sci. Res. 2016, 7, 719–723. [Google Scholar] [CrossRef]
- Şoica, C.; Dehelean, C.; Peev, C.; Aluas, M.; Zupkó, I.; Kása, P., Jr.; Alexa, E. Physico-chemical comparison of betulinic acid, betulin and birch bark extract and in vitro investigation of their cytotoxic effects towards skin epidermoid carcinoma (A431), breast carcinoma (MCF7) and cervix adenocarcinoma (HeLa) cell lines. Nat. Prod. Res. 2012, 26, 968–974. [Google Scholar] [CrossRef]
- Khan, M.J.; Saraf, S.; Saraf, S. Anti-inflammatory and associated analgesic activities of HPLC standardized alcoholic extract of known ayurvedic plant Schleichera oleosa. J. Ethnopharmacol. 2017, 197, 257–265. [Google Scholar] [CrossRef]
- Bai, Y.H.; Feng, Y.Q.; Mao, D.B.; Xu, C.P. Optimization for betulin production from mycelial culture of Inonotus obliquus by orthogonal design and evaluation of its antioxidant activity. J. Taiwan Inst. Chem. Eng. 2012, 43, 663–669. [Google Scholar] [CrossRef]
- Felföldi-Gáva, A.; Szarka, S.; Simándi, B.; Blazics, B.; Simon, B.; Kéry, Á. Supercritical fluid extraction of Alnus glutinosa (L.) Gaertn. J. Supercrit. Fluids 2012, 61, 55–61. [Google Scholar] [CrossRef]
- Ko, B.S.; Kang, S.; Moon, B.R.; Ryuk, J.A.; Park, S. A 70% Ethanol Extract of Mistletoe Rich in Betulin, Betulinic Acid, and Oleanolic Acid Potentiated β-Cell Function and Mass and Enhanced Hepatic Insulin Sensitivity. Evid.-Based Complement. Altern. Med. 2016, 2016, 7836823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penkov, D.; Dimitrova, S.; Andonova, V.; Milieva, E.; Murdjeva, M.; Stanimirova, I.; Draganov, M.; Kassarova, M. Biological activity of bulgarian folia betulae dry extract. Int. J. Pharm. Pharm. Sci. 2015, 7, 94–99. [Google Scholar]
- Misra, T.N.; Singh, R.S.; Pandey, H.S.; Singh, B.K.; Pandey, R.P. Constituents of Asteracantha longifolia. Fitoterapia 2001, 72, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Laiolo, J.; Barbieri, C.L.; Joray, M.B.; Lanza, P.A.; Palacios, S.M.; Vera, D.M.A.; Carpinella, M.C. Plant extracts and betulin from Ligaria cuneifolia inhibit P-glycoprotein function in leukemia cells. Food Chem. Toxicol. 2021, 147, 111922. [Google Scholar] [CrossRef]
- Zhou, Y.; Weng, X.; Dou, R.; Tan, X.; Zhang, T.; Fang, J.; Wu, X. Betulin from Hedyotis hedyotidea ameliorates concanavalin A-induced and T cell-mediated autoimmune hepatitis in mice. Acta Pharmacol. Sin. 2017, 38, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Zehra, B.; Ahmed, A.; Sarwar, R.; Khan, A.; Farooq, U.; Ali, S.A.; Al-Harrasi, A. Apoptotic and antimetastatic activities of betulin isolated from Quercus incana against non-small cell lung cancer cells. Cancer Manag. Res. 2019, 11, 1667–1683. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Arora, S.; Singh, R. Isolation, characterization and biological activities of betulin from Acacia nilotica bark. Sci. Rep. 2022, 12, 9370. [Google Scholar] [CrossRef]
- Hridya, V.; Godson, P.; Chandrasekar, N. Chromatographic identification of two biologically important triterpenoids from the chloroform extract of Rhizophora mucronata. Acta Chromatogr. 2012, 24, 123–129. [Google Scholar] [CrossRef]
- Azaat, A.; Babojian, G.; Issa, N. Phytochemical Screening, Antioxidant and Anticancer Activities of Euphorbia hyssopifolia L. against MDA-MB-231 Breast Cancer Cell Line. J. Turk. Chem. Soc. Sect. A Chem. 2022, 9, 295–310. [Google Scholar] [CrossRef]
- Radhakrishna, S.; Kumari, S.P. GCMS Analysis of total terpenoids from Baliospermum montanum and its antimicrobial activity. IAETSD J. Adv. Res. Appl. Sci. 2018, 5, 94–101. [Google Scholar]
- Lee, S.O.; Lee, M.H.; Lee, K.R.; Lee, E.O.; Lee, H.J. Fomes fomentarius ethanol extract exerts inhibition of cell growth and motility induction of apoptosis via targeting AKT in human breast cancer MDA-MB-231 cells. Int. J. Mol. Sci. 2019, 20, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, M.T.; Buzzi, F.D.C.; Cechinel Filho, V.; Hess, S.; Delle Monache, F.; Niero, R. Phytochemical and antinociceptive properties of Matayba elaeagnoides Radlk. barks. Z. Fur Naturforsch. Sect. C J. Biosci. 2007, 62, 550–554. [Google Scholar] [CrossRef] [Green Version]
- Qaisar, M.N.; Uzair, M.; Imran, M.; Chaudhary, B.A.; Hussain, S.N. New α-glucosidase inhibitors from Croton bonplandianum Croton bonplandianum Baill (Euphorbiaceae). Trop. J. Pharm. Res. 2016, 15, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, Y.; Guo, G.; Li, J.; Fang, X.; Zhao, L. Enhanced and green extraction betulin from Celtis sinensis leaves using hydrophobic deep eutectic solvent. Biomass Convers. Biorefinery 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Ishikawa, R.B.; Leitão, M.M.; Kassuya, R.M.; Macorini, L.F.; Moreira, F.M.F.; Cardoso, C.A.L.; Coelho, R.G.; Pott, A.; Gelfuso, G.M.; Croda, J.; et al. Anti-inflammatory, antimycobacterial and genotoxic evaluation of Doliocarpus dentatus. J. Ethnopharmacol. 2017, 204, 18–25. [Google Scholar] [CrossRef]
- Ahmadu, A.A.; Delehouzé, C.; Haruna, A.; Mustapha, L.; Lawal, B.A.; Udobre, A.; Baratte, B.; Triscornia, C.; Autret, A.; Robert, T.; et al. Betulin, a newly characterized compound in Acacia auriculiformis bark, is a multi-target protein kinase inhibitor. Molecules 2021, 26, 4599. [Google Scholar] [CrossRef]
- Ju, A.; Cho, Y.C.; Cho, S. Methanol extracts of Xanthium sibiricum roots inhibit inflammatory responses via the inhibition of nuclear factor-κB (NF-κB) and signal transducer and activator of transcription 3 (STAT3) in murine macrophages. J. Ethnopharmacol. 2015, 174, 74–81. [Google Scholar] [CrossRef]
- Razwinani, M.; Motaung, K.S. The influence of friedelin, resinone, tingenone and betulin of compounds on chondrogenic differentiation of porcine adipose-derived mesenchymal stem cells (pADMSCs). Biochimie 2022, 196, 234–242. [Google Scholar] [CrossRef]
- Wexler, R.; Elton, T.; Pleister, A.; Feldman, D. Cardiomyopathy: An Overview. Am. Fam. Physician 2009, 79, 778–784. [Google Scholar]
- Ayyappan, J.P.; Lizardo, K.; Wang, S.; Yurkow, E.; Nagajyothi, J.F. Inhibition of SREBP Improves Cardiac Lipidopathy, Improves Endoplasmic Reticulum Stress, and Modulates Chronic Chagas Cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e014255. [Google Scholar] [CrossRef]
- Yu, C.; Cai, X.; Liu, X.; Liu, J.; Zhu, N. Betulin Alleviates Myocardial Ischemia–Reperfusion Injury in Rats via Regulating the Siti1/NLRP3/NF-κB Signaling Pathway. Inflammation 2021, 44, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Geng, L.; Zhou, L.; Pei, X.; Yang, Z.; Ding, Z. Betulin alleviates on myocardial inflammation in diabetes mice via regulating Siti1/NLRP3/NF-κ-B pathway. Int. Immunopharmacol. 2020, 85, 106653. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.J.; Li, J.G.; Qi, W.; Qiu, W.W.; Li, P.S.; Li, B.L.; Song, B.L. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 2011, 13, 44–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, Y.; Yan, H.; Gao, F.; Xi, C.; Li, H.; Wang, Y. Betulin attenuates atherosclerosis in apoE−/− mice by up-regulating ABCA1 and ABCG1. Acta Pharmacol. Sin. 2016, 37, 1337–1348. [Google Scholar] [CrossRef] [Green Version]
- Muceniece, R.; Namniece, J.; Nakurte, I.; Jekabsons, K.; Riekstina, U.; Jansone, B. Pharmacological research on natural substances in Latvia: Focus on lunasin, betulin, polyprenol and phlorizin. Pharmacol. Res. 2016, 113, 760–770. [Google Scholar] [CrossRef]
- Ma, C.; Long, H. Protective effect of betulin on cognitive decline in streptozotocin (STZ)-induced diabetic rats. Neurotoxicology 2016, 57, 104–111. [Google Scholar] [CrossRef]
- Alsaadi, J.H.H. Isolation, Purification and Identification of Active Chemical Compound Lup-20(29)-ene-3, 28-diol (Betulin) from Tetradium daniellii Leaves and Study the hypoglycemic Effect on Rabbits. Univ. Thi-Qar J. Sci. 2016, 6, 54–61. [Google Scholar] [CrossRef]
- Ratna Wulan, D.; Priyo Utomo, E.; Mahdi, C. Antidiabetic Activity of Ruellia tuberosa L., Role of α -Amylase Inhibitor: In Silico, In Vitro, and In Vivo Approaches. Biochem. Res. Int. 2015, 2015, 349261. [Google Scholar] [CrossRef] [Green Version]
- Wulan, D.R.; Utomo, E.P.; Mahdi, C. Molecular modeling of Ruellia tuberosa L compounds as a-amylase inhibitor: An in silico comparation between human and rat enzyme model. Bioinformation 2014, 10, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Thengyai, S.; Thiantongin, P.; Sontimuang, C.; Ovatlarnporn, C.; Puttarak, P. α-Glucosidase and α-amylase inhibitory activities of medicinal plants in Thai antidiabetic recipes and bioactive compounds from Vitex glabrata R. Br. stem bark. J. Herb. Med. 2020, 19, 100302. [Google Scholar] [CrossRef]
- Gurupriya, S.; Cathrine, L. Molecular docking studies of isolated compounds andrographolide and betulin from methanolic leaves extract of Andrographis echioides as alpha-amylase and alpha-glucosidase activators. Int. J. Appl. Pharm. 2021, 13, 121–129. [Google Scholar] [CrossRef]
- Yuca, H.; Özbek, H.; Demirezer, L.; Güvenalp, Z. Assessment of α-glucosidase and α-amylase inhibitory potential of Paliurus spina-christi Mill. and its terpenic compounds. Med. Chem. Res. 2022, 31, 1393–1399. [Google Scholar] [CrossRef]
- Ilyina, A.; Arredondo-Valdés, R.; Farkhutdinov, S.; Segura-Ceniceros, E.P.; Martínez-Hernández, J.L.; Zaynullin, R.; Kunakova, R. Effect of Betulin-containing Extract from Birch Tree Bark on α-Amylase Activity In vitro and on Weight Gain of Broiler Chickens In vivo. Plant Foods Hum. Nutr. 2014, 69, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Wardecki, T.; Werner, P.; Thomas, M.; Templin, M.F.; Schmidt, G.; Brandner, J.M.; Merfort, I. Influence of Birch Bark Triterpenes on Keratinocytes and Fibroblasts from Diabetic and Nondiabetic Donors. J. Nat. Prod. 2016, 79, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P.V. Therapeutic Perspectives of Molecules from Urtica dioica Extracts for Cancer Treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [Green Version]
- Tuli, H.S.; Sak, K.; Gupta, D.S.; Kaur, G.; Aggarwal, D.; Parashar, N.C.; Choudhary, R.; Yerer, M.B.; Kaur, J.; Kumar, M.; et al. Anti-inflammatory and anticancer properties of birch bark-derived betulin: Recent developments. Plants 2021, 10, 2663. [Google Scholar] [CrossRef]
- Novío, S.; Cartea, M.E.; Soengas, P.; Freire-Garabal, M.; Núñez-Iglesias, M.J. Effects of Brassicaceae isothiocyanates on prostate cancer. Molecules 2016, 21, 626. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.S.; Si, H.R.; Dae, K.K.; Jin, G.L.; Yong, Y.L.; Soon, S.H.; Sung, W.K.; Jeong, H.P. Anti-cancer effect of betulin on a human lung cancer cell line: A pharmacoproteomic approach using 2 D SDS PAGE coupled with nano-HPLC tandem mass spectrometry. Planta Med. 2009, 75, 127–131. [Google Scholar] [CrossRef]
- Gauthier, C.; Legault, J.; Lavoie, S.; Rondeau, S.; Tremblay, S.; Pichette, A. Synthesis and cytotoxicity of bidesmosidic betulin and betulinic acid saponins. J. Nat. Prod. 2009, 72, 72–81. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Feflea, S.; Molnár, J.; Zupko, I.; Soica, C. Betulin as an antitumor agent tested in vitro on A431, HeLa and MCF7, and as an angiogenic inhibitor in vivo in the CAM assay. Nat. Prod. Commun. 2012, 7, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, X.; Jiang, D.; Lin, Y.; Wang, Y.; Li, Q.; Liu, L.; Jin, Y.H. Betulin induces reactive oxygen species-dependent apoptosis in human gastric cancer SGC7901 cells. Arch. Pharm. Res. 2016, 39, 1257–1265. [Google Scholar] [CrossRef]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulin is a potent anti-tumor agent that is enhanced by cholesterol. PLoS ONE 2009, 4, e1. [Google Scholar] [CrossRef] [Green Version]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Juszczak, M.; Grabarska, A.; Sifringer, M.; Kaczor, J.; Kandefer-Szerszeń, M. Betulin Elicits Anti-Cancer Effects in Tumour Primary Cultures and Cell Lines in vitro. Basic Clin. Pharmacol. Toxicol. 2009, 105, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Mun, J.G.; Jeon, H.D.; Kee, J.Y. Betulin inhibits lung metastasis by inducing cell cycle arrest, autophagy, and apoptosis of metastatic colorectal cancer cells. Nutrients 2020, 12, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehelean, C.A.; Feflea, S.; Gheorgheosu, D.; Ganta, S.; Cimpean, A.M.; Muntean, D.; Amiji, M.M. Anti-angiogenic and anti-cancer evaluation of betulin nanoemulsion in chicken chorioallantoic membrane and skin carcinoma in Balb/c mice. J. Biomed. Nanotechnol. 2013, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.H.; Duan, X.Z.; Pan, J.Y.; Li, W.M.; Zhang, X.D.; Zhang, Y.S. Involvement of protein kinase C-δ activation in betulin-induced apoptosis of neuroblastoma. Trop. J. Pharm. Res. 2017, 16, 2097–2105. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.-H.; Choi, J.-E.; Lim, S.-C. Protection of betulin against cadmium-induced apoptosis in hepatoma cells. Toxicology 2006, 220, 1–12. [Google Scholar] [CrossRef]
- Yim, N.H.; Jung, Y.P.; Kim, A.; Kim, T.; Ma, J.Y. Induction of apoptotic cell death by betulin in multidrug-resistant human renal carcinoma cells. Oncol. Rep. 2015, 34, 1058–1064. [Google Scholar] [CrossRef] [Green Version]
- Yin, F.; Feng, F.; Wang, L.; Wang, X.; Li, Z.; Cao, Y. SREBP-1 inhibitor Betulin enhances the antitumor effect of Sorafenib on hepatocellular carcinoma via restricting cellular glycolytic activity. Cell Death Dis. 2019, 10, 672. [Google Scholar] [CrossRef] [Green Version]
- Kvasnica, M.; Sarek, J.; Klinotova, E.; Dzubak, P.; Hajduch, M. Synthesis of phthalates of betulinic acid and betulin with cytotoxic activity. Bioorganic Med. Chem. 2005, 13, 3447–3454. [Google Scholar] [CrossRef]
- Gao, H.; Wu, L.; Kuroyanagi, M.; Harada, K.; Kawahara, N.; Nakane, T.; Umehara, K.; Hirasawa, A.; Nakamura, Y. Antitumor-promoting constituents from Chaenomeles sinensis Koehne and their activities in JB6 mouse epidermal cells. Chem. Pharm. Bull. 2003, 51, 1318–1321. [Google Scholar] [CrossRef] [Green Version]
- Taiyi, S.J. Caspase-9 activation-critical for betulin-induced apoptosis of human hepatoma cells. Chem. Res. Chin. Univ. 2010, 26, 792–797. [Google Scholar]
- Zhang, J.; Zhou, B.; Sun, J.; Chen, H.; Yang, Z. Betulin ameliorates 7,12-dimethylbenz(a)anthracene-induced rat mammary cancer by modulating MAPK and AhR/Nrf-2 signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, C.; Cai, Z.; Zhao, F.; He, L.; Lou, X.; Qi, X. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol. Lett. 2018, 15, 7319–7327. [Google Scholar] [CrossRef]
- Cheng, W.; Ji, S.; Zhang, H.; Han, Z.; Liu, Q.; Wang, J.; Ping, H. mTOR activation is critical for betulin treatment in renal cell carcinoma cells. Biochem. Biophys. Res. Commun. 2017, 482, 1030–1036. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, H.Y.; Hsieh, C.P.; Huang, Y.F.; Chang, I.L. Betulin inhibits mTOR and induces autophagy to promote apoptosis in human osteosarcoma cell lines. Environ. Toxicol. 2020, 35, 879–887. [Google Scholar] [CrossRef]
- Yu, J.; Li, M.; Zhan, D.; Shi, C.; Fang, L.; Ban, C.; Zheng, W.; Veeraraghavan, V.; Mohan, S.; Tang, X. Inhibitory effects of triterpenoid betulin on inflammatory mediators inducible nitric oxide synthase, cyclooxygenase-2, tumor necrosis factor-alpha, interleukin-6, and proliferating cell nuclear antigen in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Pharmacogn. Mag. 2020, 16, 836. [Google Scholar] [CrossRef]
- Yang, Q.; Fei, Z.; Huang, C. Betulin terpenoid targets OVCAR-3 human ovarian carcinoma cells by inducing mitochondrial mediated apoptosis, G2/M phase cell cycle arrest, inhibition of cell migration and invasion and modulating mTOR/PI3K/AKT signalling pathway. Cell. Mol. Biol. 2021, 67, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.M.; Zan, T.; Li, H.Y.; Han, J.F.; Liu, Z.M.; Huang, J.; Dong, L.H.; Zhang, H.N. Betulin inhibits lipopolysaccharide/D-galactosamine-induced acute liver injury in mice through activating PPAR-γ. Biomed. Pharmacother. 2018, 106, 941–945. [Google Scholar] [CrossRef]
- Buko, V.; Kuzmitskaya, I.; Kirko, S.; Belonovskaya, E.; Naruta, E.; Lukivskaya, O.; Shlyahtun, A.; Ilyich, T.; Zakreska, A.; Zavodnik, I. Betulin attenuated liver damage by prevention of hepatic mitochondrial dysfunction in rats with alcoholic steatohepatitis. Physiol. Int. 2019, 106, 323–334. [Google Scholar] [CrossRef]
- Bai, T.; Yang, Y.; Yao, Y.-L.; Sun, P.; Lian, L.-H.; Wu, Y.-L.; Nan, J.-X. Betulin alleviated ethanol-induced alcoholic liver injury via SIRT1/AMPK signaling pathway. Pharmacol. Res. 2016, 105, 1–12. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Plewka, K.; Kandefer-Szerszeń, M. Betulin, betulinic acid and butein are inhibitors of acetaldehyde-induced activation of liver stellate cells. Pharmacol. Rep. 2011, 63, 1109–1123. [Google Scholar] [CrossRef]
- Wan, Y.; Jiang, S.; Lian, L.H.; Bai, T.; Cui, P.H.; Sun, X.T.; Jin, X.J.; Wu, Y.L.; Nan, J.X. Betulinic acid and betulin ameliorate acute ethanol-induced fatty liver via TLR4 and STAT3 in vivo and in vitro. Int. Immunopharmacol. 2013, 17, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Kandefer-Szerszeń, M. Protective effects of betulin and betulinic acid against ethanol-induced cytotoxicity in HepG2 cells. Pharmacol. Rep. 2005, 57, 588–595. [Google Scholar] [PubMed]
- Eisa, N.H.; El-Sherbiny, M.; Abo El-Magd, N.F. Betulin alleviates cisplatin-induced hepatic injury in rats: Targeting apoptosis and Nek7-independent NLRP3 inflammasome pathways. Int. Immunopharmacol. 2021, 99, 107925. [Google Scholar] [CrossRef]
- Dou, J.Y.; Jiang, Y.C.; Hu, Z.H.; Yao, K.C.; Yuan, M.H.; Bao, X.X.; Zhou, M.J.; Liu, Y.; Li, Z.X.; Lian, L.H.; et al. Betulin Targets Lipin1/2-Meidated P2X7 Receptor as a Therapeutic Approach to Attenuate Lipid Accumulation and Metaflammation. Biomol. Ther. 2022, 30, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszeń, M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signaling. Toxicology 2011, 280, 152–163. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2014, 19, 1871–1882. [Google Scholar] [CrossRef]
- Guo, M.Y.; Li, W.Y.; Zhang, Z.; Qiu, C.; Li, C.; Deng, G. Betulin suppresses S. aureus-induced mammary gland inflammatory injury by regulating PPAR-γ in mice. Int. Immunopharmacol. 2015, 29, 824–831. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, Q.; Hu, X.; Shen, H.; Li, F. Betulin attenuates kidney injury in septic rats through inhibiting TLR4/NF-κB signaling pathway. Life Sci. 2016, 144, 185–193. [Google Scholar] [CrossRef]
- Pfarr, K.; Danciu, C.; Arlt, O.; Neske, C.; Dehelean, C.; Pfeilschifter, J.M.; Radeke, H.H. Simultaneous and dose dependent melanoma cytotoxic and immune stimulatory activity of betulin. PLoS ONE 2015, 10, e0118802. [Google Scholar] [CrossRef]
- Ra, H.J.; Lee, H.J.; Jo, H.S.; Nam, D.C.; Lee, Y.B.; Kang, B.H.; Moon, D.K.; Kim, D.H.; Lee, C.J.; Hwang, S.C. Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat. Korean J. Physiol. Pharmacol. 2017, 21, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.Y.; Sadhasivam, S.; Lin, F.H. The dose dependent effects of betulin on porcine chondrocytes. Process Biochem. 2009, 44, 678–684. [Google Scholar] [CrossRef]
- Kamaraj, Y.; Dhayalan, S.; Chinnaiyan, U.; Kumaresan, V.; Subramaniyan, S.; Kumar, D.; Muniyandi, K.; Punamalai, G. Triterpenoid compound betulin attenuates allergic airway inflammation by modulating antioxidants, inflammatory cytokines and tissue transglutaminase in ovalbumin-induced asthma mice model. J. Pharm. Pharmacol. 2021, 73, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, S.; Naumann, K.; Pollok, S.; Wardecki, T.; Vidal-y-Sy, S.; Nascimento, J.M.; Boerries, M.; Schmidt, G.; Brandner, J.M.; Merfort, I. From a traditional medicinal plant to a rational drug: Understanding the clinically proven wound healing efficacy of birch bark extract. PLoS ONE 2014, 9, e86147. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Niu, S.; Sun, Y.; Liang, Y.; Zhao, J.; Zhang, T.; Zhang, J. Anti-inflammatory action of betulin and its potential as a dissociated glucocorticoid receptor modulator. Food Chem. Toxicol. 2021, 157, 112539. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Lin, F.-H.; Sadhasivam, S.; Savitha, S. Antioxidant effects of betulin on porcine chondrocyte behavior in gelatin/C6S/C4S/HA modified tricopolymer scaffold. Mater. Sci. Eng. C 2010, 30, 597–604. [Google Scholar] [CrossRef]
- Ouyang, T.; Yin, H.; Yang, J.; Liu, Y.; Ma, S. Tissue regeneration effect of betulin via inhibition of ROS/MAPKs/NF-ĸB axis using zebrafish model. Biomed. Pharmacother. 2022, 153, 113420. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Jin, J.; Hu, W.; Chen, Q.; Yang, J.; Wu, Y.; Zhou, Y.; Sun, L.; Gao, W.; Zhang, X.; et al. Betulin Alleviates the Inflammatory Response in Mouse Chondrocytes and Ameliorates Osteoarthritis via AKT/Nrf2/HO-1/NF-κB Axis. Front. Pharmacol. 2021, 12, 754038. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Ci, X.; Zhou, J.; Lv, H.; Yu, Q.; Peng, L.; Hua, S. Betulin exhibits anti-inflammatory activity in lps-stimulated macrophages and endotoxin-shocked mice through an ampk/akt/nrf2-dependent mechanism. Cell Death Dis. 2017, 8, e2798. [Google Scholar] [CrossRef] [Green Version]
- Szuster-Ciesielska, A.; Pilipów, K.; Kandefer-Szerszeń, M. Protective effect of betulin and betulinic acid on acetaminophen and ethanol-induced cytotoxicity and reactive oxygen species production in HepG2 cells. J. Pre-Clin. Clin. Res. 2010, 4, 96–100. [Google Scholar]
- Kruszniewska-Rajs, C.; Strzałka-Mrozik, B.; Kimsa-Dudek, M.; Synowiec-Wojtarowicz, A.; Chrobak, E.; Bębenek, E.; Boryczka, S.; Głuszek, S.; Gola, J.M. The Influence of Betulin and Its Derivatives EB5 and ECH147 on the Antioxidant Status of Human Renal Proximal Tubule Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 2524. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.S.; Choi, B.Y. Molecular and chemical regulation of the keap1-Nrf2 signaling pathway. Molecules 2014, 19, 10074–10089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The anti-inflammatory and anti-oxidant mechanisms of the keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Lee, P.J.; Park, H.J.; Yoo, H.M.; Cho, N. Betulin Protects HT-22 Hippocampal Cells against ER Stress through Induction of Heme Oxygenase-1 and Inhibition of ROS Production. Nat. Prod. Commun. 2019, 14, 1934578X19896684. [Google Scholar] [CrossRef] [Green Version]
- Arivazhagan, L.; Subramanian, S.P. Tangeretin, a citrus flavonoid attenuates oxidative stress and protects hepatocellular architecture in rats with 7, 12-dimethylbenz(a)anthracene induced experimental mammary carcinoma. J. Funct. Foods 2015, 15, 339–353. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Liu, H.; Song, Z.; Chen, W. Phytochemical compounds targeting on Nrf2 for chemoprevention in colorectal cancer. Eur. J. Pharmacol. 2020, 887, 173588. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta- Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Loboda, A.; Rojczyk-Golebiewska, E.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Jozkowicz, A.; Dulak, J. Targeting Nrf2-mediated gene transcription by triterpenoids and their derivatives. Biomol. Ther. 2012, 20, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Loboda, A.; Jazwa, A.; Grochot-Przeczek, A.; Rutkowski, A.J.; Cisowski, J.; Agarwal, A.; Jozkowicz, A.; Dulak, J. Heme oxygenase-1 and the vascular bed: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2008, 10, 1767–1812. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Z.; Lindholm, P.F.; Sarna, S.K. NF-κB activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology 2003, 124, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monaco, C.; Andreakos, E.; Kiriakidis, S.; Mauri, C.; Bicknell, C.; Foxwell, B.; Cheshire, N.; Paleolog, E.; Feldmann, M. Canonical pathway of nuclear factor κB activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 5634–5639. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, G.; Sung, B.; Aggarwal, B.B. Nuclear factor-κB activation: From bench to bedside. Exp. Biol. Med. 2008, 233, 21–31. [Google Scholar] [CrossRef]
- Wu, Q.; Li, H.; Qiu, J.; Feng, H. Betulin protects mice from bacterial pneumonia and acute lung injury. Microb. Pathog. 2014, 75, 21–28. [Google Scholar] [CrossRef]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, M.; Eisa, N.H.; Abo El-Magd, N.F.; Elsherbiny, N.M.; Said, E.; Khodir, A.E. Anti-inflammatory/anti-apoptotic impact of betulin attenuates experimentally induced ulcerative colitis: An insight into TLR4/NF-kB/caspase signalling modulation. Environ. Toxicol. Pharmacol. 2021, 88, 103750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Zhao, Q.F.; Fang, N.N.; Yu, J.G. Betulin inhibits pro-inflammatory cytokines expression through activation STAT3 signaling pathway in human cardiac cells. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 455–460. [Google Scholar]
- Clark, R.B. The role of PPARs in inflammation and immunity. J. Leukoc. Biol. 2002, 71, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.A.; Bordji, K.; Boyault, S.; Bianchi, A.; Gouze, E.; Bécuwe, P.; Dauça, M.; Netter, P.; Terlain, B. PPAR-γ ligands modulate effects of LPS in stimulated rat synovial fibroblasts. Am. J. Physiol.- Cell Physiol. 2002, 282, C125–C133. [Google Scholar] [CrossRef]
- Chunhua, M.; Long, H.; Zhu, W.; Liu, Z.; Jie, R.; Zhang, Y.; Wang, Y. Betulin inhibited cigarette smoke-induced COPD in mice. Biomed. Pharmacother. 2017, 85, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Mierla, A.L.; Minelli, A. Nrf2 and NF-κB and their concerted modulation in cancer pathogenesis and progression. Cancers 2010, 2, 483–497. [Google Scholar] [CrossRef]
- Bellezza, I.; Grottelli, S.; Gatticchi, L.; Mierla, A.L.; Minelli, A. α-Tocopheryl succinate pre-treatment attenuates quinone toxicity in prostate cancer PC3 cells. Gene 2014, 539, 1–7. [Google Scholar] [CrossRef]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Liu, J.P.; Mei, J.H.; Li, S.J.; Shi, L.Q.; Lin, Z.H.; Xie, B.Y.; Sun, W.G.; Wang, Z.Y.; Yang, X.L.; et al. Betulin isolated from Pyrola incarnata Fisch. inhibited lipopolysaccharide (LPS)-induced neuroinflammation with the guidance of computer-aided drug design. Bioorganic Med. Chem. Lett. 2020, 30, 127193. [Google Scholar] [CrossRef]
- Shaul, Y.D.; Seger, R. The MEK/ERK cascade: From signaling specificity to diverse functions. Biochim. Biophys. Acta-Mol. Cell Res. 2007, 1773, 1213–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, M.; Chen, W.; Cobb, M.H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimienta, G.; Pascual, J. Canonical and alternative MAPK signaling. Cell Cycle 2007, 6, 2628–2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares-Silva, M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The mitogen-activated protein kinase (MAPK) pathway: Role in immune evasion by trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas, B.D.; Abell, A.N.; Johnson, G.L. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene 2007, 26, 3159–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a11254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, Q.; Yu, L.; Zhu, J.; Cao, Y.; Gao, X. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J. Ethnopharmacol. 2021, 264, 113249. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Zong, X.; Song, D.; Wang, T.; Xia, X.; Hu, W.; Han, F.; Wang, Y. LFP-20, a porcine lactoferrin peptide, ameliorates LPS-induced inflammation via the MyD88/NF-κB and MyD88/MAPK signaling pathways. Dev. Comp. Immunol. 2015, 52, 123–131. [Google Scholar] [CrossRef]
- O’Sullivan, A.W.; Wang, J.H.; Redmond, H.P. NF-κB and P38 MAPK Inhibition Improve Survival in Endotoxin Shock and in a Cecal Ligation and Puncture Model of Sepsis in Combination With Antibiotic Therapy. J. Surg. Res. 2009, 152, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, X.; Chen, G.; Jiang, L.; Wang, Z.; Fang, Q.; Liu, X.; Wang, J.; Zhang, Y.; Wu, W.; et al. MD-2 as the target of a novel small molecule, L6H21, in the attenuation of LPS-induced inflammatory response and sepsis. Br. J. Pharmacol. 2015, 172, 4391–4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizerska-Kowalska, M.; Sławinska-Brych, A.; Kaławaj, K.; Zurek, A.; Pawinska, B.; Rzeski, W.; Zdzisinska, B. Betulin promotes differentiation of human osteoblasts in vitro and exerts an osteoinductive effect on the HfOB 1.19 cell line through activation of JNK, ERK1/2, and mTOR kinases. Molecules 2019, 24, 2637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.J.; Lee, Y.; Hwang, H.G.; Sung, S.H.; Lee, M.; Son, Y.J. Betulin suppresses osteoclast formation via down-regulating NFATc1. J. Clin. Med. 2018, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Su, C.H.; Lin, C.Y.; Tsai, C.H.; Lee, H.P.; Lo, L.C.; Huang, W.C.; Wu, Y.C.; Hsieh, C.L.; Tang, C.H. Betulin suppresses TNF-α and IL-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κB pathway. J. Funct. Foods 2021, 86, 104729. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Cicenas, J.; Zalyte, E.; Rimkus, A.; Dapkus, D.; Noreika, R.; Urbonavicius, S. JNK, p38, ERK, and SGK1 inhibitors in cancer. Cancers 2018, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.E.; Salzwedel, K.; Allaway, G.P. Bevirimat: A Novel Maturation Inhibitor for the Treatment of HIV-1 Infection. Antivir. Chem. Chemother. 2008, 19, 107–113. [Google Scholar] [CrossRef]
- Pârvănescu, R.D.; Watz, C.G.; Moacă, E.A.; Vlaia, L.; Marcovici, I.; Macașoi, I.G.; Borcan, F.; Olariu, I.; Coneac, G.; Drăghici, G.A.; et al. Oleogel formulations for the topical delivery of betulin and lupeol in skin injuries—Preparation, physicochemical characterization, and pharmaco-toxicological evaluation. Molecules 2021, 26, 4174. [Google Scholar] [CrossRef]
- Schwieger-Briel, A.; Kiritsi, D.; Schempp, C.; Has, C.; Schumann, H. Betulin-based oleogel to improve wound healing in dystrophic epidermolysis bullosa: A prospective controlled proof-of-concept study. Dermatol. Res. Pract. 2017, 2017, 5068969. [Google Scholar] [CrossRef] [Green Version]
- Shikov, A.N.; Djachuk, G.I.; Sergeev, D.V.; Pozharitskaya, O.N. Birch bark extract as therapy for chronic hepatitis C—A pilot study. Phytomedicine 2011, 18, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Huyke, C.; Laszczyk, M.; Scheffler, A.; Ernst, R.; Schempp, C.M. Treatment of actinic keratoses with birch bark extract: A pilot study. JDDG-J. Ger. Soc. Dermatol. 2006, 4, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Huyke, C.; Reuter, J.; Rödig, M.; Kersten, A.; Laszczyk, M.; Scheffler, A.; Nashan, D.; Schempp, C. Treatment of actinic keratoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. JDDG-J. Ger. Soc. Dermatol. 2009, 7, 128–133. [Google Scholar] [CrossRef]
- Kern, J.S.; Schwieger-Briel, A.; Löwe, S.; Sumeray, M.; Davis, C.; Martinez, A.E. Oleogel-S10 Phase 3 study “EASE” for epidermolysis bullosa: Study design and rationale. Trials 2019, 20, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, A.; Murrell, D.F.; Bruckner, A.L.; Kern, J.; Maher, L.; Cunningham, T. BG01: Safety and efficacy of oleogel-S10 (birch triterpenes) for epidermolysis bullosa: Results of a 12-month interim analysis of the open-label phase from the EASE study. Br. J. Dermatol. 2022, 187, 89. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Brandner, J.M.; Schumann, H.; Bross, F.; Fimmers, R.; Böttger, K.; Scheffler, A.; Podmelle, F. Accelerated reepithelialization by triterpenes: Proof of concept in the healing of surgical skin lesions. Ski. Pharmacol. Physiol. 2015, 28, 1–11. [Google Scholar] [CrossRef]
- Frew, Q.; Rennekampff, H.O.; Dziewulski, P.; Moiemen, N.; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef]
- Mao, D.B.; Feng, Y.Q.; Bai, Y.H.; Xu, C.P. Novel biotransformation of betulin to produce betulone by Rhodotorula mucilaginosa. J. Taiwan Inst. Chem. Eng. 2012, 43, 825–829. [Google Scholar] [CrossRef]
- Singh, R. Microbial Biotransformation: A Process for Chemical Alterations. J. Bacteriol. Mycol. Open Access 2017, 4, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Chrobak, E.; Bȩbenek, E.; Kadela-Tomanek, M.; Latocha, M.; Jelsch, C.; Wenger, E.; Boryczka, S. Betulin phosphonates; synthesis, structure, and cytotoxic activity. Molecules 2016, 21, 1123. [Google Scholar] [CrossRef] [Green Version]
- Król, S.K.; Bębenek, E.; Sławińska-Brych, A.; Dmoszyńska-Graniczka, M.; Boryczka, S.; Stepulak, A. Synthetic betulin derivatives inhibit growth of glioma cells in vitro. Anticancer Res. 2020, 40, 6151–6158. [Google Scholar] [CrossRef] [PubMed]
- Boryczka, S.; Bebenek, E.; Wietrzyk, J.; Kempińska, K.; Jastrzebska, M.; Kusz, J.; Nowak, M. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 2013, 18, 4526–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamansarov, E.Y.; Skvortsov, D.A.; Lopukhov, A.V.; Kovalev, S.V.; Evteev, S.A.; Petrov, R.A.; Klyachko, N.L.; Zyk, N.V.; Beloglazkina, E.K.; Ivanenkov, Y.A.; et al. New ASGPR-targeted ligands based on glycoconjugated natural triterpenoids. Russ. Chem. Bull. 2019, 68, 2331–2338. [Google Scholar] [CrossRef]
- Shcherban, N.D.; Mäki-Arvela, P.; Aho, A.; Sergiienko, S.A.; Skoryk, M.A.; Kolobova, E.; Simakova, I.L.; Eränen, K.; Smeds, A.; Hemming, J.; et al. Preparation of Betulone Via Betulin Oxidation Over Ru Nanoparticles Deposited on Graphitic Carbon Nitride. Catal. Lett. 2019, 3, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Dubey, K.K. An efficient process for the transformation of betulin to betulinic acid by a strain of Bacillus megaterium. 3 Biotech 2017, 7, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grishko, V.V.; Tarasova, E.V.; Ivshina, I.B. Biotransformation of betulin to betulone by growing and resting cells of the actinobacterium Rhodococcus rhodochrous IEGM 66. Process Biochem. 2013, 48, 1640–1644. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.; Liu, J.; Xu, T.Y.; Fang, R.S.; Chen, Q.H.; He, G.Q. A novel one-step microbial transformation of betulin to betulinic acid catalysed by Cunninghamella blakesleeana. Food Chem. 2013, 136, 73–79. [Google Scholar] [CrossRef]
- Liu, H.; Lei, X.L.; Li, N.; Zong, M.H. Highly regioselective synthesis of betulone from betulin by growing cultures of marine fungus Dothideomycete sp. HQ 316564. J. Mol. Catal. B Enzym. 2013, 88, 32–35. [Google Scholar] [CrossRef]
- Li, J.; Jiang, B.; Chen, C.; Fan, B.; Huang, H.; Chen, G. Biotransformation of betulin by Mucor subtilissimus to discover anti-inflammatory derivatives. Phytochemistry 2019, 166, 112076. [Google Scholar] [CrossRef]
- Chen, Q.H.; Liu, J.; Zhang, H.F.; He, G.Q.; Fu, M.L. The betulinic acid production from betulin through biotransformation by fungi. Enzym. Microb. Technol. 2009, 45, 175–180. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Z.; Luo, S.; James, M.O.; Wang, Y. Phase II metabolism of betulin by rat and human UDP-glucuronosyltransferases and sulfotransferases. Chem. Biol. Interact. 2019, 302, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Yang, J.; Jin, M.; Du, Y.; Sun, Q.; Cao, L.; Xu, H. Safety assessment and antioxidant evaluation of betulin by LC-MS combined with free radical assays. Anal. Biochem. 2019, 587, 113460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Jin, M.; Wang, Q.; Sun, Q.; Du, Y.; Cao, L.; Xu, H. UHPLC-Q-TOF-MS/MS based screening and identification of the metabolites in vivo after oral administration of betulin. Fitoterapia 2018, 127, 29–41. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, N.; Wang, Z.; Liu, Y.; Wang, Y.; Ding, W.; Zhang, D.; Wang, Y.; Yan, X. Development and validation of an LC-ESI/MS/MS method with precolumn derivatization for the determination of betulin in rat plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 939, 38–44. [Google Scholar] [CrossRef]
- Jäger, S.; Laszczyk, M.; Scheffler, A. A Preliminary Pharmacokinetic Study of Betulin, the Main Pentacyclic Triterpene from Extract of Outer Bark of Birch (Betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef] [Green Version]
- Pozharitskaya, O.N.; Karlina, M.V.; Shikov, A.N.; Kosman, V.M.; Makarov, V.G.; Casals, E.; Rosenholm, J.M. Pharmacokinetics and Tissue Disposition of Nanosystem-Entrapped Betulin After Endotracheal Administration to Rats. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 327–332. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; Sharif, A.M.; Azhar, E.I.; Rateb, M.E. Olive-derived triterpenes suppress SARS-CoV-2 main protease: A promising scaffold for future therapeutics. Molecules 2021, 26, 2654. [Google Scholar] [CrossRef]
- Sharapov, A.D.; Fatykhov, R.F.; Khalymbadzha, I.A.; Zyryanov, G.V.; Chupakhin, O.N.; Tsurkan, M.V. Plant Coumarins with Anti-HIV Activity: Isolation and Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 2839. [Google Scholar] [CrossRef]
- Pokharkar, O.; Lakshmanan, H.; Zyryanov, G.; Tsurkan, M. In Silico Evaluation of Antifungal Compounds from Marine Sponges against COVID-19-Associated Mucormycosis. Mar. Drugs 2022, 20, 215. [Google Scholar] [CrossRef] [PubMed]
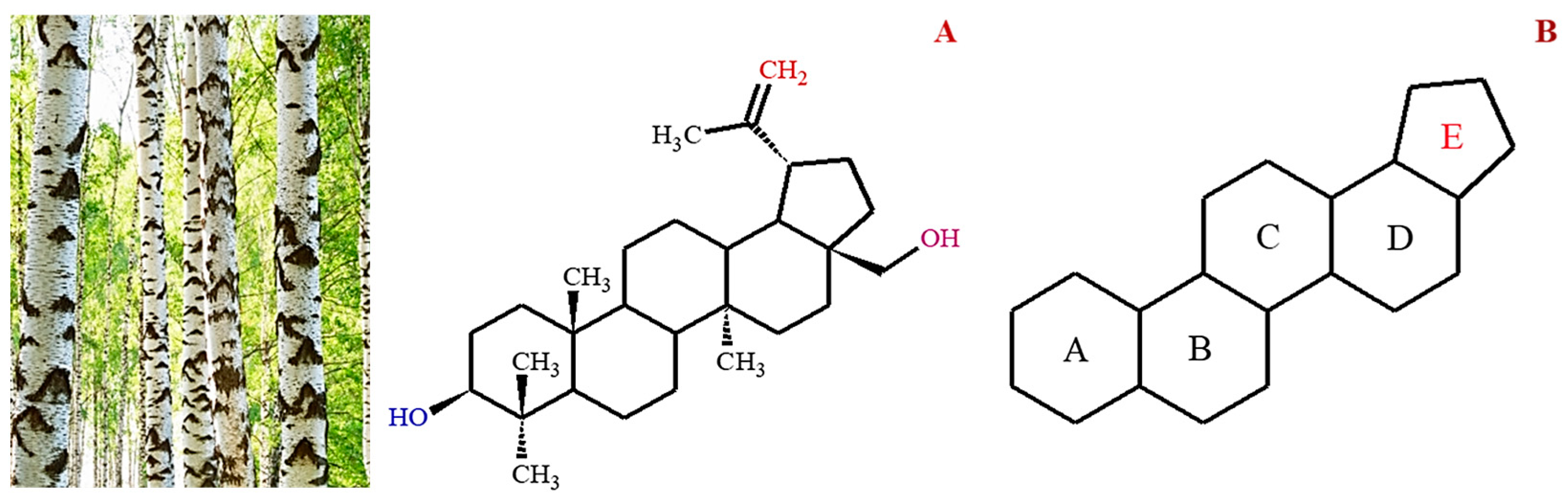



| Sources | Parts | Solvent | Isolation Techniques | References |
|---|---|---|---|---|
| Anemone raddeana | Root | Ethanol | Solvent extraction | [10] |
| Pyrola decorate H. | Leaves | Acetidin/ethanol | Solvent extraction (maceration) | [11] |
| Ougenia Dalbergioides | Bark | Methanol | Solvent extraction | [12] |
| Paecilomyces WE3-F | Mycelial culture | Dichloromethane | Solvent extraction | [13] |
| Betula pendula Roth | Bark | Ethanol | Solvent extraction | [14] |
| Pterodon emarginatus | Stem bark | Ethanol | Solvent extraction | [15] |
| Betula pendula Betula pubescens | Bark, leaves | Ethanol | Pressurized liquid extraction | [16] |
| Tectona grandis | Stem bark | Methanol | Solvent extraction | [17] |
| B. pendula Roth | Outer bark | Chloroform/dichloromethane/ methanol | Solvent extraction (maceration) | [18] |
| Schleichera oleosa (Lour) | Bark | Ethanol | Solvent extraction | [19] |
| Inonotus obliquus | Mycelial culture | Isopropanol | Ultrasonic extraction | [20] |
| Alnus glutinosa (L.) Gaertn | Alder bark | CO2/ethanol | Supercritical fluid extraction | [21] |
| Viscum album coloratum | - | Ethanol | Solvent extraction | [22] |
| Betula pendula, Roth | Leaves | Ethanol | Solvent extraction (maceration) | [23] |
| Asteracantha longifolia | Leaves Stem | Ethanol | Solvent extraction | [24] |
| Ligaria cuneifolia | Aerial parts | Ethanol | Solvent extraction (maceration) | [25] |
| Hedyotis hedyotidea | Stem | - | - | [26] |
| Quercus incana | Leaves | Methanol | Solvent extraction | [27] |
| Acacia nilotica | Bark | Methanol | Solvent extraction (maceration) | [28] |
| Rhizophora mucronata | Leaves | Chloroform | Solvent extraction (Soxhlet) | [29] |
| Euphorbia hyssopifolia L. | Dried latex | Methanol | Solvent extraction (maceration) | [30] |
| Baliospermum montanum | Leaves | Methanol | Solvent extraction (Soxhlet) | [31] |
| Fomes fomentarius | Mycelium | Ethanol | Solvent extraction | [32] |
| Matayba elaeagnoides | Bark | Methanol | Solvent extraction (maceration) | [33] |
| Croton bonplandianum | - | Dichloromethane | Solvent extraction (maceration) | [34] |
| Celtis sinensis | Leaves | Hydrophobic DES | DES extraction | [35] |
| Doliocarpus dentatus | Leaves | Ethanolic extract | Solvent extraction (maceration) | [36] |
| Acacia auriculiformis | Stem bark | Ethyl acetate soluble fraction | Solvent extraction | [37] |
| Xanthium sibiricum | Roots | Methanol | - | [38] |
| Pleurostylia capensis | Bark/Root | Dichloromethane/methanol | Solvent extraction | [39] |
| Experimental Model | Dose/Concentration | Pharmacological Indicators | Mechanism of Action | References | |
|---|---|---|---|---|---|
| - | Wistar Rats (Ethanol-induced alcoholic steatohepatitis 4 g/kg for 8 weeks) | 50 and 100 mg/kg b.wt. | TG ↓ ALP ↓ AST ↓ ALT ↓ TNF-α ↓ IL-1β ↓ TGF-β ↓ TBARs ↓ GSH ↑ ROS ↓ | None | [88] |
| Hepatic stellate cells (LX-2 cells) ethanol 50 mM) | Male C57BL/6 mice (Ethanol 5 g/kg b.wt. 10 days) | 6.25–25 μM 20 and 50 mg/kg | SREBP1 ↓ TG ↓ ALT ↓ AST ↓ p65 ↓ collagen-I ↓ α-SMA ↓ | Sirt1/LKB1/AMPK signaling pathway | [89] |
| AML-12 cells (Ethanol 50 mM) | Male C57BL/6 mice (5 g/kg b.wt. EtOH for 4 weeks) | 0–25 μM 20 and 50 mg/kg | SREBP 1 ↓ Lipin1 ↓ Lipin2 ↑ ALT ↓ AST ↓ IL-1α ↓ TG ↓ PPAR-α ↑ FASN ↓ PPAR-γ ↓ IL-1β↓ IL-6 ↓ TNF-α ↓ IL-18 ↓ caspase-1 ↓ | blocking of P2X7/NLRP3 signaling pathway | [94] |
| Hepatic stellate cells (HSC-T6, EtOH 50 mM) | Male C57BL/6 mice (EtOH 5 mg/kg) | 12.5–25 μM 20 or 50 mg/kg | ALT ↓ AST ↓ TG ↓ CYP2E1 ↓ SREBP-1c ↓ TLR4 ↓ p-STAT3 ↑ Collagen-I ↓ α-SMA ↓ | TLR4 and STAT3 pathway | [91] |
| Rat liver stellate cell CFSC-2G (EtOH 50 mM) | - | 10 μM | procollagen I ↓ TNF-α ↓ MMP2 ↓ TGF-β ↓ TIMP1/2 ↓ α-SMA ↓ | inhibited NFκB and MAPKs signaling pathway | [95] |
| - | LPS/D-galactosamine-induced acute liver injury BALB/c mice | 2–8 mg/kg b.wt. | MPO ↓ AST ↓ ALT ↓ TNF-α ↓ IL-1β ↓ PPARγ ↑ | inhibiting the NFκB signaling pathway | [87] |
| Con A-stimulated splenocytes | Concanavalin A-challenged C57BL/6J mice | 0–32 μg/mL 20 mg/kg | IFN-γ ↓ IL-4 ↓ IL-6 ↓ IL-10 ↓ IL-17 ↓ IL-2 ↓ ALT ↓ AST ↓ TNF-α ↓ | None | [26] |
| - | Sprague Dawley rats (Cisplatin 10 mg/kg) | 8 mg/kg | caspase 3 ↓ caspase 9 ↓ tBilirubin↓ albumin ↑ caspase 8 ↓ MDA ↓ TAC ↑ IL-1 ↓ AST ↓ ALT ↓ caspase 1 ↓ p53 ↓ Bax ↓ Bcl-2 ↑ | NLRP3 pathway | [93] |
| Experimental Model | Dose/Concentration | Pharmacological Indicator | Molecular Mechanism | References | |
|---|---|---|---|---|---|
| - | λ-carrageenan-induced paw edema in male ICR mice | 30 and 90 mg/kg | SOD ↑, GPx ↑ GR ↑ MDA ↓ NO ↓ | None | [101] |
| - | Ovalbumin-induced asthma in female BALB/c mice | 10 mg/kg | ROS ↓ SOD ↑ CAT ↑ GSH ↑ NO2 ↓ NO3 ↓ MDA ↓ IL-4 ↓ MMP-9 ↓ p65 ↓ TGF-β1 ↓ IL-5 ↓ IgE ↓ TNF-α ↓ IFN-γ ↑ TIMP-1 ↓ tTG ↓ IL-13 ↓ p-IκB-α ↓ TREM-1 ↓ | Inhibited NFκB signaling axis | [102] |
| primary human keratinocytes | - | 0.87 and 4.34 μg/mL | MIP-1α ↑ COX-2 ↑ IL-6 ↑ IL-8 ↑ IFN-γ ↑ IP-10 ↑ TNF-α ↑ RhoA ↑ MIP-1 β ↑ basic FGF ↑ RANTES ↑ | Modulated RNA stability involving p38 MAPK | [103] |
| HepG2 U397 macrophages | - | 0.5–10 µM | G6P ↓ PEPCK ↑ TNF-α ↓ IL-1β ↓ | Glucocorticoid receptor-mediated pathway | [104] |
| thapsigargin induced endoplasmic reticulum stress in Hippocampal neuronal cells (HT-22) | - | 10 µM | ROS ↓ HO-1 ↑ Bcl2 ↑ CHOP ↓ Caspase12 ↓ Cleaved caspase 3 ↓ | mediated HO-1 induction | [32] |
| porcine chondrocytes | - | 0.32 µg/L (4 weeks) | TGF-β1 ↑ BMP-7 ↓ IGF-1 ↑ type II collagen ↑ aggrecan ↑ decorin ↑ MMPs ↓ IL-1β ↓ | None | [105] |
| - | Wild-type AB strain zebrafish | 0.125–0.5 mg/mL | ROS ↓ IL-1β ↓ TNF-α ↓ Caspase 3 ↓ p38 α ↓ ERK1/2↓ | ROS/MAPKs/NFĸB signaling axis | [106] |
| IL-1β Induced chondrocytes | C57BL/6 male wild-type (WT) mice | 20 mg/kg/day i.p 0–200 μM | iNOS ↓ TNFα ↓ NO ↓ COX2 ↓ IL-6 ↓ PGE-2 ↓ Aggrecan ↑ Collagen II ↑ ADAMTS5 ↓ MMP13 ↓ | inhibited NFκB activation | [107] |
| Pathological Condition | Inclusion Criteria for Study Group | Duration | Compound | Mode of Administration | Intervention | Outcome of Study | References |
|---|---|---|---|---|---|---|---|
| Chronic hepatitis C: a pilot study | Patients between 20–71 years with serologically confirmed chronic hepatitis (ALT levels 1.5-fold than the upper normal limits before commencing the study) [n = 42] | 12 weeks | Birch bark extract (betulin 75% and betulinic acid 3.5%) | Oral | 8 doses of gelatine capsules/day (160 mg standardized extract per day) per capsule, 20 mg dry ethanol birch bark extract | Decreased level of ALT, HCV RNA, fatigue, and abdominal pain with the absence of dyspepsia | [161] |
| Treatment of actinic keratosis: a pilot study | Patients with between 1–10 flat actinic keratoses, barely hyperkeratotic, located in typical UV exposure hotspots [n = 28] | 2 months | Birch bark ointment (betulin 80%) | Topical | Two treatment groups: Birch bark ointment only Combination therapy (Birch bark salve and cryotherapy) | clearing of lesions and anti-inflammatory | [162] |
| Randomized monocentric phase II study on actinic keratosis | patients older than 18 years having ≤ 10 actinic keratosis of both sexes [n = 15] | 3 months | Betulin based oleogel | Topical | Two treatment groups: Betulin-based oleogel (2× daily) combination therapy with cryotherapy and betulin-based oleogel | Anti-inflammatory and antitumor activity | [163] |
| Phase III double-blind, randomized placebo-controlled trial on epidermolysis bullosa (EB) NCT03068780 | Children with hereditary epidermolysis bullosa (≥4 years) and adults with EB target wounds (10 to 50 cm2 partial thickness wounds, aged between 21 days and 9 months) [n = 223] | 90 days | Oleogel-S10 betulin 72–88% (Episalvan) | Topical | Treatment: Oleogel-S10 (90% sunflower oil, 10% birch bark extract) Placebo: sunflower oil gel | Enhanced wound healing | [164,165] |
| Split-thickness skin graft transplant: an open, blind-evaluated, controlled, prospective, randomized phase II trial | Inpatients (18–95 years) needing skin grafting because of trauma, chronic venous ulcers, burns, or surgical excision [n = 24] | 14 days | Oleogel-S10 | Topical | Two treatment groups: Oleogel-S10 + Mepilex dressing and Mepilex dressing only | Faster wound healing and reepithelization of split-thickness skin graft | [166] |
| Randomized, intra-individually controlled, open, blind evaluated, polycentric phase III study on superficial partial thickness burns (EudraCT No. 2012-000362-38) | Adults who have had two comparable burn wounds of greater than 40 cm2 and less than 12.5% of their total body surface area (TBSA) within 48 h after injury or a single superficial partial thickness burn wound of more than 80 cm2 and less than 25% of TBSA [n = 57]. | 3 months with 12 months post-injury follow-up | Oleogel-S10 (Episalvan) | Topical | Two intervention groups: Oleogel-S10 and Octenilin® wound gel (Octenidine hydrochloride gel) | Accelerated wound healing and improved reepithelization | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adepoju, F.O.; Duru, K.C.; Li, E.; Kovaleva, E.G.; Tsurkan, M.V. Pharmacological Potential of Betulin as a Multitarget Compound. Biomolecules 2023, 13, 1105. https://doi.org/10.3390/biom13071105
Adepoju FO, Duru KC, Li E, Kovaleva EG, Tsurkan MV. Pharmacological Potential of Betulin as a Multitarget Compound. Biomolecules. 2023; 13(7):1105. https://doi.org/10.3390/biom13071105
Chicago/Turabian StyleAdepoju, Feyisayo O., Kingsley C. Duru, Erguang Li, Elena G. Kovaleva, and Mikhail V. Tsurkan. 2023. "Pharmacological Potential of Betulin as a Multitarget Compound" Biomolecules 13, no. 7: 1105. https://doi.org/10.3390/biom13071105
APA StyleAdepoju, F. O., Duru, K. C., Li, E., Kovaleva, E. G., & Tsurkan, M. V. (2023). Pharmacological Potential of Betulin as a Multitarget Compound. Biomolecules, 13(7), 1105. https://doi.org/10.3390/biom13071105







