The Neurobiological Underpinnings of Obsessive-Compulsive Symptoms in Psychosis, Translational Issues for Treatment-Resistant Schizophrenia
Abstract
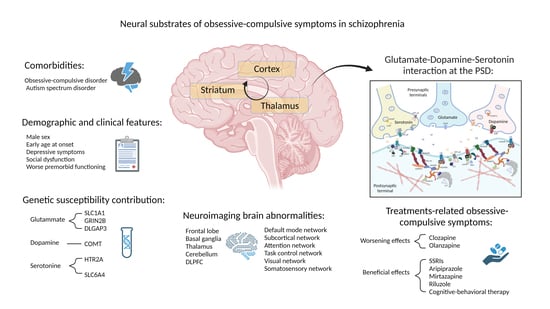
:1. Introduction
- (1)
- Do schizophrenia core symptoms (positive, negative, and cognitive) share common dysfunctional brain networks with OCD relevant to dopamine–glutamate–serotonin interplay?
- (2)
- Do schizophrenia and OCD pathophysiology share some presynaptic and postsynaptic mechanisms and do genetic findings support a potential overlapping?
- (3)
- How does dopamine–glutamate–serotonin interaction support translational research to identify new therapeutic strategies?
2. Obsessive-Compulsive Symptoms in Schizophrenia
2.1. Clinical Framework for Multiple Neurotransmitters’ Implication of OCS in Schizophrenia
2.2. Genetic Findings of OCD in Schizophrenia: Relevance for Serotonergic, Dopaminergic, and Glutamatergic Systems
2.3. Neuroimaging Studies: Points of Convergence between OCD and Schizophrenia, Potential Biological Underpinnings for OCS in Schizophrenia
2.4. Pharmacological Studies in Schizophrenia Patients with OCS Comorbidity: The Putative Connection between Serotoninergic, Dopaminergic, and Glutamatergic Neurotransmission
| Drug | Action on Neurotransmitter’s Pattern | Clinical Effects | Reference |
|---|---|---|---|
| Amisulpride | D2R antagonism | Reduction in OCS occurrence in schizophrenia patients | [95] |
| Aripiprazole | Serotonin and dopamine partial agonism | Neutral or anti-obsessive effects in patients treated with clozapine | [95,96,104] |
| Clozapine | Serotonin antagonism | Obsessional thinking and hoarding behavior, but not compulsions probably related to clozapine plasma concentration | [92,102] |
| Riluzole | Antiglutamatergic action | Anti-obsessive effects in treating refractory OCS | [124] |
3. Schizophrenia and OCS: Current Pathophysiology Hypotheses and Relevance for Glutamate, Dopamine, Serotonin, and Their Interplay
3.1. Neural Circuits
3.1.1. Glutamate and OCS in Schizophrenia
- -
- The affective circuit, from ventromedial-PFC and ACC to the nucleus accumbens (NAc) and the thalamus, relevant for affective and reward processing.
- -
- The dorsal cognitive circuit, from the dorsolateral-PFC to the caudate nucleus and the thalamus, relevant for executive functions.
- -
- The ventral cognitive circuit, from the anterolateral OFC to the anterior part of the putamen and thalamus, relevant for motor preparation and response inhibition [185].
3.1.2. Dopamine and Compulsions: Relevance for OCS in Schizophrenia
3.1.3. Serotonin and the Occurrence of Obsessive Symptoms in Schizophrenia
3.2. Neurotransmission Overlapping: Relevance for OCS in Schizophrenia
4. Postsynaptic Density: Implication for the Transdiagnostic Dimensions of Compulsions
4.1. SAPAP Proteins: Relevance for OCS in Schizophrenia
SAPAP3 and Its Involvement in Synaptic Plasticity and Repetitive Behavior
5. OCS in Schizophrenia, and Brain Neuromodulation in the Context of Dopamine–Glutamate–Serotonin Interaction
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poyurovsky, M.; Koran, L.M. Obsessive-compulsive disorder (OCD) with schizotypy vs. schizophrenia with OCD: Diagnostic dilemmas and therapeutic implications. J. Psychiatr. Res. 2005, 39, 399–408. [Google Scholar] [CrossRef]
- Poyurovsky, M.; Weizman, A.; Weizman, R. Obsessive-compulsive disorder in schizophrenia: Clinical characteristics and treatment. CNS Drugs 2004, 18, 989–1010. [Google Scholar] [CrossRef]
- Achim, A.M.; Maziade, M.; Raymond, E.; Olivier, D.; Mérette, C.; Roy, M.A. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr. Bull. 2011, 37, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Hadi, E.; Greenberg, Y.; Sirota, P. Obsessive-compulsive symptoms in schizophrenia: Prevalence, clinical features and treatment. A literature review. World J. Biol. Psychiatry 2012, 13, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.H.; Zarghami, M.; Moudi, S.; Mohammadpour, A.R. Frequency and severity of obsessive-compulsive symptoms/disorders, violence and suicidal in schizophrenic patients. Iran. Red Crescent Med. J. 2012, 14, 345–351. [Google Scholar] [PubMed]
- Sahoo, S.; Grover, S.; Nehra, R. Comparison of neurocognitive domains in patients with schizophrenia with and without co-morbid obsessive compulsive disorder. Schizophr. Res. 2018, 201, 151–158. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Chen, V.C.; Yang, Y.H.; Chen, K.J.; Lee, Y.C.; Lu, M.L. Risk of schizophrenia among people with obsessive-compulsive disorder: A nationwide population-based cohort study. Schizophr. Res. 2019, 209, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Iasevoli, F.; Giordano, S.; Balletta, R.; Latte, G.; Formato, M.V.; Prinzivalli, E.; De Berardis, D.; Tomasetti, C.; de Bartolomeis, A. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 65, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.R.; Raballo, A. Obsessive-compulsive symptoms in the schizophrenia-spectrum: Current developments in psychopathology research. Curr. Opin. Psychiatry 2023, 36, 166–171. [Google Scholar] [CrossRef]
- Zink, M. Comorbid Obsessive-Compulsive Symptoms in Schizophrenia: Insight into Pathomechanisms Facilitates Treatment. Adv. Med. 2014, 2014, 317980. [Google Scholar] [CrossRef]
- Meltzer, H.Y. Treatment-resistant schizophrenia—The role of clozapine. Curr. Med. Res. Opin. 1997, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Agid, O.; Baldwin, M.L.; Howes, O.; Lindenmayer, J.P.; Marder, S.; Olfson, M.; Potkin, S.G.; Correll, C.U. Clinical Guidance on the Identification and Management of Treatment-Resistant Schizophrenia. J. Clin. Psychiatry 2019, 80, 2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, J.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry 1988, 45, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Elkis, H.; Buckley, P.F. Treatment-Resistant Schizophrenia. Psychiatr. Clin. N. Am. 2016, 39, 239–265. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.; Agid, O.; de Bartolomeis, A.; van Beveren, N.J.; Birnbaum, M.L.; Bloomfield, M.A.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef]
- Tibbo, P.; Warneke, L. Obsessive-compulsive disorder in schizophrenia: Epidemiologic and biologic overlap. J. Psychiatry Neurosci. JPN 1999, 24, 15–24. [Google Scholar]
- Xue, J.; Qian, D.; Zhang, B.; Yang, J.; Li, W.; Bao, Y.; Qiu, S.; Fu, Y.; Wang, S.; Yuan, T.F.; et al. Midbrain dopamine neurons arbiter OCD-like behavior. Proc. Natl. Acad. Sci. USA 2022, 119, e2207545119. [Google Scholar] [CrossRef]
- Kariuki-Nyuthe, C.; Gomez-Mancilla, B.; Stein, D.J. Obsessive compulsive disorder and the glutamatergic system. Curr. Opin. Psychiatry 2014, 27, 32–37. [Google Scholar] [CrossRef]
- Stewart, S.E.; Mayerfeld, C.; Arnold, P.D.; Crane, J.R.; O’Dushlaine, C.; Fagerness, J.A.; Yu, D.; Scharf, J.M.; Chan, E.; Kassam, F.; et al. Meta-analysis of association between obsessive-compulsive disorder and the 3′ region of neuronal glutamate transporter gene SLC1A1. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2013, 162b, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Zaboski, B.A., 2nd; Merritt, O.A.; Schrack, A.P.; Gayle, C.; Gonzalez, M.; Guerrero, L.A.; Dueñas, J.A.; Soreni, N.; Mathews, C.A. Hoarding: A meta-analysis of age of onset. Depress. Anxiety 2019, 36, 552–564. [Google Scholar] [CrossRef]
- Schirmbeck, F.; Zink, M. Comorbid obsessive-compulsive symptoms in schizophrenia: Contributions of pharmacological and genetic factors. Front. Pharmacol. 2013, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Shioiri, T.; Shinada, K.; Kuwabara, H.; Someya, T. Early prodromal symptoms and diagnoses before first psychotic episode in 219 inpatients with schizophrenia. Psychiatry Clin. Neurosci. 2007, 61, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Niendam, T.A.; Berzak, J.; Cannon, T.D.; Bearden, C.E. Obsessive compulsive symptoms in the psychosis prodrome: Correlates of clinical and functional outcome. Schizophr. Res. 2009, 108, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Haan, L.; Sterk, B.; Wouters, L.; Linszen, D.H. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr. Bull. 2013, 39, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Fonseka, T.M.; Richter, M.A.; Müller, D.J. Second generation antipsychotic-induced obsessive-compulsive symptoms in schizophrenia: A review of the experimental literature. Curr. Psychiatry Rep. 2014, 16, 510. [Google Scholar] [CrossRef]
- Tiryaki, A.; Ozkorumak, E. Do the obsessive-compulsive symptoms have an effect in schizophrenia? Compr. Psychiatry 2010, 51, 357–362. [Google Scholar] [CrossRef]
- Kim, S.W.; Shin, I.S.; Kim, J.M.; Youn, T.; Yang, S.J.; Hwang, M.Y.; Yoon, J.S. The 5-HT2 receptor profiles of antipsychotics in the pathogenesis of obsessive-compulsive symptoms in schizophrenia. Clin. Neuropharmacol. 2009, 32, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Üçok, A.; Kıvrak Tihan, A.; Karadayı, G.; Tükel, R. Obsessive compulsive symptoms are related to lower quality of life in patients with Schizophrenia. Int. J. Psychiatry Clin. Pract. 2014, 18, 243–247. [Google Scholar] [CrossRef]
- Devi, S.; Rao, N.P.; Badamath, S.; Chandrashekhar, C.R.; Janardhan Reddy, Y.C. Prevalence and clinical correlates of obsessive-compulsive disorder in schizophrenia. Compr. Psychiatry 2015, 56, 141–148. [Google Scholar] [CrossRef]
- Cunill, R.; Huerta-Ramos, E.; Castells, X. The effect of obsessive-compulsive symptomatology on executive functions in schizophrenia: A systematic review and meta-analysis. Psychiatry Res. 2013, 210, 21–28. [Google Scholar] [CrossRef]
- Meier, S.M.; Petersen, L.; Pedersen, M.G.; Arendt, M.C.; Nielsen, P.R.; Mattheisen, M.; Mors, O.; Mortensen, P.B. Obsessive-compulsive disorder as a risk factor for schizophrenia: A nationwide study. JAMA Psychiatry 2014, 71, 1215–1221. [Google Scholar] [CrossRef] [Green Version]
- Bürgy, M. Obsession in the strict sense: A helpful psychopathological phenomenon in the differential diagnosis between obsessive-compulsive disorder and schizophrenia. Psychopathology 2007, 40, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Poyurovsky, M.; Faragian, S.; Pashinian, A.; Heidrach, L.; Fuchs, C.; Weizman, R.; Koran, L. Clinical characteristics of schizotypal-related obsessive-compulsive disorder. Psychiatry Res. 2008, 159, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.Y.; Morgan, J.E.; Losconzcy, M.F. Clinical and neuropsychological profiles of obsessive-compulsive schizophrenia: A pilot study. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Bottas, A.; Cooke, R.G.; Richter, M.A. Comorbidity and pathophysiology of obsessive-compulsive disorder in schizophrenia: Is there evidence for a schizo-obsessive subtype of schizophrenia? J. Psychiatry Neurosci. JPN 2005, 30, 187–193. [Google Scholar]
- Reznik, I.; Mester, R.; Kotler, M.; Weizman, A. Obsessive-compulsive schizophrenia: A new diagnostic entity? J. Neuropsychiatry Clin. Neurosci. 2001, 13, 115–116. [Google Scholar] [CrossRef]
- Reznik, I.; Kotler, M.; Weizman, A. Obsessive and compulsive symptoms in schizophrenia patients--from neuropsychology to clinical typology and classification. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 254–255. [Google Scholar] [CrossRef]
- Poyurovsky, M.; Zohar, J.; Glick, I.; Koran, L.M.; Weizman, R.; Tandon, R.; Weizman, A. Obsessive-compulsive symptoms in schizophrenia: Implications for future psychiatric classifications. Compr. Psychiatry 2012, 53, 480–483. [Google Scholar] [CrossRef]
- Oulis, P.; Konstantakopoulos, G.; Lykouras, L.; Michalopoulou, P.G. Differential diagnosis of obsessive-compulsive symptoms from delusions in schizophrenia: A phenomenological approach. World J. Psychiatry 2013, 3, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Joo, Y.H.; Nam, H.J.; Lim, M.; Cho, E.Y.; Jung, M.H.; Choi, J.S.; Kim, B.; Kang, D.H.; Oh, S.; et al. Association of the glutamate transporter gene SLC1A1 with atypical antipsychotics-induced obsessive-compulsive symptoms. Arch. Gen. Psychiatry 2009, 66, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.; Oh, S.; Cho, E.Y.; Nam, H.J.; Yoo, J.H.; Park, T.; Joo, Y.H.; Kwon, J.S.; Hong, K.S. Interaction between genetic variants of DLGAP3 and SLC1A1 affecting the risk of atypical antipsychotics-induced obsessive-compulsive symptoms. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2011, 156b, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.M.; Lu, J.; Rodriguiz, R.M.; Trotta, N.C.; Peca, J.; Ding, J.D.; Feliciano, C.; Chen, M.; Adams, J.P.; Luo, J.; et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 2007, 448, 894–900. [Google Scholar] [CrossRef] [Green Version]
- Shmelkov, S.V.; Hormigo, A.; Jing, D.; Proenca, C.C.; Bath, K.G.; Milde, T.; Shmelkov, E.; Kushner, J.S.; Baljevic, M.; Dincheva, I.; et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat. Med. 2010, 16, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Karayiorgou, M.; Altemus, M.; Galke, B.L.; Goldman, D.; Murphy, D.L.; Ott, J.; Gogos, J.A. Genotype determining low catechol-O-methyltransferase activity as a risk factor for obsessive-compulsive disorder. Proc. Natl. Acad. Sci. USA 1997, 94, 4572–4575. [Google Scholar] [CrossRef]
- Williams, H.J.; Owen, M.J.; O’Donovan, M.C. Is COMT a susceptibility gene for schizophrenia? Schizophr. Bull. 2007, 33, 635–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinopoli, V.M.; Burton, C.L.; Kronenberg, S.; Arnold, P.D. A review of the role of serotonin system genes in obsessive-compulsive disorder. Neurosci. Biobehav. Rev. 2017, 80, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Golimbet, V.; Korovaitseva, G.; Lezheiko, T.; Abramova, L.I.; Kaleda, V.G. The serotonin transporter gene 5-HTTLPR polymorphism is associated with affective psychoses but not with schizophrenia: A large-scale study in the Russian population. J. Affect. Disord. 2017, 208, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, D.J.; Lee, H.J.; Choi, J.E.; Kim, Y.K. No association of serotonin transporter polymorphism (5-HTTVNTR and 5-HTTLPR) with characteristics and treatment response to atypical antipsychotic agents in schizophrenic patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Terzić, T.; Kastelic, M.; Dolžan, V.; Plesničar, B.K. Influence of 5-HT1A and 5-HTTLPR genetic variants on the schizophrenia symptoms and occurrence of treatment-resistant schizophrenia. Neuropsychiatr. Dis. Treat. 2015, 11, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, S.H.; Akilli, A.; Bagcioglu, E.; Ozdemir Erdogan, M.; Coskun, K.S.; Alpaslan, A.H.; Subasi, B.; Arikan Terzi, E.S. Association of schizophrenia with T102C (rs6313) and 1438 A/G (rs6311) polymorphisms of HTR2A gene. Acta Neuropsychiatr. 2013, 25, 342–348. [Google Scholar] [CrossRef]
- Voyiaziakis, E.; Evgrafov, O.; Li, D.; Yoon, H.J.; Tabares, P.; Samuels, J.; Wang, Y.; Riddle, M.A.; Grados, M.A.; Bienvenu, O.J.; et al. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Mol. Psychiatry 2011, 16, 108–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.Z.; Lipsky, R.H.; Zhu, G.; Akhtar, L.A.; Taubman, J.; Greenberg, B.D.; Xu, K.; Arnold, P.D.; Richter, M.A.; Kennedy, J.L.; et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006, 78, 815–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatt, J.M.; Burton, K.L.; Williams, L.M.; Schofield, P.R. Specific and common genes implicated across major mental disorders: A review of meta-analysis studies. J. Psychiatr. Res. 2015, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, H.; Yue, W. Shared genetic loci and causal relations between schizophrenia and obsessive-compulsive disorder. Schizophrenia 2023, 9, 20. [Google Scholar] [CrossRef]
- Jagannathan, K.; Calhoun, V.D.; Gelernter, J.; Stevens, M.C.; Liu, J.; Bolognani, F.; Windemuth, A.; Ruaño, G.; Assaf, M.; Pearlson, G.D. Genetic associations of brain structural networks in schizophrenia: A preliminary study. Biol. Psychiatry 2010, 68, 657–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.L.; Wang, B.J.; Yao, J. Association between the SLC6A4 gene and schizophrenia: An updated meta-analysis. Neuropsychiatr. Dis. Treat. 2019, 15, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauls, D.L.; Abramovitch, A.; Rauch, S.L.; Geller, D.A. Obsessive-compulsive disorder: An integrative genetic and neurobiological perspective. Nat. Reviews. Neurosci. 2014, 15, 410–424. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, W.; Yi, Z.; Lu, W.; Wu, Z.; Chen, J.; Yu, S.; Fang, Y.; Zhang, C. Influence of polymorphisms in genes SLC1A1, GRIN2B, and GRIK2 on clozapine-induced obsessive-compulsive symptoms. Psychopharmacology 2013, 230, 49–55. [Google Scholar] [CrossRef]
- Venkatasubramanian, G.; Rao, N.P.; Behere, R.V. Neuroanatomical, neurochemical, and neurodevelopmental basis of obsessive-compulsive symptoms in schizophrenia. Indian J. Psychol. Med. 2009, 31, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Callicott, J.H.; Mattay, V.S.; Verchinski, B.A.; Marenco, S.; Egan, M.F.; Weinberger, D.R. Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. Am. J. Psychiatry 2003, 160, 2209–2215. [Google Scholar] [CrossRef]
- Bleich-Cohen, M.; Hendler, T.; Weizman, R.; Faragian, S.; Weizman, A.; Poyurovsky, M. Working memory dysfunction in schizophrenia patients with obsessive-compulsive symptoms: An fMRI study. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2014, 29, 160–166. [Google Scholar] [CrossRef]
- Bleich-Cohen, M.; Poyurovsky, M.; Hendler, T.; Weizman, R.; Sharon, H. Does co-morbid obsessive-compulsive disorder modify the abnormal language processing in schizophrenia patients? An FMRI study. Front. Hum. Neurosci. 2014, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.M.; Qiu, L.L.; Huang, H.X.; Zuo, X.; Zhou, Z.H.; Wang, S.; Liu, H.S.; Tian, L. Comparison of resting-state spontaneous brain activity between treatment-naive schizophrenia and obsessive-compulsive disorder. BMC Psychiatry 2021, 21, 544. [Google Scholar] [CrossRef] [PubMed]
- Luttenbacher, I.; Phillips, A.; Kazemi, R.; Hadipour, A.L.; Sanghvi, I.; Martinez, J.; Adamson, M.M. Transdiagnostic role of glutamate and white matter damage in neuropsychiatric disorders: A Systematic Review. J. Psychiatr. Res. 2022, 147, 324–348. [Google Scholar] [CrossRef]
- Rosenberg, D.R.; MacMaster, F.P.; Keshavan, M.S.; Fitzgerald, K.D.; Stewart, C.M.; Moore, G.J. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 1096–1103. [Google Scholar] [CrossRef]
- MacMaster, F.P.; O’Neill, J.; Rosenberg, D.R. Brain imaging in pediatric obsessive-compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 1262–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, B.P.; Rauch, S.L.; Jensen, J.E.; Pope, H.G., Jr. A critical review of magnetic resonance spectroscopy studies of obsessive-compulsive disorder. Biol. Psychiatry 2013, 73, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumitani, S.; Harada, M.; Kubo, H.; Ohmori, T. Proton magnetic resonance spectroscopy reveals an abnormality in the anterior cingulate of a subgroup of obsessive-compulsive disorder patients. Psychiatry Res. 2007, 154, 85–92. [Google Scholar] [CrossRef]
- Yücel, M.; Harrison, B.J.; Wood, S.J.; Fornito, A.; Wellard, R.M.; Pujol, J.; Clarke, K.; Phillips, M.L.; Kyrios, M.; Velakoulis, D.; et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch. Gen. Psychiatry 2007, 64, 946–955. [Google Scholar] [CrossRef] [Green Version]
- Adler, C.M.; McDonough-Ryan, P.; Sax, K.W.; Holland, S.K.; Arndt, S.; Strakowski, S.M. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J. Psychiatr. Res. 2000, 34, 317–324. [Google Scholar] [CrossRef]
- Bartha, R.; Stein, M.B.; Williamson, P.C.; Drost, D.J.; Neufeld, R.W.; Carr, T.J.; Canaran, G.; Densmore, M.; Anderson, G.; Siddiqui, A.R. A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. Am. J. Psychiatry 1998, 155, 1584–1591. [Google Scholar] [CrossRef]
- Pujol, J.; Soriano-Mas, C.; Alonso, P.; Cardoner, N.; Menchón, J.M.; Deus, J.; Vallejo, J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch. Gen. Psychiatry 2004, 61, 720–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perani, D.; Garibotto, V.; Gorini, A.; Moresco, R.M.; Henin, M.; Panzacchi, A.; Matarrese, M.; Carpinelli, A.; Bellodi, L.; Fazio, F. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. NeuroImage 2008, 42, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Schirmbeck, F.; Mier, D.; Esslinger, C.; Rausch, F.; Englisch, S.; Eifler, S.; Meyer-Lindenberg, A.; Kirsch, P.; Zink, M. Increased orbitofrontal cortex activation associated with “pro-obsessive” antipsychotic treatment in patients with schizophrenia. J. Psychiatry Neurosci. JPN 2015, 40, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deicken, R.F.; Zhou, L.; Schuff, N.; Weiner, M.W. Proton magnetic resonance spectroscopy of the anterior cingulate region in schizophrenia. Schizophr. Res. 1997, 27, 65–71. [Google Scholar] [CrossRef]
- Pittenger, C.; Bloch, M.H.; Williams, K. Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacol. Ther. 2011, 132, 314–332. [Google Scholar] [CrossRef] [Green Version]
- Karcher, N.R.; Rogers, B.P.; Woodward, N.D. Functional Connectivity of the Striatum in Schizophrenia and Psychotic Bipolar Disorder. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging 2019, 4, 956–965. [Google Scholar] [CrossRef]
- Arnold, P.D.; Macmaster, F.P.; Richter, M.A.; Hanna, G.L.; Sicard, T.; Burroughs, E.; Mirza, Y.; Easter, P.C.; Rose, M.; Kennedy, J.L.; et al. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Res. 2009, 172, 136–139. [Google Scholar] [CrossRef] [Green Version]
- Yücel, M.; Wood, S.J.; Wellard, R.M.; Harrison, B.J.; Fornito, A.; Pujol, J.; Velakoulis, D.; Pantelis, C. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust. N. Z. J. Psychiatry 2008, 42, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, D.R.; Mirza, Y.; Russell, A.; Tang, J.; Smith, J.M.; Banerjee, S.P.; Bhandari, R.; Rose, M.; Ivey, J.; Boyd, C.; et al. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 1146–1153. [Google Scholar] [CrossRef]
- Cavedini, P.; Bassi, T.; Zorzi, C.; Bellodi, L. The advantages of choosing antiobsessive therapy according to decision-making functioning. J. Clin. Psychopharmacol. 2004, 24, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Denys, D.; Zohar, J.; Westenberg, H.G. The role of dopamine in obsessive-compulsive disorder: Preclinical and clinical evidence. J. Clin. Psychiatry 2004, 65 (Suppl. 14), 11–17. [Google Scholar]
- Denys, D.; van der Wee, N.; Janssen, J.; De Geus, F.; Westenberg, H.G. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biol. Psychiatry 2004, 55, 1041–1045. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Li, Q.; Zhang, X.; Liu, L.; Yan, J.; Zhang, D.; Yan, H.; Yue, W. Altered Resting-State Brain Activity in Schizophrenia and Obsessive-Compulsive Disorder Compared With Non-psychiatric Controls: Commonalities and Distinctions Across Disorders. Front. Psychiatry 2021, 12, 681701. [Google Scholar] [CrossRef]
- Wang, Y.M.; Yang, Z.Y.; Cai, X.L.; Zhou, H.Y.; Zhang, R.T.; Yang, H.X.; Liang, Y.S.; Zhu, X.Z.; Madsen, K.H.; Sørensen, T.A.; et al. Identifying Schizo-Obsessive Comorbidity by Tract-Based Spatial Statistics and Probabilistic Tractography. Schizophr. Bull. 2020, 46, 442–453. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Diwadkar, V.; Rosenberg, D.R. Developmental biomarkers in schizophrenia and other psychiatric disorders: Common origins, different trajectories? Epidemiol. E Psichiatr. Soc. 2005, 14, 188–193. [Google Scholar] [CrossRef] [Green Version]
- de Bartolomeis, A.; Latte, G.; Tomasetti, C.; Iasevoli, F. Glutamatergic postsynaptic density protein dysfunctions in synaptic plasticity and dendritic spines morphology: Relevance to schizophrenia and other behavioral disorders pathophysiology, and implications for novel therapeutic approaches. Mol. Neurobiol. 2014, 49, 484–511. [Google Scholar] [CrossRef] [PubMed]
- Grados, M.A.; Specht, M.W.; Sung, H.M.; Fortune, D. Glutamate drugs and pharmacogenetics of OCD: A pathway-based exploratory approach. Expert Opin. Drug Discov. 2013, 8, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Haxby, J.V. Neuropsychological evaluation of adults with Down’s syndrome: Patterns of selective impairment in non-demented old adults. J. Ment. Defic. Res. 1989, 33 Pt 3, 193–210. [Google Scholar] [CrossRef]
- Khullar, A.; Chue, P.; Tibbo, P. Quetiapine and obsessive-compulsive symptoms (OCS): Case report and review of atypical antipsychotic-induced OCS. J. Psychiatry Neurosci. JPN 2001, 26, 55–59. [Google Scholar]
- Lykouras, L.; Alevizos, B.; Michalopoulou, P.; Rabavilas, A. Obsessive-compulsive symptoms induced by atypical antipsychotics. A review of the reported cases. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 333–346. [Google Scholar] [CrossRef]
- Lin, S.K.; Su, S.F.; Pan, C.H. Higher plasma drug concentration in clozapine-treated schizophrenic patients with side effects of obsessive/compulsive symptoms. Ther. Drug Monit. 2006, 28, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Scheltema Beduin, A.A.; Swets, M.; Machielsen, M.; Korver, N. Obsessive-compulsive symptoms in patients with schizophrenia: A naturalistic cross-sectional study comparing treatment with clozapine, olanzapine, risperidone, and no antipsychotics in 543 patients. J. Clin. Psychiatry 2012, 73, 1395–1402. [Google Scholar] [CrossRef]
- Schönfelder, S.; Schirmbeck, F.; Waltereit, R.; Englisch, S.; Zink, M. Aripiprazole improves olanzapine-associated obsessive compulsive symptoms in schizophrenia. Clin. Neuropharmacol. 2011, 34, 256–257. [Google Scholar] [CrossRef]
- Schirmbeck, F.; Esslinger, C.; Rausch, F.; Englisch, S.; Meyer-Lindenberg, A.; Zink, M. Antiserotonergic antipsychotics are associated with obsessive-compulsive symptoms in schizophrenia. Psychol. Med. 2011, 41, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- Schirmbeck, F.; Zink, M. Clozapine-induced obsessive-compulsive symptoms in schizophrenia: A critical review. Curr. Neuropharmacol. 2012, 10, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Sa, A.R.; Hounie, A.G.; Sampaio, A.S.; Arrais, J.; Miguel, E.C.; Elkis, H. Obsessive-compulsive symptoms and disorder in patients with schizophrenia treated with clozapine or haloperidol. Compr. Psychiatry 2009, 50, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Üçok, A.; Ceylan, M.E.; Tihan, A.K.; Lapçin, S.; Ger, C.; Tükel, R. Obsessive compulsive disorder and symptoms may have different effects on schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 429–433. [Google Scholar] [CrossRef]
- Fernandez-Egea, E.; Worbe, Y.; Bernardo, M.; Robbins, T.W. Distinct risk factors for obsessive and compulsive symptoms in chronic schizophrenia. Psychol. Med. 2018, 48, 2668–2675. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Baytunca, B.; Yu, X.; Öngür, D. Schizo-Obsessive Disorder: The Epidemiology, Diagnosis, and Treatment of Comorbid Schizophrenia and OCD. Curr. Treat. Options Psychiatry 2016, 3, 235–245. [Google Scholar] [CrossRef]
- Schirmbeck, F.; Rausch, F.; Englisch, S.; Eifler, S.; Esslinger, C.; Meyer-Lindenberg, A.; Zink, M. Differential effects of antipsychotic agents on obsessive-compulsive symptoms in schizophrenia: A longitudinal study. J. Psychopharmacol. 2013, 27, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Parkin, K.; Chen, S.; Biria, M.; Plaistow, J.; Beckwith, H.; Jarratt-Barnham, I.; Segarra, N.; Worbe, Y.; Fineberg, N.A.; Cardinal, R.N.; et al. Clozapine-related obsessive-compulsive symptoms and their impact on wellbeing: A naturalistic longitudinal study. Psychol. Med. 2022, 53, 2936–2945. [Google Scholar] [CrossRef]
- Englisch, S.; Esslinger, C.; Inta, D.; Weinbrenner, A.; Peus, V.; Gutschalk, A.; Schirmbeck, F.; Zink, M. Clozapine-induced obsessive-compulsive syndromes improve in combination with aripiprazole. Clin. Neuropharmacol. 2009, 32, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.D.; Barr, A.M.; Lu, C.; Stewart, S.E.; White, R.F.; Honer, W.G.; Procyshyn, R.M. Clozapine-Associated Obsessive-Compulsive Symptoms and Their Management: A Systematic Review and Analysis of 107 Reported Cases. Psychother. Psychosom. 2020, 89, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Stryjer, R.; Dambinsky, Y.; Timinsky, I.; Green, T.; Kotler, M.; Weizman, A.; Spivak, B. Escitalopram in the treatment of patients with schizophrenia and obsessive-compulsive disorder: An open-label, prospective study. Int. Clin. Psychopharmacol. 2013, 28, 96–98. [Google Scholar] [CrossRef]
- Biondi, M.; Fedele, L.; Arcangeli, T.; Pancheri, P. Development of obsessive-compulsive symptoms during clozapine treatment in schizophrenia and its positive response to clomipramine. Psychother. Psychosom. 1999, 68, 111–112. [Google Scholar] [CrossRef]
- Poyurovsky, M.; Hermesh, H.; Weizman, A. Fluvoxamine treatment in clozapine-induced obsessive-compulsive symptoms in schizophrenic patients. Clin. Neuropharmacol. 1996, 19, 305–313. [Google Scholar] [CrossRef]
- Buchanan, R.W.; Kirkpatrick, B.; Bryant, N.; Ball, P.; Breier, A. Fluoxetine augmentation of clozapine treatment in patients with schizophrenia. Am. J. Psychiatry 1996, 153, 1625–1627. [Google Scholar] [CrossRef]
- Hwang, M.Y.; Martin, A.M.; Lindenmayer, J.P.; Stein, D.; Hollander, E. Treatment of schizophrenia with obsessive-compulsive features with serotonin reuptake inhibitors. Am. J. Psychiatry 1993, 150, 1127. [Google Scholar] [CrossRef]
- Poyurovsky, M.; Isakov, V.; Hromnikov, S.; Modai, I.; Rauchberger, B.; Schneidman, M.; Weizman, A. Fluvoxamine treatment of obsessive-compulsive symptoms in schizophrenic patients: An add-on open study. Int. Clin. Psychopharmacol. 1999, 14, 95–100. [Google Scholar] [CrossRef]
- Gahr, M.; Rehbaum, K.; Connemann, B.J. Clozapine-associated development of second-onset obsessive compulsive symptoms in schizophrenia: Impact of clozapine serum levels and fluvoxamine add-on. Pharmacopsychiatry 2014, 47, 118–120. [Google Scholar] [CrossRef]
- Andrade, C. Serotonin reuptake inhibitor treatment of obsessive-compulsive symptoms in clozapine-medicated schizophrenia. J. Clin. Psychiatry 2012, 73, e1362–e1364. [Google Scholar] [CrossRef] [Green Version]
- Marazziti, D.; Hollander, E.; Lensi, P.; Ravagli, S.; Cassano, G.B. Peripheral markers of serotonin and dopamine function in obsessive-compulsive disorder. Psychiatry Res. 1992, 42, 41–51. [Google Scholar] [CrossRef]
- Bloch, M.H.; Landeros-Weisenberger, A.; Kelmendi, B.; Coric, V.; Bracken, M.B.; Leckman, J.F. A systematic review: Antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol. Psychiatry 2006, 11, 622–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poyurovsky, M.; Glick, I.; Koran, L.M. Lamotrigine augmentation in schizophrenia and schizoaffective patients with obsessive-compulsive symptoms. J. Psychopharmacol. 2010, 24, 861–866. [Google Scholar] [CrossRef]
- Juven-Wetzler, A.; Fostick, L.; Cwikel-Hamzany, S.; Balaban, E.; Zohar, J. Treatment with Ziprasidone for schizophrenia patients with OCD. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2014, 24, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.L.; Matz, J.; Schennach, R.; Müller, N.; Dehning, S. Ziprasidone for obsessive compulsive disorder in schizophrenia. Ther. Adv. Psychopharmacol. 2013, 3, 115–116. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Shin, I.S.; Kim, J.M.; Yang, S.J.; Hwang, M.Y.; Yoon, J.S. Amisulpride improves obsessive-compulsive symptoms in schizophrenia patients taking atypical antipsychotics: An open-label switch study. J. Clin. Psychopharmacol. 2008, 28, 349–352. [Google Scholar] [CrossRef]
- Glick, I.D.; Poyurovsky, M.; Ivanova, O.; Koran, L.M. Aripiprazole in schizophrenia patients with comorbid obsessive-compulsive symptoms: An open-label study of 15 patients. J. Clin. Psychiatry 2008, 69, 1856–1859. [Google Scholar] [CrossRef]
- Angelucci, F.; Ricci, V.; Martinotti, G.; Caltagirone, C.; Bria, P. Paliperidone for treatment of obsessive compulsive resistant symptoms in schizophrenia: A case report. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1277–1278. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.Y.; Yum, S.Y.; Losonczy, M.F.; Mitchell, G.; Kwon, J.S. Schizophrenia with obsessive compulsive features. Psychiatry 2006, 3, 34–41. [Google Scholar] [PubMed]
- Kokurcan, A.; Nazlı, Ş.B. Clinical correlates of obsessive-compulsive disorder comorbidity in patients with schizophrenia. Indian J. Psychiatry 2020, 62, 51–58. [Google Scholar] [CrossRef]
- MacCabe, J.H.; Marks, I.M.; Murray, R.M. Behavior therapy attenuates clozapine-induced obsessions and compulsions. J. Clin. Psychiatry 2002, 63, 1179–1180. [Google Scholar] [CrossRef] [Green Version]
- Coric, V.; Taskiran, S.; Pittenger, C.; Wasylink, S.; Mathalon, D.H.; Valentine, G.; Saksa, J.; Wu, Y.T.; Gueorguieva, R.; Sanacora, G.; et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: An open-label trial. Biol. Psychiatry 2005, 58, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Chakraborty, K. Glutamatergic dysfunction--newer targets for anti-obsessional drugs. Recent Pat. CNS Drug Discov. 2007, 2, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Baldwin, D.; Abelli, M.; Bolea-Alamanac, B.; Bourin, M.; Chamberlain, S.R.; Cinosi, E.; Davies, S.; Domschke, K.; Fineberg, N.; et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry 2017, 18, 162–214. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S. Disorder-specific genetic factors in obsessive-compulsive disorder: A comprehensive meta-analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171b, 325–332. [Google Scholar] [CrossRef]
- Kim, E.; Howes, O.D.; Park, J.W.; Kim, S.N.; Shin, S.A.; Kim, B.H.; Turkheimer, F.E.; Lee, Y.S.; Kwon, J.S. Altered serotonin transporter binding potential in patients with obsessive-compulsive disorder under escitalopram treatment: [11C]DASB PET study. Psychol. Med. 2016, 46, 357–366. [Google Scholar] [CrossRef]
- Nikolaus, S.; Antke, C.; Beu, M.; Müller, H.W. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders--results from in vivo imaging studies. Rev. Neurosci. 2010, 21, 119–139. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Reviews. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Goodman, W.K.; McDougle, C.J.; Price, L.H.; Riddle, M.A.; Pauls, D.L.; Leckman, J.F. Beyond the serotonin hypothesis: A role for dopamine in some forms of obsessive compulsive disorder? J. Clin. Psychiatry 1990, 51, 36–43. [Google Scholar] [PubMed]
- Ollmann, T.; Lénárd, L.; Péczely, L.; Berta, B.; Kertes, E.; Zagorácz, O.; Hormay, E.; László, K.; Szabó, Á.; Gálosi, R.; et al. Effect of D1- and D2-like Dopamine Receptor Antagonists on the Rewarding and Anxiolytic Effects of Neurotensin in the Ventral Pallidum. Biomedicines 2022, 10, 2104. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, G.; Chiappini, S.; Pettorruso, M.; Mosca, A.; Miuli, A.; Di Carlo, F.; D’Andrea, G.; Collevecchio, R.; Di Muzio, I.; Sensi, S.L.; et al. Therapeutic Potentials of Ketamine and Esketamine in Obsessive-Compulsive Disorder (OCD), Substance Use Disorders (SUD) and Eating Disorders (ED): A Review of the Current Literature. Brain Sci. 2021, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Vincent, J.P.; Mazella, J. Involvement of the neurotensin receptor-3 in the neurotensin-induced migration of human microglia. J. Neurosci. 2003, 23, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Olver, J.S.; O’Keefe, G.; Jones, G.R.; Burrows, G.D.; Tochon-Danguy, H.J.; Ackermann, U.; Scott, A.M.; Norman, T.R. Dopamine D(1) receptor binding in the anterior cingulate cortex of patients with obsessive-compulsive disorder. Psychiatry Res. 2010, 183, 85–88. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Khanna, S.; Chakrabarty, K.; Mahadevan, A.; Christopher, R.; Shankar, S.K. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology 2009, 34, 2489–2496. [Google Scholar] [CrossRef] [Green Version]
- Collaborative, I.O.C.D.F.G. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol. Psychiatry 2018, 23, 1181–1188. [Google Scholar] [CrossRef] [Green Version]
- Poltavskaya, E.G.; Fedorenko, O.Y.; Kornetova, E.G.; Loonen, A.J.M.; Kornetov, A.N.; Bokhan, N.A.; Ivanova, S.A. Study of Early Onset Schizophrenia: Associations of GRIN2A and GRIN2B Polymorphisms. Life 2021, 11, 997. [Google Scholar] [CrossRef]
- Li, J.M.; Lu, C.L.; Cheng, M.C.; Luu, S.U.; Hsu, S.H.; Chen, C.H. Genetic analysis of the DLGAP1 gene as a candidate gene for schizophrenia. Psychiatry Res. 2013, 205, 13–17. [Google Scholar] [CrossRef]
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B., Jr.; Charney, D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 1994, 51, 199–214. [Google Scholar] [CrossRef]
- Javitt, D.C.; Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 1991, 148, 1301–1308. [Google Scholar] [CrossRef]
- Kim, J.S.; Kornhuber, H.H.; Schmid-Burgk, W.; Holzmüller, B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci. Lett. 1980, 20, 379–382. [Google Scholar] [CrossRef]
- Yui, K.; Ikemoto, S.; Ishiguro, T.; Goto, K. Studies of amphetamine or methamphetamine psychosis in Japan: Relation of methamphetamine psychosis to schizophrenia. Ann. N. Y. Acad. Sci. 2000, 914, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, G.K.; Marek, G.J. Serotonin model of schizophrenia: Emerging role of glutamate mechanisms. Brain Res. Brain Res. Rev. 2000, 31, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Remington, G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am. J. Psychiatry 1996, 153, 466–476. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; Ciccarelli, M.; De Simone, G.; Mazza, B.; Barone, A.; Vellucci, L. Canonical and Non-Canonical Antipsychotics’ Dopamine-Related Mechanisms of Present and Next Generation Molecules: A Systematic Review on Translational Highlights for Treatment Response and Treatment-Resistant Schizophrenia. Int. J. Mol. Sci. 2023, 24, 5945. [Google Scholar] [CrossRef]
- Stahl, S.M. Beyond the dopamine hypothesis to the NMDA glutamate receptor hypofunction hypothesis of schizophrenia. CNS Spectr. 2007, 12, 265–268. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Buonaguro, E.F.; Iasevoli, F. Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: From receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology 2013, 225, 1–19. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; De Simone, G.; Ciccarelli, M.; Castiello, A.; Mazza, B.; Vellucci, L.; Barone, A. Antipsychotics-Induced Changes in Synaptic Architecture and Functional Connectivity: Translational Implications for Treatment Response and Resistance. Biomedicines 2022, 10, 3183. [Google Scholar] [CrossRef]
- Cepeda, C.; Buchwald, N.A.; Levine, M.S. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc. Natl. Acad. Sci. USA 1993, 90, 9576–9580. [Google Scholar] [CrossRef]
- Cepeda, C.; Levine, M.S. Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev. Neurosci. 1998, 20, 1–18. [Google Scholar] [CrossRef]
- Parent, A.; Hazrati, L.N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Brain Res. Rev. 1995, 20, 91–127. [Google Scholar] [CrossRef] [PubMed]
- Gurney, K.; Prescott, T.J.; Redgrave, P. A computational model of action selection in the basal ganglia. I. A new functional anatomy. Biol. Cybern. 2001, 84, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, C.C.; Miller, P.E.; Hemmings, H.C., Jr.; Walaas, S.I.; Greengard, P. DARPP-32, a dopamine- and adenosine 3’:5’-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J. Neurosci. 1984, 4, 111–124. [Google Scholar] [CrossRef]
- Ouimet, C.C.; Langley-Gullion, K.C.; Greengard, P. Quantitative immunocytochemistry of DARPP-32-expressing neurons in the rat caudatoputamen. Brain Res. 1998, 808, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Farhan, M.; Xu, J.; Lazarovici, P.; Zheng, W. The involvement of DARPP-32 in the pathophysiology of schizophrenia. Oncotarget 2017, 8, 53791–53803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonito-Oliva, A.; DuPont, C.; Madjid, N.; Ögren, S.O.; Fisone, G. Involvement of the Striatal Medium Spiny Neurons of the Direct Pathway in the Motor Stimulant Effects of Phencyclidine. Int. J. Neuropsychopharmacol. 2016, 19, pyv134. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, M.; Mizukami, K.; Iwakiri, M.; Asada, T. Immunohistochemical and immunoblot analysis of Dopamine and cyclic AMP-regulated phosphoprotein, relative molecular mass 32,000 (DARPP-32) in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1177–1181. [Google Scholar] [CrossRef]
- Kunii, Y.; Yabe, H.; Wada, A.; Yang, Q.; Nishiura, K.; Niwa, S. Altered DARPP-32 expression in the superior temporal gyrus in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1139–1143. [Google Scholar] [CrossRef]
- Pozzi, L.; Håkansson, K.; Usiello, A.; Borgkvist, A.; Lindskog, M.; Greengard, P.; Fisone, G. Opposite regulation by typical and atypical anti-psychotics of ERK1/2, CREB and Elk-1 phosphorylation in mouse dorsal striatum. J. Neurochem. 2003, 86, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Valjent, E.; Bertran-Gonzalez, J.; Bowling, H.; Lopez, S.; Santini, E.; Matamales, M.; Bonito-Oliva, A.; Hervé, D.; Hoeffer, C.; Klann, E.; et al. Haloperidol regulates the state of phosphorylation of ribosomal protein S6 via activation of PKA and phosphorylation of DARPP-32. Neuropsychopharmacology 2011, 36, 2561–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Novère, N.; Li, L.; Girault, J.A. DARPP-32: Molecular integration of phosphorylation potential. Cell. Mol. Life Sci. CMLS 2008, 65, 2125–2127. [Google Scholar] [CrossRef] [PubMed]
- Walaas, S.I.; Aswad, D.W.; Greengard, P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature 1983, 301, 69–71. [Google Scholar] [CrossRef] [PubMed]
- King, M.M.; Huang, C.Y.; Chock, P.B.; Nairn, A.C.; Hemmings, H.C., Jr.; Chan, K.F.; Greengard, P. Mammalian brain phosphoproteins as substrates for calcineurin. J. Biol. Chem. 1984, 259, 8080–8083. [Google Scholar] [CrossRef]
- Halpain, S.; Girault, J.A.; Greengard, P. Activation of NMDA receptors induces dephosphorylation of DARPP-32 in rat striatal slices. Nature 1990, 343, 369–372. [Google Scholar] [CrossRef]
- Paul, S.; Snyder, G.L.; Yokakura, H.; Picciotto, M.R.; Nairn, A.C.; Lombroso, P.J. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J. Neurosci. 2000, 20, 5630–5638. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Kurup, P.; Nairn, A.C.; Lombroso, P.J. Synaptic NMDA Receptor Activation Induces Ubiquitination and Degradation of STEP(61). Mol. Neurobiol. 2018, 55, 3096–3111. [Google Scholar] [CrossRef]
- Sukoff Rizzo, S.J.; Lotarski, S.M.; Stolyar, P.; McNally, T.; Arturi, C.; Roos, M.; Finley, J.E.; Reinhart, V.; Lanz, T.A. Behavioral characterization of striatal-enriched protein tyrosine phosphatase (STEP) knockout mice. Genes Brain Behav. 2014, 13, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Szlachta, M.; Kuśmider, M.; Pabian, P.; Solich, J.; Kolasa, M.; Żurawek, D.; Dziedzicka-Wasylewska, M.; Faron-Górecka, A. Repeated Clozapine Increases the Level of Serotonin 5-HT(1A)R Heterodimerization with 5-HT(2A) or Dopamine D(2) Receptors in the Mouse Cortex. Front. Mol. Neurosci. 2018, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Marcellino, D.; Ciruela, F.; Agnati, L.F.; Fuxe, K. Dopamine D2 and 5-hydroxytryptamine 5-HT(2A) receptors assemble into functionally interacting heteromers. Biochem. Biophys. Res. Commun. 2010, 401, 605–610. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Narvaez, M.; Oflijan, J.; Agnati, L.F.; Fuxe, K. Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes. Biochem. Biophys. Res. Commun. 2014, 443, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, S.; Polit, A.; Kędracka-Krok, S.; Wędzony, K.; Maćkowiak, M.; Dziedzicka-Wasylewska, M. Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim. Biophys. Acta 2010, 1803, 1347–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bartolomeis, A.; Vellucci, L.; Barone, A.; Manchia, M.; De Luca, V.; Iasevoli, F.; Correll, C.U. Clozapine’s multiple cellular mechanisms: What do we know after more than fifty years? A systematic review and critical assessment of translational mechanisms relevant for innovative strategies in treatment-resistant schizophrenia. Pharmacol. Ther. 2022, 236, 108236. [Google Scholar] [CrossRef]
- Maroteaux, L.; Béchade, C.; Roumier, A. Dimers of serotonin receptors: Impact on ligand affinity and signaling. Biochimie 2019, 161, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łukasiewicz, S.; Faron-Górecka, A.; Kędracka-Krok, S.; Dziedzicka-Wasylewska, M. Effect of clozapine on the dimerization of serotonin 5-HT(2A) receptor and its genetic variant 5-HT(2A)H425Y with dopamine D(2) receptor. Eur. J. Pharmacol. 2011, 659, 114–123. [Google Scholar] [CrossRef]
- Fribourg, M.; Moreno, J.L.; Holloway, T.; Provasi, D.; Baki, L.; Mahajan, R.; Park, G.; Adney, S.K.; Hatcher, C.; Eltit, J.M.; et al. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 2011, 147, 1011–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreault, M.L.; Hasbi, A.; Alijaniaram, M.; Fan, T.; Varghese, G.; Fletcher, P.J.; Seeman, P.; O’Dowd, B.F.; George, S.R. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: Increased high affinity state following amphetamine and in schizophrenia. J. Biol. Chem. 2010, 285, 36625–36634. [Google Scholar] [CrossRef] [Green Version]
- Hasbi, A.; O’Dowd, B.F.; George, S.R. Dopamine D1-D2 receptor heteromer signaling pathway in the brain: Emerging physiological relevance. Mol. Brain 2011, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Kruse, M.S.; Prémont, J.; Krebs, M.O.; Jay, T.M. Interaction of dopamine D1 with NMDA NR1 receptors in rat prefrontal cortex. Eur. Neuropsychopharmacol. 2009, 19, 296–304. [Google Scholar] [CrossRef]
- Laruelle, M.; Frankle, W.G.; Narendran, R.; Kegeles, L.S.; Abi-Dargham, A. Mechanism of action of antipsychotic drugs: From dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin. Ther. 2005, 27, S16–S24. [Google Scholar] [CrossRef]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Obeso, I.; Rothwell, J.C.; Obeso, J.A. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat. Reviews. Neurosci. 2015, 16, 719–732. [Google Scholar] [CrossRef]
- Maia, T.V.; Frank, M.J. From reinforcement learning models to psychiatric and neurological disorders. Nat. Neurosci. 2011, 14, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Mataix-Cols, D.; van den Heuvel, O.A. Common and distinct neural correlates of obsessive-compulsive and related disorders. Psychiatr. Clin. N. Am. 2006, 29, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Milad, M.R.; Rauch, S.L. Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn. Sci. 2012, 16, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APA. Diagnostic And Statistical Manual Of Mental Disorders: DSM-5-TR (Fifth Edition, Text Revision); American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Ashby, F.G.; Turner, B.O.; Horvitz, J.C. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn. Sci. 2010, 14, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Thorn, C.A.; Atallah, H.; Howe, M.; Graybiel, A.M. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron 2010, 66, 781–795. [Google Scholar] [CrossRef] [Green Version]
- Balleine, B.W.; Liljeholm, M.; Ostlund, S.B. The integrative function of the basal ganglia in instrumental conditioning. Behav. Brain Res. 2009, 199, 43–52. [Google Scholar] [CrossRef]
- Barnes, T.D.; Kubota, Y.; Hu, D.; Jin, D.Z.; Graybiel, A.M. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 2005, 437, 1158–1161. [Google Scholar] [CrossRef]
- Graybiel, A.M. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 2008, 31, 359–387. [Google Scholar] [CrossRef] [Green Version]
- White, N.M. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behav. Brain Res. 2009, 199, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, H.J.; van den Heuvel, O.A.; Cath, D.C.; Voorn, P.; Veltman, D.J. Does an imbalance between the dorsal and ventral striatopallidal systems play a role in Tourette’s syndrome? A neuronal circuit approach. Brain Dev. 2003, 25, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, O.A.; Veltman, D.J.; Groenewegen, H.J.; Dolan, R.J.; Cath, D.C.; Boellaard, R.; Mesina, C.T.; van Balkom, A.J.; van Oppen, P.; Witter, M.P.; et al. Amygdala activity in obsessive-compulsive disorder with contamination fear: A study with oxygen-15 water positron emission tomography. Psychiatry Res. 2004, 132, 225–237. [Google Scholar] [CrossRef] [Green Version]
- van den Heuvel, O.A.; Veltman, D.J.; Groenewegen, H.J.; Witter, M.P.; Merkelbach, J.; Cath, D.C.; van Balkom, A.J.; van Oppen, P.; van Dyck, R. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch. Gen. Psychiatry 2005, 62, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Adler, N.; Kaufmann, C.; Kathmann, N. Amygdala hyperactivation during symptom provocation in obsessive-compulsive disorder and its modulation by distraction. NeuroImage. Clin. 2014, 4, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.; Kaufmann, C.; Müsch, K.; Kischkel, E.; Kathmann, N. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology 2010, 47, 728–738. [Google Scholar] [CrossRef]
- de Wit, S.J.; van der Werf, Y.D.; Mataix-Cols, D.; Trujillo, J.P.; van Oppen, P.; Veltman, D.J.; van den Heuvel, O.A. Emotion regulation before and after transcranial magnetic stimulation in obsessive compulsive disorder. Psychol. Med. 2015, 45, 3059–3073. [Google Scholar] [CrossRef] [Green Version]
- Cardoner, N.; Harrison, B.J.; Pujol, J.; Soriano-Mas, C.; Hernández-Ribas, R.; López-Solá, M.; Real, E.; Deus, J.; Ortiz, H.; Alonso, P.; et al. Enhanced brain responsiveness during active emotional face processing in obsessive compulsive disorder. World J. Biol. Psychiatry 2011, 12, 349–363. [Google Scholar] [CrossRef]
- Via, E.; Cardoner, N.; Pujol, J.; Alonso, P.; López-Solà, M.; Real, E.; Contreras-Rodríguez, O.; Deus, J.; Segalàs, C.; Menchón, J.M.; et al. Amygdala activation and symptom dimensions in obsessive-compulsive disorder. Br. J. Psychiatry J. Ment. Sci. 2014, 204, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Geller, D.A.; Biederman, J.; Griffin, S.; Jones, J.; Lefkowitz, T.R. Comorbidity of juvenile obsessive-compulsive disorder with disruptive behavior disorders. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1637–1646. [Google Scholar] [CrossRef]
- de Wit, S.J.; Alonso, P.; Schweren, L.; Mataix-Cols, D.; Lochner, C.; Menchón, J.M.; Stein, D.J.; Fouche, J.P.; Soriano-Mas, C.; Sato, J.R.; et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am. J. Psychiatry 2014, 171, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.L.; Diehl, C.; Schleifer, C.; Tamminga, C.A.; Keshavan, M.S.; Sweeney, J.A.; Clementz, B.A.; Hill, S.K.; Pearlson, G.; Yang, G.; et al. Schizophrenia Exhibits Bi-directional Brain-Wide Alterations in Cortico-Striato-Cerebellar Circuits. Cereb. Cortex 2019, 29, 4463–4487. [Google Scholar] [CrossRef]
- Welsh, R.C.; Chen, A.C.; Taylor, S.F. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr. Bull. 2010, 36, 713–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, N.D.; Karbasforoushan, H.; Heckers, S. Thalamocortical dysconnectivity in schizophrenia. Am. J. Psychiatry 2012, 169, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Anticevic, A.; Cole, M.W.; Repovs, G.; Murray, J.D.; Brumbaugh, M.S.; Winkler, A.M.; Savic, A.; Krystal, J.H.; Pearlson, G.D.; Glahn, D.C. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb. Cortex 2014, 24, 3116–3130. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Zhong, M.; Zhu, X.; Gan, J.; Liu, W.; Niu, C.; Liao, H.; Zhang, H.; Yi, J.; Tan, C. Resting-state functional connectivity between right anterior insula and right orbital frontal cortex correlate with insight level in obsessive-compulsive disorder. NeuroImage. Clin. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Szalisznyó, K.; Silverstein, D.N.; Tóth, J. Neural dynamics in co-morbid schizophrenia and OCD: A computational approach. J. Theor. Biol. 2019, 473, 80–94. [Google Scholar] [CrossRef]
- Ghahremani, D.G.; Lee, B.; Robertson, C.L.; Tabibnia, G.; Morgan, A.T.; De Shetler, N.; Brown, A.K.; Monterosso, J.R.; Aron, A.R.; Mandelkern, M.A.; et al. Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 7316–7324. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, R.C.; Gleich, T.; Buchert, R.; Schlagenhauf, F.; Kühn, S.; Gallinat, J. Interactions between glutamate, dopamine, and the neuronal signature of response inhibition in the human striatum. Hum. Brain Mapp. 2015, 36, 4031–4040. [Google Scholar] [CrossRef] [Green Version]
- de Bartolomeis, A.; Vellucci, L.; De Simone, G.; Mazza, B.; Barone, A.; Ciccarelli, M. Dysregulated Signaling at Postsynaptic Density: A Systematic Review and Translational Appraisal for the Pathophysiology, Clinics, and Antipsychotics’ Treatment of Schizophrenia. Cells 2023, 12, 574. [Google Scholar] [CrossRef]
- Tomasetti, C.; Iasevoli, F.; Buonaguro, E.F.; De Berardis, D.; Fornaro, M.; Fiengo, A.L.; Martinotti, G.; Orsolini, L.; Valchera, A.; Di Giannantonio, M.; et al. Treating the Synapse in Major Psychiatric Disorders: The Role of Postsynaptic Density Network in Dopamine-Glutamate Interplay and Psychopharmacologic Drugs Molecular Actions. Int. J. Mol. Sci. 2017, 18, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreault, M.L.; Graham, D.; Scattolon, S.; Wang, Y.; Szechtman, H.; Foster, J.A. Cotreatment with the kappa opioid agonist U69593 enhances locomotor sensitization to the D2/D3 dopamine agonist quinpirole and alters dopamine D2 receptor and prodynorphin mRNA expression in rats. Psychopharmacology 2007, 194, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Eilam, D.; Szechtman, H. Biphasic effect of D-2 agonist quinpirole on locomotion and movements. Eur. J. Pharmacol. 1989, 161, 151–157. [Google Scholar] [CrossRef]
- Usiello, A.; Baik, J.H.; Rougé-Pont, F.; Picetti, R.; Dierich, A.; LeMeur, M.; Piazza, P.V.; Borrelli, E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature 2000, 408, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Szechtman, H.; Talangbayan, H.; Eilam, D. Environmental and behavioral components of sensitization induced by the dopamine agonist quinpirole. Behav. Pharmacol. 1993, 4, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Szechtman, H.; Talangbayan, H.; Canaran, G.; Dai, H.; Eilam, D. Dynamics of behavioral sensitization induced by the dopamine agonist quinpirole and a proposed central energy control mechanism. Psychopharmacology 1994, 115, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Szechtman, H.; Culver, K.; Eilam, D. Role of dopamine systems in obsessive-compulsive disorder (OCD): Implications from a novel psychostimulant-induced animal model. Pol. J. Pharmacol. 1999, 51, 55–61. [Google Scholar]
- Szechtman, H.; Eckert, M.J.; Tse, W.S.; Boersma, J.T.; Bonura, C.A.; McClelland, J.Z.; Culver, K.E.; Eilam, D. Compulsive checking behavior of quinpirole-sensitized rats as an animal model of Obsessive-Compulsive Disorder(OCD): Form and control. BMC Neurosci. 2001, 2, 4. [Google Scholar] [CrossRef]
- Szechtman, H.; Ahmari, S.E.; Beninger, R.J.; Eilam, D.; Harvey, B.H.; Edemann-Callesen, H.; Winter, C. Obsessive-compulsive disorder: Insights from animal models. Neurosci. Biobehav. Rev. 2017, 76 Pt B, 254–279. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Song, Z.; Tian, Y.; Tian, W.; Zhu, C.; Ji, G.; Luo, Y.; Chen, S.; Wang, L.; Mao, Y.; et al. Basolateral amygdala input to the medial prefrontal cortex controls obsessive-compulsive disorder-like checking behavior. Proc. Natl. Acad. Sci. USA 2019, 116, 3799–3804. [Google Scholar] [CrossRef] [Green Version]
- Asaoka, N.; Nishitani, N.; Kinoshita, H.; Nagai, Y.; Hatakama, H.; Nagayasu, K.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. An Adenosine A(2A) Receptor Antagonist Improves Multiple Symptoms of Repeated Quinpirole-Induced Psychosis. eNeuro 2019, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar, A.D.P.; Casanova, J.P.; Andrés, M.E.; Fuentealba, J.A. Crosstalk Between Kappa Opioid and Dopamine Systems in Compulsive Behaviors. Front. Pharmacol. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejeda, H.A.; Wu, J.; Kornspun, A.R.; Pignatelli, M.; Kashtelyan, V.; Krashes, M.J.; Lowell, B.B.; Carlezon, W.A., Jr.; Bonci, A. Pathway- and Cell-Specific Kappa-Opioid Receptor Modulation of Excitation-Inhibition Balance Differentially Gates D1 and D2 Accumbens Neuron Activity. Neuron 2017, 93, 147–163. [Google Scholar] [CrossRef] [Green Version]
- Koeltzow, T.E.; Austin, J.D.; Vezina, P. Behavioral sensitization to quinpirole is not associated with increased nucleus accumbens dopamine overflow. Neuropharmacology 2003, 44, 102–110. [Google Scholar] [CrossRef]
- Escobar, A.P.; Cornejo, F.A.; Olivares-Costa, M.; González, M.; Fuentealba, J.A.; Gysling, K.; España, R.A.; Andrés, M.E. Reduced dopamine and glutamate neurotransmission in the nucleus accumbens of quinpirole-sensitized rats hints at inhibitory D2 autoreceptor function. J. Neurochem. 2015, 134, 1081–1090. [Google Scholar] [CrossRef] [Green Version]
- Sesia, T.; Bizup, B.; Grace, A.A. Evaluation of animal models of obsessive-compulsive disorder: Correlation with phasic dopamine neuron activity. Int. J. Neuropsychopharmacol. 2013, 16, 1295–1307. [Google Scholar] [CrossRef] [Green Version]
- Culver, K.E.; Szechtman, H.; Levant, B. Altered dopamine D2-like receptor binding in rats with behavioral sensitization to quinpirole: Effects of pre-treatment with Ro 41-1049. Eur. J. Pharmacol. 2008, 592, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.; Ahmari, S.E. A Framework for Understanding the Emerging Role of Corticolimbic-Ventral Striatal Networks in OCD-Associated Repetitive Behaviors. Front. Syst. Neurosci. 2015, 9, 171. [Google Scholar] [CrossRef] [Green Version]
- Zike, I.D.; Chohan, M.O.; Kopelman, J.M.; Krasnow, E.N.; Flicker, D.; Nautiyal, K.M.; Bubser, M.; Kellendonk, C.; Jones, C.K.; Stanwood, G.; et al. OCD candidate gene SLC1A1/EAAT3 impacts basal ganglia-mediated activity and stereotypic behavior. Proc. Natl. Acad. Sci. USA 2017, 114, 5719–5724. [Google Scholar] [CrossRef]
- Denys, D.; de Vries, F.; Cath, D.; Figee, M.; Vulink, N.; Veltman, D.J.; van der Doef, T.F.; Boellaard, R.; Westenberg, H.; van Balkom, A.; et al. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 2013, 23, 1423–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klanker, M.; Feenstra, M.; Denys, D. Dopaminergic control of cognitive flexibility in humans and animals. Front. Neurosci. 2013, 7, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, C.J.; Zuccolo, A.J.; Edwards, E.V.; Mascaro-Blanco, A.; Alvarez, K.; Stoner, J.; Chang, K.; Cunningham, M.W. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol. 2015, 25, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Endres, D.; Pollak, T.A.; Bechter, K.; Denzel, D.; Pitsch, K.; Nickel, K.; Runge, K.; Pankratz, B.; Klatzmann, D.; Tamouza, R.; et al. Immunological causes of obsessive-compulsive disorder: Is it time for the concept of an “autoimmune OCD” subtype? Transl. Psychiatry 2022, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Crider, A. Perseveration in schizophrenia. Schizophr. Bull. 1997, 23, 63–74. [Google Scholar] [CrossRef]
- Avery, M.C.; Krichmar, J.L. Improper activation of D1 and D2 receptors leads to excess noise in prefrontal cortex. Front. Comput. Neurosci. 2015, 9, 31. [Google Scholar] [CrossRef]
- Hesse, S.; Müller, U.; Lincke, T.; Barthel, H.; Villmann, T.; Angermeyer, M.C.; Sabri, O.; Stengler-Wenzke, K. Serotonin and dopamine transporter imaging in patients with obsessive-compulsive disorder. Psychiatry Res. 2005, 140, 63–72. [Google Scholar] [CrossRef]
- Reimold, M.; Smolka, M.N.; Zimmer, A.; Batra, A.; Knobel, A.; Solbach, C.; Mundt, A.; Smoltczyk, H.U.; Goldman, D.; Mann, K.; et al. Reduced availability of serotonin transporters in obsessive-compulsive disorder correlates with symptom severity—A [11C]DASB PET study. J. Neural Transm. 2007, 114, 1603–1609. [Google Scholar] [CrossRef]
- Zitterl, W.; Aigner, M.; Stompe, T.; Zitterl-Eglseer, K.; Gutierrez-Lobos, K.; Wenzel, T.; Zettinig, G.; Hornik, K.; Pirker, W.; Thau, K. Changes in thalamus-hypothalamus serotonin transporter availability during clomipramine administration in patients with obsessive-compulsive disorder. Neuropsychopharmacology 2008, 33, 3126–3134. [Google Scholar] [CrossRef] [Green Version]
- Nakao, T.; Okada, K.; Kanba, S. Neurobiological model of obsessive-compulsive disorder: Evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin. Neurosci. 2014, 68, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, N.A.; Velez, L.P.; Masten, V.L.; Dulawa, S.C. Essential role for orbitofrontal serotonin 1B receptors in obsessive-compulsive disorder-like behavior and serotonin reuptake inhibitor response in mice. Biol. Psychiatry 2011, 70, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Piñeyro, G.; Blier, P. Autoregulation of serotonin neurons: Role in antidepressant drug action. Pharmacol. Rev. 1999, 51, 533–591. [Google Scholar]
- El Mansari, M.; Blier, P. Mechanisms of action of current and potential pharmacotherapies of obsessive-compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Szechtman, H.; Harvey, B.H.; Woody, E.Z.; Hoffman, K.L. The Psychopharmacology of Obsessive-Compulsive Disorder: A Preclinical Roadmap. Pharmacol. Rev. 2020, 72, 80–151. [Google Scholar] [CrossRef] [PubMed]
- Geddes, S.D.; Assadzada, S.; Lemelin, D.; Sokolovski, A.; Bergeron, R.; Haj-Dahmane, S.; Béïque, J.C. Target-specific modulation of the descending prefrontal cortex inputs to the dorsal raphe nucleus by cannabinoids. Proc. Natl. Acad. Sci. USA 2016, 113, 5429–5434. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, S.; Nishizawa, K.; Sakai, Y.; Kawaguchi, Y.; Kato, S.; Uchigashima, M.; Watanabe, M.; Yamanaka, K.; Enomoto, K.; Chiken, S.; et al. Monitoring and Updating of Action Selection for Goal-Directed Behavior through the Striatal Direct and Indirect Pathways. Neuron 2018, 99, 1302–1314.E5. [Google Scholar] [CrossRef] [Green Version]
- Rompre, P.P.; Miliaressis, E. Pontine and mesencephalic substrates of self-stimulation. Brain Res. 1985, 359, 246–259. [Google Scholar] [CrossRef]
- Cohen, J.Y.; Amoroso, M.W.; Uchida, N. Serotonergic neurons signal reward and punishment on multiple timescales. eLife 2015, 4, e06346. [Google Scholar] [CrossRef]
- Yagishita, S. Transient and sustained effects of dopamine and serotonin signaling in motivation-related behavior. Psychiatry Clin. Neurosci. 2020, 74, 91–98. [Google Scholar] [CrossRef]
- Shah, U.H.; González-Maeso, J. Serotonin and Glutamate Interactions in Preclinical Schizophrenia Models. ACS Chem. Neurosci. 2019, 10, 3068–3077. [Google Scholar] [CrossRef]
- Marek, G.J.; Wright, R.A.; Schoepp, D.D.; Monn, J.A.; Aghajanian, G.K. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther. 2000, 292, 76–87. [Google Scholar]
- Ibi, D.; de la Fuente Revenga, M.; Kezunovic, N.; Muguruza, C.; Saunders, J.M.; Gaitonde, S.A.; Moreno, J.L.; Ijaz, M.K.; Santosh, V.; Kozlenkov, A.; et al. Antipsychotic-induced Hdac2 transcription via NF-κB leads to synaptic and cognitive side effects. Nat. Neurosci. 2017, 20, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Muguruza, C.; Miranda-Azpiazu, P.; Díez-Alarcia, R.; Morentin, B.; González-Maeso, J.; Callado, L.F.; Meana, J.J. Evaluation of 5-HT2A and mGlu2/3 receptors in postmortem prefrontal cortex of subjects with major depressive disorder: Effect of antidepressant treatment. Neuropharmacology 2014, 86, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Carlsson, A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia--implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990, 13, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Annies, R.; Hönack, D. Comparison of competitive and uncompetitive NMDA receptor antagonists with regard to monoaminergic neuronal activity and behavioural effects in rats. Eur. J. Pharmacol. 1993, 242, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Whitton, P.S.; Biggs, C.S.; Pearce, B.R.; Fowler, L.J. Regional effects of MK-801 on dopamine and its metabolites studied by in vivo microdialysis. Neurosci. Lett. 1992, 142, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.O.; Kemel, M.L.; Gauchy, C.; Desban, M.; Glowinski, J. Glycine potentiates the NMDA-induced release of dopamine through a strychnine-insensitive site in the rat striatum. Eur. J. Pharmacol. 1989, 166, 567–570. [Google Scholar] [CrossRef]
- Wang, J.K. Presynaptic glutamate receptors modulate dopamine release from striatal synaptosomes. J. Neurochem. 1991, 57, 819–822. [Google Scholar] [CrossRef]
- Clow, D.W.; Jhamandas, K. Characterization of L-glutamate action on the release of endogenous dopamine from the rat caudate-putamen. J. Pharmacol. Exp. Ther. 1989, 248, 722–728. [Google Scholar]
- Iasevoli, F.; Tomasetti, C.; Buonaguro, E.F.; de Bartolomeis, A. The glutamatergic aspects of schizophrenia molecular pathophysiology: Role of the postsynaptic density, and implications for treatment. Curr. Neuropharmacol. 2014, 12, 219–238. [Google Scholar] [CrossRef] [Green Version]
- Borovac, J.; Bosch, M.; Okamoto, K. Regulation of actin dynamics during structural plasticity of dendritic spines: Signaling messengers and actin-binding proteins. Mol. Cell. Neurosci. 2018, 91, 122–130. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Tomasetti, C. Calcium-dependent networks in dopamine-glutamate interaction: The role of postsynaptic scaffolding proteins. Mol. Neurobiol. 2012, 46, 275–296. [Google Scholar] [CrossRef] [Green Version]
- Meshul, C.K.; Tan, S.E. Haloperidol-induced morphological alterations are associated with changes in calcium/calmodulin kinase II activity and glutamate immunoreactivity. Synapse 1994, 18, 205–217. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; Aloj, L.; Ambesi-Impiombato, A.; Bravi, D.; Caracò, C.; Muscettola, G.; Barone, P. Acute administration of antipsychotics modulates Homer striatal gene expression differentially. Brain Res. Mol. Brain Res. 2002, 98, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Lum, J.S.; Fernandez, F.; Matosin, N.; Andrews, J.L.; Huang, X.F.; Ooi, L.; Newell, K.A. Neurodevelopmental Expression Profile of Dimeric and Monomeric Group 1 mGluRs: Relevance to Schizophrenia Pathogenesis and Treatment. Sci. Rep. 2016, 6, 34391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bartolomeis, A.; Avagliano, C.; Vellucci, L.; D’Ambrosio, L.; Manchia, M.; D’Urso, G.; Buonaguro, E.F.; Iasevoli, F. Translating preclinical findings in clinically relevant new antipsychotic targets: Focus on the glutamatergic postsynaptic density. Implications for treatment resistant schizophrenia. Neurosci. Biobehav. Rev. 2019, 107, 795–827. [Google Scholar] [CrossRef]
- Barone, A.; Signoriello, S.; Latte, G.; Vellucci, L.; Giordano, G.; Avagliano, C.; Buonaguro, E.F.; Marmo, F.; Tomasetti, C.; Iasevoli, F.; et al. Modulation of glutamatergic functional connectivity by a prototypical antipsychotic: Translational inference from a postsynaptic density immediate-early gene-based network analysis. Behav. Brain Res. 2021, 404, 113160. [Google Scholar] [CrossRef]
- Sheng, M.; Hoogenraad, C.C. The postsynaptic architecture of excitatory synapses: A more quantitative view. Annu. Rev. Biochem. 2007, 76, 823–847. [Google Scholar] [CrossRef] [Green Version]
- Roussignol, G.; Ango, F.; Romorini, S.; Tu, J.C.; Sala, C.; Worley, P.F.; Bockaert, J.; Fagni, L. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J. Neurosci. 2005, 25, 3560–3570. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.K.; Tang, C.; Verpelli, C.; Narayanan, R.; Stearns, M.H.; Xu, R.M.; Li, H.; Sala, C.; Hayashi, Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 2009, 137, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Sheng, M. PDZ domain proteins of synapses. Nat. Reviews. Neurosci. 2004, 5, 771–781. [Google Scholar] [CrossRef]
- Funke, L.; Dakoji, S.; Bredt, D.S. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu. Rev. Biochem. 2005, 74, 219–245. [Google Scholar] [CrossRef]
- Gilman, S.R.; Iossifov, I.; Levy, D.; Ronemus, M.; Wigler, M.; Vitkup, D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 2011, 70, 898–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celikel, T.; Marx, V.; Freudenberg, F.; Zivkovic, A.; Resnik, E.; Hasan, M.T.; Licznerski, P.; Osten, P.; Rozov, A.; Seeburg, P.H.; et al. Select overexpression of homer1a in dorsal hippocampus impairs spatial working memory. Front. Neurosci. 2007, 1, 97–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lominac, K.D.; Oleson, E.B.; Pava, M.; Klugmann, M.; Schwarz, M.K.; Seeburg, P.H.; During, M.J.; Worley, P.F.; Kalivas, P.W.; Szumlinski, K.K. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J. Neurosci. 2005, 25, 11586–11594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietschel, M.; Mattheisen, M.; Frank, J.; Treutlein, J.; Degenhardt, F.; Breuer, R.; Steffens, M.; Mier, D.; Esslinger, C.; Walter, H.; et al. Genome-wide association-, replication-, and neuroimaging study implicates HOMER1 in the etiology of major depression. Biol. Psychiatry 2010, 68, 578–585. [Google Scholar] [CrossRef]
- Tomasetti, C.; Dell’Aversano, C.; Iasevoli, F.; Marmo, F.; de Bartolomeis, A. The acute and chronic effects of combined antipsychotic-mood stabilizing treatment on the expression of cortical and striatal postsynaptic density genes. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 184–197. [Google Scholar] [CrossRef]
- Dell’aversano, C.; Tomasetti, C.; Iasevoli, F.; de Bartolomeis, A. Antipsychotic and antidepressant co-treatment: Effects on transcripts of inducible postsynaptic density genes possibly implicated in behavioural disorders. Brain Res. Bull. 2009, 79, 123–129. [Google Scholar] [CrossRef]
- Tomasetti, C.; Dell’Aversano, C.; Iasevoli, F.; de Bartolomeis, A. Homer splice variants modulation within cortico-subcortical regions by dopamine D2 antagonists, a partial agonist, and an indirect agonist: Implication for glutamatergic postsynaptic density in antipsychotics action. Neuroscience 2007, 150, 144–158. [Google Scholar] [CrossRef]
- Ambesi-Impiombato, A.; Panariello, F.; Dell’aversano, C.; Tomasetti, C.; Muscettola, G.; de Bartolomeis, A. Differential expression of Homer 1 gene by acute and chronic administration of antipsychotics and dopamine transporter inhibitors in the rat forebrain. Synapse 2007, 61, 429–439. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, H.; Li, C. SAPAP Scaffold Proteins: From Synaptic Function to Neuropsychiatric Disorders. Cells 2022, 11, 3815. [Google Scholar] [CrossRef]
- Kirov, G.; Pocklington, A.J.; Holmans, P.; Ivanov, D.; Ikeda, M.; Ruderfer, D.; Moran, J.; Chambert, K.; Toncheva, D.; Georgieva, L.; et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry 2012, 17, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Stewart, S.E.; Yu, D.; Scharf, J.M.; Neale, B.M.; Fagerness, J.A.; Mathews, C.A.; Arnold, P.D.; Evans, P.D.; Gamazon, E.R.; Davis, L.K.; et al. Genome-wide association study of obsessive-compulsive disorder. Mol. Psychiatry 2013, 18, 788–798. [Google Scholar] [CrossRef] [Green Version]
- Gazzellone, M.J.; Zarrei, M.; Burton, C.L.; Walker, S.; Uddin, M.; Shaheen, S.M.; Coste, J.; Rajendram, R.; Schachter, R.J.; Colasanto, M.; et al. Uncovering obsessive-compulsive disorder risk genes in a pediatric cohort by high-resolution analysis of copy number variation. J. Neurodev. Disord. 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, A.H.; Rasmussen, H.B.; Silahtaroglu, A. The DLGAP family: Neuronal expression, function and role in brain disorders. Mol. Brain 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, J.M.; Wang, D.; Feng, G. Differential mRNA expression and protein localization of the SAP90/PSD-95-associated proteins (SAPAPs) in the nervous system of the mouse. J. Comp. Neurol. 2004, 472, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Kindler, S.; Rehbein, M.; Classen, B.; Richter, D.; Böckers, T.M. Distinct spatiotemporal expression of SAPAP transcripts in the developing rat brain: A novel dendritically localized mRNA. Brain Res. Mol. Brain Res. 2004, 126, 14–21. [Google Scholar] [CrossRef]
- Kim, E.; Naisbitt, S.; Hsueh, Y.P.; Rao, A.; Rothschild, A.; Craig, A.M.; Sheng, M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J. Cell Biol. 1997, 136, 669–678. [Google Scholar] [CrossRef]
- Naisbitt, S.; Kim, E.; Tu, J.C.; Xiao, B.; Sala, C.; Valtschanoff, J.; Weinberg, R.J.; Worley, P.F.; Sheng, M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 1999, 23, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Yao, I.; Hata, Y.; Hirao, K.; Deguchi, M.; Ide, N.; Takeuchi, M.; Takai, Y. Synamon, a novel neuronal protein interacting with synapse-associated protein 90/postsynaptic density-95-associated protein. J. Biol. Chem. 1999, 274, 27463–27466. [Google Scholar] [CrossRef] [Green Version]
- Sala, C.; Vicidomini, C.; Bigi, I.; Mossa, A.; Verpelli, C. Shank synaptic scaffold proteins: Keys to understanding the pathogenesis of autism and other synaptic disorders. J. Neurochem. 2015, 135, 849–858. [Google Scholar] [CrossRef]
- De Rosa, A.; Fontana, A.; Nuzzo, T.; Garofalo, M.; Di Maio, A.; Punzo, D.; Copetti, M.; Bertolino, A.; Errico, F.; Rampino, A.; et al. Machine Learning algorithm unveils glutamatergic alterations in the post-mortem schizophrenia brain. Schizophrenia 2022, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.T.; Peça, J.; Feng, G. Functional consequences of mutations in postsynaptic scaffolding proteins and relevance to psychiatric disorders. Annu. Rev. Neurosci. 2012, 35, 49–71. [Google Scholar] [CrossRef] [Green Version]
- Scannevin, R.H.; Huganir, R.L. Postsynaptic organization and regulation of excitatory synapses. Nat. Reviews. Neurosci. 2000, 1, 133–141. [Google Scholar] [CrossRef]
- Irie, M.; Hata, Y.; Takeuchi, M.; Ichtchenko, K.; Toyoda, A.; Hirao, K.; Takai, Y.; Rosahl, T.W.; Südhof, T.C. Binding of neuroligins to PSD-95. Science 1997, 277, 1511–1515. [Google Scholar] [CrossRef]
- Mondin, M.; Labrousse, V.; Hosy, E.; Heine, M.; Tessier, B.; Levet, F.; Poujol, C.; Blanchet, C.; Choquet, D.; Thoumine, O. Neurexin-neuroligin adhesions capture surface-diffusing AMPA receptors through PSD-95 scaffolds. J. Neurosci. 2011, 31, 13500–13515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bats, C.; Groc, L.; Choquet, D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 2007, 53, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Park, M. Shank postsynaptic scaffolding proteins in autism spectrum disorder: Mouse models and their dysfunctions in behaviors, synapses, and molecules. Pharmacol. Res. 2022, 182, 106340. [Google Scholar] [CrossRef]
- Du, Y.; Weed, S.A.; Xiong, W.C.; Marshall, T.D.; Parsons, J.T. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol. Cell. Biol. 1998, 18, 5838–5851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, J.J.; Schob, C.; Rehbein, M.; Gkogkas, C.G.; Richter, D.; Kindler, S. Synthesis of two SAPAP3 isoforms from a single mRNA is mediated via alternative translational initiation. Sci. Rep. 2012, 2, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanus, C.; Schuman, E.M. Proteostasis in complex dendrites. Nat. Reviews. Neurosci. 2013, 14, 638–648. [Google Scholar] [CrossRef]
- Cajigas, I.J.; Tushev, G.; Will, T.J.; tom Dieck, S.; Fuerst, N.; Schuman, E.M. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron 2012, 74, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Ade, K.K.; Caffall, Z.; Ilcim Ozlu, M.; Eroglu, C.; Feng, G.; Calakos, N. Circuit-selective striatal synaptic dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder. Biol. Psychiatry 2014, 75, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamothe, H.; Schreiweis, C.; Mondragón-González, L.S.; Rebbah, S.; Lavielle, O.; Mallet, L.; Burguière, E. The Sapap3 (−/−) mouse reconsidered as a comorbid model expressing a spectrum of pathological repetitive behaviours. Transl. Psychiatry 2023, 13, 26. [Google Scholar] [CrossRef]
- Bienvenu, O.J.; Wang, Y.; Shugart, Y.Y.; Welch, J.M.; Grados, M.A.; Fyer, A.J.; Rauch, S.L.; McCracken, J.T.; Rasmussen, S.A.; Murphy, D.L.; et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150b, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Zhang, N.; Hansen, J.; Gerges, N.Z.; Pak, D.T.; Sheng, M.; Lee, S.H. GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling. Nat. Neurosci. 2012, 15, 1655–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Zhou, Q.; Shang, Y.; Li, H.; Peng, M.; Ke, X.; Weng, Z.; Zhang, R.; Huang, X.; Li, S.S.C.; et al. Synaptic Targeting and Function of SAPAPs Mediated by Phosphorylation-Dependent Binding to PSD-95 MAGUKs. Cell Rep. 2017, 21, 3781–3793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celano, E.; Tiraboschi, E.; Consogno, E.; D’Urso, G.; Mbakop, M.P.; Gennarelli, M.; de Bartolomeis, A.; Racagni, G.; Popoli, M. Selective regulation of presynaptic calcium/calmodulin-dependent protein kinase II by psychotropic drugs. Biol. Psychiatry 2003, 53, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; LaPalombara, Z.; Ahmari, S.E. Monoamine abnormalities in the SAPAP3 knockout model of obsessive-compulsive disorder-related behaviour. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2018, 373, 20170023. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Noh, H.J.; Bae, S.H.; Kim, Y.S.; Lew, H.; Lim, J.; Kim, S.J.; Hong, K.S.; Rah, J.C.; Kim, C.H. Clozapine generates obsessive compulsive disorder-like behavior in mice. Mol. Brain 2020, 13, 84. [Google Scholar] [CrossRef]
- Zai, G.; Barta, C.; Cath, D.; Eapen, V.; Geller, D.; Grünblatt, E. New insights and perspectives on the genetics of obsessive-compulsive disorder. Psychiatr. Genet. 2019, 29, 142–151. [Google Scholar] [CrossRef]
- Rosson, S.; de Filippis, R.; Croatto, G.; Collantoni, E.; Pallottino, S.; Guinart, D.; Brunoni, A.R.; Dell’Osso, B.; Pigato, G.; Hyde, J.; et al. Brain stimulation and other biological non-pharmacological interventions in mental disorders: An umbrella review. Neurosci. Biobehav. Rev. 2022, 139, 104743. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, P.; Tang, Y.; Zhang, C.; Lin, L.; Gao, J.; Wang, Z. Transcranial direct current stimulation improve symptoms and modulates cortical inhibition in obsessive-compulsive disorder: A TMS-EEG study. J. Affect. Disord. 2022, 298 Pt A, 558–564. [Google Scholar] [CrossRef]
- Gao, T.; Du, J.; Tian, S.; Liu, W. A meta-analysis of the effects of non-invasive brain stimulation on obsessive-compulsive disorder. Psychiatry Res. 2022, 312, 114530. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef] [Green Version]
- D’Urso, G.; Toscano, E.; Barone, A.; Palermo, M.; Dell’Osso, B.; Di Lorenzo, G.; Mantovani, A.; Martinotti, G.; Fornaro, M.; Iasevoli, F.; et al. Transcranial direct current stimulation for bipolar depression: Systematic reviews of clinical evidence and biological underpinnings. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 121, 110672. [Google Scholar] [CrossRef]
- Tian, D.; Izumi, S.I. Transcranial Magnetic Stimulation and Neocortical Neurons: The Micro-Macro Connection. Front. Neurosci. 2022, 16, 866245. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Lampe, C.; Antal, A.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur. J. Neurosci. 2006, 23, 1651–1657. [Google Scholar] [CrossRef]
- Kuo, H.I.; Paulus, W.; Batsikadze, G.; Jamil, A.; Kuo, M.F.; Nitsche, M.A. Chronic Enhancement of Serotonin Facilitates Excitatory Transcranial Direct Current Stimulation-Induced Neuroplasticity. Neuropsychopharmacology 2016, 41, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Wang, F.; Wang, L.; Zhang, Y.; Yamamoto, R.; Sugai, T.; Zhang, Q.; Wang, Z.; Kato, N. Increase in cortical pyramidal cell excitability accompanies depression-like behavior in mice: A transcranial magnetic stimulation study. J. Neurosci. 2011, 31, 16464–16472. [Google Scholar] [CrossRef] [Green Version]
- Gornerova, N.; Brunovsky, M.; Klirova, M.; Novak, T.; Zaytseva, Y.; Koprivova, J.; Bravermanova, A.; Horacek, J. The effect of low-frequency rTMS on auditory hallucinations, EEG source localization and functional connectivity in schizophrenia. Neurosci. Lett. 2023, 794, 136977. [Google Scholar] [CrossRef] [PubMed]
- Brunelin, J.; Mondino, M.; Gassab, L.; Haesebaert, F.; Gaha, L.; Suaud-Chagny, M.F.; Saoud, M.; Mechri, A.; Poulet, E. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am. J. Psychiatry 2012, 169, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Lisoni, J.; Baldacci, G.; Nibbio, G.; Zucchetti, A.; Butti Lemmi Gigli, E.; Savorelli, A.; Facchi, M.; Miotto, P.; Deste, G.; Barlati, S.; et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial Direct Current Stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: Results of a randomized double-blind sham-controlled trial. J. Psychiatr. Res. 2022, 155, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Meiron, O.; David, J.; Yaniv, A. Left prefrontal transcranial direct-current stimulation reduces symptom-severity and acutely enhances working memory in schizophrenia. Neurosci. Lett. 2021, 755, 135912. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Brunoni, A.R.; Anastasia, A.; Micillo, M.; de Bartolomeis, A.; Mantovani, A. Polarity-dependent effects of transcranial direct current stimulation in obsessive-compulsive disorder. Neurocase 2016, 22, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.; Brunoni, A.R.; Goerigk, S.; Batistuzzo, M.C.; Costa, D.; Diniz, J.B.; Padberg, F.; D’Urso, G.; Miguel, E.C.; Shavitt, R.G. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for obsessive-compulsive disorder: A randomized, sham-controlled trial. Neuropsychopharmacology 2021, 46, 1028–1034. [Google Scholar] [CrossRef]
- Mantovani, A.; Neri, F.; D’Urso, G.; Mencarelli, L.; Tatti, E.; Momi, D.; Menardi, A.; Sprugnoli, G.; Santarnecchi, E.; Rossi, S. Functional connectivity changes and symptoms improvement after personalized, double-daily dosing, repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: A pilot study. J. Psychiatr. Res. 2021, 136, 560–570. [Google Scholar] [CrossRef]
- Whitney, K.A.; Fastenau, P.S.; Evans, J.D.; Lysaker, P.H. Comparative neuropsychological function in obsessive-compulsive disorder and schizophrenia with and without obsessive-compulsive symptoms. Schizophr. Res. 2004, 69, 75–83. [Google Scholar] [CrossRef]
- Baytunca, B.; Kalyoncu, T.; Ozel, I.; Erermiş, S.; Kayahan, B.; Öngur, D. Early Onset Schizophrenia Associated With Obsessive-Compulsive Disorder: Clinical Features and Correlates. Clin. Neuropharmacol. 2017, 40, 243–245. [Google Scholar] [CrossRef]
- Moreira, P.S.; Marques, P.; Soriano-Mas, C.; Magalhães, R.; Sousa, N.; Soares, J.M.; Morgado, P. The neural correlates of obsessive-compulsive disorder: A multimodal perspective. Transl. Psychiatry 2017, 7, e1224. [Google Scholar] [CrossRef]
- Fuxe, K.; Guidolin, D.; Agnati, L.F.; Borroto-Escuela, D.O. Dopamine heteroreceptor complexes as therapeutic targets in Parkinson’s disease. Expert Opin. Ther. Targets 2015, 19, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Delille, H.K.; Becker, J.M.; Burkhardt, S.; Bleher, B.; Terstappen, G.C.; Schmidt, M.; Meyer, A.H.; Unger, L.; Marek, G.J.; Mezler, M. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology 2012, 62, 2184–2191. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.O.; Bustillo, J.R. Glutamatergic dysfunction in Schizophrenia. Transl. Psychiatry 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; Barone, A.; Buonaguro, E.F.; Tomasetti, C.; Vellucci, L.; Iasevoli, F. The Homer1 family of proteins at the crossroad of dopamine-glutamate signaling: An emerging molecular “Lego” in the pathophysiology of psychiatric disorders. A systematic review and translational insight. Neurosci. Biobehav. Rev. 2022, 136, 104596. [Google Scholar] [CrossRef]
- Mäki-Marttunen, V.; Andreassen, O.A.; Espeseth, T. The role of norepinephrine in the pathophysiology of schizophrenia. Neurosci. Biobehav. Rev. 2020, 118, 298–314. [Google Scholar] [CrossRef]
- Benes, F.M. The GABA system in schizophrenia: Cells, molecules and microcircuitry. Schizophr. Res. 2015, 167, 1–3. [Google Scholar] [CrossRef]
- Correll, C.U. Current Treatment Options and Emerging Agents for Schizophrenia. J. Clin. Psychiatry 2020, 81, 26548. [Google Scholar] [CrossRef]
- Dalack, G.W.; Becks, L.; Hill, E.; Pomerleau, O.F.; Meador-Woodruff, J.H. Nicotine withdrawal and psychiatric symptoms in cigarette smokers with schizophrenia. Neuropsychopharmacology 1999, 21, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.A.; Burgess, S.; Suckling, J.; Lalousis, P.A.; Batool, F.; Griffiths, S.L.; Palmer, E.; Karwath, A.; Barsky, A.; Gkoutos, G.V.; et al. Inflammation and Brain Structure in Schizophrenia and Other Neuropsychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry 2022, 79, 498–507. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Barone, A.; Vellucci, L.; Mazza, B.; Austin, M.C.; Iasevoli, F.; Ciccarelli, M. Linking Inflammation, Aberrant Glutamate-Dopamine Interaction, and Post-synaptic Changes: Translational Relevance for Schizophrenia and Antipsychotic Treatment: A Systematic Review. Mol. Neurobiol. 2022, 59, 6460–6501. [Google Scholar] [CrossRef]
- Meyer, J. Inflammation, Obsessive-Compulsive Disorder, and Related Disorders. Curr. Top. Behav. Neurosci. 2021, 49, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Gidziela, A.; Ahmadzadeh, Y.I.; Michelini, G.; Allegrini, A.G.; Agnew-Blais, J.; Lau, L.Y.; Duret, M.; Procopio, F.; Daly, E.; Ronald, A.; et al. A meta-analysis of genetic effects associated with neurodevelopmental disorders and co-occurring conditions. Nat. Hum. Behav. 2023, 7, 642–656. [Google Scholar] [CrossRef] [PubMed]

| Neurotransmitter | Gene | Protein | Genetic Variant | Functional Meaning | Reference |
|---|---|---|---|---|---|
| Glutamate | SLC1A1 | EAAC1 | rs2228622–rs3780413–rs3780412 | Susceptibility of atypical antipsychotics-induced obsessive-compulsive symptoms | [40] |
| DLGAP3 | SAPAP3 | rs7525948 | The interaction with rs2228622 polymorphism of the SLC1A1 gene increases the risk of developing atypical antipsychotics-induced obsessive-compulsive symptoms | [41] | |
| SLC1A1 and GRIN2B | EAAC1 and NR2B | rs2228622 and rs890 | Susceptibility to OCS in patients treated with clozapine | [58] | |
| Dopamine | COMT | COMT | Val158/Val158 Val158/Met158 Met158/Met158 | Met158/Met158 appears to act in a recessive manner and increase the susceptibility to developing OCD | [44] |
| Serotonin | 5HTR | 5-HT2A | rs6311-rs6313 | Susceptibility to OCD | [46] |
| Neuroimaging Techniques | Neurotransmitters Pattern | Diagnosis Groups | Brain Regions | Functional/Anatomical Alteration | Reference |
|---|---|---|---|---|---|
| 1H-MRS | Glutamate | OCD | ACC | Decrease in N-acetylaspartate resonance peaks | [67] |
| OCD | ACC and striatum | Decrease in glutamate peaks Increased in Glx peaks | [67] [57] | ||
| Schizophrenia | ACC | Decrease in N-acetylaspartate resonance peaks | [75] | ||
| SPECT | Dopamine | OCD | Left caudate nucleus | Decrease in D2R binding | [83] |
| PET | Dopamine | OCD | Striatum | Decrease in [11C]Raclopride, a selective D2R antagonist, uptake | [73] |
| Serotonine | Polar, dorsolateral, and medial frontal cortex | Decrease in [11C]MDL, a selective 5-HT2A antagonist, uptake | |||
| Resting state fMRI | - | OCD and schizophrenia | Hippocampus and the left posterior cingulate cortex | Abnormal local spontaneous neural activity | [84] |
| Trait-based spatial statistics and probabilistic tractography | - | Schizo-obsessive | Right sagittal layer and left crescent of the fornix/stria terminalis | Reduced fractional anisotropy and increased radial diffusivity resulted in altered connections in the default mode network, subcortical network, attention network, task control network, visual network, somatosensory network, and cerebellum | [85] |
| fMRI | - | Clozapine/olanzapine-induced OCS in schizophrenia | CSTC loop, left parahippocampal gyrus, globus pallidus, and the right precentral gyrus | Increased brain region activation | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellucci, L.; Ciccarelli, M.; Buonaguro, E.F.; Fornaro, M.; D’Urso, G.; De Simone, G.; Iasevoli, F.; Barone, A.; de Bartolomeis, A. The Neurobiological Underpinnings of Obsessive-Compulsive Symptoms in Psychosis, Translational Issues for Treatment-Resistant Schizophrenia. Biomolecules 2023, 13, 1220. https://doi.org/10.3390/biom13081220
Vellucci L, Ciccarelli M, Buonaguro EF, Fornaro M, D’Urso G, De Simone G, Iasevoli F, Barone A, de Bartolomeis A. The Neurobiological Underpinnings of Obsessive-Compulsive Symptoms in Psychosis, Translational Issues for Treatment-Resistant Schizophrenia. Biomolecules. 2023; 13(8):1220. https://doi.org/10.3390/biom13081220
Chicago/Turabian StyleVellucci, Licia, Mariateresa Ciccarelli, Elisabetta Filomena Buonaguro, Michele Fornaro, Giordano D’Urso, Giuseppe De Simone, Felice Iasevoli, Annarita Barone, and Andrea de Bartolomeis. 2023. "The Neurobiological Underpinnings of Obsessive-Compulsive Symptoms in Psychosis, Translational Issues for Treatment-Resistant Schizophrenia" Biomolecules 13, no. 8: 1220. https://doi.org/10.3390/biom13081220