Non-Invasive Retinal Blood Vessel Wall Measurements with Polarization-Sensitive Optical Coherence Tomography for Diabetes Assessment: A Quantitative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Imaging Protocol
2.2. Subject Recruitment and Study Approval
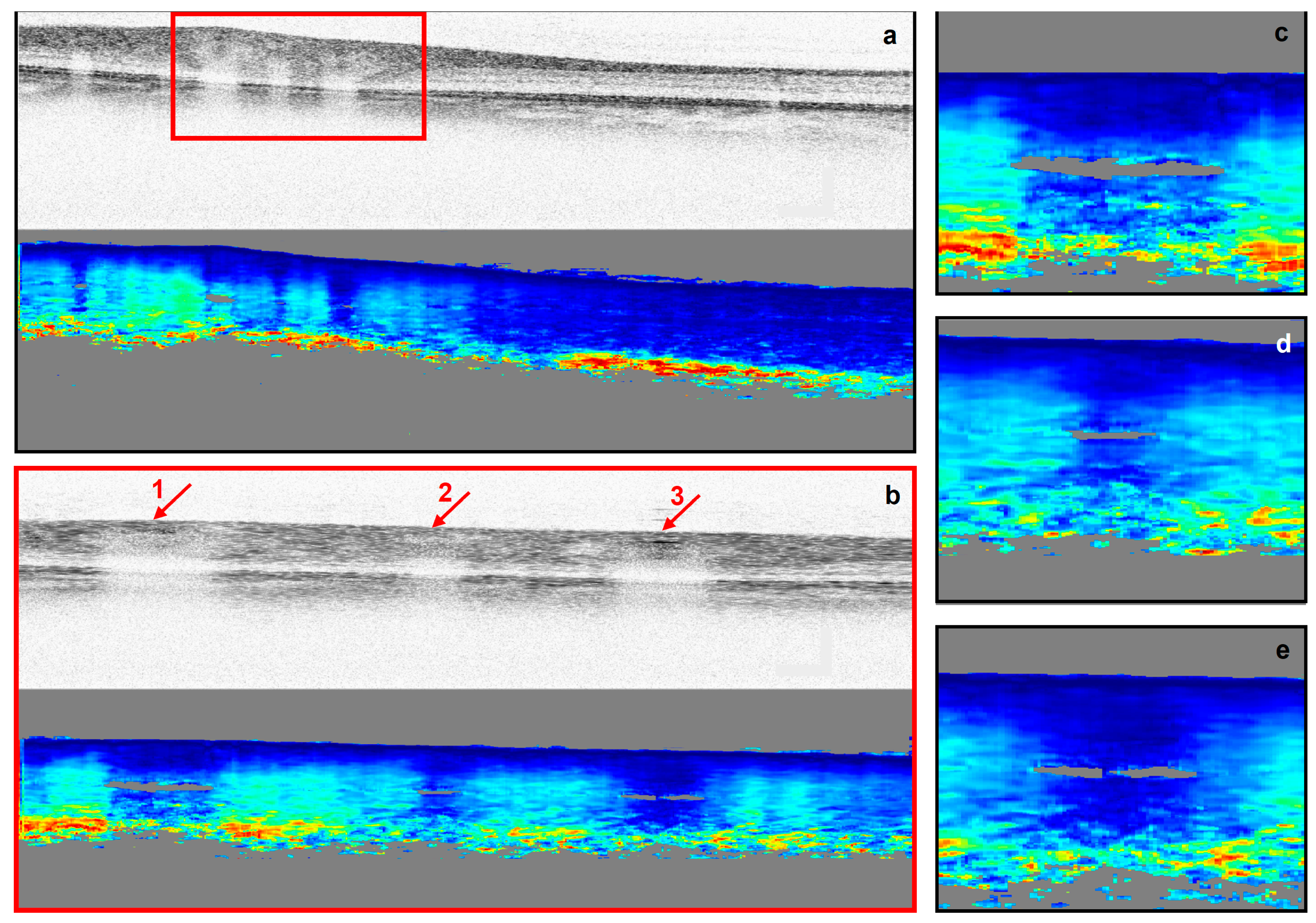
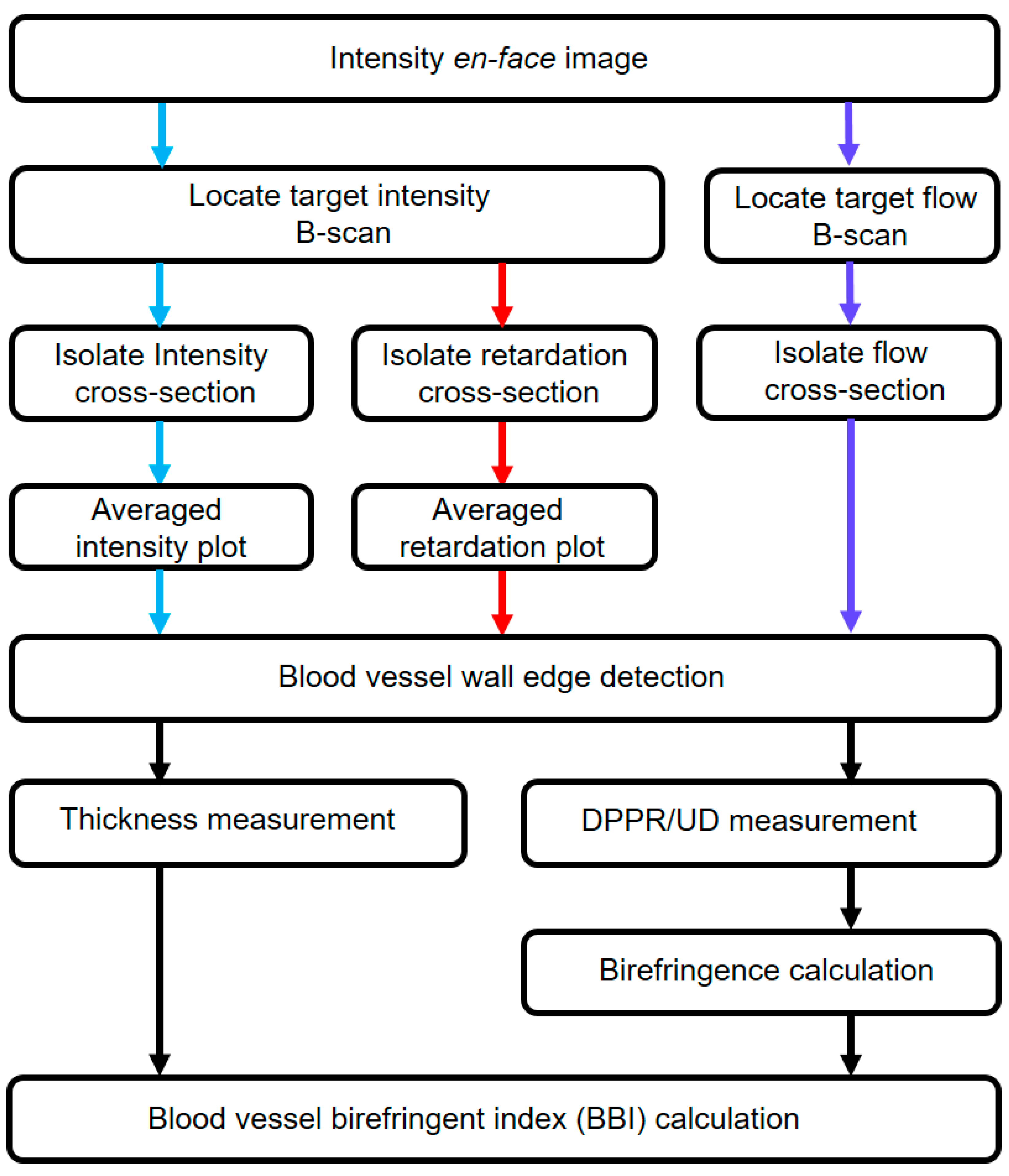
2.3. Retardation and Image Analysis
2.4. Statistical Analysis
3. Results
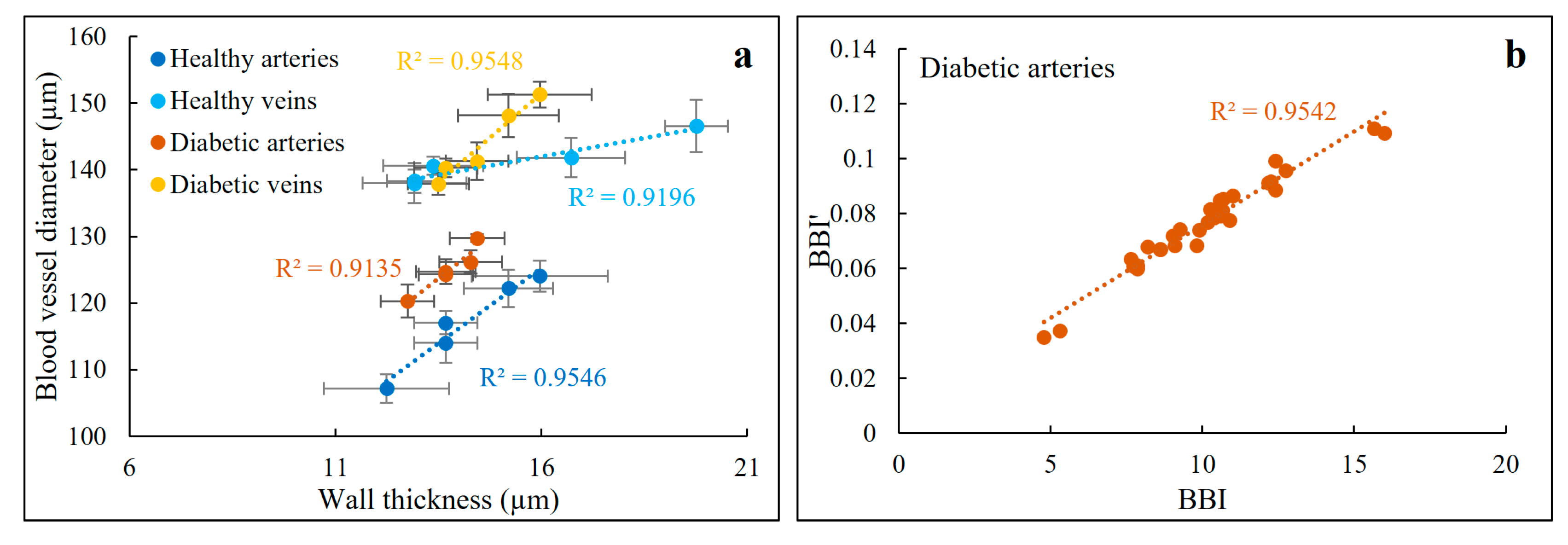
3.1. Thickness Behavior versus Blood Vessel Diameter
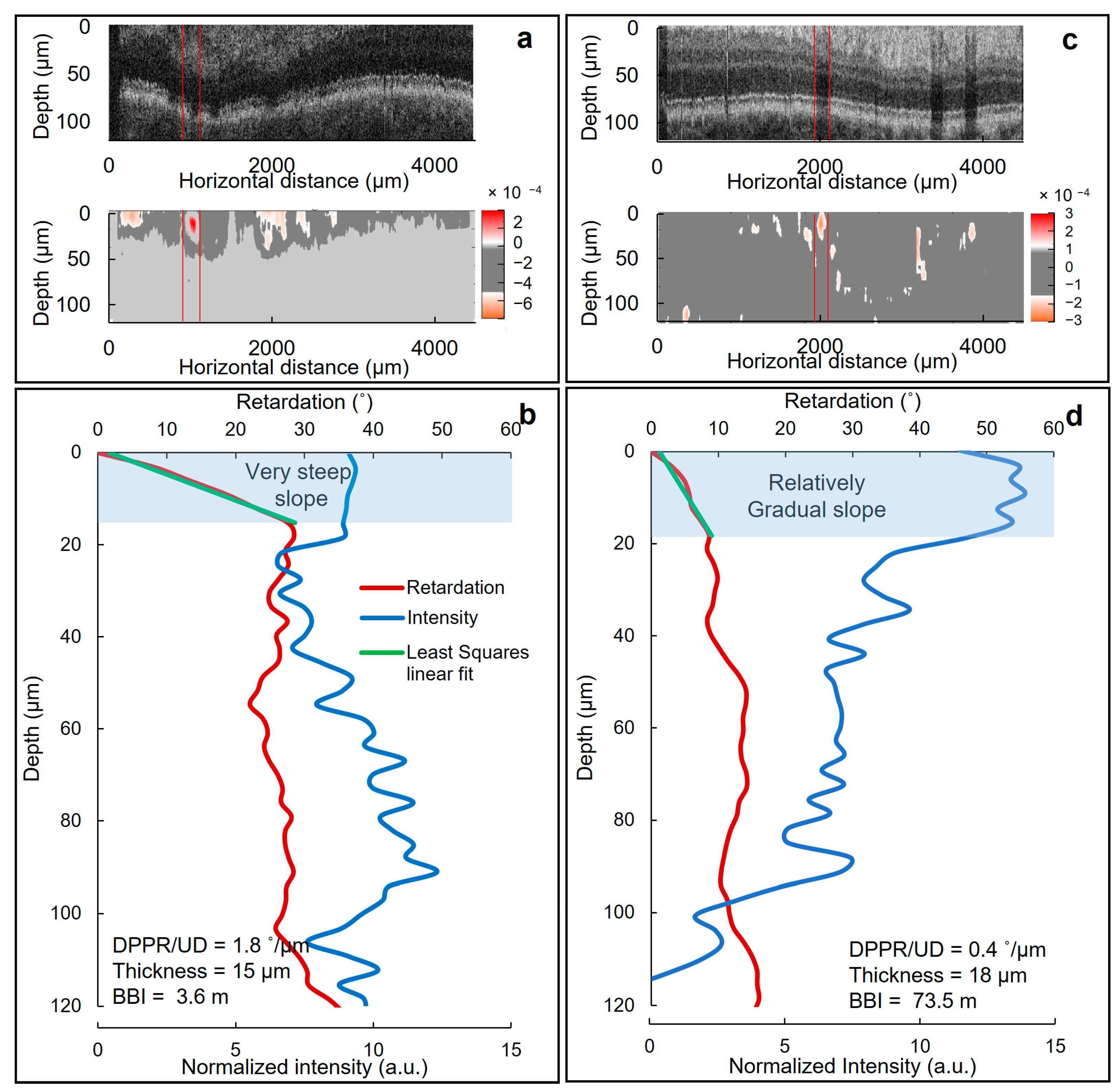
3.2. SNR and the Birefringence of the Blood Vessel Wall
3.3. Thickness and DPPR/UD Comparison in Normal and Diabetic Blood Vessels
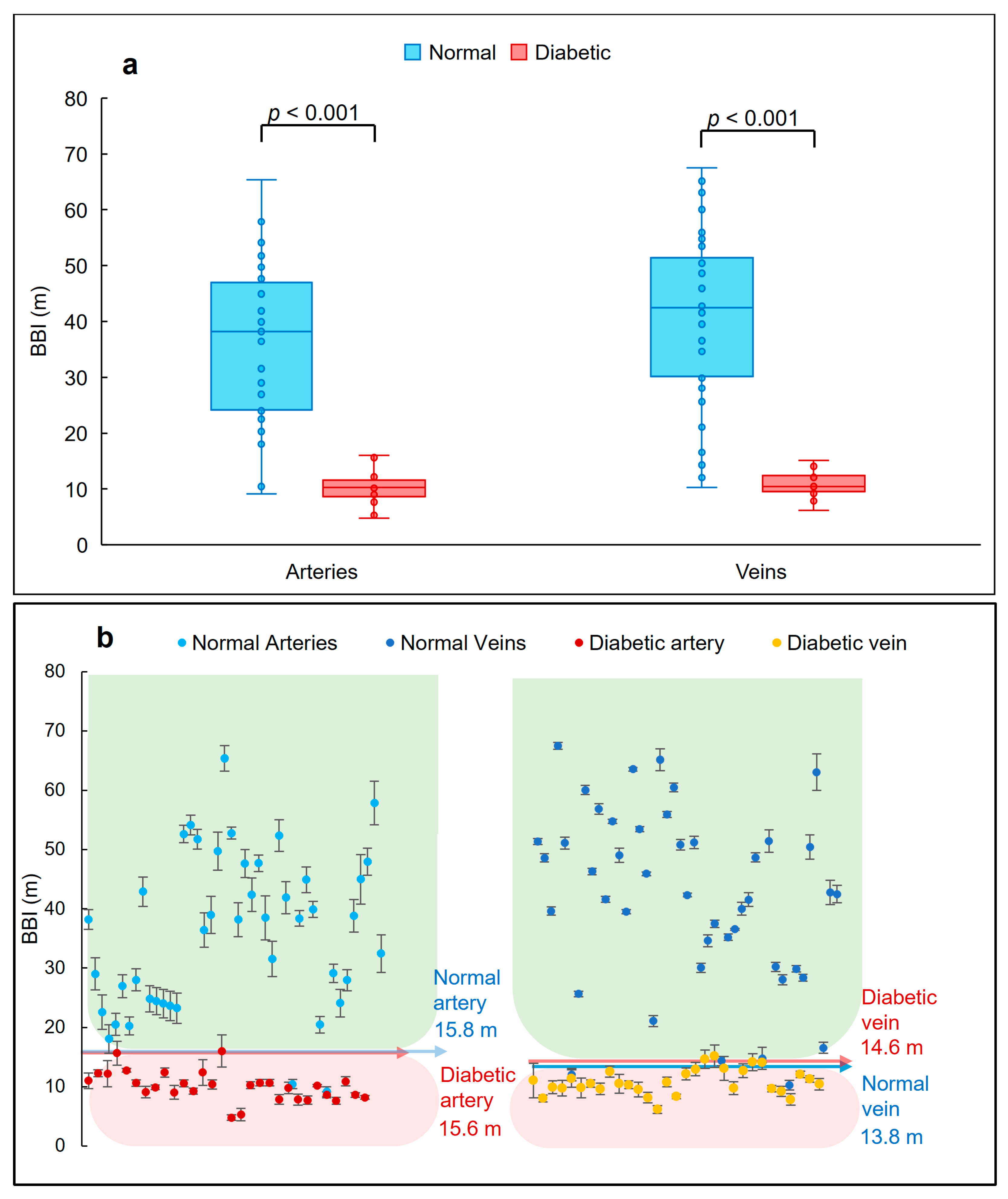
3.4. BBI Measurements
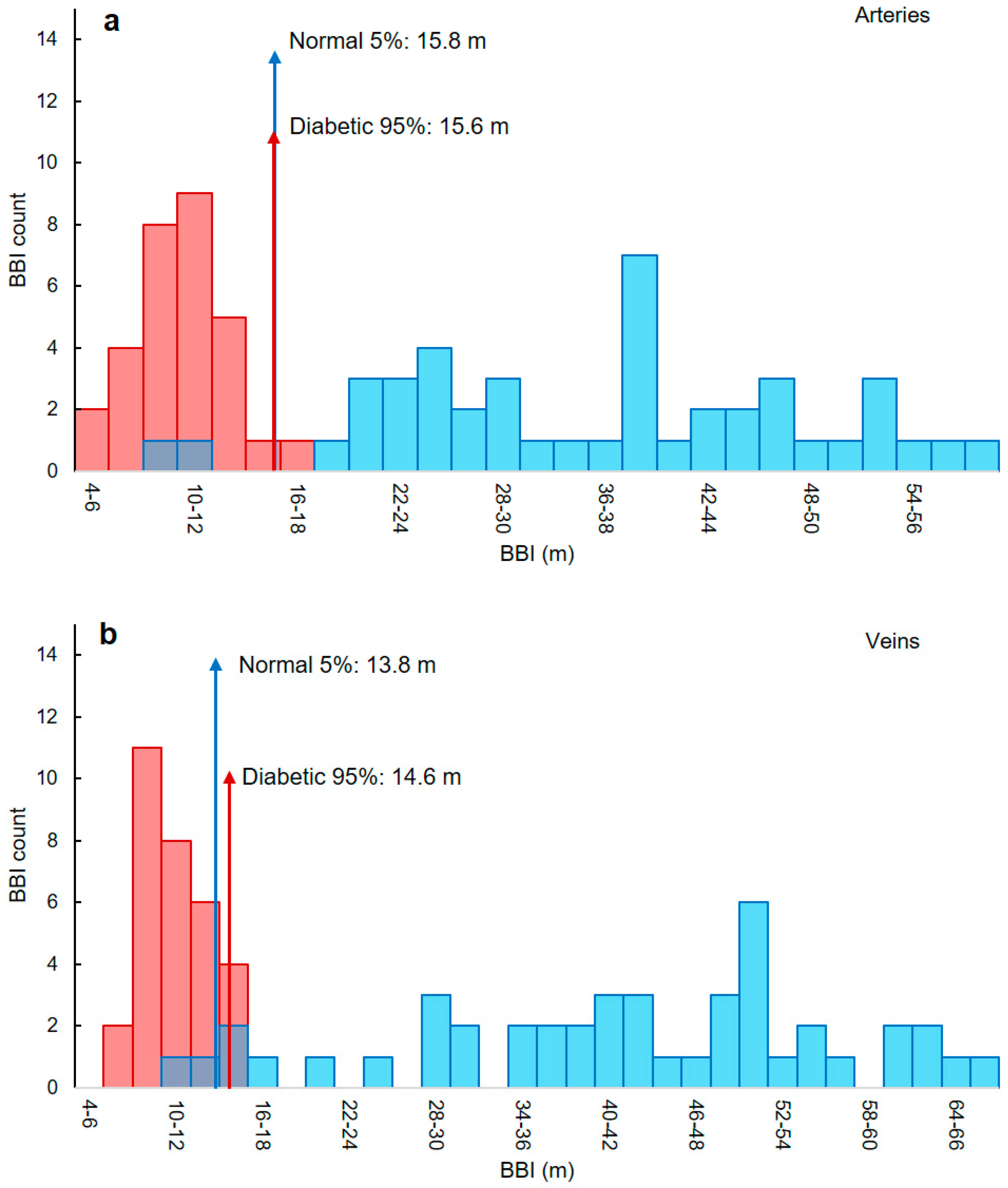
3.5. Classifying Thresholds Based on 95th and 5th Percentiles
3.6. BBI Values along a Vessel
3.7. Repeatability
3.8. Sensitivity, Specificity and Accuracy
4. Discussion
4.1. Strengths
4.2. Weaknesses
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GHDx. Global Burden of Disease Study 2019 (GBD 2019) Reference Life Table; Institute for Health Metrics and Evaluation (IHME): Seattle, DC, USA, 2021; Available online: https://ghdx.healthdata.org/record/ihme-data/global-burden-disease-study-2019-gbd-2019-reference-life-table (accessed on 7 August 2023).
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
- King, G.L. The Role of Hyperglycaemia and Hyperinsulinaemia in Causing Vascular Dysfunction in Diabetes. Ann. Med. 1996, 28, 427–432. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.M.; Eliasson, B.; Gudbjörnsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Magliano, D.J.; Rancière, F.; Harding, J.L.; Nanayakkara, N.; Shaw, J.E.; Carstensen, B. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997–2011. Diabetologia 2018, 61, 1055–1063. [Google Scholar] [CrossRef]
- Steinarsson, A.O.; Rawshani, A.; Gudbjörnsdottir, S.; Franzén, S.; Svensson, A.-M.; Sattar, N. Short-term progression of cardiometabolic risk factors in relation to age at type 2 diabetes diagnosis: A longitudinal observational study of 100,606 individuals from the Swedish National Diabetes Register. Diabetologia 2018, 61, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Cleary, P.A.; Dahms, W.; Goldstein, D.; Malone, J.; Tamborlane, W.V. Beneficial effects of intensive therapy of diabetes during adolescence: Outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J. Pediatr. 2001, 139, 804–812. [Google Scholar]
- TODAY Study Group. A Clinical Trial to Maintain Glycemic Control in Youth with Type 2 Diabetes. N. Engl. J. Med. 2012, 366, 2247–2256. [Google Scholar] [CrossRef]
- Bell, R.A.; Mayer-Davis, E.J.; Beyer, J.W.; D’Agostino, R.B., Jr.; Lawrence, J.M.; Linder, B.; Liu, L.L.; Marcovina, S.M.; Rodriguez, B.L.; Williams, D.; et al. Diabetes in Non-Hispanic White Youth: Prevalence, incidence, and clinical characteristics: The SEARCH for Diabetes in Youth Study. Diabetes Care 2009, 32 (Suppl. 2), S102–S111. [Google Scholar] [CrossRef]
- Kotliar, K.; Nagel, E.; Vilser, W.; Seidova, S.-F.; Lanzl, I.; Kofoed, P.K.; Munch, I.C.; Sander, B.; Holfort, S.K.; Sillesen, H.; et al. Microstructural Alterations of Retinal Arterial Blood Column along the Vessel Axis in Systemic Hypertension. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2165–2172. [Google Scholar] [CrossRef]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic Stiffness, Blood Pressure Progression, and Incident Hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Berkowitz, D.E.; Cohen, R.A.; Figueroa, C.A.; Harrison, D.G.; Humphrey, J.D.; Larson, D.F.; Leopold, J.A.; Mecham, R.P.; Ruiz-Opazo, N.; et al. A Special Report on the NHLBI Initiative to Study Cellular and Molecular Mechanisms of Arterial Stiffness and Its Association with Hypertension. Circ. Res. 2017, 121, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, R.E.; Werner, K.E.; Yum, V.; Lu, C.; Tat, V.; Memon, M.; No, Y.; Ask, K.; Dickhout, J.G. Endoplasmic reticulum stress inhibition reduces hypertension through the preservation of resistance blood vessel structure and function. J. Hypertens. 2016, 34, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Inrig, J.K. Antihypertensive Agents in Hemodialysis Patients: A Current Perspective. Semin. Dial. 2010, 23, 290–297. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, G.E.; Brennan, G.M.; Johnston, G.D.; McDermott, B.J.; McGrath, L.T.; Henry, W.R.; Andrews, J.W.; Hayes, J.R. Impaired endothelium-dependent and independent vasodilation in patients with Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1992, 35, 771–776. [Google Scholar] [CrossRef]
- Williams, S.B.; Cusco, J.A.; Roddy, M.-A.; Johnstone, M.T.; Creager, M.A. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1996, 27, 567–574. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Gräfe, M.G.O.; van de Kreeke, J.A.; Willemse, J.; Braaf, B.; de Jong, Y.; Tan, H.S.; Verbraak, F.D.; de Boer, J.F. Subretinal Fibrosis Detection Using Polarization Sensitive Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2020, 9, 13. [Google Scholar] [CrossRef]
- Marjanovic, E.J.; Sharma, V.; Smith, L.; Pinder, C.; Moore, T.L.; Manning, J.B.; Dinsdale, G.; Berks, M.; Newton, V.L.; Wilkinson, S.; et al. Polarisation-sensitive optical coherence tomography measurement of retardance in fibrosis, a non-invasive biomarker in patients with systemic sclerosis. Sci. Rep. 2022, 12, 2893. [Google Scholar] [CrossRef]
- Willemse, J.; Wener, R.R.; Feroldi, F.; Vaselli, M.; Kwakkel-van Erp, J.M.; van de Graaf, E.A.; Thunnissen, E.; de Boer, J.F. Polarization-sensitive optical coherence tomography in end-stage lung diseases: An ex vivo pilot study. Biomed. Optics Express 2021, 12, 6796–6813. [Google Scholar] [CrossRef]
- Vaselli, M.; Kalverda, K.A.M.; Bonta, P.; De Boer, J.; Annema, J. In vivo PS-OCT to detect fibrotic lung disease. Eur. Respir. J. 2021, 58 (Suppl. 65), OA234. [Google Scholar]
- Kuo, W.C.; Hsiung, M.W.; Shyu, J.J.; Chou, N.K.; Yang, P.N. Assessment of arterial characteristics in human atherosclerosis by extracting optical properties from polarization-sensitive optical coherence tomography. Opt. Express 2008, 16, 8117–8125. [Google Scholar] [CrossRef] [PubMed]
- Afsharan, H.; Hackmann, M.J.; Wang, Q.; Navaeipour, F.; Jayasree, S.V.K.; Zawadzki, R.J.; Silva, D.; Joo, C.; Cense, B. Polarization properties of retinal blood vessel walls measured with polarization sensitive optical coherence tomography. Biomed. Opt. Express 2021, 12, 4340–4362. [Google Scholar] [CrossRef]
- Afsharan, H.; Anilkumar, V.; Silva, D.; Dwivedi, G.; Joo, C.; Cense, B. Trans-Ocular Hypertension Assessment. 2023. Available online: https://www.researchsquare.com/article/rs-2536703/v1 (accessed on 7 August 2023).
- Cense, B. Optical Coherence Tomography for Retinal Imaging; University of Twente: Enschede, The Netherlands, 2005. [Google Scholar]
- Mujat, M.; Park, B.H.; Cense, B.; Chen, T.C.; de Boer, J.F. Autocalibration of spectral-domain optical coherence tomography spectrometers for in vivo quantitative retinal nerve fiber layer birefringence determination. J. Biomed. Opt. 2007, 12, 041205. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.F.; Milner, T.E. Review of polarization sensitive optical coherence tomography and Stokes vector determination. J. Biomed. Opt. 2002, 7, 359–372. [Google Scholar] [CrossRef]
- Cense, B.; Maddipatla, R.; Lozano, F.J.C.; Joo, C. Two concepts for ultra-high-resolution polarization-sensitive optical coherence tomography with a single camera. J. Opt. Soc. Am. A 2022, 39, 1295–1308. [Google Scholar] [CrossRef]
- Cense, B.; Miller, D.T.; King, B.J.; Theelen, T.; Elsner, A.E. Measuring polarization changes in the human outer retina with polarization-sensitive optical coherence tomography. J. Biophotonics 2018, 11, e201700134. [Google Scholar] [CrossRef]
- Park, B.H.; Pierce, M.C.; Cense, B.; de Boer, J.F. Real-time multi-functional optical coherence tomography. Opt. Express 2003, 11, 782–793. [Google Scholar] [CrossRef]
- Park, B.H.; Saxer, C.; Srinivas, S.M.; Nelson, J.S.; de Boer, J.F. In vivo burn depth determination by high-speed fiber-based polarization sensitive optical coherence tomography. J. Biomed. Opt. 2001, 6, 474–479. [Google Scholar] [CrossRef] [PubMed]
- White, B.R.; Pierce, M.C.; Nassif, N.; Cense, B.; Park, B.H.; Tearney, G.J.; Bouma, B.E.; Chen, T.C.; de Boer, J.F. In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical Doppler tomography. Opt. Express 2003, 11, 3490–3497. [Google Scholar] [CrossRef]
- Cense, B.; Chen, T.C.; Park, B.H.; Pierce, M.C.; de Boer, J.F. In vivo birefringence and thickness measurements of the human retinal nerve fiber layer using polarization-sensitive optical coherence tomography. J. Biomed. Opt. 2004, 9, 121–126. [Google Scholar] [CrossRef]
- Park, B.H.; Pierce, M.C.; Cense, B.; Yun, S.-H.; Mujat, M.; Tearney, G.J.; Bouma, B.E.; de Boer, J.F. Real-time fiber-based multi-functional spectral-domain optical coherence tomography at 1.3 µm. Opt. Express 2005, 13, 3931–3944. [Google Scholar] [CrossRef]
- Cense, B.; Mujat, M.; Chen, T.C.; Park, B.H.; de Boer, J.F. Polarization-sensitive spectral-domain optical coherence tomography using a single line scan camera. Opt. Express 2007, 15, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Cense, B.; Chen, T.C.; Park, B.H.; Pierce, M.C.; de Boer, J.F. Thickness and Birefringence of Healthy Retinal Nerve Fiber Layer Tissue Measured with Polarization-Sensitive Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Götzinger, E.; Pircher, M.; Baumann, B.; Schmoll, T.; Sattmann, H.; Leitgeb, R.A.; Hitzenberger, C.K. Speckle noise reduction in high speed polarization sensitive spectral domain optical coherence tomography. Opt. Express 2011, 19, 14568–14584. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.; Harper, D.J.; Eugui, P.; Gesperger, J.; Lichtenegger, A.; Merkle, C.W.; Augustin, M.; Woehrer, A. Improved accuracy of quantitative birefringence imaging by polarization sensitive OCT with simple noise correction and its application to neuroimaging. J. Biophotonics 2021, 14, e202000323. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.M.; Barczak, A.J.; DeHoff, P.; Srinivasan, S.; Etheridge, A.; Galas, D.; Das, S.; Erle, D.J.; Laurent, L.C. Comparison of reproducibility, accuracy, sensitivity, and specificity of miRNA quantification platforms. Cell Rep. 2019, 29, 4212–4222.e5. [Google Scholar] [CrossRef]
- Streiner, D.L.; Norman, G.R. “Precision” and “Accuracy”: Two Terms That Are Neither. J. Clin. Epidemiol. 2006, 59, 327–330. [Google Scholar] [CrossRef]
- Bedggood, P.; Metha, A. Adaptive optics imaging of the retinal microvasculature. Clin. Exp. Optom. 2020, 103, 112–122. [Google Scholar] [CrossRef]
- Martinez-Quinones, P.; McCarthy, C.G.; Watts, S.W.; Klee, N.S.; Komic, A.; Calmasini, F.B.; Priviero, F.; Warner, A.; Chenghao, Y.; Wenceslau, C.F. Hypertension Induced Morphological and Physiological Changes in Cells of the Arterial Wall. Am. J. Hypertens. 2018, 31, 1067–1078. [Google Scholar] [CrossRef]
- Wong, T.Y.; Shankar, A.; Klein, R.; Klein, B.E.K.; Hubbard, L.D. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ 2004, 329, 79. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Żmijewska, A.; Wawrzyniak, Z.M.; Dąbrowska, A.; Szaflik, J.P. Adaptive Optics (rtx1) High-Resolution Imaging of Photoreceptors and Retinal Arteries in Patients with Diabetic Retinopathy. J. Diabetes Res. 2019, 2019, 9548324. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Prattichizzo, F.; Ceriello, A. The link between diabetes and atherosclerosis. Eur. J. Prev. Cardiol. 2020, 26 (Suppl. 2), 15–24. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Spagnoli, L.G.; Bonanno, E.; Sangiorgi, G.; Mauriello, A. Role of Inflammation in Atherosclerosis. J. Nucl. Med. 2007, 48, 1800–1815. [Google Scholar] [CrossRef]
- Tuleta, I.; Frangogiannis, N.G. Diabetic fibrosis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2021, 1867, 166044. [Google Scholar] [CrossRef]
- Muraoka, Y.; Tsujikawa, A.; Kumagai, K.; Akiba, M.; Ogino, K.; Murakami, T.; Akagi-Kurashige, Y.; Miyamoto, K.; Yoshimura, N. Age- and Hypertension-Dependent Changes in Retinal Vessel Diameter and Wall Thickness: An Optical Coherence Tomography Study. Am. J. Ophthalmol. 2013, 156, 706–714.e2. [Google Scholar] [CrossRef]
- Zhu, T.P.; Tong, Y.H.; Zhan, H.J.; Ma, J. Update on retinal vessel structure measurement with spectral-domain optical coherence tomography. Microvasc. Res. 2014, 95, 7–14. [Google Scholar] [CrossRef]
- Chui, T.Y.P.; Gast, T.J.; Burns, S.A. Imaging of Vascular Wall Fine Structure in the Human Retina Using Adaptive Optics Scanning Laser Ophthalmoscopy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7115–7124. [Google Scholar] [CrossRef]
- Rim, T.H.; Choi, Y.S.; Kim, S.S.; Kang, M.-J.; Oh, J.; Park, S.; Byeon, S.H. Retinal vessel structure measurement using spectral-domain optical coherence tomography. Eye 2016, 30, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.A.; Elsner, A.E.; Chui, T.Y.; Van Nasdale, D.A., Jr.; Clark, C.A.; Gast, T.J.; Malinovsky, V.E.; Phan, A.-D.T. In vivo adaptive optics microvascular imaging in diabetic patients without clinically severe diabetic retinopathy. Biomed. Opt. Express 2014, 5, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Moss, S.E.; Wong, T.Y.; Hubbard, L.; Cruickshanks, K.J.; Palta, M. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX: The Wisconsin epidemiologic study of diabetic retinopathy. Arch. Ophthalmol. 2004, 122, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, V.S.E.; Sabanayagam, C.; Tai, E.S.; Lee, J.; Lamoureux, E.; Sun, C.; Kawasaki, R.; Wong, T.Y. Retinal vascular caliber and diabetes in a multiethnic Asian population. Microcirculation 2009, 16, 534–543. [Google Scholar] [CrossRef]
- Guan, K.; Hudson, C.; Wong, T.; Kisilevsky, M.; Nrusimhadevara, R.K.; Lam, W.C.; Mandelcorn, M.; Devenyi, R.G.; Flanagan, J.G. Retinal hemodynamics in early diabetic macular edema. Diabetes 2006, 55, 813–818. [Google Scholar] [CrossRef]
- Abdul-Rahman, A.; Morgan, W.; Vukmirovic, A.; Mehnert, A.; Obreschow, D.; Yu, D.-Y. Empirical retinal venous pulse wave velocity using modified photoplethysmography. BMC Res. Notes 2023, 16, 48. [Google Scholar] [CrossRef]
- Goldenberg, D.; Shahar, J.; Loewenstein, A.; Goldstein, M. Diameters of retinal blood vessels in a healthy cohort as measured by spectral domain optical coherence tomography. RETINA 2013, 33, 1888–1894. [Google Scholar] [CrossRef]
- Desissaire, S.; Pollreisz, A.; Sedova, A.; Hajdu, D.; Datlinger, F.; Steiner, S.; Vass, C.; Schwarzhans, F.; Fischer, G.; Pircher, M.; et al. Analysis of retinal nerve fiber layer birefringence in patients with glaucoma and diabetic retinopathy by polarization sensitive OCT. Biomed. Opt. Express 2020, 11, 5488–5505. [Google Scholar] [CrossRef]
- Motschi, A.R.; Schwarzhans, F.; Desissaire, S.; Steiner, S.; Bogunović, H.; Roberts, P.K.; Vass, C.; Hitzenberger, C.K.; Pircher, M. Quantitative assessment of depolarization by the retinal pigment epithelium in healthy and glaucoma subjects measured over a large field of view. PLoS ONE 2022, 17, e0278679. [Google Scholar] [CrossRef]
- Li, C.; Chua, J.; Schwarzhans, F.; Husain, R.; Girard, M.J.A.; Majithia, S.; Tham, Y.-C.; Cheng, C.-Y.; Aung, T.; Fischer, G.; et al. Assessing the external validity of machine learning-based detection of glaucoma. Sci. Rep. 2023, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Normal (N = 10) | Diabetes (N = 5) |
|---|---|---|
| Age ± SD, y | 49 ± 11 | 47 ± 8 |
| Age range, y | 30 to 64 | 33 to 59 |
| Eyes, N | 18 | 10 |
| Sum of vessels analyzed (artery/vein) | 44/45 | 30/31 |
| Sex (female/male), N | 4/6 | 2/3 |
| Height, mean ± SD, cm | 178 ± 8 | 173 ± 9 |
| Body mass index ± SD, kg/m2 | 25.3 ± 2.1 | 27.3 ± 2.2 |
| Smoking status | ||
| Never, N (%) | 7 (70%) | 4 (80%) |
| Former, N (%) | 2 (20%) | 1 (20%) |
| Current, N (%) | 1 (10%) | 0 (0%) |
| Clinical characteristics | ||
| Type I/II | N/A | 0/5 |
| Controlled/uncontrolled | N/A | 5/0 |
| Disease duration ± SD, y | N/A | 3.5 ± 1.3 |
| Disease duration, range, y | N/A | 1.5 to 7 |
| Diabetic retinopathy | N/A | 0 |
| Hypertension | N/A | 0 |
| BBI Average Values (m) | Average Birefringence (×10−4) | Average Thickness (µm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Artery | Vein | All | Artery | Vein | All | Artery | Vein | All | |
| Diabetic ± SD | 10.1 ± 1.8 | 10.8 ± 2.1 | 10.5 ± 1.9 | 12.4 ± 1.7 | 11.8 ± 1.1 | 12.1 ± 1.4 | 14 ± 1 | 15 ± 1 | 15 ± 1 |
| Normal ± SD | 35.8 ± 4.5 | 41.8 ± 3.6 | 38.8 ± 4.1 | 6.4 ± 1.2 | 5.9 ± 0.8 | 6.2 ± 1.0 | 15 ± 2 | 15 ± 2 | 15 ± 2 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.68 | <0.05 | <0.05 |
| BBI Thresholds (m) | ||
|---|---|---|
| Diabetic | Normal | |
| Artery | <15.6 | >15.8 |
| Vein | <13.8 | >13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afsharan, H.; Silva, D.; Joo, C.; Cense, B. Non-Invasive Retinal Blood Vessel Wall Measurements with Polarization-Sensitive Optical Coherence Tomography for Diabetes Assessment: A Quantitative Study. Biomolecules 2023, 13, 1230. https://doi.org/10.3390/biom13081230
Afsharan H, Silva D, Joo C, Cense B. Non-Invasive Retinal Blood Vessel Wall Measurements with Polarization-Sensitive Optical Coherence Tomography for Diabetes Assessment: A Quantitative Study. Biomolecules. 2023; 13(8):1230. https://doi.org/10.3390/biom13081230
Chicago/Turabian StyleAfsharan, Hadi, Dilusha Silva, Chulmin Joo, and Barry Cense. 2023. "Non-Invasive Retinal Blood Vessel Wall Measurements with Polarization-Sensitive Optical Coherence Tomography for Diabetes Assessment: A Quantitative Study" Biomolecules 13, no. 8: 1230. https://doi.org/10.3390/biom13081230
APA StyleAfsharan, H., Silva, D., Joo, C., & Cense, B. (2023). Non-Invasive Retinal Blood Vessel Wall Measurements with Polarization-Sensitive Optical Coherence Tomography for Diabetes Assessment: A Quantitative Study. Biomolecules, 13(8), 1230. https://doi.org/10.3390/biom13081230





