Deleterious Interaction between the Neurosteroid (3α,5α)3-Hydroxypregnan-20-One (3α,5α-THP) and the Mu-Opioid System Activation during Forced Swim Stress in Rats
Abstract
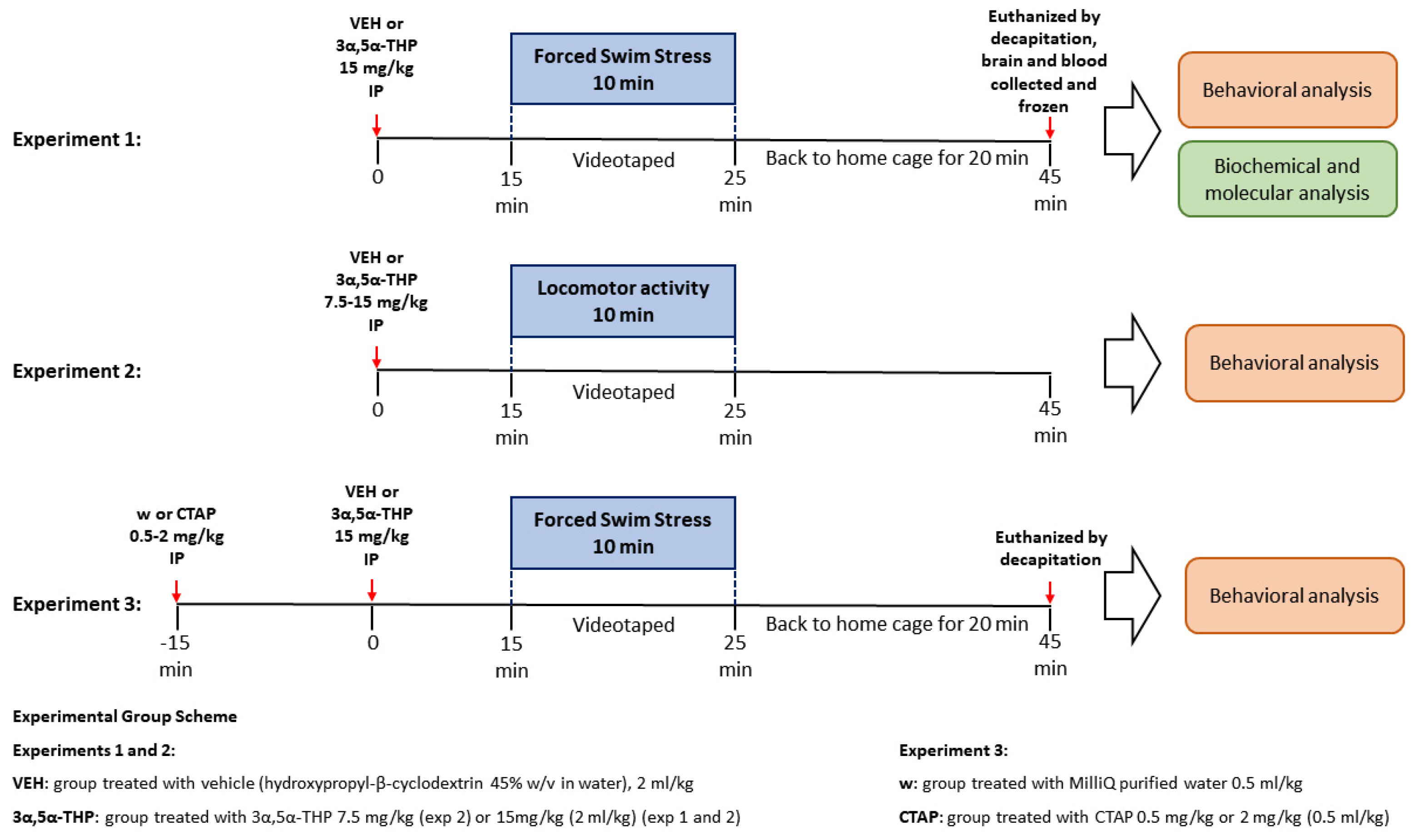
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Administration
2.2.1. 3α,5α-THP
2.2.2. CTAP
2.3. Behavioral Tests
2.3.1. Forced Swim Stress (FSS)
2.3.2. Locomotor Activity
2.4. Behavioral Analysis
- (1)
- Definition of submersion. Submersion was scored when the entire body of the animal, including the head, was upright but underwater, with no possibility of the rat breathing. If the rat showed distress underwater for longer than 2 s, it was removed from the cylinder immediately. Submersion was considered an involuntary passive behavior.
- (2)
- Definition of immobility. A rat was considered immobile when floating passively, keeping its head above the surface, making only the necessary movements to maintain balance. For the analysis, immobility behavior was measured starting from the first bout of immobility lasting longer than 1 s. Immobility was considered a passive behavior.
- (3)
- Definition of swimming. Swimming was identified as active swimming movements, more than were necessary to maintain the head above the water. Swimming was considered an active behavior.
- (4)
- Definition of climbing. Climbing was defined as the animal making upward-directed movements of the forepaws against the walls of the cylinders, trying to escape. Climbing was considered an active behavior.
- (5)
- Definition of exploration. Exploration was identified if the animal intentionally swam underwater, exploring the environment looking for a way to escape. Exploration was considered an active behavior.
- (6)
- Angle during immobility. The angle (α) during immobility was defined as the position of the animal with respect to the water surface, as previously described by Chen et al., 2015 [31]. One frame was taken out from each video 5 min after the beginning of the test, at the first moment the rat stopped swimming and was in full profile view. The angle (α) was calculated by drawing a triangle between the water surface, the base of the rat tail and the intersection of the rat body with the water using Snip & Sketch tool on Microsoft Windows 10 Enterprise.
2.5. Biomarker Measurement
2.5.1. Sample Collection
2.5.2. Immunoblotting
2.5.3. ELISA
2.6. Statistical Analysis
3. Results
3.1. Experiment 1: FSS Behavioral Analysis
3.1.1. 3α,5α-THP Induced Submersion Behavior in Male and Female Rats Exposed to FSS
3.1.2. 3α,5α-THP Also Increased the Duration of Immobility in Male and Female Rats during the Swim Stress
3.1.3. 3α,5α-THP Affected the Position of the Body in Water during Immobility
3.1.4. 3α,5α-THP Decreased the Total Swimming Time in Male and Females Rats during FSS
3.1.5. 3α,5α-THP Did Not Affect Climbing in Male and Female Rats during FSS
3.1.6. 3α,5α-THP Reduced Exploring Behavior in Female, but Not in Male Rats
3.2. Experiment 2: Locomotor Activity Analysis
3α,5α-THP Did Not Affect Locomotor Activity in Male and Female Rats
3.3. Experiment 1: FSS Biochemical and Molecular Analysis
3.3.1. 3α,5α-THP Exacerbated the Increase in β-Endorphins Induced by FSS
3.3.2. Changes in β-EP Levels Are Induced Specifically by Swim Stress
3.3.3. 3α,5α-THP Altered MOP Receptors Differently, Depending on Sex and Type of Stress
3.4. Experiment 3: FSS Behavioral Analysis Following Mu Opioid Receptors Inhibition with CTAP Prior to 3α,5α-THP Administration
3.4.1. CTAP Administration Prior to 3α,5α-THP Treatment Prevented Submersion Behavior Induced by 3α,5α-THP in Male, but Not in Female Rats
3.4.2. The Higher Dose of CTAP Prior to 3α,5α-THP Administration Reduced the Number of Immobility Episodes but Increased the Immobility Time in Male and Female Rats
3.4.3. CTAP Administration Prior to 3α,5α-THP Treatment Did Not Affect the Body Position in the Water during Immobility
3.4.4. CTAP Following 3α,5α-THP Treatment Reduced Swimming Time in Both Male and Female Rats
3.4.5. CTAP High Dose Administration Prior to 3α,5α-THP Treatment Reduced Climbing Behavior in Male Rats, but Did Not Change the 3α,5α-THP-Induced Effect in Female Rats
3.4.6. CTAP Administration Prior to 3α,5α-THP Treatment Effect on Exploration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Beall, D. The isolation of progesterone and 3:20-allopregnanolone from ox adrenals. Biochem. J. 1938, 32, 1957–1960. [Google Scholar] [CrossRef] [Green Version]
- Meltzer-Brody, S.; Colquhoun, H.; Riesenberg, R.; Epperson, C.N.; Deligiannidis, K.M.; Rubinow, D.R.; Li, H.; Sankoh, A.J.; Clemson, C.; Schacterle, A.; et al. Brexanolone injection in post-partum depression: Two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 2018, 392, 1058–1070. [Google Scholar] [CrossRef]
- American Psychiatric Association. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: Dsm-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Boero, G.; Porcu, P.; Morrow, A.L. Pleiotropic actions of allopregnanolone underlie therapeutic benefits in stress-related disease. Neurobiol. Stress 2020, 12, 100203. [Google Scholar] [CrossRef]
- Owens, M.J.; Ritchie, J.C.; Nemeroff, C.B. 5a-Pregnane-3a,21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: Comparison with alprazolam. Brain Res. 1992, 573, 353–355. [Google Scholar] [CrossRef]
- Patchev, V.; B.V.Sc, A.H.; Holsboer, F.; Almeida, O. The Neurosteroid Tetrahydroprogesterone Attenuates the Endocrine Response to Stress and Exerts Glucocorticoid-like Effects on Vasopressin Gene Transcription in the Rat Hypothalamus. Neuropsychopharmacology 1996, 15, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Patchev, V.; Shoaib, M.; Holsboer, F.; Almeida, O. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 1994, 62, 265–271. [Google Scholar] [CrossRef]
- Balan, I.; Aurelian, L.; Schleicher, R.; Boero, G.; O’buckley, T.; Morrow, A.L. Neurosteroid allopregnanolone (3α,5α-THP) inhibits inflammatory signals induced by activated MyD88-dependent toll-like receptors. Transl. Psychiatry 2021, 11, 145. [Google Scholar] [CrossRef]
- Balan, I.; Beattie, M.C.; O’buckley, T.K.; Aurelian, L.; Morrow, A.L. Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain. Sci. Rep. 2019, 9, 1220. [Google Scholar] [CrossRef] [Green Version]
- Balan, I.; Aurelian, L.; Williams, K.S.; Campbell, B.; Meeker, R.B.; Morrow, A.L. Inhibition of human macrophage activation via pregnane neurosteroid interactions with toll-like receptors: Sex differences and structural requirements. Front. Immunol. 2022, 13, 940095. [Google Scholar] [CrossRef]
- Balan, I.; Patterson, R.; Boero, G.; Krohn, H.; O’Buckley, T.K.; Meltzer-Brody, S.; Morrow, A.L. Brexanolone therapeutics in post-partum depression involves inhibition of systemic inflammatory pathways. Ebiomedicine 2023, 89, 104473. [Google Scholar] [CrossRef]
- Boero, G.; Tyler, R.E.; Todd, C.A.; O’Buckley, T.K.; Balan, I.; Besheer, J.; Morrow, A.L. (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP) regulation of hypothalamic and extrahypothalamic corticotropin releasing factor (CRF): Sexual dimorphism and brain region specificity in Sprague Dawley rats. Neuropharmacology 2021, 186, 108463. [Google Scholar] [CrossRef]
- Boero, G.; Tyler, R.E.; O’buckley, T.K.; Balan, I.; Besheer, J.; Morrow, A.L. (3α,5α)3-Hydroxypregnan-20-one (3α,5α-THP) Regulation of the HPA Axis in the Context of Different Stressors and Sex. Biomolecules 2022, 12, 1134. [Google Scholar] [CrossRef]
- Crawley, J.N.; Glowa, J.R.; Majewska, M.D.; Paul, S.M. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986, 398, 382–385. [Google Scholar] [CrossRef]
- Devaud, L.L.; Morrow, A.L. Interactions between neuroactive steroids and ethanol at gabaa receptors: Effects of ethanol withdrawal. In Stress, Gender and Alcohol-Seeking Behavior, Niaaa Research Monograph No. 29; Hunt, W.A., Zakhari, S., Eds.; U.S. Gov. Printing Office: Washington, DC, USA, 1995; pp. 219–240. [Google Scholar]
- Mendelson, W.B.; Martin, J.V.; Perlis, M.; Wagner, R.; Majewska, M.D.; Paul, S.M. Sleep induction by an adrenal steroid in the rat. Psychopharmacology 1987, 93, 226–229. [Google Scholar] [CrossRef]
- Reddy, D.; Zeng, Y.-C. Differential anesthetic activity of ketamine and the GABAergic neurosteroid allopregnanolone in mice lacking progesterone receptor A and B subtypes. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Drolet, G.; Dumont, C.; Gosselin, I.; Kinkead, R.; Laforest, S.; Trottier, J.-F. Role of endogenous opioid system in the regulation of the stress response. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 729–741. [Google Scholar] [CrossRef]
- Constantopoulos, A.; Papadaki-Papandreou, U.; Papaconstantinou, E. Increased beta-endorphin but not Leu-enkephalin in plasma due to preoperative stress. Cell. Mol. Life Sci. 1995, 51, 16–18. [Google Scholar]
- Eberwine, J.H.; Roberts, J.L. Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J. Biol. Chem. 1984, 259, 2166–2170. [Google Scholar] [CrossRef]
- Hellbach, S.; Gärtner, P.; Deicke, J.; Fischer, D.; Hassan, A.H.S.; Almeida, O.F.X. Inherent glucocorticoid response potential of isolated hypothalamic neuroendocrine neurons. FASEB J. 1998, 12, 199–207. [Google Scholar] [CrossRef]
- Van Bockstaele, E.; Valentino, R.J. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology 2001, 158, 331–342. [Google Scholar] [CrossRef]
- Shields, G.S.; Sazma, M.A.; Yonelinas, A.P. The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neurosci. Biobehav. Rev. 2016, 68, 651–668. [Google Scholar] [CrossRef] [Green Version]
- Bandura, A.; Cioffi, D.; Taylor, C.B.; Brouillard, M.E. Perceived self-efficacy in coping with cognitive stressors and opioid activation. J. Pers. Soc. Psychol. 1988, 55, 479–488. [Google Scholar] [CrossRef]
- Laredo, S.A.; Steinman, M.Q.; Robles, C.F.; Ferrer, E.; Ragen, B.J.; Trainor, B.C. Effects of defeat stress on behavioral flexibility in males and females: Modulation by the mu-opioid receptor. Eur. J. Neurosci. 2015, 41, 434–441. [Google Scholar] [CrossRef]
- Lim, A.T.; Funder, J.W. Stress-Induced Changes in Plasma, Pituitary and Hypothalamic Immunoreactive β-Endorphin: Effects of Diurnal Variation, Adrenalectomy, Corticosteroids, and Opiate Agonists and Antagonists. Neuroendocrinology 1983, 36, 225–234. [Google Scholar] [CrossRef]
- Brunton, P.J.; McKay, A.J.; Ochędalski, T.; Piastowska, A.; Rębas, E.; Lachowicz, A.; Russell, J.A. Central Opioid Inhibition of Neuroendocrine Stress Responses in Pregnancy in the Rat Is Induced by the Neurosteroid Allopregnanolone. J. Neurosci. 2009, 29, 6449–6460. [Google Scholar] [CrossRef]
- Nequin, L.G.; Alvarez, J.; Schwartz, N.B. Measurement of Serum Steroid and Gonadotropin Levels and Uterine and Ovarian Variables throughout 4 Day and 5 Day Estrous Cycles in the Rat1. Biol. Reprod. 1979, 20, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.E. Neuroendocrine control of the ovarian cycle of the rat. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Neill, J.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 2327–2388. [Google Scholar]
- Bartlett, M.J.; So, L.Y.; Szabò, L.; Skinner, D.P.; Parent, K.L.; Heien, M.L.; Vanderah, T.W.; Polt, R.; Sherman, S.J.; Falk, T. Highly-selective µ-opioid receptor antagonism does not block L-DOPA-induced dyskinesia in a rodent model. BMC Res. Notes 2020, 13, 149. [Google Scholar] [CrossRef]
- Chen, L.; Faas, G.C.; Ferando, I.; Mody, I. Novel insights into the behavioral analysis of mice subjected to the forced-swim test. Transl. Psychiatry 2015, 5, e551. [Google Scholar] [CrossRef] [Green Version]
- Facchinetti, F.; Petraglia, F.; Genazzani, A.R. Localization and Expression of the Three Opioid Systems. Semin. Reprod. Med. 1987, 5, 103–113. [Google Scholar] [CrossRef]
- Khisti, R.T.; Chopde, C.T.; Jain, S.P. Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacol. Biochem. Behav. 2000, 67, 137–143. [Google Scholar] [CrossRef]
- Commons, K.G.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci. 2017, 8, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Dunn, A.J.; Swiergiel, A.H.; Palamarchouk, V. Brain Circuits Involved in Corticotropin-Releasing Factor-Norepinephrine Interactions during Stress. Ann. N. Y. Acad. Sci. 2004, 1018, 25–34. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.-D.; Zubieta, J.-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463. [Google Scholar] [CrossRef]
- Su, T.-P.; London, E.D.; Jaffe, J.H. Steroid Binding at σ Receptors Suggests a Link Between Endocrine, Nervous, and Immune Systems. Science 1988, 240, 219–221. [Google Scholar] [CrossRef]
- Maurice, T.; Phan, V.-L.; Urani, A.; Kamei, H.; Noda, Y.; Nabeshima, T. Neuroactive Neurosteroids as Endogenous Effectors for the Sigma1 (.SIGMA.1) Receptor. Pharmacological Evidence and Therapeutic Opportunities. Jpn. J. Pharmacol. 1999, 81, 125–155. [Google Scholar] [CrossRef] [PubMed]
- Monnet, F.P.; Mahé, V.; Robel, P.; Baulieu, E.E. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc. Natl. Acad. Sci. USA 1995, 92, 3774–3778. [Google Scholar] [CrossRef]
- Urani, A.; Privat, A.; Maurice, T. The modulation by neurosteroids of the scopolamine-induced learning impairment in mice involves an interaction with sigma1 (σ1) receptors. Brain Res. 1998, 799, 64–77. [Google Scholar] [CrossRef]
- Dawson-Basoa, M.E.; Gintzler, A.R. Estrogen and progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain 1996, 64, 608–615. [Google Scholar] [CrossRef]
- Piva, F.; Limonta, P.; Dondi, D.; Pimpinelli, F.; Martini, L.; Maggi, R. Effects of steroids on the brain opioid system. J. Steroid Biochem. Mol. Biol. 1995, 53, 343–348. [Google Scholar] [CrossRef]
- Dennerstein, L.; Morse, C.; Burrows, G.; Oats, J.; Brown, J.; Smith, M. Menstrual migraine: A double-blind trial of percutaneous estradiol. Gynecol. Endocrinol. 1988, 2, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, A.; Stomati, M.; Catarsi, S.; Genazzani, A.D.; Criscuolo, M.; Petraglia, F. Effects of sex steroid hormones on the neuroendocrine system. Eur. J. Contracept. Reprod. Health Care 1997, 2, 63–69. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Petraglia, F.; Facchinetti, F.; Facchini, V.; Volpe, A.; Alessandrini, G. Increase of proopiomelanocortin-related peptides during subjective menopausal flushes. Am. J. Obstet. Gynecol. 1984, 149, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Luisi, M.; Lenzi, E.; Centofanti, M.; Begliuomini, S.; Freschi, L.; Ninni, F.; Genazzani, A. Progesterone and progestins: Effects on brain, allopregnanolone and β-endorphin. J. Steroid Biochem. Mol. Biol. 2006, 102, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A. Prefrontal Cortical Opioids and Dysregulated Motivation: A Network Hypothesis. Trends Neurosci. 2016, 39, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Regier, P.S.; Claxton, A.B.; Zlebnik, N.E.; Carroll, M.E. Cocaine-, caffeine-, and stress-evoked cocaine reinstatement in high vs. low impulsive rats: Treatment with allopregnanolone. Drug Alcohol Depend. 2014, 143, 58–64. [Google Scholar] [CrossRef]
- Schmoutz, C.D.; Guerin, G.F.; Runyon, S.P.; Dhungana, S.; Goeders, N.E. A therapeutic combination of metyrapone and oxazepam increases brain levels of GABA-active neurosteroids and decreases cocaine self-administration in male rats. Behav. Brain Res. 2015, 291, 108–111. [Google Scholar] [CrossRef]
- Anker, J.J.; Holtz, N.A.; Zlebnik, N.; Carroll, M.E. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology 2009, 203, 63–72. [Google Scholar] [CrossRef]
- Anker, J.J.; Zlebnik, N.E.; Carroll, M.E. Differential effects of allopregnanolone on the escalation of cocaine self-administration and sucrose intake in female rats. Psychopharmacology 2010, 212, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Morrow, A.L.; Boero, G.; Porcu, P. A Rationale for Allopregnanolone Treatment of Alcohol Use Disorders: Basic and Clinical Studies. Alcohol. Clin. Exp. Res. 2020, 44, 320–339. [Google Scholar] [CrossRef]
- Besheer, J.; Lindsay, T.G.; O’buckley, T.K.; Hodge, C.W.; Morrow, A.L. Pregnenolone and Ganaxolone Reduce Operant Ethanol Self-Administration in Alcohol-Preferring P Rats. Alcohol. Clin. Exp. Res. 2010, 34, 2044–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ornelas, L.C.; Boero, G.; Van Voorhies, K.; O’Buckley, T.K.; Besheer, J.; Morrow, A.L. Pharmacological administration of 3α,5α-THP into the nucleus accumbens core increases 3α,5α-THP expression and reduces alcohol self-administration. Alcohol. Clin. Exp. Res. 2023, 47, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Milivojevic, V.; Charron, L.; Fogelman, N.; Hermes, G.; Sinha, R. Pregnenolone Reduces Stress-Induced Craving, Anxiety, and Autonomic Arousal in Individuals with Cocaine Use Disorder. Biomolecules 2022, 12, 1593. [Google Scholar] [CrossRef] [PubMed]
- Milivojevic, V.; Sullivan, L.; Tiber, J.; Fogelman, N.; Simpson, C.; Hermes, G.; Sinha, R. Pregnenolone effects on provoked alcohol craving, anxiety, HPA axis, and autonomic arousal in individuals with alcohol use disorder. Psychopharmacology 2022, 240, 101–114. [Google Scholar] [CrossRef] [PubMed]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boero, G.; McFarland, M.H.; Tyler, R.E.; O’Buckley, T.K.; Chéry, S.L.; Robinson, D.L.; Besheer, J.; Morrow, A.L. Deleterious Interaction between the Neurosteroid (3α,5α)3-Hydroxypregnan-20-One (3α,5α-THP) and the Mu-Opioid System Activation during Forced Swim Stress in Rats. Biomolecules 2023, 13, 1205. https://doi.org/10.3390/biom13081205
Boero G, McFarland MH, Tyler RE, O’Buckley TK, Chéry SL, Robinson DL, Besheer J, Morrow AL. Deleterious Interaction between the Neurosteroid (3α,5α)3-Hydroxypregnan-20-One (3α,5α-THP) and the Mu-Opioid System Activation during Forced Swim Stress in Rats. Biomolecules. 2023; 13(8):1205. https://doi.org/10.3390/biom13081205
Chicago/Turabian StyleBoero, Giorgia, Minna H. McFarland, Ryan E. Tyler, Todd K. O’Buckley, Samantha L. Chéry, Donita L. Robinson, Joyce Besheer, and A. Leslie Morrow. 2023. "Deleterious Interaction between the Neurosteroid (3α,5α)3-Hydroxypregnan-20-One (3α,5α-THP) and the Mu-Opioid System Activation during Forced Swim Stress in Rats" Biomolecules 13, no. 8: 1205. https://doi.org/10.3390/biom13081205
APA StyleBoero, G., McFarland, M. H., Tyler, R. E., O’Buckley, T. K., Chéry, S. L., Robinson, D. L., Besheer, J., & Morrow, A. L. (2023). Deleterious Interaction between the Neurosteroid (3α,5α)3-Hydroxypregnan-20-One (3α,5α-THP) and the Mu-Opioid System Activation during Forced Swim Stress in Rats. Biomolecules, 13(8), 1205. https://doi.org/10.3390/biom13081205





