Emergent Peptides of the Antifibrotic Arsenal: Taking Aim at Myofibroblast Promoting Pathways
Abstract
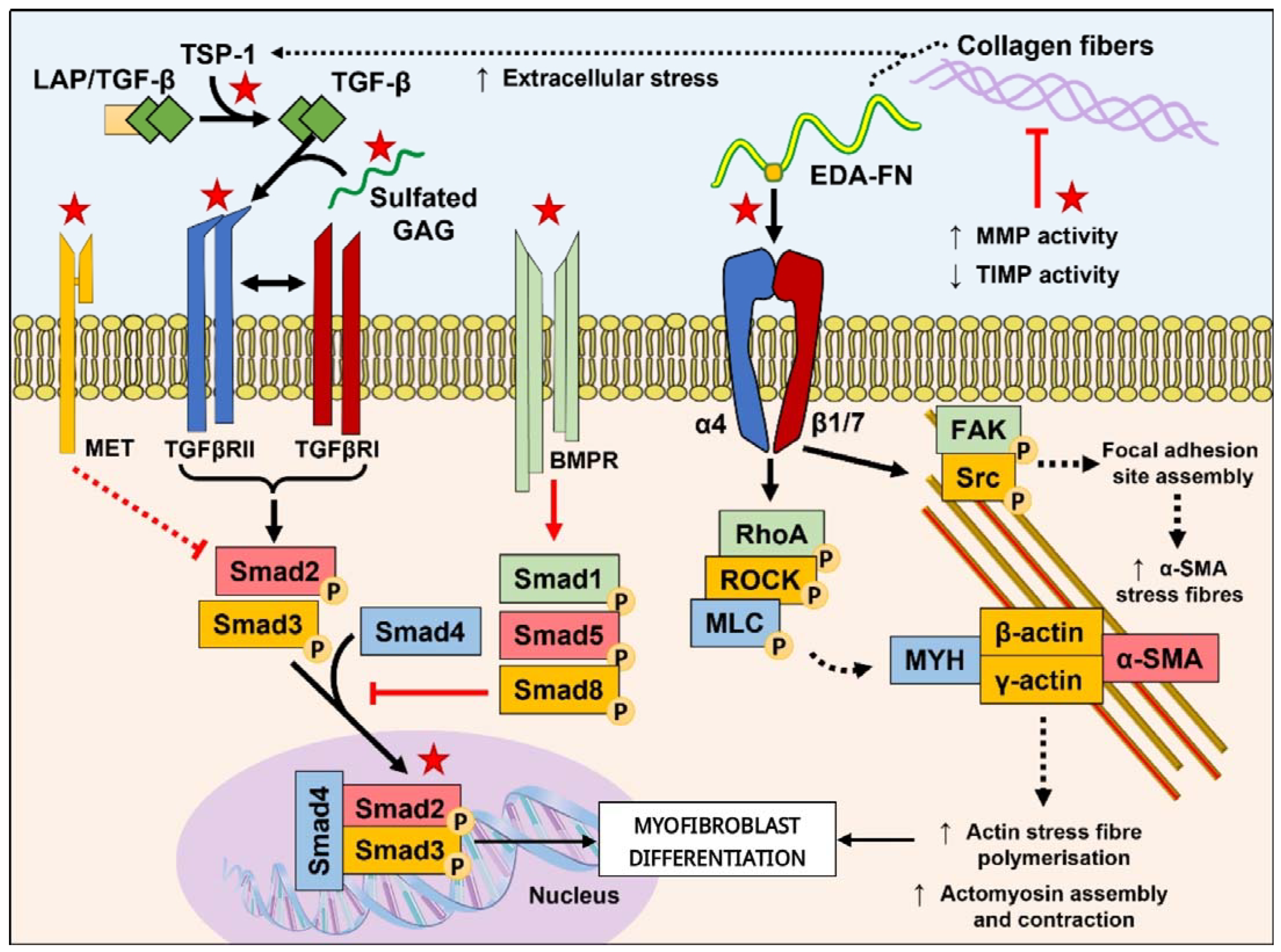
1. Introduction
2. Direct Targeting of Myofibroblast Pathways with Peptides
2.1. Peptides Targeting the TGF-β Pathway
2.2. Peptides Targeting the ECM and Mechanotransduction
3. Peptide Mediators of Auxiliary Fibrosis-Promoting Mechanisms
4. Prospect of Non-Mammalian Sourced Peptides as Anti-Fibrotics
5. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast: A key cell for wound healing and fibrocontractive diseases. Prog. Clin. Biol. Res. 1981, 54, 183–194. [Google Scholar]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Takihara, T.; Chambers, R.A.; Veraldi, K.L.; Larregina, A.T.; Feghali-Bostwick, C.A. A peptide derived from endostatin ameliorates organ fibrosis. Sci. Transl. Med. 2012, 4, 136ra171. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Watanabe, T.; Nishimoto, T.; Takihara, T.; Mlakar, L.; Nguyen, X.X.; Sanderson, M.; Su, Y.; Chambers, R.A.; Feghali-Bostwick, C. E4 engages uPAR and enolase-1 and activates urokinase to exert antifibrotic effects. JCI Insight 2021, 6, e144935. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Iyer, S.R.; Harders, G.E.; Pan, S.; Chen, H.H.; Ichiki, T.; Burnett, J.C., Jr.; Sangaralingham, S.J. C53: A novel particulate guanylyl cyclase B receptor activator that has sustained activity in vivo with anti-fibrotic actions in human cardiac and renal fibroblasts. J. Mol. Cell. Cardiol. 2019, 130, 140–150. [Google Scholar] [CrossRef]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Evans, R.A.; Tian, Y.A.C.; Steadman, R.; Phillips, A.O. TGF-β1-mediated fibroblast–myofibroblast terminal differentiation—The role of smad proteins. Exp. Cell Res. 2003, 282, 90–100. [Google Scholar] [CrossRef]
- Frangogiannis, N. Transforming growth factor-beta in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Midgley, A.C.; Rogers, M.; Hallett, M.B.; Clayton, A.; Bowen, T.; Phillips, A.O.; Steadman, R. Transforming growth factor-beta1 (TGF-beta1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J. Biol. Chem. 2013, 288, 14824–14838. [Google Scholar] [CrossRef] [PubMed]
- Poosti, F.; Soebadi, M.A.; Crijns, H.; De Zutter, A.; Metzemaekers, M.; Berghmans, N.; Vanheule, V.; Albersen, M.; Opdenakker, G.; Van Damme, J.; et al. Inhibition of renal fibrosis with a human CXCL9-derived glycosaminoglycan-binding peptide. Clin. Transl. Immunol. 2022, 11, e1370. [Google Scholar] [CrossRef]
- Yuan, Q.; Ren, Q.; Li, L.; Tan, H.; Lu, M.; Tian, Y.; Huang, L.; Zhao, B.; Fu, H.; Hou, F.F.; et al. A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-beta signaling. Nat. Commun. 2022, 13, 438. [Google Scholar] [CrossRef]
- Li, Y.; Ji, X.; Yao, W.; Pan, H.; Li, P.; Liu, Y.; Yuan, J.; Xu, Q.; Ni, C. M10 peptide attenuates silica-induced pulmonary fibrosis by inhibiting Smad2 phosphorylation. Toxicol. Appl. Pharmacol. 2019, 376, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Y.; Zhang, P.; Zou, H. A HGFderived peptide suppresses EMT in human lens epithelial cells via the TGFbeta/Smad and Akt/mTOR signaling pathways. Mol. Med. Rep. 2020, 22, 551–558. [Google Scholar] [CrossRef]
- Salido-Medina, A.B.; Gil, A.; Exposito, V.; Martinez, F.; Redondo, J.M.; Hurle, M.A.; Nistal, J.F.; Garcia, R. BMP7-based peptide agonists of BMPR1A protect the left ventricle against pathological remodeling induced by pressure overload. Biomed. Pharmacother. 2022, 149, 112910. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, C.C.; Montenegro, S.E.; Jo, G.; Kusumaningrum, N.; Lee, S.H.; Chung, J.H.; Mun, J.H. Adiponectin-Based Peptide (ADP355) Inhibits Transforming Growth Factor-beta1-Induced Fibrosis in Keloids. Int. J. Mol. Sci. 2020, 21, 2833. [Google Scholar] [CrossRef]
- Lyon, M.; Rushton, G.; Gallagher, J.T. The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific. J. Biol. Chem. 1997, 272, 18000–18006. [Google Scholar] [CrossRef]
- Li, P.; Oparil, S.; Novak, L.; Cao, X.; Shi, W.; Lucas, J.; Chen, Y.F. ANP signaling inhibits TGF-beta-induced Smad2 and Smad3 nuclear translocation and extracellular matrix expression in rat pulmonary arterial smooth muscle cells. J. Appl. Physiol. (1985) 2007, 102, 390–398. [Google Scholar] [CrossRef]
- Li, P.; Wang, D.; Lucas, J.; Oparil, S.; Xing, D.; Cao, X.; Novak, L.; Renfrow, M.B.; Chen, Y.F. Atrial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ. Res. 2008, 102, 185–192. [Google Scholar] [CrossRef]
- Saura, M.; Zaragoza, C.; Herranz, B.; Griera, M.; Diez-Marques, L.; Rodriguez-Puyol, D.; Rodriguez-Puyol, M. Nitric oxide regulates transforming growth factor-beta signaling in endothelial cells. Circ. Res. 2005, 97, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Dally, J.; Khan, J.S.; Voisey, A.; Charalambous, C.; John, H.L.; Woods, E.L.; Steadman, R.; Moseley, R.; Midgley, A.C. Hepatocyte Growth Factor Mediates Enhanced Wound Healing Responses and Resistance to Transforming Growth Factor-beta(1)-Driven Myofibroblast Differentiation in Oral Mucosal Fibroblasts. Int. J. Mol. Sci. 2017, 18, 1843. [Google Scholar] [CrossRef]
- Midgley, A.C.; Wei, Y.; Zhu, D.; Gao, F.; Yan, H.; Khalique, A.; Luo, W.; Jiang, H.; Liu, X.; Guo, J.; et al. Multifunctional Natural Polymer Nanoparticles as Antifibrotic Gene Carriers for CKD Therapy. J. Am. Soc. Nephrol. 2020, 31, 2292–2311. [Google Scholar] [CrossRef]
- Meng, X.M.; Chung, A.C.; Lan, H.Y. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin. Sci. 2013, 124, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Cool, B.L.; Laderoute, K.R.; Foretz, M.; Viollet, B.; Simonson, M.S. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J. Biol. Chem. 2008, 283, 10461–10469. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000Res 2016, 5, F1000. [Google Scholar] [CrossRef]
- Goffin, J.M.; Pittet, P.; Csucs, G.; Lussi, J.W.; Meister, J.J.; Hinz, B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J. Cell Biol. 2006, 172, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Masters and servants of the force: The role of matrix adhesions in myofibroblast force perception and transmission. Eur. J. Cell Biol. 2006, 85, 175–181. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Bhuiyan, S.; Shen, M.; Chelvaretnam, S.; Tan, A.Y.; Ho, G.; Hossain, M.A.; Widdop, R.E.; Samuel, C.S. Assessment of renal fibrosis and anti-fibrotic agents using a novel diagnostic and stain-free second-harmonic generation platform. FASEB J. 2021, 35, e21595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, H.; Tai, Y.; Xue, Y.; Wei, Y.; Wang, K.; Zhao, Q.; Wang, S.; Kong, D.; Midgley, A.C. Design and Evaluation of a Polypeptide that Mimics the Integrin Binding Site for EDA Fibronectin to Block Profibrotic Cell Activity. Int. J. Mol. Sci. 2021, 22, 1575. [Google Scholar] [CrossRef]
- Zhang, L.; Tai, Y.; Liu, X.; Liu, Y.; Dong, Y.; Liu, Y.; Yang, C.; Kong, D.; Qi, C.; Wang, S.; et al. Natural polymeric and peptide-loaded composite wound dressings for scar prevention. Appl. Mater. Today 2021, 25, 101186. [Google Scholar] [CrossRef]
- Xu, X.; Khoong, Y.M.; Gu, S.; Huang, X.; Ren, J.Y.; Gu, Y.H.; Li, H.; Gao, Y.; Wang, Z.; Zan, T. Investigating the potential of LSKL peptide as a novel hypertrophic scar treatment. Biomed. Pharmacother. 2020, 124, 109824. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.S.; Royce, S.G.; Hewitson, T.D.; Denton, K.M.; Cooney, T.E.; Bennett, R.G. Anti-fibrotic actions of relaxin. Br. J. Pharmacol. 2017, 174, 962–976. [Google Scholar] [CrossRef]
- Alam, F.; Gaspari, T.A.; Kemp-Harper, B.K.; Low, E.; Aw, A.; Ferens, D.; Spizzo, I.; Jefferis, A.M.; Praveen, P.; Widdop, R.E.; et al. The single-chain relaxin mimetic, B7-33, maintains the cardioprotective effects of relaxin and more rapidly reduces left ventricular fibrosis compared to perindopril in an experimental model of cardiomyopathy. Biomed. Pharmacother. 2023, 160, 114370. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, X.; Lopez-Guisa, J.M.; Collins, S.J.; Eddy, A.A. Mitogenic signaling of urokinase receptor-deficient kidney fibroblasts: Actions of an alternative urokinase receptor and LDL receptor-related protein. J. Am. Soc. Nephrol. 2004, 15, 2090–2102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alenchery, R.G.; Ajalik, R.E.; Jerreld, K.; Midekksa, F.; Zhong, S.; Alkatib, B.; Awad, H.A. PAI-1 mediates TGF-beta1-induced myofibroblast activation in tenocytes via mTOR signaling. J. Orthop. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Hattori, N.; Senoo, T.; Takayama, Y.; Masuda, T.; Nakashima, T.; Iwamoto, H.; Fujitaka, K.; Hamada, H.; Kohno, N. Inhibition of Plasminogen Activator Inhibitor-1 Attenuates Transforming Growth Factor-beta-Dependent Epithelial Mesenchymal Transition and Differentiation of Fibroblasts to Myofibroblasts. PLoS ONE 2016, 11, e0148969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Zhang, J.S.; Hou, Y.L.; Lu, W.W.; Ni, X.Q.; Lin, F.; Liu, X.Y.; Wang, X.J.; Yu, Y.R.; Jia, M.Z.; et al. Intermedin1-53 Inhibits NLRP3 Inflammasome Activation by Targeting IRE1alpha in Cardiac Fibrosis. Inflammation 2022, 45, 1568–1584. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhang, W.; Zheng, L.; Xu, M.; Rong, R.; Zhu, T.; Yang, C. Cyclic helix B peptide ameliorates renal tubulointerstitial fibrosis induced by unilateral ureter obstruction via inhibiting NLRP3 pathway. Ann. Transl. Med. 2020, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Nistri, S.; Chellini, F.; Bani, D. Human Recombinant Relaxin (Serelaxin) as Anti-fibrotic Agent: Pharmacology, Limitations and Actual Perspectives. Curr. Mol. Med. 2022, 22, 196–208. [Google Scholar] [CrossRef]
- Caceres, F.T.; Gaspari, T.A.; Samuel, C.S.; Pinar, A.A. Serelaxin inhibits the profibrotic TGF-beta1/IL-1beta axis by targeting TLR-4 and the NLRP3 inflammasome in cardiac myofibroblasts. FASEB J. 2019, 33, 14717–14733. [Google Scholar] [CrossRef]
- Xiang, Z.; Guan, X.; Ma, Z.; Shi, Q.; Panteleev, M.; Ataullakhanov, F.I. Bioactive fibrous scaffolds with programmable release of polypeptides regulate inflammation and extracellular matrix remodeling. Regen. Biomater. 2023, 10, rbad010. [Google Scholar] [CrossRef] [PubMed]
- Hogan, T.B.; Tiwari, N.; Nagaraja, M.R.; Shetty, S.K.; Fan, L.; Shetty, R.S.; Bhandary, Y.P.; Shetty, S. Caveolin-1 peptide regulates p53-microRNA-34a feedback in fibrotic lung fibroblasts. iScience 2022, 25, 104022. [Google Scholar] [CrossRef]
- Savitha, M.N.; Suvilesh, K.N.; Siddesha, J.M.; Milan Gowda, M.D.; Choudhury, M.; Velmurugan, D.; Umashankar, M.; Vishwanath, B.S. Combinatorial inhibition of Angiotensin converting enzyme, Neutral endopeptidase and Aminopeptidase N by N-methylated peptides alleviates blood pressure and fibrosis in rat model of dexamethasone-induced hypertension. Peptides 2020, 123, 170180. [Google Scholar] [CrossRef] [PubMed]
- Ock, S.; Ham, W.; Kang, C.W.; Kang, H.; Lee, W.S.; Kim, J. IGF-1 protects against angiotensin II-induced cardiac fibrosis by targeting alphaSMA. Cell Death Dis. 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Zhang, Y.; Zhu, D.; Zhao, Z.; Kim, D.H.; Kim, S.H.; Kong, D. In situ cardiac regeneration by using neuropeptide substance P and IGF-1C peptide eluting heart patches. Regen. Biomater. 2018, 5, 303–316. [Google Scholar] [CrossRef]
- Khalili, D.; Kalcher, C.; Baumgartner, S.; Theopold, U. Anti-Fibrotic Activity of an Antimicrobial Peptide in a Drosophila Model. J. Innate Immun. 2021, 13, 376–390. [Google Scholar] [CrossRef]
- Li, Q.; Peng, W.; Zhang, Z.; Pei, X.; Sun, Z.; Ou, Y. A phycocyanin derived eicosapeptide attenuates lung fibrosis development. Eur. J. Pharmacol. 2021, 908, 174356. [Google Scholar] [CrossRef]
- Xu, H.; Hong, S.; Yan, Z.; Zhao, Q.; Shi, Y.; Song, N.; Xie, J.; Jiang, X. RAP-8 ameliorates liver fibrosis by modulating cell cycle and oxidative stress. Life Sci. 2019, 229, 200–209. [Google Scholar] [CrossRef]
- Lee, J.; Byun, J.; Shim, G.; Oh, Y.K. Fibroblast activation protein activated antifibrotic peptide delivery attenuates fibrosis in mouse models of liver fibrosis. Nat. Commun. 2022, 13, 1516. [Google Scholar] [CrossRef]
- Gu, X.; Liu, Z.; Tai, Y.; Zhou, L.Y.; Liu, K.; Kong, D.; Midgley, A.C.; Zuo, X.C. Hydrogel and nanoparticle carriers for kidney disease therapy: Trends and recent advancements. Prog. Biomed. Eng. 2022, 4, 022006. [Google Scholar] [CrossRef]
- Verdino, P.; Lee, S.L.; Cooper, F.N.; Cottle, S.R.; Grealish, P.F.; Hu, C.C.; Meyer, C.M.; Lin, J.; Copeland, V.; Porter, G.; et al. Development of a long-acting relaxin analogue, LY3540378, for treatment of chronic heart failure. Br. J. Pharmacol. 2023, 180, 1965–1980. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, X.; Liu, X.; Han, Y.; Chu, Y.; Guan, Y.; Liu, H. Engineered fibrotic liver-targeted truncated transforming growth factor beta receptor type II variant for superior anti-liver fibrosis therapy. Arch. Pharm. Res. 2023, 46, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qiu, Y.; Shi, J.; Li, L.; Yuan, X.; Wu, D.; Chu, Y. Prokaryotic expression, purification and evaluation of anti-cardiac fibrosis activity of recombinant TGF-beta latency associated peptide. PeerJ 2022, 10, e12797. [Google Scholar] [CrossRef]
- Lei, Y.; Li, S.; Liu, Z.; Wan, F.; Tian, T.; Li, S.; Zhao, D.; Zeng, J. A deep-learning framework for multi-level peptide-protein interaction prediction. Nat. Commun. 2021, 12, 5465. [Google Scholar] [CrossRef]
- Corso, G.; Stärk, H.; Jing, B.; Barzilay, R.; Jaakkola, T. DiffDock: Diffusion Steps, Twists, and Turns for Molecular Docking. arXiv 2023, arXiv:2210.01776v2. [Google Scholar]
- Mallart, S.; Ingenito, R.; Bianchi, E.; Bresciani, A.; Esposito, S.; Gallo, M.; Magotti, P.; Monteagudo, E.; Orsatti, L.; Roversi, D.; et al. Identification of Potent and Long-Acting Single-Chain Peptide Mimetics of Human Relaxin-2 for Cardiovascular Diseases. J. Med. Chem. 2021, 64, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Carlson, W.D.; Keck, P.C.; Bosukonda, D.; Carlson, F.R. A Process for the Design and Development of Novel Bone Morphoge-netic Protein-7 (BMP-7) Mimetics with an Example: THR-184. Front. Pharmacol. 2022, 13, 864509. [Google Scholar] [CrossRef]
- Ayad, S.; Neylan, J.F.; Mayne, T.J.; Gouveia, D.; Swaminathan, M. Hepatocyte Growth Factor Mimetic ANG-3777 for Cardiac Surgery–Associated Acute Kidney Injury. Kidney Int. Rep. 2022, 5, 2325–2332. [Google Scholar] [CrossRef]
- Buck, M.; Solis-Herruzo, J.; Chojkier, M. C/EBPβ-Thr217 Phosphorylation Stimulates Macrophage Inflammasome Activation and Liver Injury. Sci. Rep. 2016, 6, 24268. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, M.; Men, S.; Shi, Y.; Ma, L.; An, Y.; Gao, Y.; Jin, H.; Liu, W.; Du, Z. The Inflammatory Transcription Factor C/EBPβ Plays a Critical Role in Cardiac Fibroblast Differentiation and a Rat Model of Cardiac Fibrosis Induced by Autoimmune Myocarditis. Int. Heart J. 2018, 59, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
| Peptide | Origin/Inspiration | Amino Acid Sequence | Antifibrotic Mechanism | Ref. |
|---|---|---|---|---|
| CXCL9(74-103) | CXCL9 | KKKQKNGKKHQKKKVLKVRKSQRSRQKKTT | Competitively binds heparan sulphate GAGs to inhibit its complexation with TGF-β1 | [12] |
| KP1 | Klotho | FQGTFPDGFLWAVGSAAYQTEGGWQQHGK | Competitively binds TGFβRII to inhibit TGFβRII/RI dimerization | [13] |
| C53 | CNP | DLRVDTKSRAAWARLLQEHPNARKYKGANKKGLSKGCFGLKLDRIGSMSGLGC | Facilitates prolonged cGMP production, inhibits Smad3 nuclear translocation, and promotes Smad3 degradation | [7] |
| M10 | MET | TRPASFWETS | Interacts with Smad2 to inhibit its nuclear translocation | [14] |
| H-RN | HGF | RNPRGEEGGPW | Inhibition of TGF-β2/Smad and Akt/mTOR signaling pathways | [15] |
| THR123 | BMP7 | CYFDDSSNVLCKKYRS | BMPR1A/Smad(1/5/(8)9) pathway agonist | [16] |
| THR184 | CYYDNSSSVLCKRYRS | |||
| ADP355 | Adiponectin | H(dN)IP(nV)LY(dS)FA(dS) | Activates AMPK suppression of TGF-β1 induced transcription driven by Smad3-binding cis-elements | [17] |
| Peptide | Origin /Inspiration | Amino Acid Sequence | Antifibrotic Mechanism | Ref. |
|---|---|---|---|---|
| B7-33 | H2-Relaxin | VIKLSGRELVRAQIAISGMSTWSKRSL | RXFP1 agonist; promotes MMP2 expression and inhibits TIMP1, leading to reduced collagen fiber thickness | [30] |
| E4 | Endostatin/COL18A1 | SYCETWRTEAPSATGQASSLLGGRLLGQSAASCH HAYIVLCIENSFMT | Regulates the urokinase pathway and induces MMP-1/-3 degradation of ECM | [6] |
| AF38Pep | Integrin α4/β1 (VLA-4) | VMPYISTTPAKPCTSENCGNSWYGGFKSKNENKI YFIN | Competitively binds the EDA-FN C-C’ loop to prevent recognition by integrin α4/β1 or α4/β7 | [31,32] |
| LSKL | LAP | LSKL | TSP-1 antagonist, suppresses the PI3K/Akt/mTOR pathways | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, X.; Wang, Y.; Tai, Y.; Yao, X.; Midgley, A.C. Emergent Peptides of the Antifibrotic Arsenal: Taking Aim at Myofibroblast Promoting Pathways. Biomolecules 2023, 13, 1179. https://doi.org/10.3390/biom13081179
Liu Z, Zhang X, Wang Y, Tai Y, Yao X, Midgley AC. Emergent Peptides of the Antifibrotic Arsenal: Taking Aim at Myofibroblast Promoting Pathways. Biomolecules. 2023; 13(8):1179. https://doi.org/10.3390/biom13081179
Chicago/Turabian StyleLiu, Zhen, Xinyan Zhang, Yanrong Wang, Yifan Tai, Xiaolin Yao, and Adam C. Midgley. 2023. "Emergent Peptides of the Antifibrotic Arsenal: Taking Aim at Myofibroblast Promoting Pathways" Biomolecules 13, no. 8: 1179. https://doi.org/10.3390/biom13081179
APA StyleLiu, Z., Zhang, X., Wang, Y., Tai, Y., Yao, X., & Midgley, A. C. (2023). Emergent Peptides of the Antifibrotic Arsenal: Taking Aim at Myofibroblast Promoting Pathways. Biomolecules, 13(8), 1179. https://doi.org/10.3390/biom13081179







