Abstract
Liver X receptor α (LXRα), a member of the nuclear receptor superfamily, is identified as a protein activated by ligands that interacts with the promoters of specific genes. It regulates cholesterol, bile acid, and lipid metabolism in normal physiological processes, and it participates in the development of some related diseases. However, many studies have demonstrated that LXRα is also involved in regulating numerous human malignancies. Aberrant LXRα expression is emerging as a fundamental and pivotal factor in cancer cell proliferation, invasion, apoptosis, and metastasis. Herein, we outline the expression levels of LXRα between tumor tissues and normal tissues via the Oncomine and Tumor Immune Estimation Resource (TIMER) 2.0 databases; summarize emerging insights into the roles of LXRα in the development, progression, and treatment of different human cancers and their diversified mechanisms; and highlight that LXRα can be a biomarker and therapeutic target in diverse cancers.
1. Introduction
Liver X receptor α (LXRα), a nuclear receptor superfamily member, can recruit ligands and bind to particular DNA sequences in target gene promoters [1,2]. As one of the critical regulators, LXRα has been widely correlated with certain specific genes in metabolism processes, especially cholesterol metabolism [3]. Many studies have suggested that LXRα may exert distinct functions in different tissue-specific environments. LXRα-modulated distinct downstream target genes can engender these varied roles. LXRα may be involved in various signaling pathways by repressing or inducing the expression of these genes to maintain normal physiological activity, such as the ATP-binding cassette transporter A1 (ABCA1) and Niemann–Pick type C1 [4,5]. Moreover, the high expression of LXRα in macrophages suggests that LXRα is also an essential factor for inflammation and immunity homeostasis by regulating the expression of many inflammation-associated genes through various pathways [6]. For example, LXRα can induce the expression of interferon γ and control the immunity process via macrophage phagocytosis [7]. LXRα dysfunction is present in the pathogenesis of different diseases. For example, LXRα might contribute to antiatherosclerosis by ameliorating the function of macrophages, which includes encouraging M2 polarization, reducing the release of pro-inflammatory cytokines, and increasing the release of protective molecules [8]. In addition, some studies have suggested that LXRα could contribute to the pathogenesis of liver steatosis [9], neuroinflammatory disease [10], and cardiometabolic disease [11]. In addition to these known functions, there is increasing evidence that LXRα is associated with many cancers.
In recent years, an increasing number of studies in vitro and in vivo have shown that LXRα may be an important factor in the proliferation, apoptosis, invasion, and migration of cancer cells [12]. The abnormal expression of LXRα can be observed in many cancers and has a strong relationship with the clinical outcome. Thus, LXRα may function as a diagnostic and prognostic biomarker, and it may potentially be a target for cancer therapy. However, the expression of LXRα in different cancers is controversial, the mechanism of LXRα in tumor pathophysiology is relatively complex, and the potential clinical value of LXRα in tumor therapy is uncertain. In this review, we show the expression levels of LXRα between tumor tissues and normal tissues via the Oncomine and the Tumor Immune Estimation Resource (TIMER) databases, focus on the role and mechanism of LXRα in various cancers, and discuss the conceivable performance of LXRα in cancer treatments.
2. The Physiological Function of LXRs and LXRα
As early as the 1990s, LXRs had already been cloned from mice. Two isoforms were identified, named LXRα and LXRβ because of their high expression in the liver [13,14]. They are also known as nuclear receptor subfamily 1 group H member 3 (NR1H3) and nuclear receptor subfamily 1 group H member 2 (NR1H2), respectively [15]. LXRs have the typical nuclear receptor structures, which are mainly composed of four domains: the ligand-independent activation functional domain (AF-1) at the N-terminus; the DNA-binding domain (DBD) with two zinc finger motifs; the hydrophobic ligand-binding domain (LBD), which identifies specific ligands and binds to coactivators; and the ligand-dependent activation functional domain (AF-2) at the C-terminal region [16]. LXRα is encoded by the NR1H3 gene located on chromosome 11p11.2, while the NR1H2 gene on chromosome 19q13.3 can encode LXRβ [17]. LXRα and LXRβ consist of 447 amino acids and 460 amino acids, respectively, and more than 78% of their sequences are identical, mainly located in the DBD and LBD parts [15,18]. However, their expression patterns are different in the body. LXRα is mainly expressed in cells, tissues, and organs with active cholesterol and lipid metabolisms, such as the liver, intestine, kidney, spleen, lung, adipose tissue, and macrophages, while LXRβ is expressed in almost all tissues and organs [19]. Although current studies suggest that both LXRα and LXRβ are involved in lipid metabolism, there are still some differences in their mechanisms. The current study found that LXRα makes a greater contribution to the regulation of cholesterol efflux transport pathways, while LXRβ plays a greater role in lipid metabolism [20,21].
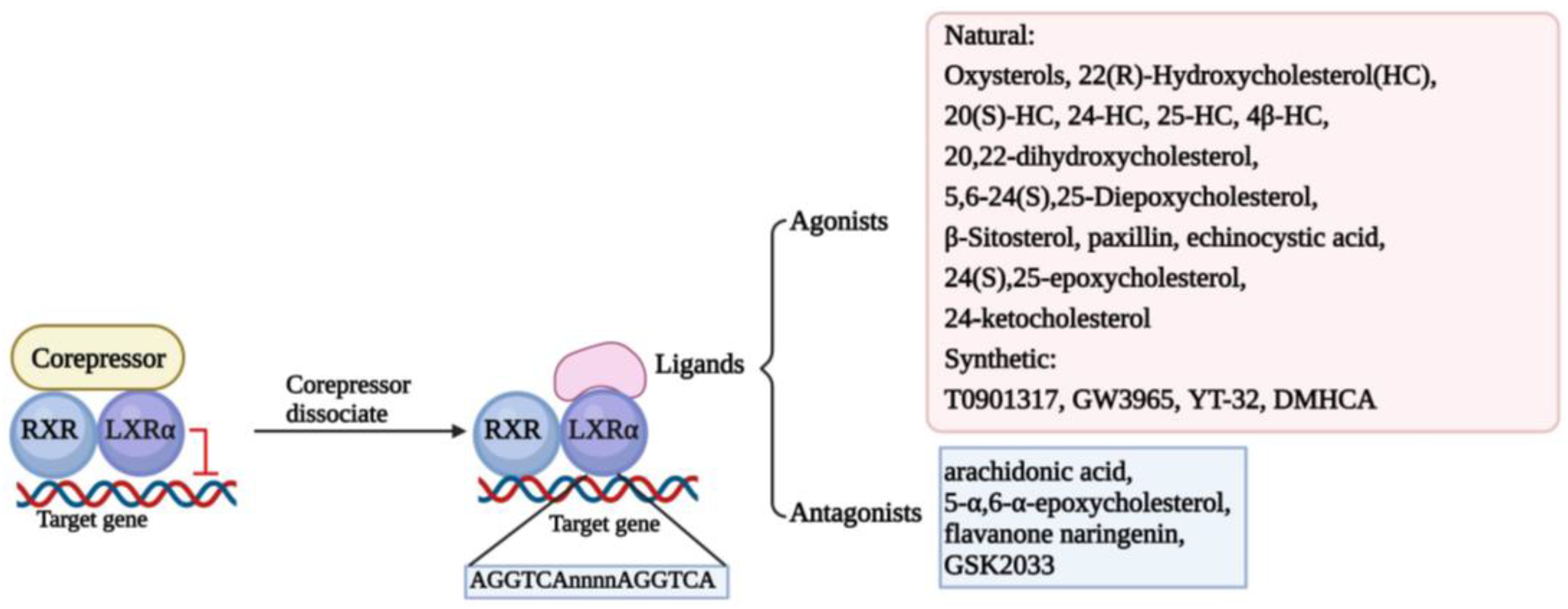
Structure determines function. The classic structure of nuclear receptors contributes to their interaction with ligands and the DNA sequence of the target gene, named LXR response elements (LXREs), finally modulating the transcription of the target gene [22]. In the screening of retinal X receptor (RXR)-binding ligands, it was found that LXRs can bind to it and form heterodimers to participate in retinol signal transduction [23]. In the absence of ligands, LXR/RXR heterodimers form complexes with classic nuclear receptor corepressors to inhibit the transcriptional activity of target genes. However, in the presence of ligands, LXR/RXR heterodimers release corepressors and recruit coactivators to transactivate the expression of target genes [16]. Thus, there is no doubt that the ligand plays an essential role in activating LXRs. The ligands of LXRs are mainly divided into two main categories, agonists and antagonists, which include natural substances and synthetic ligands [24,25]. We have summarized the ligands in Figure 1.
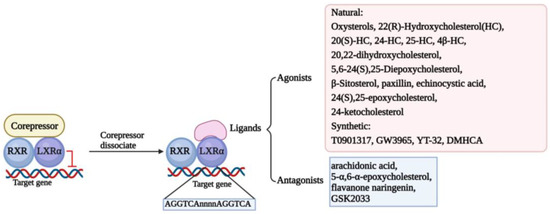
Figure 1.
Classical mechanism and multifarious ligands of LXRα. The classical transcriptional mechanism of LXRα is binding to RXR formatting heterodimer. When the LXRα/RXR interacts with corepressors, LXRα/RXR dimer activation is inhibited, and transcription of the target genes is suspended. Once the corepressor is dissociated, the ligands could activate the function of LXRα/RXR to interact with LXRE (AGGTCAnnnnAGGTCA) located in the promoter of target genes and regulate the transcription of target genes. Some ligands are depicted here, including agonists and antagonists.
Although a large number of studies have shown that the classic DNA sequence of LXREs is usually “AGGTCAnnnnAGGTCA”, some studies have also demonstrated that LXRs can share binding sites with other nuclear receptors or transcription factors in some regions of chromatin, such as tumor necrosis factor-α/nuclear factor-κB [16]. Furthermore, it has been found that LXRα binds specifically and preferentially to overlapping cAMP response elements and negative response elements (CNREs) in a monomeric form, increasing the expression levels of renin. They also found that LXRα specifically binds to CNREs in the c-myc promoter, thereby increasing c-myc transcription, suggesting that LXRα may act as a cAMP-responsive transcriptional regulator to regulate gene expression [26,27]. In addition, LXRs can also be regulated by certain transcription factors or non-coding RNAs to exert antitumor effects. For instance, it was found that the transcription factor KLF4 significantly contributes to the maintenance of vascular homeostasis by regulating the expression of LXR [28]. In addition, miR-552-3p also regulates LXRα transcription by binding to the region of AGGTCA [29]. In conclusion, LXRα may regulate downstream target genes or be regulated by upstream factors, and its activation or not may affect normal cellular activities and may be involved in tumorigenic mechanisms.
3. The Expression of LXRα in Different Tumor Tissues
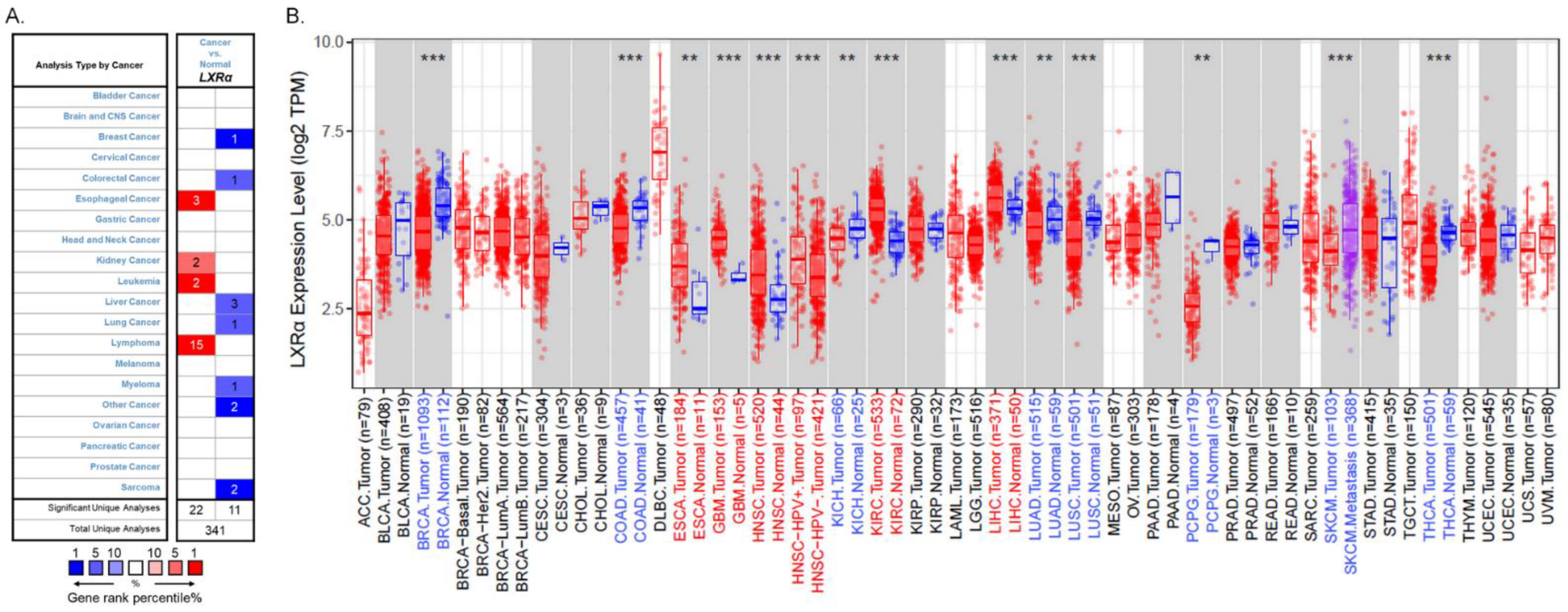
LXRs are essential in cholesterol and lipid metabolism, inflammation, and immune response. However, their role in tumorigenesis and tumor development is still unclear. Previous studies have shown that the patterns of LXRα expression between cancer and normal tissues are diverse and depend on cancer types [12]. Therefore, we used the Oncomine database (https://www.oncomine.org/, accessed on 22 August 2021) to analyze LXRα expression over a wide range of cancers [30]. The thresholds were set as a p-value of 0.001, a fold change of 1.5, a gene ranking in the top 5%, and mRNA data type. The results indicated that LXRα expression was lower in breast cancer, colorectal cancer, liver cancer, lung cancer, myeloma, and sarcoma than in normal tissues. In addition, higher expression was found in esophageal cancer, kidney cancer, leukemia, and lymphoma in some datasets (Figure 2A). The details of LXRα expression in multiple cancers are summarized in Table 1.
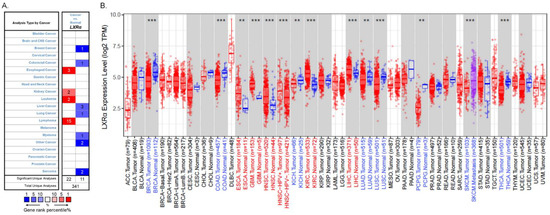
Figure 2.
LXRα expression levels in different types of human cancers: (A) increased or decreased expression of LXRα in different types of cancer tissues compared with normal tissues in the Oncomine database; (B) human LXRα expression levels in different cancers were assessed using the TIMER 2.0 database (** p < 0.01, and *** p < 0.001).
To further evaluate LXRα expression in different human cancers, we examined LXRα expression using RNA-seq data via the TIMER 2.0 database (http://timer.cistrome.org/, accessed on 22 August 2021) [31,32]. There are over 10,897 samples across 32 cancer types on aggregate from The Cancer Genome Atlas, and we can evaluate gene expression in pan-cancer analysis. The differential LXRα expression patterns between cancer and adjacent normal tissues are shown in Figure 2B. The level of LXRα was found to be significantly lower in breast invasive carcinoma (BRCA), colon adenocarcinoma (COAD), kidney chromophobe (KICH), lung adenocarcinoma (LUAD), and lung squamous cell carcinoma (LUSC) than in adjacent normal tissues. Meanwhile, the mRNA level of LXRα was significantly higher in esophageal carcinoma (ESCA), head and neck squamous cell carcinoma (HNSC), head and neck squamous carcinoma-HPV positive (HNSC-HPV positive), kidney renal clear cell carcinoma (KIRC), and hepatocellular carcinoma (HCC) compared with adjacent normal tissues. Taking the two databases together, due to the heterogeneous data collection methods and the different biological properties of cancers, the expression levels of LXRα vary in some cancers, such as liver and kidney cancers. However, the expression of LXRα is consistent in breast, colon, and lung cancers.

Table 1.
LXRα expression in cancerous tissue versus normal tissue in Oncomine.
Table 1.
LXRα expression in cancerous tissue versus normal tissue in Oncomine.
| Cancer Site | Cancer Type | p-Value | Sample Size | Fold Change | Up-/Downregulated Gene Rank | Reference |
|---|---|---|---|---|---|---|
| Breast | Intraductal cribriform breast adenocarcinoma | 1.43 × 10−12 | 3 | −1.945 | 112 (1%) | TCGA |
| Colorectal | Rectosigmoid adenocarcinoma | 4.73 × 10−5 | 10 | −1.732 | 781 (4%) | [33] |
| Esophageal | Esophageal adenocarcinoma | 4.24 × 10−18 | 75 | 2.851 | 101 (1%) | [34] |
| Barrett’s esophagus | 3.11 × 10−12 | 15 | 5.769 | 120 (1%) | [34] | |
| Esophageal adenocarcinoma | 8.15 × 10−4 | 8 | 2.488 | 345 (3%) | [35] | |
| Kidney | Clear cell renal cell carcinoma | 2.85 × 10−6 | 26 | 1.818 | 430 (3%) | [36] |
| Clear cell renal cell carcinoma | 1.31 × 10−13 | 23 | 1.625 | 443 (4%) | [37] | |
| Leukemia | Acute myeloid leukemia | 3.42 × 10−55 | 542 | 1.809 | 56 (1%) | [38] |
| Acute myeloid leukemia | 2.58 × 10−11 | 23 | 3.240 | 57 (1%) | [39] | |
| Liver | Cirrhosis | 7.34 × 10−11 | 58 | −1.814 | 341 (3%) | [40] |
| Hepatocellular carcinoma | 8.79 × 10−8 | 38 | −1.578 | 560 (5%) | [40] | |
| Liver cell dysplasia | 5.15 × 10−4 | 17 | −1.519 | 398 (3%) | [41] | |
| Lung | Lung carcinoid tumor | 3.78 × 10−8 | 20 | −7.888 | 404 (5%) | [42] |
| Lymphoma | Centroblastic lymphoma | 1.63 × 10−24 | 28 | 46.208 | 14 (1%) | [43] |
| Mantle cell lymphoma | 1.38 × 10−6 | 8 | 3.976 | 150 (2%) | [43] | |
| Diffuse large B-cell lymphoma | 6.37 × 10−8 | 32 | 8.966 | 152 (2%) | [43] | |
| Burkitt’s lymphoma | 9.81 × 10−7 | 7 | 8.495 | 368 (5%) | [43] | |
| Primary effusion lymphoma | 1.39 × 10−5 | 9 | 6.261 | 389 (5%) | [43] | |
| Diffuse large B-cell lymphoma | 2.19 × 10−32 | 44 | 15.835 | 42 (1%) | [44] | |
| Follicular lymphoma | 5.77 × 10−33 | 38 | 9.725 | 55 (1%) | [44] | |
| Activated B-cell-like diffuse large B-cell lymphoma | 1.24 × 10−15 | 17 | 18.080 | 75 (1%) | [44] | |
| Germinal center B-cell-like diffuse large B-cell lymphoma | 3.56 × 10−7 | 9 | 14.761 | 312 (2%) | [44] | |
| Diffuse large B-cell lymphoma | 2.78 × 10−4 | 6 | 3.532 | 71 (1%) | [45] | |
| Hodgkin’s lymphoma | 2.33 × 10−7 | 12 | 5.190 | 155 (1%) | [46] | |
| Diffuse large B-cell lymphoma | 3.35 × 10−6 | 11 | 5.417 | 638 (4%) | [46] | |
| Follicular lymphoma | 3.43 × 10−4 | 5 | 2.201 | 974 (5%) | [46] | |
| Unspecified peripheral T-cell lymphoma | 4.82 × 10−15 | 28 | 11.346 | 281 (2%) | [47] | |
| Angioimmunoblastic T-cell lymphoma | 1.51 × 10−6 | 6 | 25.312 | 710 (4%) | [47] | |
| Myeloma | Multiple myeloma | 1.05 × 10−6 | 74 | −1.931 | 226 (5%) | [48] |
| Sarcoma | Pleomorphic myxofibrosarcoma | 2.97 × 10−6 | 3 | −3.662 | 109 (1%) | [49] |
| Leiomyosarcoma | 3.73 × 10−6 | 26 | −8.192 | 627 (5%) | [49] | |
| Other | Vulvar intraepithelial neoplasia | 3.55 × 10−6 | 9 | −3.109 | 71 (1%) | [50] |
4. Biological Functions and Mechanisms of LXRα in Diverse Cancers
Some studies have indicated that the abnormal expression of LXRs is a potential tumor-specific signature and can be involved in the clinical characteristics of malignant tissues, suggesting the indelible role of LXRs in different types of tumors [12]. LXRα might be related to tumor cell proliferation, differentiation, progression, and metastasis, and could be regarded as a potential prognostic and diagnostic biomarker in carcinogenesis.
4.1. Gastric Cancer
Gastric cancer (GC) is one of the five most common cancers globally and one of the leading causes of cancer-related deaths worldwide. In addition to Helicobacter pylori infection, many recent studies have confirmed that the abnormal expression of oncogenes and tumor suppressor genes can lead to the abnormal differentiation and metabolism of GC cells [51].
Recently, using immunohistochemistry, Yu et al. found that the expression level of LXRα was significantly lower in GC tissue than in adjacent normal mucosas, and was related to the differentiation degree of cancer tissue. The lower the differentiation level of GC tissue, the lower the expression level of LXRα. They determined that decreased LXRα expression could potentially inhibit the differentiation of GC cells by activating the Wnt/β-catenin signaling, whereas the activation of LXRα had the opposite effect [52]. Whether LXRα could affect the invasion or epithelial–mesenchymal transition (EMT) ability of GC cells remains uncertain. However, different from the results of Yu et al., a study conducted by Ji et al. revealed that the mRNA and protein levels of LXRα were highly expressed in GC tissues and cell lines. Their results ultimately suggest that LXRα is an oncogene in GC and increases the expression of MMP-2/MMP-9 to promote invasion and EMT by regulating the activity of PI3k/Akt/NF-κB in GC cell lines [53]. Additionally, Zhang et al. revealed that the X-linked ectodermal dysplasia receptor, a tumor suppressor gene, could promote differentiation and inhibit the proliferation and migration of GC cells by increasing the expression of p65/LXRα to deactivate the Wnt/β-catenin pathway [54]. A recent study suggested that hypoxia can induce the expression of LXRα and promote the migration and invasion of GC cells [55]. Therefore, it is necessary to further clarify the expression level of LXRα in GC and to understand the role of LXRα in the pathological mechanism of GC to determine its potential clinical value.
4.2. Liver Cancer
Many factors can lead to liver cancer, such as hepatitis virus infection and liver steatosis. Recent data indicate that LXRα is correlated with multiple mechanisms to modulate the process of HCC tumorigenesis. Moriishi et al. proved that the hepatitis C virus (HCV) core protein could be degraded by the proteasome activator PA28γ and activate sterol-regulatory-element-binding protein (SREBP)-1c promoter via an LXRα/RXRα-dependent pathway, eventually accelerating the development of hepatic steatosis and HCC [56]. A subsequent study showed that LXRα was highly expressed in patients with nonalcoholic fatty liver disease and HCV infection in the liver tissues. At the same time, lipogenic targets of LXRα were also overexpressed, such as peroxisome-proliferator-activated receptor-γ (PPAR-γ), SREBP-1c, SREBP-2, and fatty acid synthase (FAS), suggesting that these genes might be involved in HCC carcinogenesis [57]. Additionally, another study suggested that HCV core protein and nonstructural protein 5A might regulate lipogenesis mediated by LXRα and contribute to liver steatosis and HCV replication [58]. Kim et al. demonstrated that HBV X protein (HBx) could interact with LXRα and facilitate the binding of LXRα to LXRE, thereby leading to the overexpression of SREBP-1c and FAS, and eventually stimulating hepatic lipid accumulation [59]. Na et al. also found these results and suggested that HBx could augment the transactivation function of LXRα by promoting CREB binding protein to interact with target gene promoters [60].
LXRα can affect the lipid metabolism of the liver and inhibit the proliferation and growth of HCC cancer cells. For example, one study indicated that the expression and activity of LXRα could be decreased by c-Fos, a component of the AP-1 transcription factor, thereby leading to changes in hepatocyte morphology, the formation of necrotic foci, and immune cell infiltration, eventually increasing proliferation, dedifferentiation, and DNA damage [61]. Previous studies have shown that the proliferation-specific regulator forkhead box M1 (FOXM1) was associated with developing HCC and upregulated the transcription of cell cycle genes, such as cyclin D1 and B1, enhancing cell cycle progression and proliferation. Hu et al. determined that LXRα could bind to the inverted repeat IR2(52-CCGTCACGTGACCT-39) region in the promoter of FOXM1 and repress the expression of FOXM1, leading to the suppression of HCC proliferation [62]. Recently, He et al. indicated that LXRα could modulate the HULC/miR-134-5p/FOXM1 axis, inhibiting HCC cell growth [63]. Moreover, another study suggested that LXRα could enhance the stability of the suppressor of cytokine signaling 3 (SOCS3) mRNA and elevate the level of SOCS3, resulting in a decline in cyclinD1 and an increase in p21 and p27, eventually leading to cell cycle arrest at the G1/S phase and inhibiting the growth of HCC cells [64].
Researchers also demonstrated that LXRα overexpression might inhibit the ability of TGFβ-induced Snail expression by deactivating Snail’s promoter, leading to the inhibition of mesenchymal differentiation, the suppression of epithelial cell proliferation, and the generation of ROS [65]. Another study showed that LXRα activation was negatively correlated with the differentiation of TGFβ-dependent cancer-associated fibroblasts by inhibiting the promoter activity of ACTA2 and limiting the growth of primary HCC [66]. Lin et al. found that LXRα activation could also increase the transcription level of miRNA-378a and enhance the potency of sorafenib in HCC [67]. In addition, recent studies have suggested that the activation of LXRα may induce the accumulation of free fatty acids in HCC cells causing lethal lipotoxicity, thereby providing an additional means for drug-resistant HCC patients [68,69]. A recent study showed that LXRα activation upregulated saturated fatty acid levels in HCC cells, while the RAF proto-oncogene serine/threonine protein kinase (Raf-1) could activate stearoyl-CoA desaturase (SCD1) to desaturate the saturated fatty acids. They then found that DFG-out Raf inhibitors could inhibit Raf-1, leading to SCD1 degradation that aroused the overload of toxic saturated fatty acids, consequently resulting in the apoptosis of HCC cells. Therefore, the combinatorial lipotoxic therapy between specific LXRα agonists and DFG-out Raf inhibitors may be exploited to develop potent cancer therapeutics [69]. Based on these studies, we conclude that LXRα is potentially a key modulator of liver cancer development and might be a potential metabolic target of liver cancer.
4.3. Pancreatic Cancer
Pancreatic cancer (PC) is one of the most lethal cancers, and the five-year survival rate of pancreatic ductal adenocarcinoma (PDAC) is only 5%. Yang et al. determined that the expression levels of LXRα, SREBP-1c, and polynucleotide kinase/phosphatase (PNKP) were reduced in PC cancer tissues compared with normal pancreatic tissues. They then identified that the LXRα/SREBP-1c/PNKP signaling pathway is involved in DNA repair in PC cells. Specifically, hydrophobic triptolide can bind to LXRα to inhibit the LXRα/SREBP-1c/PNKP axis, causing severe defects in DNA repair, leading to increased levels of intracellular DNA breaks, activating p53 to further induce apoptosis in PC cells beyond the apoptotic threshold, thus exerting an antitumor effect [70].
4.4. Colorectal Cancer
Previously, several studies elaborated that LXRα plays an essential role in lipid metabolism, modulating the transcription of genes related to the metabolism of cholesterol and fatty acids [71]. Many studies highlighted that colorectal cancer (CRC) had a very close connection with cholesterol levels; high total cholesterol (≥240 mg/dL) was positively associated with the risk of colon cancer [72,73]. Some in vivo studies illuminated that cholesterol metabolite oxysterols, as the natural ligand of LXRα, could accelerate the apoptosis of colon cancer cells and inhibit cell proliferation [74,75,76]. Some studies found that the expression of LXRα was decreased in colon cancer specimens compared with normal samples [77,78].
Uno et al. found that LXRα could bind to the central armadillo repeats (amino acid fragment 131–680) of β-catenin and inhibit its activity, leading to the decreased proliferation of colon cancer cells [79]. Another study determined that the overexpression of LXRα could block the cell cycle, induce caspase-dependent apoptosis, and impede the growth of cancer cells [77]. Vedin et al. also suggested that the activation of LXRα resulted in robust cell cycle arrest in CRC cell lines and suppressed the proliferation of cancer cells [80]. Recently, Wang et al. identified RAS protein activator-like 1 (RASAL1) as an antitumor gene that could downregulate the expression level of SCD1 and inhibit cell proliferation in CRC via the LXRα/SREBP-1c signaling pathway [81]. Based on these results, activating the expression of LXRα might be a promising treatment strategy for colon cancer.
4.5. Lung Cancer
Korehito Kashiwagi et al. investigated the expression of LXRα in small-cell lung carcinoma and normal lung tissue and found that it exists in alveolar macrophages and is not evident in lung epithelial cells [82]. This result suggests that LXRα might play a more significant role in affecting the function of macrophages, such as participating in the inflammatory signaling pathway. Indeed, many studies found that LXRα activation could reduce acute lung injury [83,84,85]. However, one study showed that LXRα activation could inhibit the migration and tubulogenesis of human umbilical vein endothelial cells, repressing the process of neoangiogenesis. Specifically, LXRα activation can affect endothelial cell proliferation, mainly by impairing vascular endothelial growth factor receptor 2 (VEGFR2) phosphorylation and weakening VEGFR2 compartments in lipid rafts/caveolae. Subsequently, LXRα activation was found to reduce tumor angiogenesis in Lewis lung carcinoma-1 cells’ tumor grafts [86]. Dai et al. indicated that LXRα and LXRβ double-ablated mice could spontaneously develop peripheral squamous cell lung cancer [87]. Recently, a retrospective study found that the percentage of LXRα-positive cells in stage II non-small cell lung cancer (NSCLC) patients was higher than that in stage III NSCLC patients (58% vs. 41%, p = 0.04), indicating that LXRα might be an influential factor in the TNM staging system for further improving NSCLC treatment [88]. These results raise the possibility that the LXRα may be involved in the growth of lung cancer and could be a potent target for lung cancer adjunctive therapies.
4.6. Renal Cell Carcinoma
Clear-cell renal-cell carcinoma (ccRCC) is the most knowledgeable subtype of RCC, constituting 75% of RCC [89]. Wang et al. suggested that LXRα expression was higher in ccRCC cancer tissues than in normal tissues and was correlated with the poor prognosis of ccRCC. They then found that LXRα overexpression could inhibit the expression of NLRP3 inflammasome in ccRCC cells and promote metastasis in vivo [90]. In conclusion, LXRα may be an oncogene related to the diagnosis and prognosis of RCC and could become a novel therapeutic target.
4.7. Breast Cancer
Previous studies identified that LXR activation could induce the expression of estrogen deactivation enzyme and estrogen sulfotransferase, exert an indirect antiproliferative function, and inhibit the growth of breast cancer in a nude mouse model [91]. Similarly, Vedin et al. showed that the antiproliferative role of LXRs was correlated with the estrogen signaling pathway using in vitro experiments. The activation of LXRs resists estrogen-induced cell proliferation by reducing estrogen receptor α at the mRNA and protein levels, but the exact mechanism remains to be further investigated [92]. Recently, a high expression of LXRs has been identified as a biomarker for a poor prognosis [93,94,95].
Tumor immune evasion is mainly caused by the abnormal metabolism of immune cells in the tumor microenvironment. The results of single-cell RNA sequencing suggested that triple-negative breast cancer (TNBC) tumor-resident immune cells showed a high expression of LXRα, and Seurat cell-cluster analysis showed that LXRα expression was most induced in the “myeloid cell” cluster, suggesting that the upregulation of LXRα may be associated with tumor cell–immune cell interactions in TNBC. They also found that TNBC cells could produce LXRα agonists in macrophages and cytotoxic CD8+ T cells. Moreover, they illustrated that the TNBC-induced activation of LXRα could inhibit macrophage M1/M2 polarization and prohibit the normal immune function of CD8+ T cells. Therefore, they deemed that LXRα inverse agonists could be used for TNBC immunotherapies [96]. Recently, Han et al. found that LXRα was lowly expressed in breast cancer tissues and implied that it was a tumor suppressor gene that could inhibit the p56 expression of the NF-κB pathway in breast cancer cells [97]. Thus, LXRα may be a prognostic biomarker for breast cancer, and targeting LXRα might provide a novel treatment strategy.
4.8. Endometrial Carcinoma and Ovarian Cancer
A recent study detected the expression of LXRα between normal endometrial tissues and endometrial carcinoma (EC) tissues and found that LXRα expression was upregulated in cancer tissues, implying that LXRα might be correlated with the development of EC. They then conducted cell viability analysis and flow cytometry in Ishikawa cells. The results showed that LXRα agonist TO901317 could inhibit cell proliferation and arrest the cell cycle by inhibiting the expression of cyclin D1 and cyclin E [98]. Therefore, LXRα activation will favor the prevention of EC and may produce potential therapeutic methods.
During treatment, ovarian cancer patients with malignant ascites generally have a poor prognosis and increased resistance to multiple drugs, such as cisplatin and paclitaxel. Kim et al. indicated that enriched cholesterol in malignant ascites could activate LXRα/β to upregulate the expression of drug efflux pump proteins and contribute to chemoresistance in ovarian cancer cells [99]. This finding will help better manage and monitor the clinical application of chemotherapeutic drugs in cancer.
4.9. Prostate Cancer
The role of LXRs in prostate cancer (PCa) cells and animal models has been widely studied [100]. Fukuchi et al. found that the T0901317-induced activation of LXRα could interrupt the normal PCa cell cycle process and inhibit the proliferation of PCa cells by upregulating the expression of p27 [101]. With more in-depth research, an increasing number of analyses have indicated that LXRα activation could induce ABCA1 expression, which could play a role in antiproliferation activity in PCa progression [102,103,104]. Therefore, targeting LXRα may be a promising direction for prostate cancer treatment in the future. Recently, Song et al. found that SULT2B1b sulfotransferase could produce sulfated oxysterols that were identified as inhibiting LXR activation and showed that SULT2B1b expression was undetectable in the clinical samples of castration-resistant prostate cancer patients. They subsequently demonstrated that a novel LXRα/ERRα/AKR1C3/ERK1/2 survival axis could activate SULT2B1b-vanished CRPC cells. Therefore, this novel axis might be utilized in exploring novel therapeutics against CRPC [105].
4.10. Other Tumors
Geyeregger et al. suggested that LXR activation could suppress the interleukin-mediated proliferation of normal and leukemic T-cell blasts. Moreover, the activation of LXRα could bring about leukemic B-cell apoptosis in chronic lymphocytic leukemia (CLL) patients [106]. This result implies that targeting LXRα may be a promising therapeutic strategy for CLL patients. LXRα is also involved in glioblastoma (GBM) tumorigenesis. A study by Fang et al. showed that the overexpression of YTHDF2, mediated by the EGFR/SRC/ERK signal pathway, could expedite the m6A-dependent mRNA depletion of LXRα and HIVEP2, which facilitated cholesterol disturbances and the invasive growth of GBM [107]. Some studies have also identified that LXRα plays an essential role in osteosarcoma. Chang et al. described that LXRα activation could upregulate some of the genes relevant to the cell cycle, such as p21 and p27, mediated by the activation of FoxO1 in Saos-2 and U2OS cells [108]. These results suggest that LXRα might be a suppressor in the invasion of osteosarcoma.
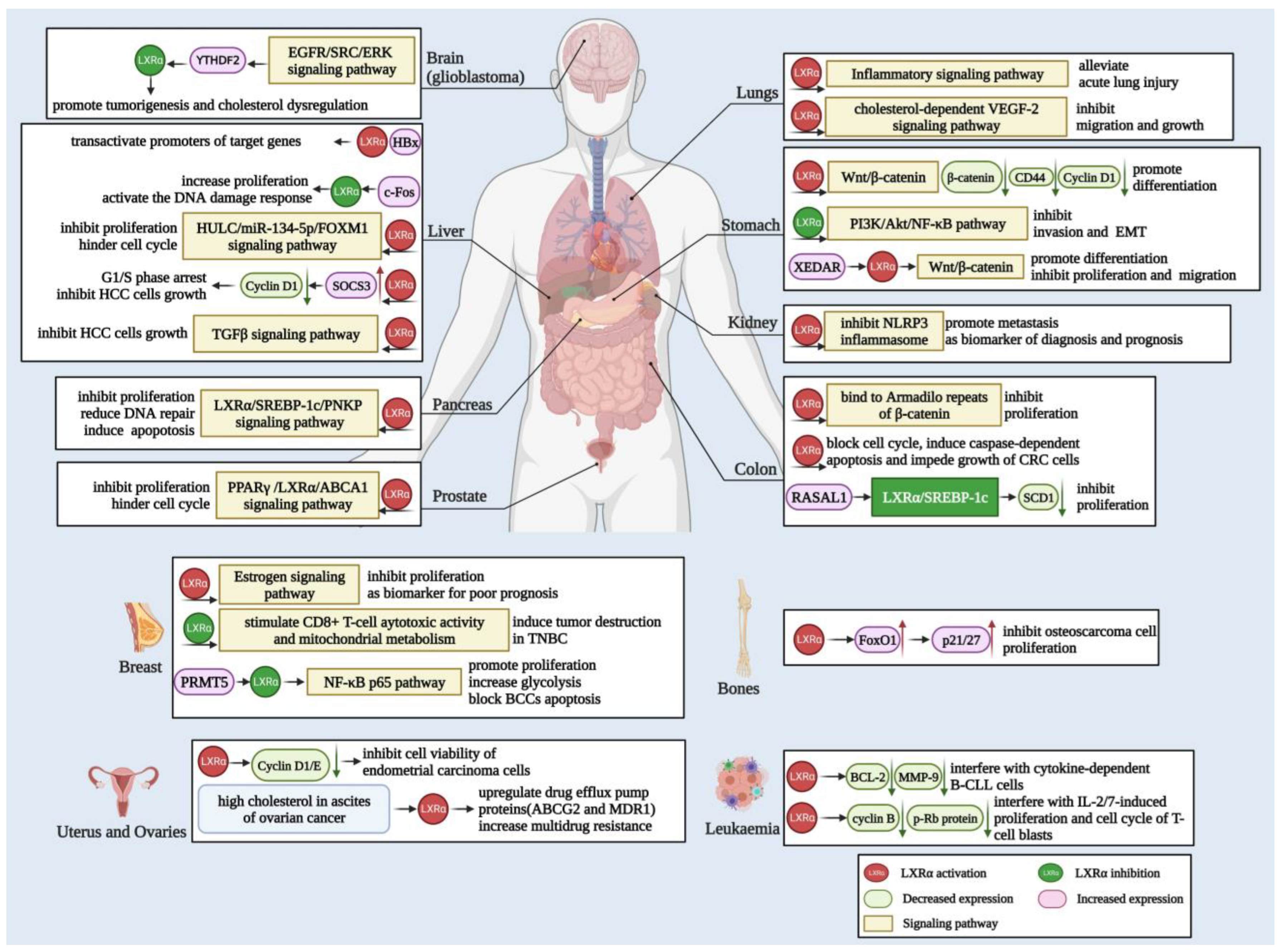
Consequently, in addition to cholesterol and lipid metabolism, LXRα also plays a vital role in many types of tumors, so targeted LXRα can make a specific contribution to the development of tumor therapy. Herein, we summarize the above information in detail in Figure 3.
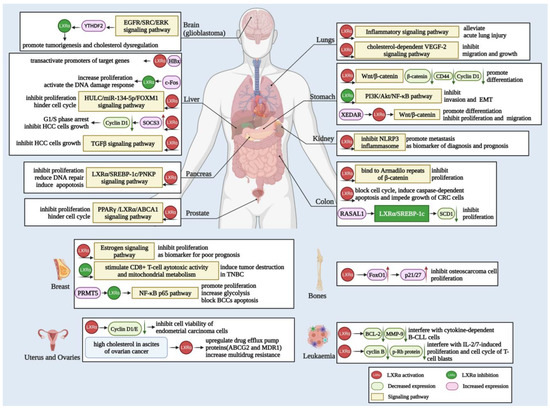
Figure 3.
Biological function and mechanisms of LXRα in various cancers. LXRα is related to the carcinogenesis of different cancers, including glioblastoma, liver, lung, stomach, kidney, colon, pancreas, prostate, breast, and other cancers depicted in the figure. LXRα mainly exerts function in cancer cells via the Wnt/β-catenin, NF-κB, EGFR/ERK, and TGFβ signaling pathways to regulate cell differentiation, migration, invasion, EMT, and apoptosis. Moreover, the activation of LXRα also modulates target genes, such as NLRP3, PPARγ, SREBF-1c, ABCA1, and other targets, leading to proliferation inhibition, cell cycle arrest, DNA repair abnormality, and other outcomes.
5. Therapeutic Insights into LXRα
As a member of the nuclear receptor superfamily, previous studies have suggested that targeting LXRα can be used to treat atherosclerosis and inflammation-related diseases. To date, many studies have revealed that abnormal lipid metabolism could promote the carcinogenesis, invasion, and metastasis of various cancers [109]. Due to the rapid growth and high biosynthesis of tumors, tumor cells need time to adapt to metabolic signals and dispel the overabundance of toxic metabolites. Because of the LXRα property of maintaining cholesterol and lipid homeostasis, it can be exploited for developing novel therapeutics against cancers via modulating the accumulation of metabolic products.
Moreover, studies have indicated that LXRα is correlated with the proliferation and invasion of many cancers and might be used as a potential therapeutic target in cancers. Recently, Shiragannavar et al. indicated that withaferin A, derived from the Withania somnifera plant, could activate the activity of LXRα, leading to the inhibition of NF-κB transcription activity, suppressing the proliferation, migration, and invasion of HCC cells. These data imply that withaferin A could be a potent anticancer compound targeting LXRα, decreasing the expression of various angiogenesis and inflammatory markers and contributing to the therapeutics of HCC [110]. One study indicated that 1, 6-O, O-diacetylbritannilactone, extracted from Inula britannica, could inhibit the proliferation of oral squamous cell carcinoma (OSCC) cells and impair the migration and invasion of OSCC cells, mediated by miR-1247-3p/LXRα/ABCA1 signaling [111]. Yang et al. suggested that lycopene could inhibit the proliferation of androgen-dependent human prostate tumor cells via the activation of the PPARγ/LXRα/ABCA1 signaling pathway [104].
In addition to the previously discovered LXR classic ligands, increasingly more natural or synthesized small molecular compounds have been found to specifically regulate LXRα activation. Several potential therapeutic agents for some cancers are shown in Table 2. Although the therapeutic methods targeting LXRα are not yet fully developed, more therapeutic achievements could be attained in the future due to the knowledge regarding the mechanisms and functions of LXRα in cancer.

Table 2.
Potential reagents to modulate LXRα activity in cancer therapy.
6. Conclusions
In this review, we summarized the potential mechanisms of LXRα that modulate multiple processes in different cancers. Because of the cancer-specific expression characteristic of LXRα, it can exert contrary functions in tumor inhibition and promotion. The expression of LXRα in most types of cancer cells is decreased (e.g., lung, breast, and kidney cancer cells), and its overexpression can suppress the EMT, metastasis, and proliferation of cancer cells; however, it can exert a contentious effect in some cancers, such as gastric cancer and liver cancer. This phenomenon may be caused by the heterogeneity of the included clinical samples in different studies, the various activated ligands of LXRα, or the different signaling pathways regulated by LXRα. However, most studies have found that the activation of LXRα can inhibit cancer pathogenesis. Although some studies have emphasized the relationship between LXRα expression and patient prognosis (e.g., breast, kidney, and prostate cancer), further understanding of the diagnostic value of LXRα is needed.
Even though numerous studies have demonstrated that LXRα expression decreases in cancers and may serve as a novel therapeutic target, its clinical application remains challenging. At present, most of the activating ligands for LXRα are non-specific and target LXRα and LXRβ at the same time; thus, more natural small-molecule substances and manual interventions that specifically target LXRα should be studied. Moreover, the excessive activation of LXRα may also affect the normal function of the cholesterol and lipid metabolism signaling pathways, which may cause unnecessary side effects. Therefore, how to specifically target LXRα against cancer without severe cholesterol metabolism abnormalities is worth considering in future investigations. In short, LXRα plays a vital role in the occurrence and development of cancer, and it could be a target for adjuvant cancer treatment. However, some problems remain to be solved, and developing more specific and effective drugs is also an urgent priority.
Author Contributions
N.H. drafted the article and did literature research. M.Y. generated the figures and the tables. L.Y. and H.T. performed language editing and revised the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (No.2022YFC2304800), the Science and Technological Supports Project of Sichuan Province (2020YFS0135), and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD20009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Willy, P.J.; Mangelsdorf, D.J. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR. Genes Dev. 1997, 11, 289–298. [Google Scholar] [CrossRef]
- Zelcer, N.; Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006, 116, 607–614. [Google Scholar] [CrossRef]
- Kalaany, N.Y.; Mangelsdorf, D.J. LXRS and FXR: The yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 2006, 68, 159–191. [Google Scholar] [CrossRef]
- Koldamova, R.; Fitz, N.F.; Lefterov, I. ATP-binding cassette transporter A1: From metabolism to neurodegeneration. Neurobiol. Dis. 2014, 72 Pt A, 13–21. [Google Scholar] [CrossRef]
- Yu, X.H.; Jiang, N.; Yao, P.B.; Zheng, X.L.; Cayabyab, F.S.; Tang, C.K. NPC1, intracellular cholesterol trafficking and atherosclerosis. Clin. Chim. Acta Int. J. Clin. Chem. 2014, 429, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Leussink, S.; Aranda-Pardos, I.; Noelia, A. Lipid metabolism as a mechanism of immunomodulation in macrophages: The role of liver X receptors. Curr. Opin. Pharmacol. 2020, 53, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, X.; Chen, Y.; Zhang, L.; Jiang, M.; Li, X.; Xiang, R.; Miao, R.; Hajjar, D.P.; Duan, Y.; et al. Identification of interferon-γ as a new molecular target of liver X receptor. Biochem. J. 2014, 459, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Savla, S.R.; Prabhavalkar, K.S.; Bhatt, L.K. Liver X receptor: A potential target in the treatment of atherosclerosis. Expert Opin. Ther. Targets 2022, 26, 645–658. [Google Scholar] [CrossRef]
- Russo-Savage, L.; Schulman, I.G. Liver X receptors and liver physiology. Biochim. Et Biophys. Acta Mol. Basis Dis. 2021, 1867, 166121. [Google Scholar] [CrossRef]
- Li, P.; Wang, G.; Zhang, X.L.; He, G.L.; Luo, X.; Yang, J.; Luo, Z.; Shen, T.T.; Yang, X.S. MicroRNA-155 Promotes Heat Stress-Induced Inflammation via Targeting Liver X Receptor α in Microglia. Front. Cell. Neurosci. 2019, 13, 12. [Google Scholar] [CrossRef]
- Voisin, M.; Gage, M.C.; Becares, N.; Shrestha, E.; Fisher, E.A.; Pineda-Torra, I.; Garabedian, M.J. LXRα Phosphorylation in Cardiometabolic Disease: Insight from Mouse Models. Endocrinology 2020, 161, bqaa089. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Wang, J.; Zhang, H. Molecular mechanism of liver X receptors in cancer therapeutics. Life Sci. 2021, 273, 119287. [Google Scholar] [CrossRef]
- Teboul, M.; Enmark, E.; Li, Q.; Wikström, A.C.; Pelto-Huikko, M.; Gustafsson, J.A. OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 2096–2100. [Google Scholar] [CrossRef]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef]
- Peet, D.J.; Janowski, B.A.; Mangelsdorf, D.J. The LXRs: A new class of oxysterol receptors. Curr. Opin. Genet. Dev. 1998, 8, 571–575. [Google Scholar] [CrossRef]
- Zhao, L.; Lei, W.; Deng, C.; Wu, Z.; Sun, M.; Jin, Z.; Song, Y.; Yang, Z.; Jiang, S.; Shen, M.; et al. The roles of liver X receptor α in inflammation and inflammation-associated diseases. J. Cell. Physiol. 2021, 236, 4807–4828. [Google Scholar] [CrossRef]
- Ma, Z.; Deng, C.; Hu, W.; Zhou, J.; Fan, C.; Di, S.; Liu, D.; Yang, Y.; Wang, D. Liver X Receptors and their Agonists: Targeting for Cholesterol Homeostasis and Cardiovascular Diseases. Curr. Issues Mol. Biol. 2017, 22, 41–64. [Google Scholar] [CrossRef] [PubMed]
- Maqdasy, S.; Trousson, A.; Tauveron, I.; Volle, D.H.; Baron, S.; Lobaccaro, J.M. Once and for all, LXRα and LXRβ are gatekeepers of the endocrine system. Mol. Asp. Med. 2016, 49, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors and lipid physiology: Opening the X-files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Filomenko, R.; Rébé, C.; Chevriaux, A.; Varin, A.; Derangère, V.; Bessède, G.; Gambert, P.; Lagrost, L.; Masson, D. Knock-down of the oxysterol receptor LXRα impairs cholesterol efflux in human primary macrophages: Lack of compensation by LXRβ activation. Biochem. Pharmacol. 2013, 86, 122–129. [Google Scholar] [CrossRef]
- Gabbi, C.; Warner, M.; Gustafsson, J.A. Minireview: Liver X receptor beta: Emerging roles in physiology and diseases. Mol. Endocrinol. 2009, 23, 129–136. [Google Scholar] [CrossRef]
- Jakobsson, T.; Treuter, E.; Gustafsson, J.; Steffensen, K.R. Liver X receptor biology and pharmacology: New pathways, challenges and opportunities. Trends Pharmacol. Sci. 2012, 33, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Apfel, R.; Benbrook, D.; Lernhardt, E.; Ortiz, M.A.; Salbert, G.; Pfahl, M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell. Biol. 1994, 14, 7025–7035. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, D.D.; Yan, D.L.; Hu, Y.; Chen, D.; Liu, Y.; Zhang, H.D.; Yu, S.R.; Cao, H.X.; Feng, J.F. Liver X receptor as a drug target for the treatment of breast cancer. Anti-Cancer Drugs 2016, 27, 373–382. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, S.; Gustafsson, J. Nuclear Receptors: Recent Drug Discovery for Cancer Therapies. Endocr. Rev. 2019, 40, 1207–1249. [Google Scholar] [CrossRef]
- Tamura, K.; Chen, Y.E.; Horiuchi, M.; Chen, Q.; Daviet, L.; Yang, Z.; Lopez-Ilasaca, M.; Mu, H.; Pratt, R.E.; Dzau, V.J. LXRalpha functions as a cAMP-responsive transcriptional regulator of gene expression. Proc. Natl. Acad. Sci. USA 2000, 97, 8513–8518. [Google Scholar] [CrossRef]
- Tamura, K.; Chen, Y.E.; Tanaka, Y.; Sakai, M.; Tsurumi, Y.; Koide, Y.; Kihara, M.; Pratt, R.E.; Horiuchi, M.; Umemura, S.; et al. Nuclear receptor LXRalpha is involved in cAMP-mediated human renin gene expression. Mol. Cell. Endocrinol. 2004, 224, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Martin, M.; Zhang, J.; Huang, H.Y.; Bai, L.; Zhang, J.; Kang, J.; He, M.; Li, J.; Maurya, M.R.; et al. Krüppel-Like Factor 4 Regulation of Cholesterol-25-Hydroxylase and Liver X Receptor Mitigates Atherosclerosis Susceptibility. Circulation 2017, 136, 1315–1330. [Google Scholar] [CrossRef]
- Fan, L.; Lai, R.; Ma, N.; Dong, Y.; Li, Y.; Wu, Q.; Qiao, J.; Lu, H.; Gong, L.; Tao, Z.; et al. miR-552-3p modulates transcriptional activities of FXR and LXR to ameliorate hepatic glycolipid metabolism disorder. J. Hepatol. 2021, 74, 8–19. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pandey, A.; Chinnaiyan, A.M. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–w514. [Google Scholar] [CrossRef]
- Kaiser, S.; Park, Y.K.; Franklin, J.L.; Halberg, R.B.; Yu, M.; Jessen, W.J.; Freudenberg, J.; Chen, X.; Haigis, K.; Jegga, A.G.; et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007, 8, R131. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, Y.Y.; Park, E.S.; Cho, J.Y.; Izzo, J.G.; Zhang, D.; Kim, S.B.; Lee, J.H.; Bhutani, M.S.; Swisher, S.G.; et al. Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS ONE 2010, 5, e15074. [Google Scholar] [CrossRef]
- Kimchi, E.T.; Posner, M.C.; Park, J.O.; Darga, T.E.; Kocherginsky, M.; Karrison, T.; Hart, J.; Smith, K.D.; Mezhir, J.J.; Weichselbaum, R.R.; et al. Progression of Barrett’s metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res. 2005, 65, 3146–3154. [Google Scholar] [CrossRef] [PubMed]
- Yusenko, M.V.; Kuiper, R.P.; Boethe, T.; Ljungberg, B.; van Kessel, A.G.; Kovacs, G. High-resolution DNA copy number and gene expression analyses distinguish chromophobe renal cell carcinomas and renal oncocytomas. BMC Cancer 2009, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Otu, H.; Spentzos, D.; Kolia, S.; Inan, M.; Beecken, W.D.; Fellbaum, C.; Gu, X.; Joseph, M.; Pantuck, A.J.; et al. Gene signatures of progression and metastasis in renal cell cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 5730–5739. [Google Scholar] [CrossRef]
- Haferlach, T.; Kohlmann, A.; Wieczorek, L.; Basso, G.; Kronnie, G.T.; Béné, M.C.; De Vos, J.; Hernández, J.M.; Hofmann, W.K.; Mills, K.I.; et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: Report from the International Microarray Innovations in Leukemia Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2529–2537. [Google Scholar] [CrossRef]
- Andersson, A.; Ritz, C.; Lindgren, D.; Edén, P.; Lassen, C.; Heldrup, J.; Olofsson, T.; Råde, J.; Fontes, M.; Porwit-Macdonald, A.; et al. Microarray-based classification of a consecutive series of 121 childhood acute leukemias: Prediction of leukemic and genetic subtype as well as of minimal residual disease status. Leukemia 2007, 21, 1198–1203. [Google Scholar] [CrossRef]
- Mas, V.R.; Maluf, D.G.; Archer, K.J.; Yanek, K.; Kong, X.; Kulik, L.; Freise, C.E.; Olthoff, K.M.; Ghobrial, R.M.; McIver, P.; et al. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol. Med. 2009, 15, 85–94. [Google Scholar] [CrossRef]
- Wurmbach, E.; Chen, Y.B.; Khitrov, G.; Zhang, W.; Roayaie, S.; Schwartz, M.; Fiel, I.; Thung, S.; Mazzaferro, V.; Bruix, J.; et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology 2007, 45, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Richards, W.G.; Staunton, J.; Li, C.; Monti, S.; Vasa, P.; Ladd, C.; Beheshti, J.; Bueno, R.; Gillette, M.; et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl. Acad. Sci. USA 2001, 98, 13790–13795. [Google Scholar] [CrossRef] [PubMed]
- Basso, K.; Margolin, A.A.; Stolovitzky, G.; Klein, U.; Dalla-Favera, R.; Califano, A. Reverse engineering of regulatory networks in human B cells. Nat. Genet. 2005, 37, 382–390. [Google Scholar] [CrossRef]
- Compagno, M.; Lim, W.K.; Grunn, A.; Nandula, S.V.; Brahmachary, M.; Shen, Q.; Bertoni, F.; Ponzoni, M.; Scandurra, M.; Califano, A.; et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 2009, 459, 717–721. [Google Scholar] [CrossRef]
- Storz, M.N.; van de Rijn, M.; Kim, Y.H.; Mraz-Gernhard, S.; Hoppe, R.T.; Kohler, S. Gene expression profiles of cutaneous B cell lymphoma. J. Investig. Dermatol. 2003, 120, 865–870. [Google Scholar] [CrossRef]
- Brune, V.; Tiacci, E.; Pfeil, I.; Döring, C.; Eckerle, S.; van Noesel, C.J.; Klapper, W.; Falini, B.; von Heydebreck, A.; Metzler, D.; et al. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J. Exp. Med. 2008, 205, 2251–2268. [Google Scholar] [CrossRef]
- Piccaluga, P.P.; Agostinelli, C.; Califano, A.; Rossi, M.; Basso, K.; Zupo, S.; Went, P.; Klein, U.; Zinzani, P.L.; Baccarani, M.; et al. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J. Clin. Investig. 2007, 117, 823–834. [Google Scholar] [CrossRef]
- Zhan, F.; Hardin, J.; Kordsmeier, B.; Bumm, K.; Zheng, M.; Tian, E.; Sanderson, R.; Yang, Y.; Wilson, C.; Zangari, M.; et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood 2002, 99, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Taylor, B.S.; Banerji, S.; Ramos, A.H.; Lagos-Quintana, M.; Decarolis, P.L.; Shah, K.; Socci, N.D.; Weir, B.A.; Ho, A.; et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat. Genet. 2010, 42, 715–721. [Google Scholar] [CrossRef]
- Santegoets, L.A.; Seters, M.; Helmerhorst, T.J.; Heijmans-Antonissen, C.; Hanifi-Moghaddam, P.; Ewing, P.C.; van Ijcken, W.F.; van der Spek, P.J.; van der Meijden, W.I.; Blok, L.J. HPV related VIN: Highly proliferative and diminished responsiveness to extracellular signals. Int. J. Cancer 2007, 121, 759–766. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Wang, R.; Tan, X.; Huang, C.; Chen, G.; Chen, Z. LXRα Promotes the Differentiation of Human Gastric Cancer Cells through Inactivation of Wnt/β-catenin Signaling. J. Cancer 2019, 10, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, B.; Zhao, G. Liver X receptor α (LXRα) promoted invasion and EMT of gastric cancer cells by regulation of NF-κB activity. Hum. Cell 2017, 30, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Sun, X.; Li, S.; Sun, Y.; Zhai, H. Tumor Suppressor Gene XEDAR Promotes Differentiation and Suppresses Proliferation and Migration of Gastric Cancer Cells Through Upregulating the RELA/LXRα Axis and Deactivating the Wnt/β-Catenin Pathway. Cell Transplant. 2021, 30, 963689721996346. [Google Scholar] [CrossRef]
- Guo, R.; Yang, B. Hypoxia-Induced LXRα Contributes to the Migration and Invasion of Gastric Cancer Cells. Folia Biol. 2021, 67, 91–101. [Google Scholar]
- Moriishi, K.; Mochizuki, R.; Moriya, K.; Miyamoto, H.; Mori, Y.; Abe, T.; Murata, S.; Tanaka, K.; Miyamura, T.; Suzuki, T.; et al. Critical role of PA28gamma in hepatitis C virus-associated steatogenesis and hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 1661–1666. [Google Scholar] [CrossRef]
- Lima-Cabello, E.; García-Mediavilla, M.V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Lozano-Rodríguez, T.; Fernández-Bermejo, M.; Olcoz, J.L.; González-Gallego, J.; García-Monzón, C.; Sánchez-Campos, S. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin. Sci. 2011, 120, 239–250. [Google Scholar] [CrossRef]
- García-Mediavilla, M.V.; Pisonero-Vaquero, S.; Lima-Cabello, E.; Benedicto, I.; Majano, P.L.; Jorquera, F.; González-Gallego, J.; Sánchez-Campos, S. Liver X receptor α-mediated regulation of lipogenesis by core and NS5A proteins contributes to HCV-induced liver steatosis and HCV replication. Lab. Investig. A J. Tech. Methods Pathol. 2012, 92, 1191–1202. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.H.; Kim, H.H.; Cheong, J. Hepatitis B virus X protein induces lipogenic transcription factor SREBP1 and fatty acid synthase through the activation of nuclear receptor LXRalpha. Biochem. J. 2008, 416, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Na, T.Y.; Shin, Y.K.; Roh, K.J.; Kang, S.A.; Hong, I.; Oh, S.J.; Seong, J.K.; Park, C.K.; Choi, Y.L.; Lee, M.O. Liver X receptor mediates hepatitis B virus X protein-induced lipogenesis in hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2009, 49, 1122–1131. [Google Scholar] [CrossRef]
- Bakiri, L.; Hamacher, R.; Graña, O.; Guío-Carrión, A.; Campos-Olivas, R.; Martinez, L.; Dienes, H.P.; Thomsen, M.K.; Hasenfuss, S.C.; Wagner, E.F. Liver carcinogenesis by FOS-dependent inflammation and cholesterol dysregulation. J. Exp. Med. 2017, 214, 1387–1409. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, D.; Zhang, Y.; Lou, G.; Huang, G.; Chen, B.; Shen, X.; Gao, M.; Gong, W.; Zhou, P.; et al. LXRα-mediated downregulation of FOXM1 suppresses the proliferation of hepatocellular carcinoma cells. Oncogene 2014, 33, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, T.; He, W.; Jiang, S.; Zhong, D.; Xu, Z.; Wei, Q.; Zhang, Y.; Shi, C. Liver X receptor inhibits the growth of hepatocellular carcinoma cells via regulating HULC/miR-134-5p/FOXM1 axis. Cell Signal 2020, 74, 109720. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhang, Y.; Chen, S.; Ni, Z.; He, J.; Li, X.; Li, B.; Zhao, K.; Yang, F.; Zeng, Y.; et al. Induction of SOCS3 by liver X receptor suppresses the proliferation of hepatocellular carcinoma cells. Oncotarget 2017, 8, 64083–64094. [Google Scholar] [CrossRef]
- Bellomo, C.; Caja, L.; Fabregat, I.; Mikulits, W.; Kardassis, D.; Heldin, C.H.; Moustakas, A. Snail mediates crosstalk between TGFβ and LXRα in hepatocellular carcinoma. Cell Death Differ. 2018, 25, 885–903. [Google Scholar] [CrossRef]
- Morén, A.; Bellomo, C.; Tsubakihara, Y.; Kardassis, D.; Mikulits, W.; Heldin, C.H.; Moustakas, A. LXRα limits TGFβ-dependent hepatocellular carcinoma associated fibroblast differentiation. Oncogenesis 2019, 8, 36. [Google Scholar] [CrossRef]
- Lin, Z.; Xia, S.; Liang, Y.; Ji, L.; Pan, Y.; Jiang, S.; Wan, Z.; Tao, L.; Chen, J.; Lin, C.; et al. LXR activation potentiates sorafenib sensitivity in HCC by activating microRNA-378a transcription. Theranostics 2020, 10, 8834–8850. [Google Scholar] [CrossRef]
- Rudalska, R.; Harbig, J.; Snaebjornsson, M.T.; Klotz, S.; Zwirner, S.; Taranets, L.; Heinzmann, F.; Kronenberger, T.; Forster, M.; Cui, W.; et al. LXRα activation and Raf inhibition trigger lethal lipotoxicity in liver cancer. Nat. Cancer 2021, 2, 201–217. [Google Scholar] [CrossRef]
- Rudalska, R.; Zender, L.; Dauch, D. Exploiting lipotoxicity for the treatment of liver cancer. Br. J. Cancer 2021, 125, 1459–1461. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, B.; Cao, Z.; Xu, X.; Huo, Z.; Zhang, P.; Xiang, S.; Zhao, Z.; Lv, C.; Meng, M.; et al. The lipogenic LXR-SREBF1 signaling pathway controls cancer cell DNA repair and apoptosis and is a vulnerable point of malignant tumors for cancer therapy. Cell Death Differ. 2020, 27, 2433–2450. [Google Scholar] [CrossRef]
- Chuu, C.P. Modulation of liver X receptor signaling as a prevention and therapy for colon cancer. Med. Hypotheses 2011, 76, 697–699. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Berrington de González, A.; Freedman, N.D.; Huxley, R.; Mok, Y.; Jee, S.H.; Samet, J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Broadbent, H.; Law, P.J.; Sud, A.; Palin, K.; Tuupanen, S.; Gylfe, A.; Hänninen, U.A.; Cajuso, T.; Tanskanen, T.; Kondelin, J.; et al. Mendelian randomisation implicates hyperlipidaemia as a risk factor for colorectal cancer. Int. J. Cancer 2017, 140, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Chiarpotto, E.; Sottero, B.; Maina, M.; Mascia, C.; Guina, T.; Gamba, P.; Gargiulo, S.; Testa, G.; Leonarduzzi, G.; et al. Evidence of cell damage induced by major components of a diet-compatible mixture of oxysterols in human colon cancer CaCo-2 cell line. Biochimie 2013, 95, 632–640. [Google Scholar] [CrossRef]
- Roussi, S.; Winter, A.; Gosse, F.; Werner, D.; Zhang, X.; Marchioni, E.; Geoffroy, P.; Miesch, M.; Raul, F. Different apoptotic mechanisms are involved in the antiproliferative effects of 7beta-hydroxysitosterol and 7beta-hydroxycholesterol in human colon cancer cells. Cell Death Differ. 2005, 12, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Warns, J.; Marwarha, G.; Freking, N.; Ghribi, O. 27-hydroxycholesterol decreases cell proliferation in colon cancer cell lines. Biochimie 2018, 153, 171–180. [Google Scholar] [CrossRef]
- Lo Sasso, G.; Bovenga, F.; Murzilli, S.; Salvatore, L.; Di Tullio, G.; Martelli, N.; D’Orazio, A.; Rainaldi, S.; Vacca, M.; Mangia, A.; et al. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology 2013, 144, 1497–1507.e13. [Google Scholar] [CrossRef]
- Sharma, B.; Gupta, V.; Dahiya, D.; Kumar, H.; Vaiphei, K.; Agnihotri, N. Clinical relevance of cholesterol homeostasis genes in colorectal cancer. Biochim. Et Biophys. Acta. Mol. Cell Biol. Lipids 2019, 1864, 1314–1327. [Google Scholar] [CrossRef]
- Uno, S.; Endo, K.; Jeong, Y.; Kawana, K.; Miyachi, H.; Hashimoto, Y.; Makishima, M. Suppression of beta-catenin signaling by liver X receptor ligands. Biochem. Pharmacol. 2009, 77, 186–195. [Google Scholar] [CrossRef]
- Vedin, L.L.; Gustafsson, J.; Steffensen, K.R. The oxysterol receptors LXRα and LXRβ suppress proliferation in the colon. Mol. Carcinog. 2013, 52, 835–844. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Li, X.; Zhang, C.; Peng, L. RASAL1 induces to downregulate the SCD1, leading to suppression of cell proliferation in colon cancer via LXRα/SREBP1c pathway. Biol. Res. 2019, 52, 60. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Yanagida, M.; Matsui, D.; Tanaka, M.; Sugimoto, K.; Chen, H.; Ichikawa-Tomikawa, N.; Marubashi, S.; Suzuki, H.; Chiba, H. Expression of liver X receptors in normal and refractory carcinoma tissues of the human lung and pancreas. Histol. Histopathol. 2018, 33, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Zhang, G.; Zhang, X.; Jiang, W. Chikusetsusaponin V attenuates lipopolysaccharide-induced acute lung injury in mice by modulation of the NF-κB and LXRα. Int. Immunopharmacol. 2019, 70, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Fu, Y.; Lu, X.; Zhang, Z.; Zhang, W.; Cao, Y.; Zhang, N. Protective Effects of Platycodin D on Lipopolysaccharide-Induced Acute Lung Injury by Activating LXRα-ABCA1 Signaling Pathway. Front. Immunol. 2016, 7, 644. [Google Scholar] [CrossRef]
- Gong, H.; He, J.; Lee, J.H.; Mallick, E.; Gao, X.; Li, S.; Homanics, G.E.; Xie, W. Activation of the liver X receptor prevents lipopolysaccharide-induced lung injury. J. Biol. Chem. 2009, 284, 30113–30121. [Google Scholar] [CrossRef]
- Noghero, A.; Perino, A.; Seano, G.; Saglio, E.; Lo Sasso, G.; Veglio, F.; Primo, L.; Hirsch, E.; Bussolino, F.; Morello, F. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2280–2288. [Google Scholar] [CrossRef]
- Dai, Y.B.; Miao, Y.F.; Wu, W.F.; Li, Y.; D’Errico, F.; Su, W.; Burns, A.R.; Huang, B.; Maneix, L.; Warner, M.; et al. Ablation of Liver X receptors α and β leads to spontaneous peripheral squamous cell lung cancer in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 7614–7619. [Google Scholar] [CrossRef]
- Melloni, G.; Muriana, P.; Bandiera, A.; Fontana, R.; Maggioni, D.; Russo, V.; Doglioni, C.; Zannini, P. Prognostic role of liver X receptor-alpha in resected stage II and III non-small-cell lung cancer. Clin. Respir. J. 2018, 12, 241–246. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Wang, K.; Xu, T.; Ruan, H.; Xiao, H.; Liu, J.; Song, Z.; Cao, Q.; Bao, L.; Liu, D.; Wang, C.; et al. LXRα promotes cell metastasis by regulating the NLRP3 inflammasome in renal cell carcinoma. Cell Death Dis. 2019, 10, 159. [Google Scholar] [CrossRef]
- Gong, H.; Guo, P.; Zhai, Y.; Zhou, J.; Uppal, H.; Jarzynka, M.J.; Song, W.C.; Cheng, S.Y.; Xie, W. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol. Endocrinol. 2007, 21, 1781–1790. [Google Scholar] [CrossRef]
- Vedin, L.L.; Lewandowski, S.A.; Parini, P.; Gustafsson, J.A.; Steffensen, K.R. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis 2009, 30, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luquis, O.; Madden, K.; N’Dri, N.M.; Berg, R.; Olopade, O.F.; Ngwa, W.; Abuidris, D.; Mittal, S.; Lyn-Cook, B.; Mohammed, S.I. LXR/RXR pathway signaling associated with triple-negative breast cancer in African American women. Breast Cancer 2019, 11, 1–12. [Google Scholar] [CrossRef]
- Hutchinson, S.A.; Websdale, A.; Cioccoloni, G.; Røberg-Larsen, H.; Lianto, P.; Kim, B.; Rose, A.; Soteriou, C.; Pramanik, A.; Wastall, L.M.; et al. Liver x receptor alpha drives chemoresistance in response to side-chain hydroxycholesterols in triple negative breast cancer. Oncogene 2021, 40, 2872–2883. [Google Scholar] [CrossRef] [PubMed]
- Lianto, P.; Hutchinson, S.A.; Moore, J.B.; Hughes, T.A.; Thorne, J.L. Characterization and prognostic value of LXR splice variants in triple-negative breast cancer. iScience 2021, 24, 103212. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.J.; Valfort, A.C.; Steinauer, N.; Chatterjee, A.; Abuirqeba, S.; Majidi, S.; Sengupta, M.; Di Paolo, R.J.; Shornick, L.P.; Zhang, J.; et al. LXR-inverse agonism stimulates immune-mediated tumor destruction by enhancing CD8 T-cell activity in triple negative breast cancer. Sci. Rep. 2019, 9, 19530. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wei, L.; Wu, B. PRMT5 Promotes Aerobic Glycolysis and Invasion of Breast Cancer Cells by Regulating the LXRα/NF-κBp65 Pathway. OncoTargets Ther. 2020, 13, 3347–3357. [Google Scholar] [CrossRef]
- Fang, F.; Li, D.; Zhao, L.; Li, Y.; Zhang, T.; Cui, B. Expression of NR1H3 in endometrial carcinoma and its effect on the proliferation of Ishikawa cells in vitro. OncoTargets Ther. 2019, 12, 685–697. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.; Dhanasekaran, D.N.; Song, Y.S. Activation of LXRɑ/β by cholesterol in malignant ascites promotes chemoresistance in ovarian cancer. BMC Cancer 2018, 18, 1232. [Google Scholar] [CrossRef] [PubMed]
- Bousset, L.; Rambur, A.; Fouache, A.; Bunay, J.; Morel, L.; Lobaccaro, J.A.; Baron, S.; Trousson, A.; de Joussineau, C. New Insights in Prostate Cancer Development and Tumor Therapy: Modulation of Nuclear Receptors and the Specific Role of Liver X Receptors. Int. J. Mol. Sci. 2018, 19, 2545. [Google Scholar] [CrossRef]
- Fukuchi, J.; Hiipakka, R.A.; Kokontis, J.M.; Hsu, S.; Ko, A.L.; Fitzgerald, M.L.; Liao, S. Androgenic suppression of ATP-binding cassette transporter A1 expression in LNCaP human prostate cancer cells. Cancer Res. 2004, 64, 7682–7685. [Google Scholar] [CrossRef]
- Yang, C.M.; Lu, Y.L.; Chen, H.Y.; Hu, M.L. Lycopene and the LXRα agonist T0901317 synergistically inhibit the proliferation of androgen-independent prostate cancer cells via the PPARγ-LXRα-ABCA1 pathway. J. Nutr. Biochem. 2012, 23, 1155–1162. [Google Scholar] [CrossRef]
- Trasino, S.E.; Kim, Y.S.; Wang, T.T. Ligand, receptor, and cell type-dependent regulation of ABCA1 and ABCG1 mRNA in prostate cancer epithelial cells. Mol. Cancer Ther. 2009, 8, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Lu, I.H.; Chen, H.Y.; Hu, M.L. Lycopene inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARγ-LXRα-ABCA1 pathway. J. Nutr. Biochem. 2012, 23, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Song, C.S.; Chatterjee, B. Prostate Cancer Stimulation by a Novel Liver X Receptor (LXRa)-Estrogen Related Receptor (ERRa) Axis. J. Endocr. Soc. 2021, 5, A1030. [Google Scholar] [CrossRef]
- Geyeregger, R.; Shehata, M.; Zeyda, M.; Kiefer, F.W.; Stuhlmeier, K.M.; Porpaczy, E.; Zlabinger, G.J.; Jäger, U.; Stulnig, T.M. Liver X receptors interfere with cytokine-induced proliferation and cell survival in normal and leukemic lymphocytes. J. Leukoc. Biol. 2009, 86, 1039–1048. [Google Scholar] [CrossRef]
- Fang, R.; Chen, X.; Zhang, S.; Shi, H.; Ye, Y.; Shi, H.; Zou, Z.; Li, P.; Guo, Q.; Ma, L.; et al. EGFR/SRC/ERK-stabilized YTHDF2 promotes cholesterol dysregulation and invasive growth of glioblastoma. Nat. Commun. 2021, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Zhao, Y.F.; Cao, Y.L.; Gu, X.F.; Li, Z.Q.; Wang, S.Q.; Miao, J.H.; Zhan, H.S. Liver X receptor α inhibits osteosarcoma cell proliferation through up-regulation of FoxO1. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013, 32, 180–186. [Google Scholar] [CrossRef]
- Long, J.; Zhang, C.J.; Zhu, N.; Du, K.; Yin, Y.F.; Tan, X.; Liao, D.F.; Qin, L. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar]
- Shiragannavar, V.D.; Gowda, N.G.S.; Kumar, D.P.; Mirshahi, F.; Santhekadur, P.K. Withaferin A Acts as a Novel Regulator of Liver X Receptor-α in HCC. Front. Oncol. 2020, 10, 628506. [Google Scholar] [CrossRef]
- Zheng, S.; Li, L.; Li, N.; Du, Y.; Zhang, N. 1, 6-O, O-Diacetylbritannilactone from Inula britannica Induces Anti-Tumor Effect on Oral Squamous Cell Carcinoma via miR-1247-3p/LXRα/ABCA1 Signaling. OncoTargets Ther. 2020, 13, 11097–11109. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, J.; Fu, W. EGFR/FOXO3A/LXR-α Axis Promotes Prostate Cancer Proliferation and Metastasis and Dual-Targeting LXR-α/EGFR Shows Synthetic Lethality. Front. Oncol. 2020, 10, 1688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Zhu, N.; Long, J.; Wu, H.T.; Wang, Y.X.; Liu, B.Y.; Liao, D.F.; Qin, L. Celastrol induces lipophagy via the LXRα/ABCA1 pathway in clear cell renal cell carcinoma. Acta Pharmacol. Sin. 2021, 42, 1472–1485. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).