Serum Level of Cytokeratin 18 (M65) as a Prognostic Marker of High Cardiovascular Disease Risk in Individuals with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. The PREVEND General Population Cohort
2.2. NAFLD Definition in PREVEND
2.3. CVD Risk Prediction Assessment
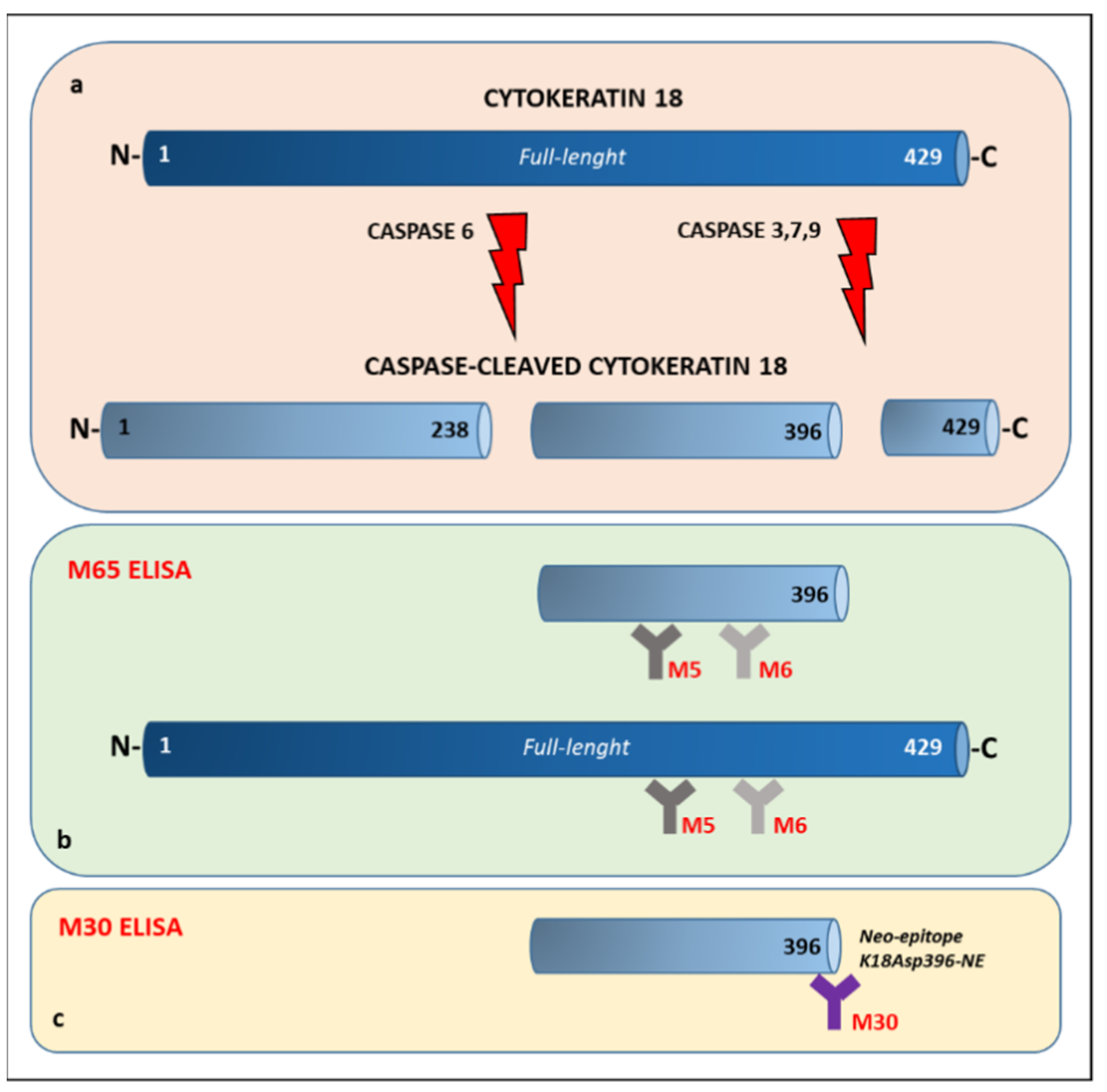
2.4. Cytokeratin 18 Assessment
2.5. Biomarkers Determinations
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Subjects
3.2. CK18 and the Risk of NAFLD (FLI ≥ 60)
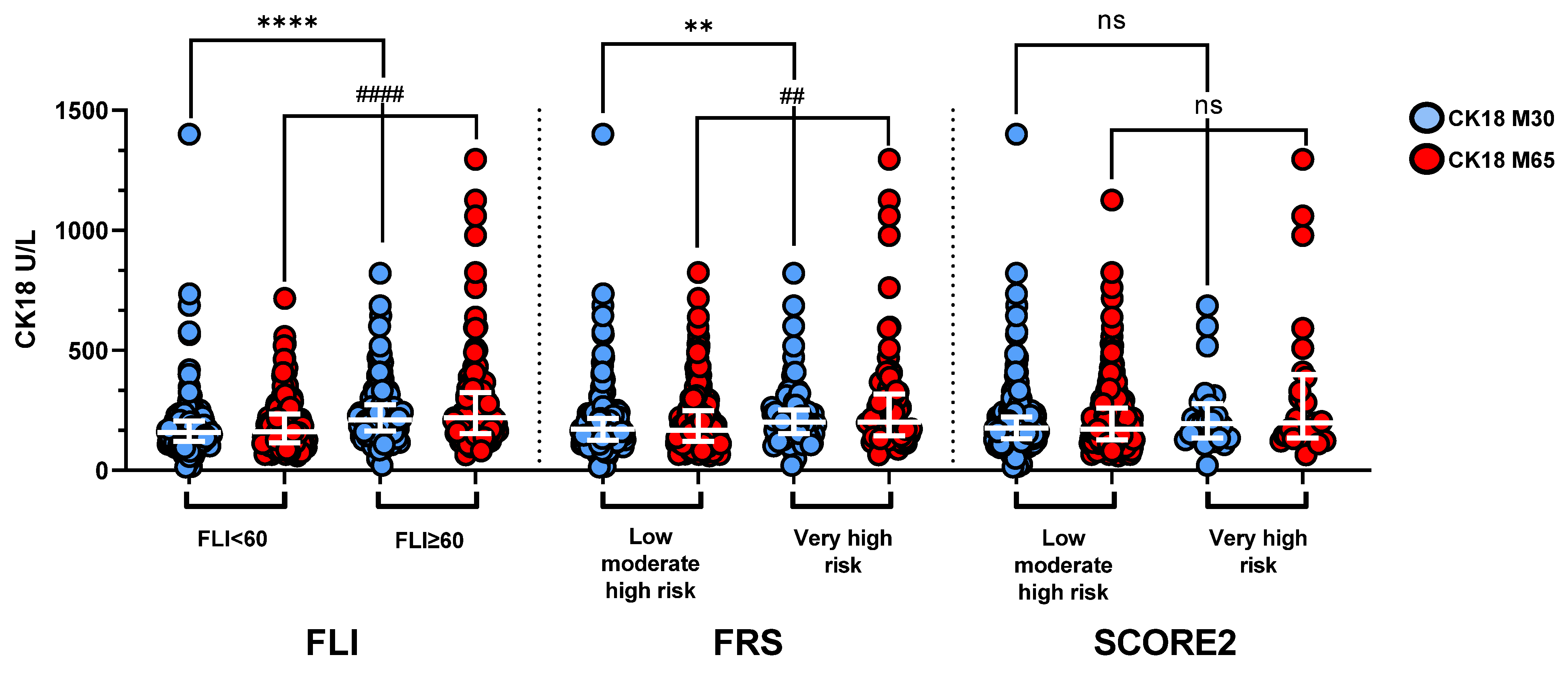
3.3. CK18 and High-Risk of Cardiovascular Disease Prediction
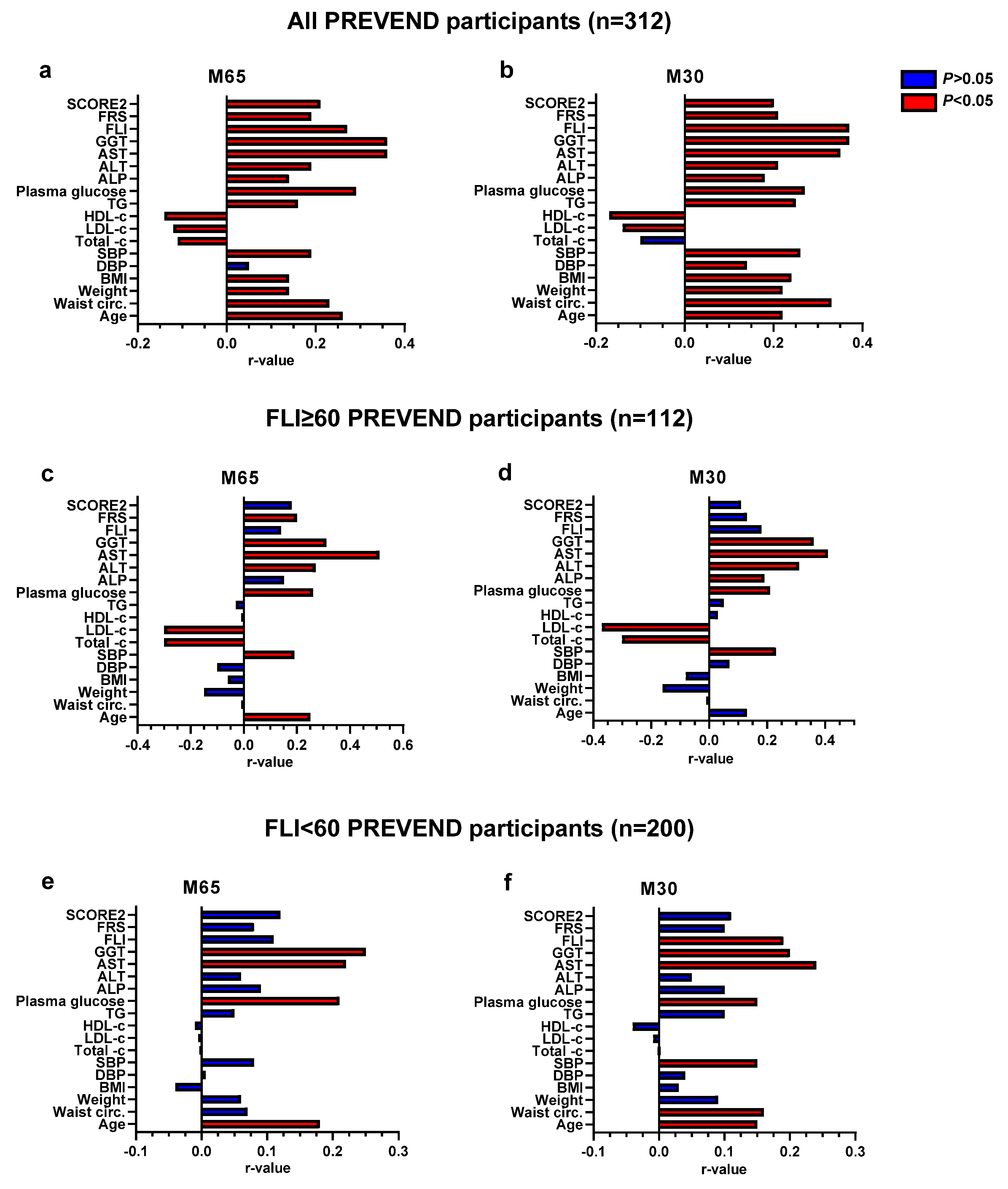
3.4. Determinants of CK18 Associations with Cardiovascular Disease Risk Scores and FLI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of Nafld and Nash: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The Economic and Clinical Burden of Nonalcoholic Fatty Liver Disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.; Zhang, X. Non-Alcoholic Fatty Liver Disease: An Expanded Review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The Global Nafld Epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Segura-Azuara, N.L.A.; Varela-Chinchilla, C.D.; Trinidad-Calderon, P.A. Mafld/Nafld Biopsy-Free Scoring Systems for Hepatic Steatosis, Nash, and Fibrosis Diagnosis. Front. Med. 2021, 8, 774079. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A Simple and Accurate Predictor of Hepatic Steatosis in the General Population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Gastaldelli, A.; Kozakova, M.; Hojlund, K.; Flyvbjerg, A.; Favuzzi, A.; Mitrakou, A.; Balkau, B.; RISC Investigators. Fatty Liver Is Associated with Insulin Resistance, Risk of Coronary Heart Disease, and Early Atherosclerosis in a Large European Population. Hepatology 2009, 49, 1537–1544. [Google Scholar] [CrossRef]
- van den Berg, E.H.; Amini, M.; Schreuder, T.C.; Dullaart, R.P.; Faber, K.N.; Alizadeh, B.Z.; Blokzijl, H. Prevalence and Determinants of Non-Alcoholic Fatty Liver Disease in Lifelines: A Large Dutch Population Cohort. PLoS ONE 2017, 12, e0171502. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Adams, L.A.; Canbay, A.; Syn, W.K. Extrahepatic Complications of Nonalcoholic Fatty Liver Disease. Hepatology 2014, 59, 1174–1197. [Google Scholar] [CrossRef]
- Targher, G.; Tilg, H.; Byrne, C.D. Non-Alcoholic Fatty Liver Disease: A Multisystem Disease Requiring a Multidisciplinary and Holistic Approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef]
- van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; de Borst, M.H.; Wolak-Dinsmore, J.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Non-Alcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: Role of Circulating Branched-Chain Amino Acids. Nutrients 2019, 11, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targher, G.; Day, C.P.; Bonora, E. Risk of Cardiovascular Disease in Patients with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, A.; Wree, A.; Feldstein, A.E. Biomarkers of Liver Cell Death. J. Hepatol. 2014, 60, 1063–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, T.; Toi, M.; Linder, S. Detection of Epithelial Cell Death in the Body by Cytokeratin 18 Measurement. Biomed. Pharmacother. 2005, 59 (Suppl. 2), S359–S362. [Google Scholar] [CrossRef]
- Schutte, B.; Henfling, M.; Kolgen, W.; Bouman, M.; Meex, S.; Leers, M.P.; Nap, M.; Bjorklund, V.; Bjorklund, P.; Bjorklund, B.; et al. Keratin 8/18 Breakdown and Reorganization During Apoptosis. Exp. Cell. Res. 2004, 297, 11–26. [Google Scholar] [CrossRef]
- Cummings, J.; Ranson, M.; Butt, F.; Moore, D.; Dive, C. Qualification of M30 and M65 Elisas as Surrogate Biomarkers of Cell Death: Long Term Antigen Stability in Cancer Patient Plasma. Cancer Chemother. Pharmacol. 2007, 60, 921–924. [Google Scholar] [CrossRef]
- Kramer, G.; Schwarz, S.; Hagg, M.; Havelka, A.M.; Linder, S. Docetaxel Induces Apoptosis in Hormone Refractory Prostate Carcinomas During Multiple Treatment Cycles. Br. J. Cancer 2006, 94, 1592–1598. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabres, M.; Abbate, M.; Montemayor, S.; Mascaro, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, M.A.; Tur, J.A.; et al. Oxidative Stress and Pro-Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef]
- Sahebkar, A.; Sancho, E.; Abello, D.; Camps, J.; Joven, J. Novel Circulating Biomarkers for Non-Alcoholic Fatty Liver Disease: A Systematic Review. J. Cell. Physiol. 2018, 233, 849–855. [Google Scholar] [CrossRef]
- Kosasih, S.; Qin, W.Z.; Rani, R.A.; Abd Hamid, N.; Soon, N.C.; Shah, S.A.; Yaakob, Y.; Ali, R.A.R. Relationship between Serum Cytokeratin-18, Control Attenuation Parameter, Nafld Fibrosis Score, and Liver Steatosis in Nonalcoholic Fatty Liver Disease. Int. J. Hepatol. 2018, 2018, 9252536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahn, L.; Franke, W.W. High Frequency of Cytokeratin-Producing Smooth Muscle Cells in Human Atherosclerotic Plaques. Differentiation 1989, 40, 55–62. [Google Scholar] [CrossRef]
- Senturk, T.; Aydinlar, A.; Yilmaz, Y.; Oral, A.Y.; Ozdabakoglu, O.; Ulukaya, E. Serial Changes in Circulating M30 Antigen, a Biomarker of Apoptosis, in Patients with Acute Coronary Syndromes: Relationship with the Severity of Coronary Artery Disease. Coron. Artery Dis. 2009, 20, 494–498. [Google Scholar] [CrossRef]
- Roth, G.A.; Lebherz-Eichinger, D.; Ankersmit, H.J.; Hacker, S.; Hetz, H.; Vukovich, T.; Perne, A.; Reiter, T.; Farr, A.; Horl, W.H.; et al. Increased Total Cytokeratin-18 Serum and Urine Levels in Chronic Kidney Disease. Clin. Chim. Acta 2011, 412, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Lin, H.C.; Hwu, D.W.; Chang, D.M.; Lin, K.C.; Lee, Y.J. Elevated Serum Cytokeratin-18 Concentration in Patients with Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease. Ann. Clin. Biochem. 2019, 56, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell Death and Diseases Related to Oxidative Stress: 4-Hydroxynonenal (Hne) in the Balance. Cell. Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, L.; Tabas, I. Role of Endoplasmic Reticulum Stress in Metabolic Disease and Other Disorders. Annu. Rev. Med. 2012, 63, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Swisa, A.; Glaser, B.; Dor, Y. Metabolic Stress and Compromised Identity of Pancreatic Beta Cells. Front. Genet. 2017, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Zhang, L.; Wu, L.; Zhang, J.; Fang, Q.; Hou, X.; Gao, Q.; Li, H.; Jia, W. Elevated Serum Level of Cytokeratin 18 M65ed Is an Independent Indicator of Cardiometabolic Disorders. J. Diabetes Res. 2020, 2020, 5198359. [Google Scholar] [CrossRef]
- Kappelle, P.J.W.H.; Gansevoort, R.T.; Hillege, J.L.; Wolffenbuttel, B.H.R.; Dullaart, R.P.F.; PREVEND Study group. Apolipoprotein B/a-I and Total Cholesterol/High-Density Lipoprotein Cholesterol Ratios Both Predict Cardiovascular Events in the General Population Independently of Nonlipid Risk Factors, Albuminuria and C-Reactive Protein. J. Intern. Med. 2011, 269, 232–242. [Google Scholar] [CrossRef]
- Damba, T.; Bourgonje, A.R.; Abdulle, A.E.; Pasch, A.; Sydor, S.; van den Berg, E.H.; Gansevoort, R.T.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; et al. Oxidative Stress Is Associated with Suspected Non-Alcoholic Fatty Liver Disease and All-Cause Mortality in the General Population. Liver Int. 2020, 40, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Garcia, E.; van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; Groothof, D.; Westenbrink, B.D.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Nonalcoholic Fatty Liver Disease, Circulating Ketone Bodies and All-Cause Mortality in a General Population-Based Cohort. Eur. J. Clin. Investig. 2021, 51, e13627. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SCORE2 working group and ESC Cardiovascular risk collaboration. Cardiovascular risk. Score2 Risk Prediction Algorithms: New Models to Estimate 10-Year Risk of Cardiovascular Disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Bosomworth, N.J. Practical Use of the Framingham Risk Score in Primary Prevention: Canadian Perspective. Can. Fam. Physician 2011, 57, 417–423. [Google Scholar] [PubMed]
- Sohn, C.; Kim, J.; Bae, W. The Framingham Risk Score, Diet, and Inflammatory Markers in Korean Men with Metabolic Syndrome. Nutr. Res. Pract. 2012, 6, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Vali, Y.; Boursier, J.; Duffin, K.; Verheij, J.; Brosnan, M.J.; Zwinderman, K.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Accuracy of Cytokeratin 18 (M30 and M65) in Detecting Non-Alcoholic Steatohepatitis and Fibrosis: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0238717. [Google Scholar] [CrossRef]
- Kawanaka, M.; Nishino, K.; Nakamura, J.; Urata, N.; Oka, T.; Goto, D.; Suehiro, M.; Kawamoto, H.; Yamada, G. Correlation between Serum Cytokeratin-18 and the Progression or Regression of Non-Alcoholic Fatty Liver Disease. Ann. Hepatol. 2015, 14, 837–844. [Google Scholar] [CrossRef]
- Pennells, L.; Kaptoge, S.; Wood, A.; Sweeting, M.; Zhao, X.; White, I.; Burgess, S.; Willeit, P.; Bolton, T.; Moons, K.G.M.; et al. Emerging Risk Factors, Collaboration. Equalization of Four Cardiovascular Risk Algorithms after Systematic Recalibration: Individual-Participant Meta-Analysis of 86 Prospective Studies. Eur. Heart J. 2019, 40, 621–631. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. Easl-Easd-Easo Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Altaf, B.; Rehman, A.; Jawed, S.; Raouf, A. Association of Liver Biomarkers and Cytokeratin-18 in Nonalcoholic Fatty Liver Disease Patients. Pak. J. Med. Sci. 2020, 36, 387–390. [Google Scholar] [CrossRef] [Green Version]
- Kasper, P.; Martin, A.; Lang, S.; Kutting, F.; Goeser, T.; Demir, M.; Steffen, H.M. Nafld and Cardiovascular Diseases: A Clinical Review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Tilg, H. Nafld and Increased Risk of Cardiovascular Disease: Clinical Associations, Pathophysiological Mechanisms and Pharmacological Implications. Gut 2020, 69, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Moon, J.S.; Byun, S.J.; Lee, J.H.; Kang, D.R.; Sung, K.C.; Kim, J.Y.; Huh, J.H. Fatty Liver Index and Development of Cardiovascular Disease in Koreans without Pre-Existing Myocardial Infarction and Ischemic Stroke: A Large Population-Based Study. Cardiovasc. Diabetol. 2020, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F. Associations of the Fatty Liver and Hepatic Steatosis Indices with Risk of Cardiovascular Disease: Interrelationship with Age. Clin. Chim. Acta 2017, 466, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Rasool, A.; Qadir, A.; Sahar, T.; Khan, M.A. Correlation of Cytokeratin-18 as a Marker of Cardiovascular Risk Assessment in Nonalcoholic Fatty Liver Disease in Nonobese and Nondiabetic Patients: Two Years Prospective Study. Asian J. Pharm. Res. Health Care 2022, 14, 177–180. [Google Scholar] [CrossRef]
- Goralska, J.; Razny, U.; Gruca, A.; Zdzienicka, A.; Micek, A.; Dembinska-Kiec, A.; Solnica, B.; Malczewska-Malec, M. Plasma Cytokeratin-18 Fragment Level Reflects the Metabolic Phenotype in Obesity. Biomolecules 2023, 13, 675. [Google Scholar] [CrossRef]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic Fatty Liver Disease and the Heart: Jacc State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.Y.; Wu, J.C.; Liu, M.; Li, M.M.; Sun, Y.; Huang, W.; Li, Y.J.; Zhang, Y.; Tang, W.; et al. Endothelial Sirt6 Is Vital to Prevent Hypertension and Associated Cardiorenal Injury through Targeting Nkx3.2-Gata5 Signaling. Circ. Res. 2019, 124, 1448–1461. [Google Scholar] [CrossRef]
- Bing, O.H.L. Hypothesis-Apoptosis May Be a Mechanism for the Transition to Heart-Failure with Chronic Pressure-Overload. J. Mol. Cell. Cardiol. 1994, 26, 943–948. [Google Scholar] [CrossRef]
- Narula, J.; Haider, N.; Virmani, R.; DiSalvo, T.G.; Kolodgie, F.D.; Hajjar, R.J.; Schmidt, U.; Semigran, M.J.; Dec, G.W.; Khaw, B.A. Apoptosis in Myocytes in End-Stage Heart Failure. N. Engl. J. Med. 1996, 335, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.C.; Liu, H.K.; Su, Y.T.; Tsai, C.C.; Wu, J.R. Serum Apoptotic Marker M30 Is Positively Correlated with Early Diastolic Dysfunction in Adolescent Obesity. PLoS ONE 2019, 14, e0217429. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, C.; Gur, M.; Seker, T.; Selek, S.; Kocyigit, A. The Predictive Value of M30 and Oxidative Stress for Left Ventricular Remodeling in Patients with Anterior St-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. Coron. Artery Dis. 2016, 27, 690–695. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 312) | FLI < 60 (n = 200) | FLI ≥ 60 (n = 112) | p-Value | |
|---|---|---|---|---|
| Demographic | ||||
| Age, yr. | 53 (46–65) | 51 (45–59.5) | 59 (50.5–69) | <0.0001 |
| Males, no. (%) | 154 (49.3) | 111 (55.5) | 43 (38.3) | 0.01 |
| Waist circumference, cm | 94 (85–104) | 87 (80–94) | 107 (102–114) | <0.0001 |
| Weight, kg | 78 (69.7–89) | 73 (65–80) | 93 (84–102) | <0.0001 |
| BMI, kg/m2 | 26.5 (23.7–29.5) | 24.6 (22.8–26.4) | 30.4 (28.8–32.9) | <0.0001 |
| Diastolic blood pressure, mm Hg | 72 (67–78) | 70 (65–76) | 76 (71–80) | <0.0001 |
| Systolic blood pressure, mm Hg | 124 (111–138) | 118 (108–130) | 134 (123–146) | <0.0001 |
| Current smoker, no. (%) | 87 (27.9) | 65 (32.5) | 22 (19.6) | 0.064 |
| Type 2 diabetes, no. (%) | 43 (13.7) | 17 (8.5) | 16 (14.2) | 0.03 |
| FRS (%) § | 11.7 (6.1–23) | 9.1 (5.3–14.9) | 18.5 (11.7–30) | <0.0001 |
| SCORE2 (%) §§ | 4 (2.1–7.2) | 3.2 (1.9–5.8) | 5.75 (3.5–9) | <0.0001 |
| Biochemical | ||||
| Total-c mg/dL | 195 (167–221.9) | 196.8 (166.2–220.4) | 189.8 (167.6–224.4) | 0.97 |
| LDL-c mg/dL # | 131.7 (104–155.7) | 132.3 (106–154.2) | 123.7 (100.2–155.8) | 0.46 |
| HDL-c mg/dL | 38.2 (31.7–47.5) | 41.9 (35.5–50.8) | 32.4 (26.9–37.1) | <0.0001 |
| TG mg/dL | 114.2 (84.1–157.6) | 95.6 (69–123.1) | 162 (120.4–215.2) | <0.0001 |
| Plasma glucose, mg/dL | 85 (77–92) | 81.4 (76–88.6) | 90 (84.6–106.2) | <0.0001 |
| ALP, U/L | 44 (35–54) | 42 (34–52.5) | 47.5 (40–56.5) | 0.0008 |
| ALT, U/L | 6.5 (5–9) | 5.8 (5–8) | 7.9 (5.6–10.1) | <0.0001 |
| AST, U/L | 18 (15–23) | 18 (14–21) | 20 (16–26) | 0.0005 |
| GGT, U/L | 21 (14–35) | 16.5 (12.5–24.5) | 36 (24–54.5) | <0.0001 |
| FLI (%) §§§ | 41.3 (16.7–75.6) | 22.2 (10.9–39.5) | 82.3 (73.4–92.5) | <0.0001 |
| CK-18/M30, U/L | 176.9 (132.8–224.5) | 158.6 (121.2–204.6) | 210.6 (163.7–272.4) | <0.0001 |
| CK-18/M65, U/L | 173.1 (128.2–263.1) | 161.1 (115.5–234.6) | 220.4 (153.6–323.2) | <0.0001 |
| All PREVEND Participants (n = 312) | |||||||||
| Discriminant Accuracy | Univariate Analysis | Multivariate Analysis | |||||||
| Predictor | AUC | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| M30 | 0.609 | 0.540–0.677 | 0.03 | 1.001 | 0.99–1.00 | 0.08 | 0.99 | 0.99–1.001 | 0.32 |
| FLI | 0.722 | 0.658–0.785 | <0.0001 | 1.03 | 1.01–1.03 | <0.0001 | 1.025 | 1.01–1.035 | <0.0001 |
| M65 | 0.608 | 0.540–0.677 | 0.03 | 1.003 | 1.001–1.004 | 0.001 | 1.002 | 0.99–1.004 | 0.05 |
| M30 > 200 | - | - | - | 2.01 | 1.22–3.32 | 0.005 | 1.10 | 0.61–2.00 | 0.73 |
| FLI | 0.722 | 0.658–0.785 | <0.0001 | 1.03 | 1.01–1.03 | <0.0001 | 1.026 | 1.016–1.035 | <0.0001 |
| M65 > 400 | - | - | - | 2.01 | 0.91–4.45 | 0.08 | 1.10 | 0.57–3.51 | 0.44 |
| FLI ≥ 60 PREVEND Participants (n = 112) | |||||||||
| Discriminant Accuracy | Univariate Analysis | Multivariate Analysis | |||||||
| Predictor | AUC | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| M30 | 0.572 | 0.466–0.679 | 0.187 | - | - | - | - | - | - |
| FLI | 0.678 | 0.580–0.777 | 0.001 | 1.06 | 1.023–1.010 | 0.001 | 1.06 | 1.020–1.100 | 0.002 |
| M65 | 0.615 | 0.511–0.719 | 0.036 | 1.002 | 1.000–1.004 | 0.03 | 1.001 | 0.999–1.004 | 0.06 |
| M30 > 200 | - | - | - | 0.52 | 0.246–1.135 | 0.102 | - | - | - |
| FLI | 0.678 | 0.580–0.777 | 0.001 | 1.06 | 1.023–1.010 | 0.001 | 1.062 | 1.023–1.102 | 0.001 |
| M65 > 400 | - | - | - | 1.58 | 0.545–0.46 | 0.39 | 1.46 | 0.48–4.47 | 0.49 |
| FLI < 60 PREVEND Participants (n = 200) | |||||||||
| Discriminant Accuracy | Univariate Analysis | Multivariate Analysis | |||||||
| Predictor | AUC | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| M30 | 0.543 | 0.447–0.639 | 0.416 | - | - | - | - | - | - |
| FLI | 0.618 | 0.520–0.716 | 0.025 | 1.024 | 1.003–1.045 | 0.02 | - | - | - |
| M65 | 0.532 | 0.434–0.630 | 0.546 | - | - | - | - | - | - |
| M30 > 200 | - | - | - | 1.109 | 0.506–2.43 | 0.79 | - | - | - |
| FLI | 0.618 | 0.520–0.716 | 0.025 | 1.024 | 1.003–1.045 | 0.02 | - | - | - |
| M65 > 400 | - | - | - | 1.50 | 0.388–0.587 | 0.55 | - | - | - |
| All PREVEND Participants (n = 312) | |||||||||
| Discriminant Accuracy | Univariate Analysis | Multivariate Analysis | |||||||
| Predictor | AUC | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| M30 | 0.554 | 0.422–0.686 | 0.39 | - | - | - | - | - | - |
| FLI | 0.637 | 0.518–0.756 | 0.029 | 1.015 | 1.001–1.029 | 0.035 | 1.009 | 0.99–1.024 | 0.21 |
| M65 | 0.608 | 0.478–0.737 | 0.085 | 1.003 | 1.001–1.004 | 0.0006 | 1.002 | 1.00–1.004 | 0.005 |
| M30 > 200 | - | - | - | 1.267 | 0.537–2.991 | 0.587 | - | - | - |
| FLI | 0.637 | 0.518–0.756 | 0.029 | 1.015 | 1.001–1.029 | 0.035 | 1.012 | 0.991.027 | 0.08 |
| M65 > 400 | - | - | - | 4.23 | 1.516–11.83 | 0.005 | 3.59 | 1.25–10.26 | 0.01 |
| FLI ≥ 60 PREVEND Participants (n = 112) | |||||||||
| Discriminant Accuracy | Univariate Analysis | Multivariate Analysis | |||||||
| Predictor | AUC | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| M30 | 0.587 | 0.384–0.790 | 0.384 | - | - | - | - | - | - |
| FLI | 0.636 | 0.467–0.805 | 0.086 | 1.045 | 0.984–1.109 | 0.147 | 1.043 | 0.975–1.115 | 0.21 |
| M65 | 0.714 | 0.524–0.904 | 0.016 | 1.003 | 1.001–1.005 | 0.001 | 1.003 | 1.001–1.005 | 0.002 |
| M30 > 200 | - | - | - | 1.592 | 0.449–5.644 | 0.471 | - | - | - |
| FLI | 0.636 | 0.467–0.805 | 0.086 | 1.045 | 0.984–1.109 | 0.147 | 1.042 | 0.98–1.108 | 0.18 |
| M65 > 400 | - | - | - | 5.584 | 1.50–20.65 | 0.009 | 5.444 | 1.44–20.53 | 0.01 |
| FLI < 60 PREVEND Participants (n = 200) | |||||||||
| Discriminant Accuracy | Univariate Analysis | Multivariate Analysis | |||||||
| Predictor | AUC | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| M30 | 0.495 | 0.346–0.644 | 0.955 | - | - | - | - | - | - |
| FLI | 0.568 | 0.424–0.712 | 0.448 | - | - | - | - | - | - |
| M65 | 0.500 | 0.376–0.625 | 0.996 | - | - | - | - | - | - |
| M30 > 200 | - | - | - | 0.555 | 0.116–2.655 | 0.461 | - | - | - |
| M65 > 400 | - | - | - | 1.618 | 0.189–13.80 | 0.659 | - | - | - |
| All PREVEND Participants (n = 312) | ||||
| Univariate Analysis | Multivariate Analysis | |||
| Variable | β (95% CI) | p-Value | β (95% CI) | p-Value |
| FRS | ||||
| M30 | 0.27 | 0.0008 | −0.009 | 0.91 |
| M65 | 0.31 | 0.00012 | 0.14 | 0.08 |
| FLI | 0.41 | <0.0001 | 0.39 | <0.0001 |
| SCORE2 | ||||
| M30 | 0.27 | 0.0006 | 0.01 | 0.86 |
| M65 | 0.34 | <0.0001 | 0.20 | 0.02 |
| FLI | 0.33 | <0.0001 | 0.30 | <0.0001 |
| FLI | ||||
| M30 | 0.53 | <0.0001 | 0.41 | 0.0001 |
| M65 | 0.44 | <0.0001 | 0.23 | 0.03 |
| FLI ≥ 60 PREVEND Participants (n = 112) | ||||
| Univariate Analysis | Multivariate Analysis | |||
| Variable | β (95% CI) | p-Value | β (95% CI) | p-Value |
| FRS | ||||
| M30 | 0.09 | 0.43 | −0.09 | 0.54 |
| M65 | 0.19 | 0.05 | 0.20 | 0.12 |
| FLI | 1.2 | 0.003 | 1.15 | 0.007 |
| SCORE2 | ||||
| M30 | 0.09 | 0.48 | −0.20 | 0.20 |
| M65 | 0.28 | 0.01 | 0.37 | 0.01 |
| FLI | 0.98 | 0.03 | 0.81 | 0.08 |
| FLI | ||||
| M30 | 0.02 | 0.27 | 0.004 | 0.88 |
| M65 | 0.03 | 0.12 | 0.032 | 0.28 |
| FLI < 60 PREVEND Participants (n = 200) | ||||
| Univariate Analysis | Multivariate Analysis | |||
| Variable | β (95% CI) | p-Value | β (95% CI) | p-Value |
| FRS | ||||
| M30 | 0.13 | 0.17 | 0.01 | 0.91 |
| M65 | 0.14 | 0.19 | 0.09 | 0.41 |
| FLI | 0.37 | <0.0001 | 0.36 | <0.0001 |
| SCORE2 | ||||
| M30 | 0.20 | 0.04 | 0.10 | 0.43 |
| M65 | 0.20 | 0.06 | 0.11 | 0.24 |
| FLI | 0.31 | <0.0001 | 0.06 | <0.0001 |
| FLI | ||||
| M30 | 0.27 | 0.01 | 0.25 | 0.02 |
| M65 | 0.14 | 0.23 | 0.04 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, S.; Bakker, S.J.L.; Juillard, C.; Dullaart, R.P.F.; Vuilleumier, N. Serum Level of Cytokeratin 18 (M65) as a Prognostic Marker of High Cardiovascular Disease Risk in Individuals with Non-Alcoholic Fatty Liver Disease. Biomolecules 2023, 13, 1128. https://doi.org/10.3390/biom13071128
Pagano S, Bakker SJL, Juillard C, Dullaart RPF, Vuilleumier N. Serum Level of Cytokeratin 18 (M65) as a Prognostic Marker of High Cardiovascular Disease Risk in Individuals with Non-Alcoholic Fatty Liver Disease. Biomolecules. 2023; 13(7):1128. https://doi.org/10.3390/biom13071128
Chicago/Turabian StylePagano, Sabrina, Stephan J. L. Bakker, Catherine Juillard, Robin P. F. Dullaart, and Nicolas Vuilleumier. 2023. "Serum Level of Cytokeratin 18 (M65) as a Prognostic Marker of High Cardiovascular Disease Risk in Individuals with Non-Alcoholic Fatty Liver Disease" Biomolecules 13, no. 7: 1128. https://doi.org/10.3390/biom13071128
APA StylePagano, S., Bakker, S. J. L., Juillard, C., Dullaart, R. P. F., & Vuilleumier, N. (2023). Serum Level of Cytokeratin 18 (M65) as a Prognostic Marker of High Cardiovascular Disease Risk in Individuals with Non-Alcoholic Fatty Liver Disease. Biomolecules, 13(7), 1128. https://doi.org/10.3390/biom13071128







