Computational Analysis Reveals Unique Binding Patterns of Oxygenated and Deoxygenated Myoglobin to the Outer Mitochondrial Membrane
Abstract
:1. Introduction
2. Methods
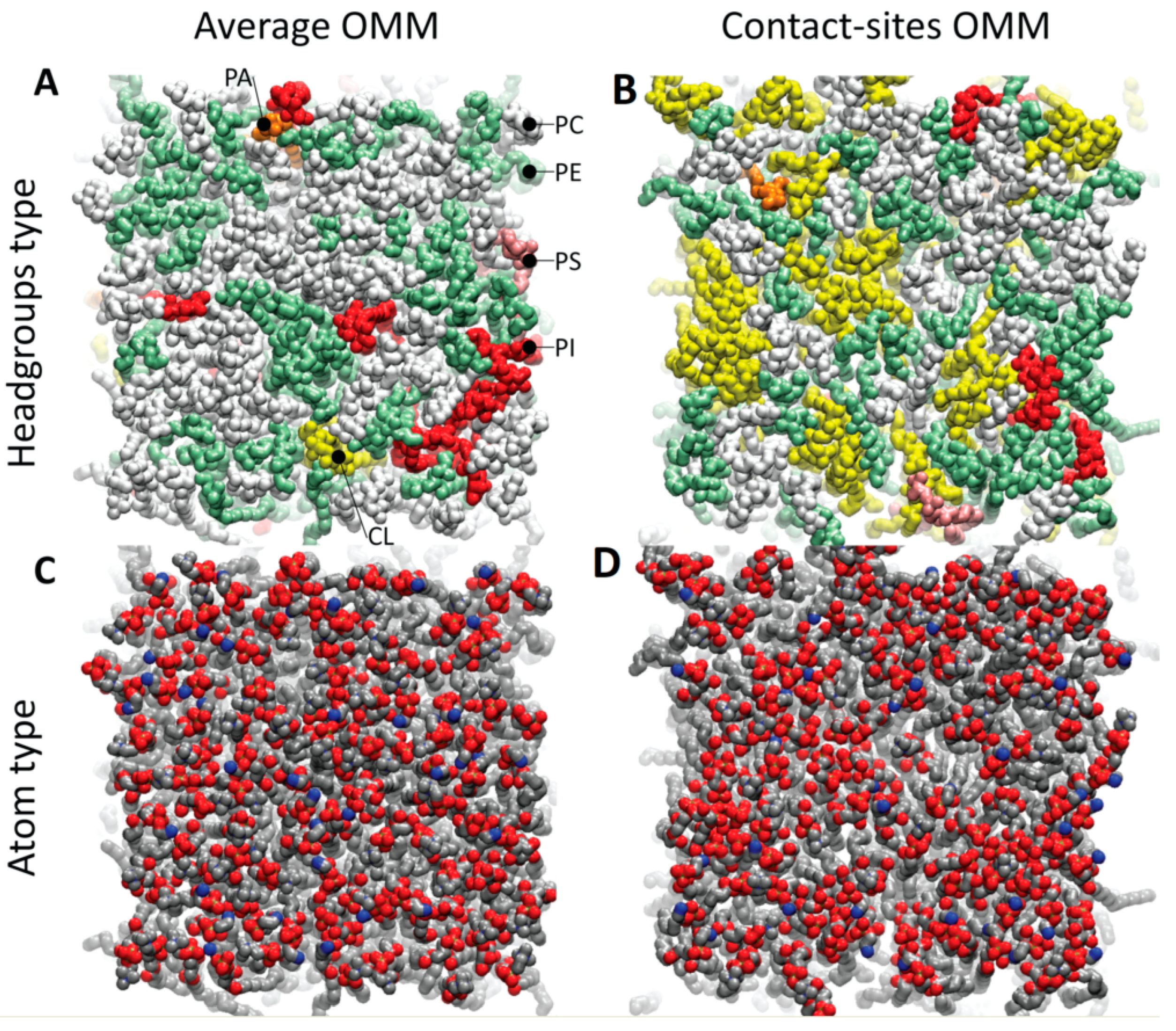
2.1. Construction of Outer Mitochondrial Membrane
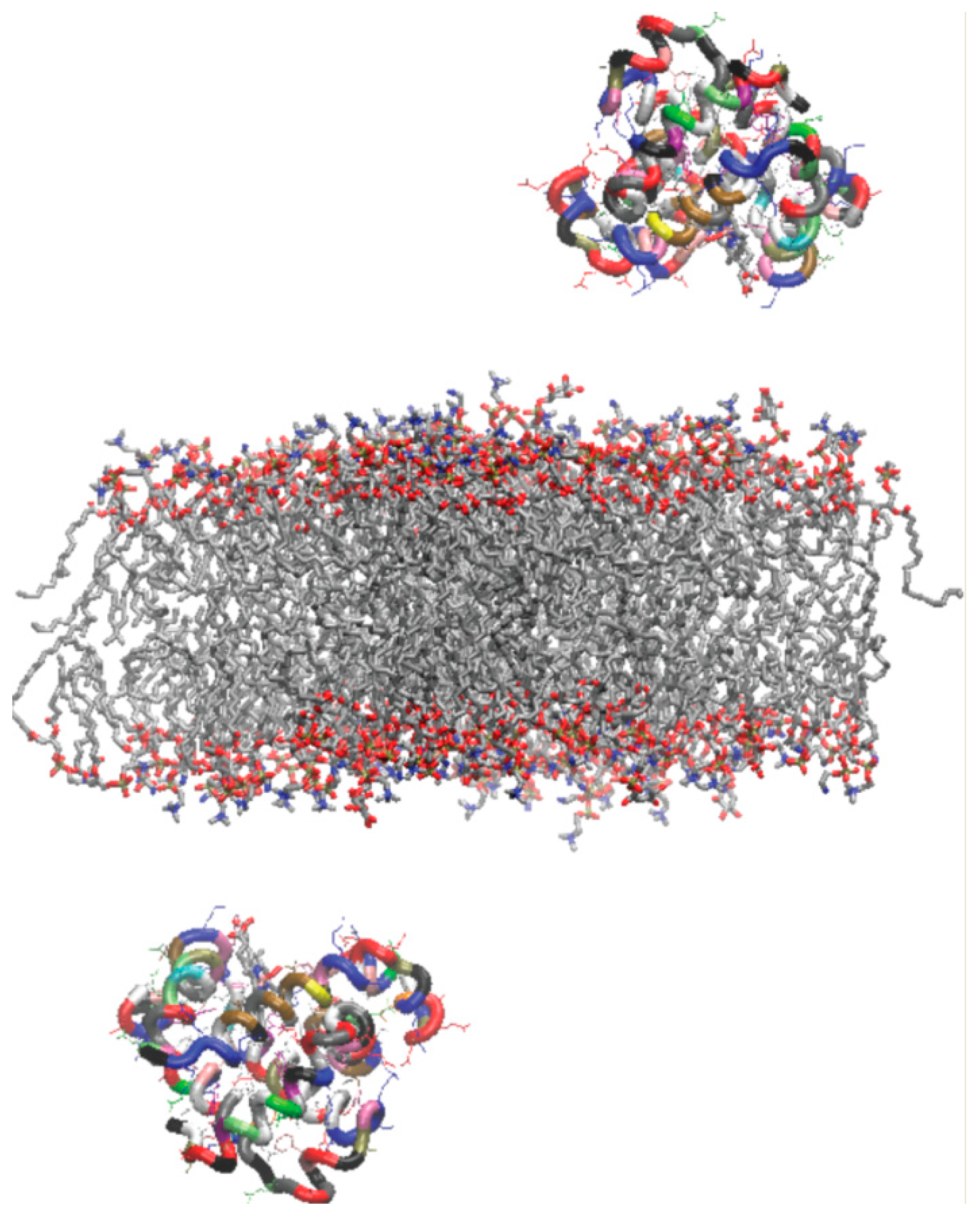
2.2. Oxy- and Deoxy-Mb Models
2.3. Molecular Dynamics Simulations
- (1)
- The slow (40 ns) approach of oxy- or deoxy-Mb toward the membrane, and establishing a stable contact under a constant force (0.01 kcal/mol/Å per atom, the total force 6.13 kcal/mol/Å-on the order of ~1.2 strong H-bonds per Å) uniformly distributed only over the backbone (alpha carbon, and the carbon, nitrogen, and oxygen of the peptide group, 4 atoms per residue in total) of the protein. It allowed the side chains, heme, and O2 to adjust freely to the contact. The amount of force used was 11.33 pN per whole protein. These settings were determined empirically, to identify Mb approaching the bilayer and establishing a stable positioning at the lipid surface over the first 20 ns under force (on average) but avoiding a significant distortion in the membrane.
- (2)
- This was followed by 40 ns relaxation without any restraints, to test the stability of contact. The “stability score” of an individual Mb–membrane attachment was quantified as the ratio of (1 + the lowest number of contacts observed) / (the largest number of contacts observed). The contributions of individual protein residues were estimated as the product of (the average frequency of the contacts of the residue with lipids) * (the correlation coefficient between the number of residue–lipid contacts and the “stability score” of the attachment).
- (3)
- The final stage is the 30 ns detachment of oxy- or deoxy-Mb from the membrane surface, using steered molecular dynamics, with the constant velocity of the harmonic restraint, to estimate the force required to break the attachment. The velocity for the detachment (0.5 Å/ns), and the strength of the harmonic restraint for the center of mass (10 kcal/mol/Å2), were chosen based on test runs, to be slow enough to minimize the variability of the force between the repetitions, but fast enough to enable a reasonable simulation time. Typically, all contacts between the protein and the membrane were lost within the first 20 ns of steering.
3. Results and Discussion
3.1. Freedom of Systems to Adjust and Variability of the Contact Arrangement
3.2. Statistics of the Initial Contacts between Mb and the Membrane
3.3. Stability of Myoglobin–Membrane Assemblies in Unrestrained Simulations
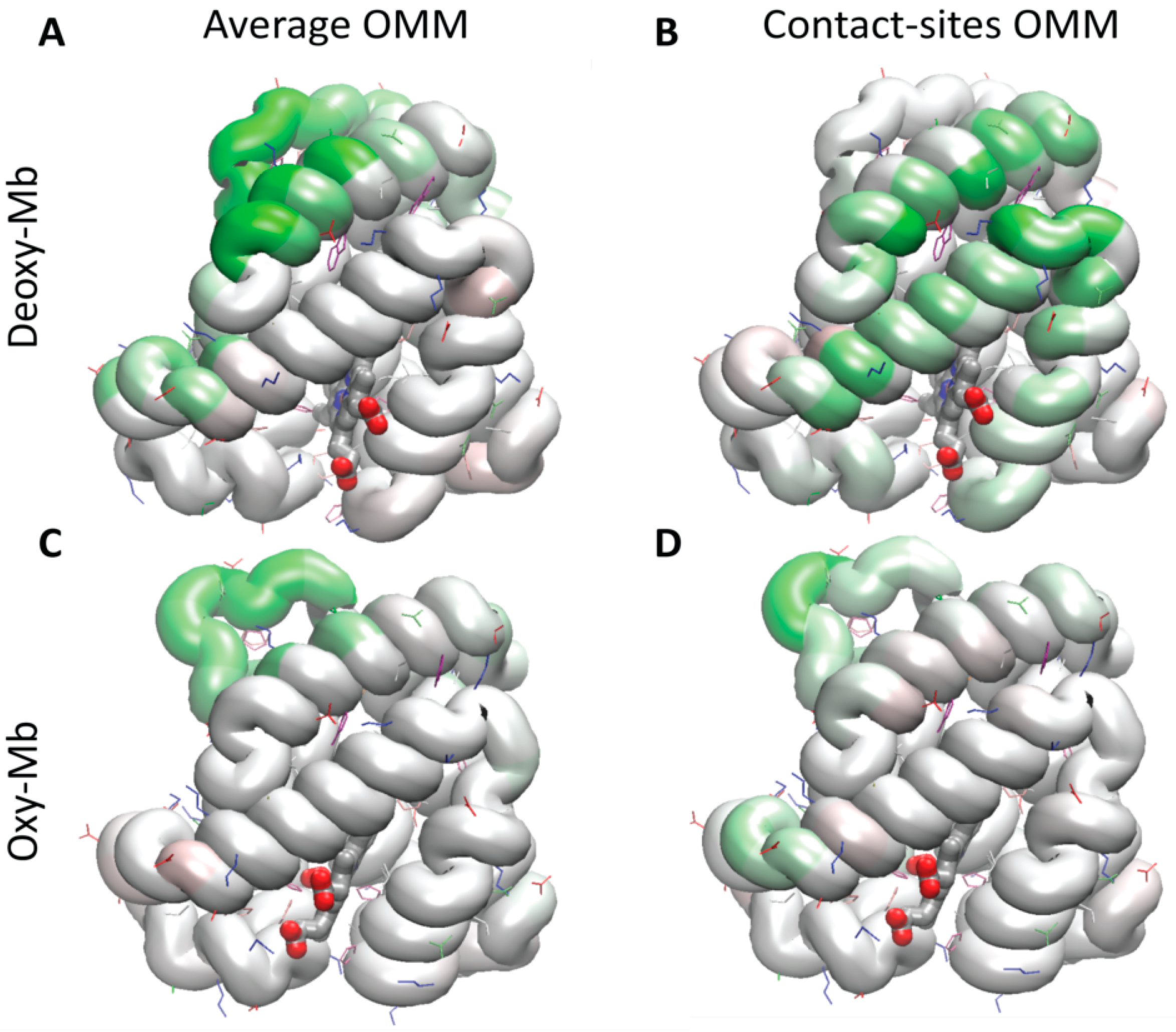
3.4. Maps of the Residues Contributing to Unrestrained Stability
3.5. Electrostatic Field of Myoglobin
3.6. Mb–Membrane Contact Strength Test in the Steered Detachment Simulations
3.7. Contributions of Specific Amino Acid and Lipid Types to the Stability of Mb–Membrane Contact in the Steered Detachment Simulations
3.8. Regions of the Protein Stabilizing Contact with the Membrane in the Steered Detachment
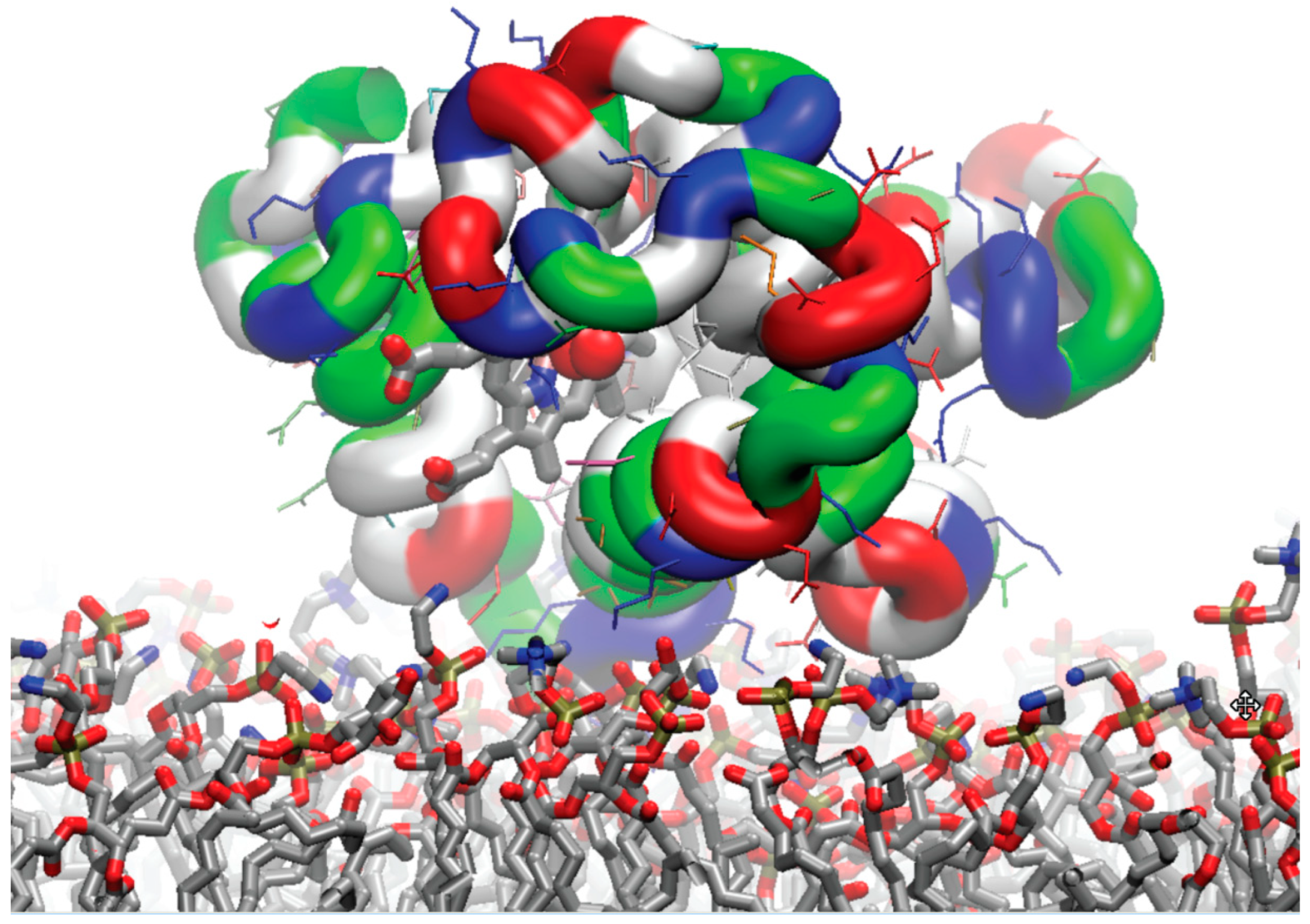
3.9. Typical Orientation of the Bound Myoglobin Relative to the Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ordway, G.A.; Garry, D.J. Myoglobin: An essential hemoprotein in striated muscle. J. Exp. Biol. 2004, 207, 3441–3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, P.J.; Jones, D.R. Physiology of diving of birds and mammals. Physiol. Rev. 1997, 77, 837–899. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, J.B. Myoglobin-facilitated oxygen diffusion: Role of myoglobin in oxygen entry into muscle. Physiol. Rev. 1970, 50, 559–636. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Choi, J.Y.; Shimojo, H.; Katsuta, S. Maintenance of myoglobin concentration in human skeletal muscle after heavy resistance training. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 347–352. [Google Scholar] [CrossRef]
- Yang, F.; Phillips, G.N., Jr. Crystal structures of CO-, deoxy- and met-myoglobins at various pH values. J. Mol. Biol. 1996, 256, 762–774. [Google Scholar] [CrossRef]
- Richardson, R.S.; Noyszewski, E.A.; Kendrick, K.F.; Leigh, J.S.; Wagner, P.D. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J. Clin. Investig. 1995, 96, 1916–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponganis, P.J.; Kreutzer, U.; Stockard, T.K.; Lin, P.C.; Sailasuta, N.; Tran, T.K.; Hurd, R.; Jue, T. Blood flow and metabolic regulation in seal muscle during apnea. J. Exp. Biol. 2008, 211, 3323–3332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merx, M.W.; Flogel, U.; Stumpe, T.; Godecke, A.; Decking, U.K.; Schrader, J. Myoglobin facilitates oxygen diffusion. FASEB J. 2001, 15, 1077–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, R.S.; Leigh, J.S.; Wagner, P.D.; Noyszewski, E.A. Cellular PO2 as a determinant of maximal mitochondrial O(2) consumption in trained human skeletal muscle. J. Appl. Physiol. 1999, 87, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mole, P.A.; Chung, Y.; Tran, T.K.; Sailasuta, N.; Hurd, R.; Jue, T. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am. J. Physiol. 1999, 277, R173–R180. [Google Scholar] [CrossRef]
- Chung, Y.; Mole, P.A.; Sailasuta, N.; Tran, T.K.; Hurd, R.; Jue, T. Control of respiration and bioenergetics during muscle contraction. Am. J. Physiol. Cell. Physiol. 2005, 288, C730–C738. [Google Scholar] [CrossRef]
- Wittenberg, B.A.; Wittenberg, J.B.; Caldwell, P.R. Role of myoglobin in the oxygen supply to red skeletal muscle. J. Biol. Chem. 1975, 250, 9038–9043. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Matthews, P.M.; Radda, G.K. Myoglobin-dependent oxidative metabolism in the hypoxic rat heart. Respir. Physiol. 1986, 63, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Driedzic, W.R. The fish heart as a model system for the study of myoglobin. Comp. Biochem. Physiol. A Comp. Physiol. 1983, 76, 487–493. [Google Scholar] [CrossRef]
- Cole, R.P. Skeletal muscle function in hypoxia: Effect of alteration of intracellular myoglobin. Respir. Physiol. 1983, 53, 1–14. [Google Scholar] [CrossRef]
- Merx, M.W.; Godecke, A.; Flogel, U.; Schrader, J. Oxygen supply and nitric oxide scavenging by myoglobin contribute to exercise endurance and cardiac function. FASEB J. 2005, 19, 1015–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jue, T.; Simond, G.; Wright, T.J.; Shih, L.; Chung, Y.; Sriram, R.; Kreutzer, U.; Davis, R.W. Effect of fatty acid interaction on myoglobin oxygen affinity and triglyceride metabolism. J. Physiol. Biochem. 2016, 73, 359–370. [Google Scholar] [CrossRef]
- Garry, D.J.; Ordway, G.A.; Lorenz, J.N.; Radford, N.B.; Chin, E.R.; Grange, R.W.; Bassel-Duby, R.; Williams, R.S. Mice without myoglobin. Nature 1998, 395, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Grange, R.W.; Meeson, A.; Chin, E.; Lau, K.S.; Stull, J.T.; Shelton, J.M.; Williams, R.S.; Garry, D.J. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am. J. Physiol. Cell. Physiol. 2001, 281, C1487–C1494. [Google Scholar] [CrossRef] [Green Version]
- Garry, D.J.; Meeson, A.; Yan, Z.; Williams, R.S. Life without myoglobin. Cell. Mol. Life Sci. 2000, 57, 896–898. [Google Scholar] [CrossRef]
- Adepu, K.K.; Bhandari, D.; Anishkin, A.; Adams, S.H.; Chintapalli, S.V. Myoglobin-Pyruvate Interactions: Binding Thermodynamics, Structure-Function Relationships, and Impact on Oxygen Release Kinetics. Int. J. Mol. Sci. 2022, 23, 8766. [Google Scholar] [CrossRef]
- Adepu, K.K.; Bhandari, D.; Anishkin, A.; Adams, S.H.; Chintapalli, S.V. Myoglobin Interaction with Lactate Rapidly Releases Oxygen: Studies on Binding Thermodynamics, Spectroscopy, and Oxygen Kinetics. Int. J. Mol. Sci. 2022, 23, 4747. [Google Scholar] [CrossRef]
- Chintapalli, S.V.; Jayanthi, S.; Mallipeddi, P.L.; Gundampati, R.; Suresh Kumar, T.K.; van Rossum, D.B.; Anishkin, A.; Adams, S.H. Novel Molecular Interactions of Acylcarnitines and Fatty Acids with Myoglobin. J. Biol. Chem. 2016, 291, 25133–25143. [Google Scholar] [CrossRef] [Green Version]
- Chintapalli, S.V.; Bhardwaj, G.; Patel, R.; Shah, N.; Patterson, R.L.; van Rossum, D.B.; Anishkin, A.; Adams, S.H. Molecular dynamic simulations reveal the structural determinants of Fatty Acid binding to oxy-myoglobin. PLoS ONE 2015, 10, e0128496. [Google Scholar] [CrossRef] [Green Version]
- Chintapalli, S.V.; Anishkin, A.; Adams, S.H. Exploring the entry route of palmitic acid and palmitoylcarnitine into myoglobin. Arch. Biochem. Biophys. 2018, 655, 56–66. [Google Scholar] [CrossRef]
- Blackburn, M.L.; Wankhade, U.D.; Ono-Moore, K.D.; Chintapalli, S.V.; Fox, R.; Rutkowsky, J.M.; Willis, B.J.; Tolentino, T.; Lloyd, K.C.K.; Adams, S.H. On the potential role of globins in brown adipose tissue: A novel conceptual model and studies in myoglobin knockout mice. Am. J. Physiology. Endocrinol. Metab. 2021, 321, E47–E62. [Google Scholar] [CrossRef]
- Christen, L.; Broghammer, H.; Rapohn, I.; Mohlis, K.; Strehlau, C.; Ribas-Latre, A.; Gebhardt, C.; Roth, L.; Krause, K.; Landgraf, K.; et al. Myoglobin-mediated lipid shuttling increases adrenergic activation of brown and white adipocyte metabolism and is as a marker of thermogenic adipocytes in humans. Clin. Transl. Med. 2022, 12, e1108. [Google Scholar] [CrossRef] [PubMed]
- Clanton, T.L. Managing the power grid: How myoglobin can regulate PO2 and energy distribution in skeletal muscle. J. Appl. Physiol. 2019, 126, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Rochon, E.R.; Corti, P. Globins and nitric oxide homeostasis in fish embryonic development. Mar. Genomics 2020, 49, 100721. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.A.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.M.; Gladwin, M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef]
- Aguirre, E.; Rodriguez-Juarez, F.; Bellelli, A.; Gnaiger, E.; Cadenas, S. Kinetic model of the inhibition of respiration by endogenous nitric oxide in intact cells. Biochim. Biophys. Acta 2010, 1797, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Koivisto, A.; Matthias, A.; Bronnikov, G.; Nedergaard, J. Kinetics of the inhibition of mitochondrial respiration by NO. FEBS Lett. 1997, 417, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Mole, P.A.; Oscai, L.B.; Holloszy, J.O. Adaptation of muscle to exercise. Increase in levels of palmityl Coa synthetase, carnitine palmityltransferase, and palmityl Coa dehydrogenase, and in the capacity to oxidize fatty acids. J. Clin. Investig. 1971, 50, 2323–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.M.; Klinkerfuss, G.H.; Terjung, R.L.; Mole, P.A.; Holloszy, J.O. Respiratory capacity of white, red, and intermediate muscle: Adaptative response to exercise. Am. J. Physiol. 1972, 222, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Constable, S.H.; Favier, R.J.; McLane, J.A.; Fell, R.D.; Chen, M.; Holloszy, J.O. Energy metabolism in contracting rat skeletal muscle: Adaptation to exercise training. Am. J. Physiol. 1987, 253, C316–C322. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Luthi, P.; Claassen, H.; Weibel, E.R.; Howald, H. The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflug. Arch. 1973, 344, 217–232. [Google Scholar] [CrossRef]
- Gollnick, P.D.; King, D.W. Effect of exercise and training on mitochondria of rat skeletal muscle. Am. J. Physiol. 1969, 216, 1502–1509. [Google Scholar] [CrossRef]
- Palmer, J.W.; Tandler, B.; Hoppel, C.L. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: Effects of procedural manipulations. Arch. Biochem. Biophys. 1985, 236, 691–702. [Google Scholar] [CrossRef]
- Manneschi, L.; Federico, A. Polarographic analyses of subsarcolemmal and intermyofibrillar mitochondria from rat skeletal and cardiac muscle. J. Neurol. Sci. 1995, 128, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, A.M.; Stevens, R.J.; Hood, D.A. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am. J. Physiol. 1993, 264, C383–C389. [Google Scholar] [CrossRef] [PubMed]
- Postnikova, G.B.; Tselikova, S.V.; Shekhovtsova, E.A. Myoglobin and mitochondria: Oxymyoglobin interacts with mitochondrial membrane during deoxygenation. Biochem. Mosc. 2009, 74, 1211–1218. [Google Scholar] [CrossRef]
- Postnikova, G.B.; Tselikova, S.V. Myoglobin and mitochondria: Kinetics of oxymyoglobin deoxygenation in mitochondria suspension. Biofizika 2005, 50, 297–306. [Google Scholar]
- Postnikova, G.B.; Shekhovtsova, E.A. Fluorescence studies on the interaction of myoglobin with mitochondria. Biochem. Mosc. 2012, 77, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Shuruta, S.A.; Amerkhanov, Z.G.; Postnikova, G.B. Effect of mitochondria on the redox reaction between oxyhemoglobin and ferricytochrome c. Biofizika 1999, 44, 1059–1062. [Google Scholar]
- Romero-Herrera, A.E.; Lehmann, H.; Joysey, K.A.; Friday, A.E. On the evolution of myoglobin. Philos. Trans. R Soc. Lond. B Biol. Sci. 1978, 283, 61–163. [Google Scholar] [CrossRef]
- Perutz, M.F.; Mathews, F.S. An x-ray study of azide methaemoglobin. J. Mol. Biol. 1966, 21, 199–202. [Google Scholar] [CrossRef]
- Vernier, G.; Chenal, A.; Vitrac, H.; Barumandzadhe, R.; Montagner, C.; Forge, V. Interactions of apomyoglobin with membranes: Mechanisms and effects on heme uptake. Protein Sci. 2007, 16, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Basu Ball, W.; Neff, J.K.; Gohil, V.M. The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett. 2018, 592, 1273–1290. [Google Scholar] [CrossRef] [Green Version]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Stefanyk, L.E.; Coverdale, N.; Roy, B.D.; Peters, S.J.; LeBlanc, P.J. Skeletal muscle type comparison of subsarcolemmal mitochondrial membrane phospholipid fatty acid composition in rat. J. Membr. Biol. 2010, 234, 207–215. [Google Scholar] [CrossRef]
- de Kroon, A.I.; Dolis, D.; Mayer, A.; Lill, R.; de Kruijff, B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim. Biophys. Acta 1997, 1325, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.A.; Rog, T.; da Silva, A.B.F.; Amaro, R.E.; Johnson, M.S.; Postila, P.A. Examining the Effect of Charged Lipids on Mitochondrial Outer Membrane Dynamics Using Atomistic Simulations. Biomolecules 2022, 12, 183. [Google Scholar] [CrossRef]
- Venable, R.M.; Sodt, A.J.; Rogaski, B.; Rui, H.; Hatcher, E.; MacKerell, A.D., Jr.; Pastor, R.W.; Klauda, J.B. CHARMM all-atom additive force field for sphingomyelin: Elucidation of hydrogen bonding and of positive curvature. Biophys. J. 2014, 107, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Pandit, K.R.; Klauda, J.B. Membrane models of E. coli containing cyclic moieties in the aliphatic lipid chain. Biochim. Biophys. Acta 2012, 1818, 1205–1210. [Google Scholar] [CrossRef] [Green Version]
- Lemmin, T.; Bovigny, C.; Lancon, D.; Dal Peraro, M. Cardiolipin Models for Molecular Simulations of Bacterial and Mitochondrial Membranes. J. Chem. Theory Comput. 2013, 9, 670–678. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Fiser, A.; Do, R.K.; Sali, A. Modeling of loops in protein structures. Protein Sci. 2000, 9, 1753–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrashekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Daigle, R.; Guertin, M.; Lague, P. Structural characterization of the tunnels of Mycobacterium tuberculosis truncated hemoglobin N from molecular dynamics simulations. Proteins 2009, 75, 735–747. [Google Scholar] [CrossRef]
- Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef] [Green Version]
- Feller, S.E.; Yuhong, Z.; Pastor, R.W. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1998, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Huber, C.T.; Morrison, M. Heterogeneity of the outer membrane of mitochondria. Biochemistry 1973, 12, 4274–4282. [Google Scholar] [CrossRef]
- Calvo, S.E.; Clauser, K.R.; Mootha, V.K. MitoCarta2.0: An updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016, 44, D1251–D1257. [Google Scholar] [CrossRef] [Green Version]
- Palade, G.E. An electron microscope study of the mitochondrial structure. J. Histochem. Cytochem. 1953, 1, 188–211. [Google Scholar] [CrossRef] [PubMed]
- Srere, P.A.; Sumegi, B. Organization of the mitochondrial matrix. Adv. Exp. Med. Biol. 1986, 194, 13–25. [Google Scholar] [CrossRef]
- Lemeshko, V.V. Electrical control of the cell energy metabolism at the level of mitochondrial outer membrane. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183493. [Google Scholar] [CrossRef] [PubMed]
- Wollweber, F.; von der Malsburg, K.; van der Laan, M. Mitochondrial contact site and cristae organizing system: A central player in membrane shaping and crosstalk. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 1481–1489. [Google Scholar] [CrossRef]
- Shviro, Y.; Zilber, I.; Shaklai, N. The interaction of hemoglobin with phosphatidylserine vesicles. Biochim. Biophys. Acta 1982, 687, 63–70. [Google Scholar] [CrossRef] [PubMed]



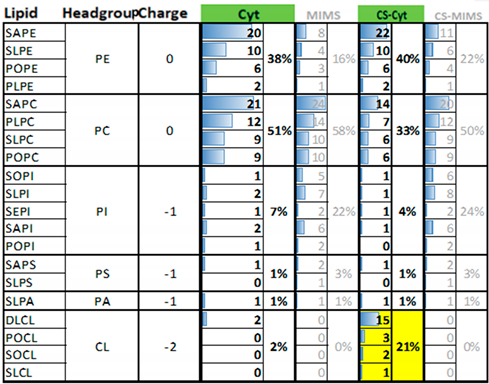
| Average OMM | Contact-Sites OMM | |||
|---|---|---|---|---|
| Independent Runs | Deoxy-Mb | Oxy-Mb | Deoxy-Mb | Oxy-Mb |
| 39 | 15 | 56 | 26 | |
| 38 | 12 | 41 | 21 | |
| 27 | 8 | 30 | 18 | |
| 17 | 5 | 29 | 10 | |
| 9 | 3 | 20 | 4 | |
| 0 | 2 | 5 | 2 | |
| 0 | 0 | 3 | 1 | |
| 0 | 0 | 2 | 0 | |
| 0 | 0 | 1 | 0 | |
| 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | |
| Average | 10.8 | 3.8 | 15.6 | 6.8 |
| CS/Avg OMM Ratio | 1.4 | 1.8 | ||
| Oxy/Deoxy Mb Ratio | 0.3 | 0.4 | ||
| Average OMM | Contact-Sites OMM | |||
|---|---|---|---|---|
| Independent Runs | Deoxy-Mb | Oxy-Mb | Deoxy-Mb | Oxy-Mb |
| 150.92 | 62.13 | 57.65 | 126.39 | |
| 80.16 | 45.48 | 53.54 | 102.9 | |
| 72.58 | 43.94 | 46.74 | 97.19 | |
| 57.09 | 39.07 | 46.73 | 93.11 | |
| 51.98 | 27.55 | 45.88 | 88.53 | |
| 40.8 | 27.29 | 41.49 | 65 | |
| 40.11 | 25.98 | 25.76 | 60.72 | |
| 37.36 | 24.83 | 16.65 | 38.61 | |
| 30.14 | 14.08 | 13.01 | 28.79 | |
| 18.49 | 13.36 | 12.55 | 24.89 | |
| 12.89 | 7.99 | |||
| 9.67 | ||||
| Average | 50.2 | 32.4 | 36.0 | 66.7 |
| CS/Avg OMM Ratio | 0.7 | 2.1 | ||
| Oxy/Deoxy Mb Ratio | 0.6 | 1.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anishkin, A.; Adepu, K.K.; Bhandari, D.; Adams, S.H.; Chintapalli, S.V. Computational Analysis Reveals Unique Binding Patterns of Oxygenated and Deoxygenated Myoglobin to the Outer Mitochondrial Membrane. Biomolecules 2023, 13, 1138. https://doi.org/10.3390/biom13071138
Anishkin A, Adepu KK, Bhandari D, Adams SH, Chintapalli SV. Computational Analysis Reveals Unique Binding Patterns of Oxygenated and Deoxygenated Myoglobin to the Outer Mitochondrial Membrane. Biomolecules. 2023; 13(7):1138. https://doi.org/10.3390/biom13071138
Chicago/Turabian StyleAnishkin, Andriy, Kiran Kumar Adepu, Dipendra Bhandari, Sean H. Adams, and Sree V. Chintapalli. 2023. "Computational Analysis Reveals Unique Binding Patterns of Oxygenated and Deoxygenated Myoglobin to the Outer Mitochondrial Membrane" Biomolecules 13, no. 7: 1138. https://doi.org/10.3390/biom13071138





