Biomolecular Minerals and Volcanic Glass Bio-Mimics to Control Adult Sand Flies, the Vector of Human Leishmania Protozoan Parasites
Abstract
1. Introduction
2. Materials and Methods
2.1. Sand Fly and Mechanical Insecticides
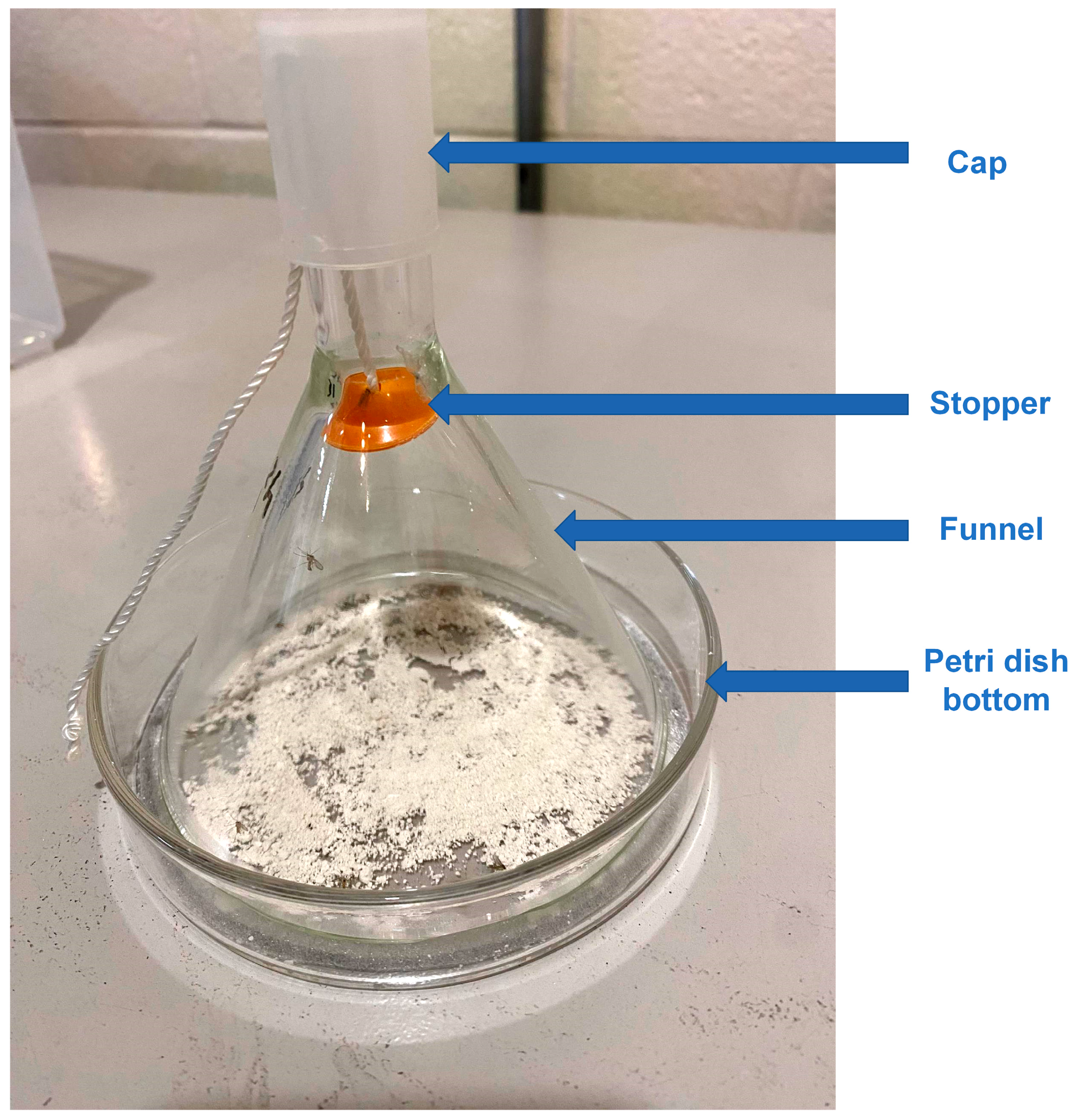
2.2. Bioassay
2.3. Bioassay with Light Source under the Petri Dish
2.4. Bioassay at High Humidity
2.5. Statistical Analysis
2.6. Scanning Microscopy
3. Results
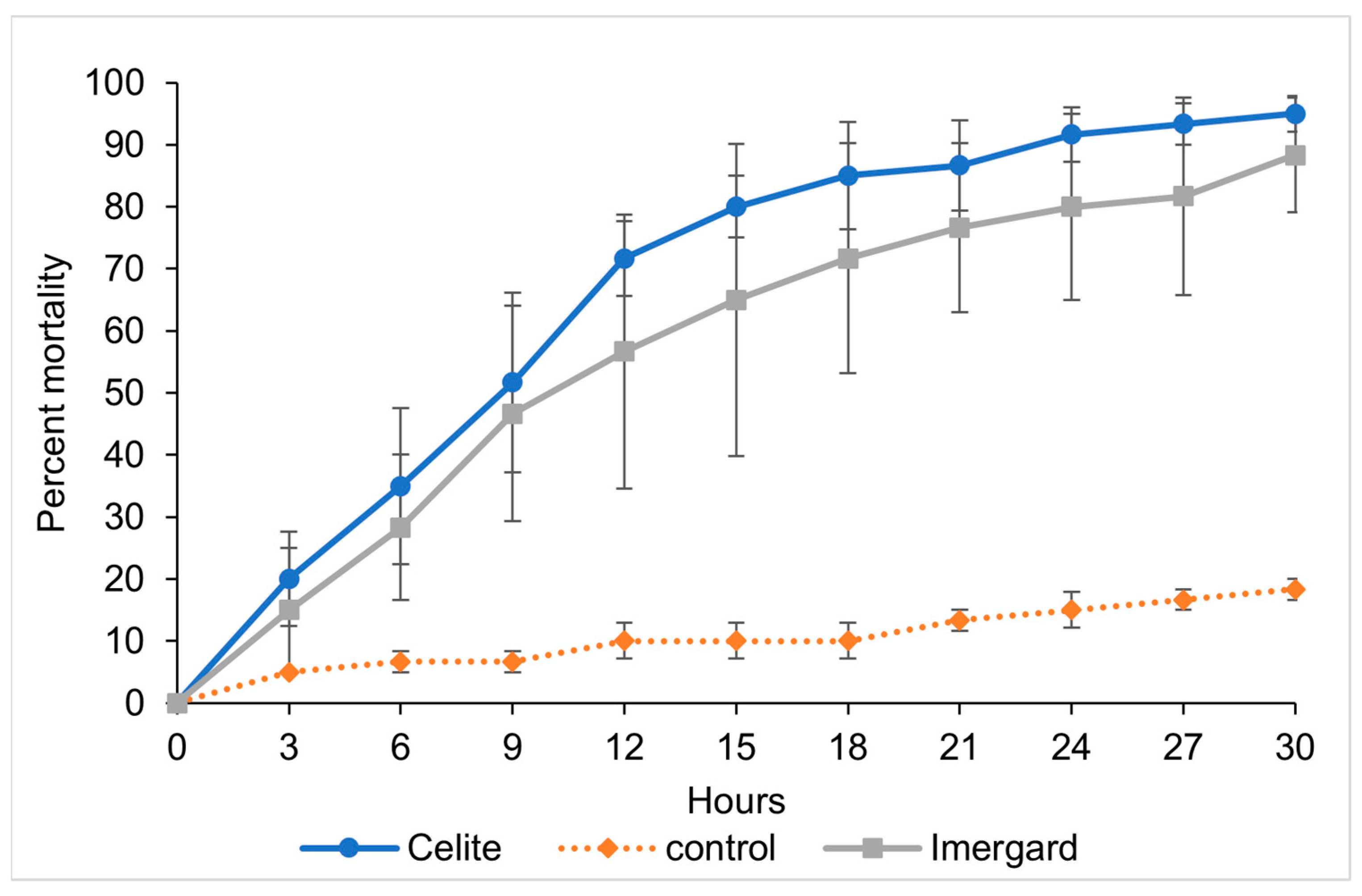
3.1. Efficacy of Imergard and Celite against Sand Flies
3.2. Effect of a Light Source on Sand Fly Mortality
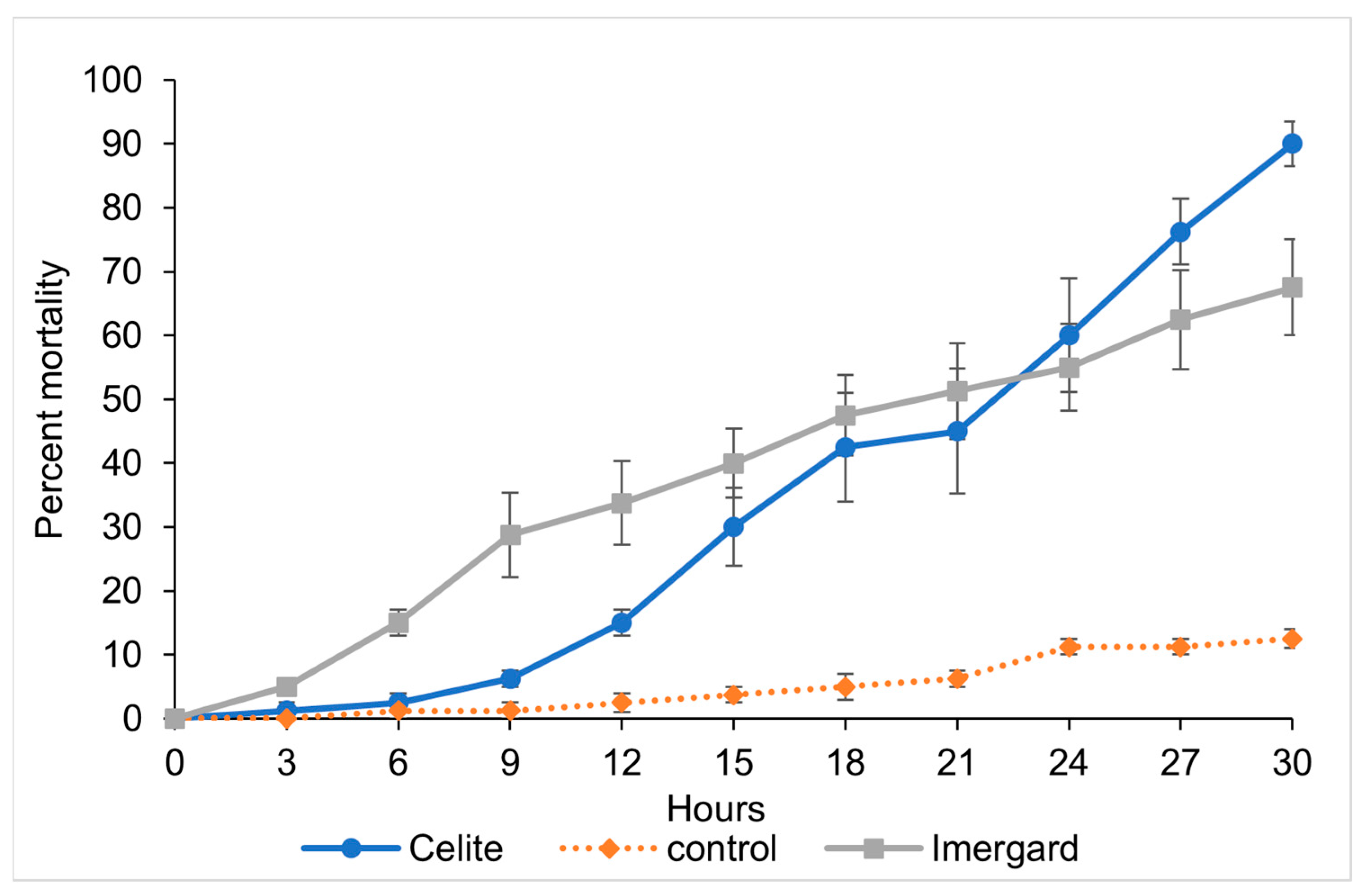
3.3. Effect of Humidity on Sand Fly Mortality against Minerals
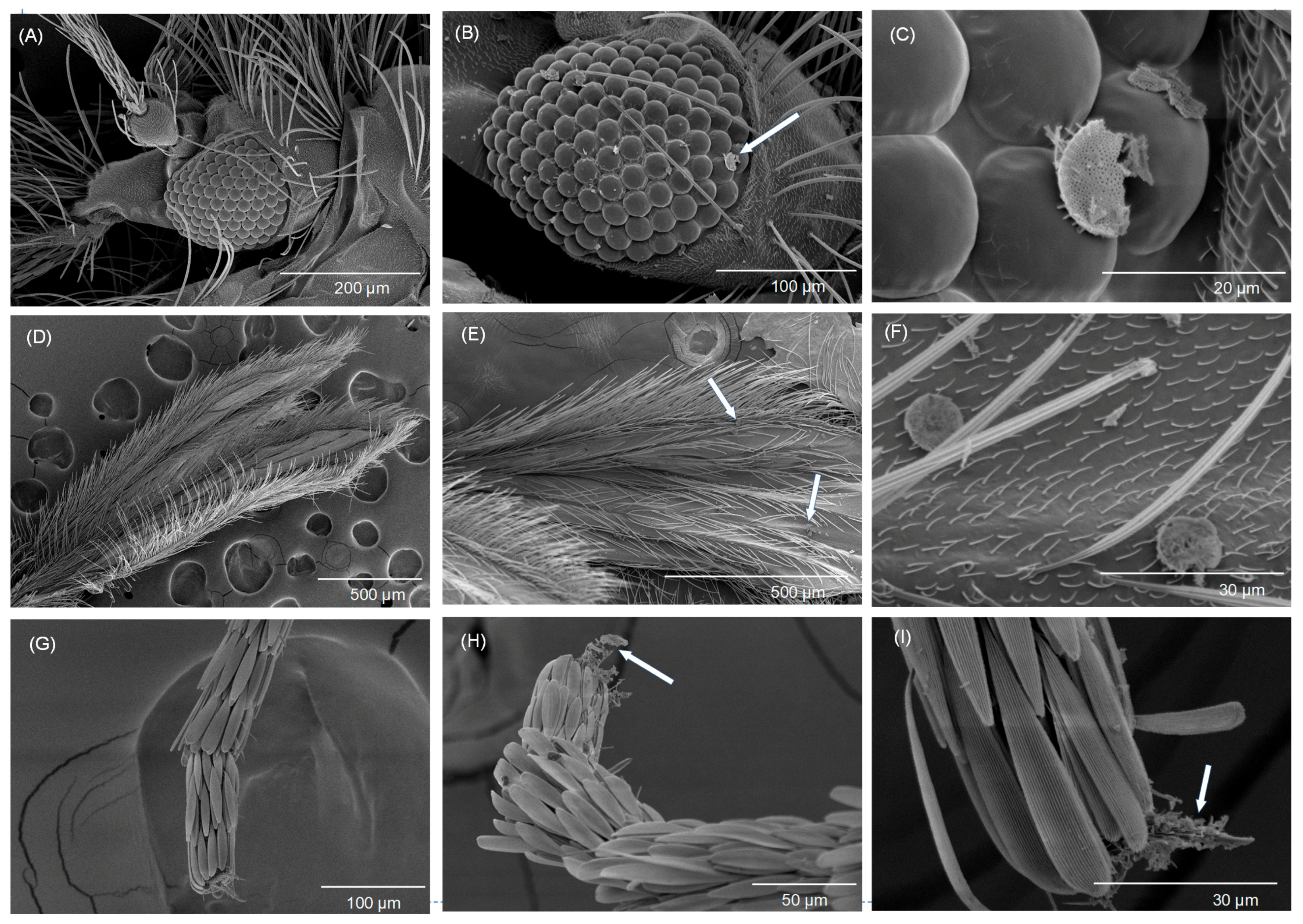
3.4. Scanning Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ready, P.D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Ready, P.D. Epidemiology of visceral leishmaniasis. Clin. Epidemiol. 2014, 6, 147–154. [Google Scholar] [CrossRef]
- Goto, H.; Lindoso, J.A.L. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev. Anti-Infect. Ther. 2010, 8, 419–433. [Google Scholar] [CrossRef] [PubMed]
- WHO. Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 28 July 2023).
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Alexander, B.; Maroli, M. Control of phlebotomine sandflies. Med. Vet. Entomol. 2003, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Faulde, M.K.; Scharninghausen, J.J.; Cavaljuga, S. Toxic and behavioural effects of different modified diatomaceous earths on the German cockroach, Blattella germanica (L.) (Orthoptera: Blattellidae) under simulated field conditions. J. Stored Prod. Res. 2006, 42, 253–263. [Google Scholar] [CrossRef]
- Doggett, S.L.; Geary, M.J.; Lilly, D.; Russell, R.C. The Efficacy of Diatomaceous Earth against the Common Bed Bug, Cimex Lectularius; Department of Medical Entomology: Sydney, Australia, 2008. [Google Scholar]
- Korunić, Z.; Liška, A.; Lucić, P.; Hamel, D.; Rozman, V. Evaluation of diatomaceous earth formulations enhanced with natural products against stored product insects. J. Stored Prod. Res. 2020, 86, 101565. [Google Scholar] [CrossRef]
- Deguenon, J.M.; Riegel, C.; Cloherty-Duvernay, E.R.; Chen, K.; Stewart, D.A.; Wang, B.; Gittins, D.; Tihomirov, L.; Apperson, C.S.; McCord, M.G.; et al. New mosquitocide derived from volcanic rock. J. Med. Entomol. 2020, 58, 458–464. [Google Scholar] [CrossRef]
- Deguenon, J.M.; Azondekon, R.; Agossa, F.R.; Padonou, G.G.; Anagonou, R.; Ahoga, J.; N’dombidje, B.; Akinro, B.; Stewart, D.A.; Wang, B. ImergardTM WP: A non-chemical alternative for an indoor residual spray, effective against pyrethroid-resistant Anopheles gambiae (sl) in Africa. Insects 2020, 11, 322. [Google Scholar] [CrossRef]
- Chen, K.; Deguenon, J.M.; Cave, G.; Denning, S.S.; Reiskind, M.H.; Watson, D.W.; Stewart, D.A.; Gittins, D.; Zheng, Y.; Liu, X.; et al. New thinking for filth fly control: Residual, non-chemical wall spray from volcanic glass. Med. Vet. Entomol. 2021, 35, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.A.; Ponnusamy, L.; Roe, R.M. Mechanical acaricides active against the blacklegged tick, Ixodes scapularis. Insects 2022, 13, 672. [Google Scholar] [CrossRef] [PubMed]
- Korunić, Z.K.; Rozman, V.; Liška, A.; Lucić, P. A review of natural insecticides based on diatomaceous earths. Poljoprivreda 2016, 22, 10–18. [Google Scholar] [CrossRef]
- Luan, K.; McCord, M.G.; West, A.J.; Cave, G.; Travanty, N.V.; Apperson, C.S.; Roe, R.M. Mosquito blood feeding prevention using an extra-low DC voltage charged cloth. Insects 2023, 14, 405. [Google Scholar] [CrossRef]
- WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticides on Treated Surfaces: Report of the WHO Informal Consultation, Geneva, 28–30 September 1998; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Hughes, A.; Matope, A.; Emery, M.; Steen, K.; Murray, G.; Ranson, H.; McCall, P.J.; Foster, G.M. A closer look at the WHO cone bioassay: Video analysis of the hidden effects of a human host on mosquito behaviour and insecticide contact. Malar. J. 2022, 21, 208. [Google Scholar] [CrossRef]
- Volf, P.; Ozbel, Y.; Akkafa, F.; Svobodová, M.; Votýpka, J.; Chang, K.P. Sand flies (Diptera: Phlebotominae) in Sanliurfa, Turkey: Relationship of Phlebotomus sergenti with the epidemic of anthroponotic cutaneous leishmaniasis. J. Med. Entomol. 2002, 39, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entom. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Korunic, Z. ReviewDiatomaceous earths, a group of natural insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Jacobson, L.R.; Studentsky, L.; Schlein, Y. Glycolytic and chitinolytic activities of Phlebotomus papatasi (Diptera: Psychodidae) from diverse ecological habitats. Folia Parasitol. 2007, 54, 301–309. [Google Scholar] [CrossRef]
- Singh, K.V. Studies on the role of climatological factors in the distribution of phlebotomine sandflies (Diptera: Psychodidae) in semi-arid areas of Rajasthan, India. J. Arid Environ. 1999, 42, 43–48. [Google Scholar] [CrossRef]
- Boussaa, S.; Guernaoui, S.; Pesson, B.; Boumezzough, A. Seasonal fluctuations of phlebotomine sand fly populations (Diptera: Psychodidae) in the urban area of Marrakech, Morocco. Acta Trop. 2005, 95, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Cave, G.L.; Richardson, E.A.; Chen, K.; Watson, D.W.; Roe, R.M. Acaricidal biominerals and mode-of-action studies against adult blacklegged ticks, Ixodes scapularis. Microorganisms 2023, 11, 1906. [Google Scholar] [CrossRef]


| Mineral | Environment | n | Slope (SE) + | LT50 (95% CL) ++ | LT80 (95% CL) ++ | χ2 +++ |
|---|---|---|---|---|---|---|
| Imergard | 29 ± 1 °C, 55% RH | 80 | 2.75 (0.20) | 13.08Aa (12.00–14.21) | 26.50Ab (23.77–30.31) | 43.65 |
| Celite | 29 ± 1 °C, 55% RH | 80 | 3.44 (0.22) | 7.57Ba (6.87–8.25) | 13.30Bb (12.28–14.49) | 44.35 |
| Imergard | 29 ± 1 °C, 55% RH, light | 80 | 2.17 (0.58) | 11.59ABa (7.06–16.46) | 28.34ACDb (19.31–75.89) | 205.64 |
| Celite | 29 ± 1 °C, 55% RH, light | 80 | 2.65 (0.30) | 8.23Ba (6.72–9.66) | 17.11Cb (14.63–20.80) | 51.62 |
| Imergard | 29 ± 1 °C, 70% RH | 80 | 1.81 (0.23) | 22.16C (18.92–27.34) | N/A | 53.90 |
| Celite | 29 ± 1 °C, 70% RH | 80 | 4.32 (1.13) | 20.82Ca (16.93–27.81) | 32.59ADa (25.25–64.80) | 418.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Deguenon, J.M.; Lawrie, R.D.; Roe, R.M. Biomolecular Minerals and Volcanic Glass Bio-Mimics to Control Adult Sand Flies, the Vector of Human Leishmania Protozoan Parasites. Biomolecules 2023, 13, 1235. https://doi.org/10.3390/biom13081235
Chen K, Deguenon JM, Lawrie RD, Roe RM. Biomolecular Minerals and Volcanic Glass Bio-Mimics to Control Adult Sand Flies, the Vector of Human Leishmania Protozoan Parasites. Biomolecules. 2023; 13(8):1235. https://doi.org/10.3390/biom13081235
Chicago/Turabian StyleChen, Kaiying, Jean Marcel Deguenon, Roger D. Lawrie, and R. Michael Roe. 2023. "Biomolecular Minerals and Volcanic Glass Bio-Mimics to Control Adult Sand Flies, the Vector of Human Leishmania Protozoan Parasites" Biomolecules 13, no. 8: 1235. https://doi.org/10.3390/biom13081235
APA StyleChen, K., Deguenon, J. M., Lawrie, R. D., & Roe, R. M. (2023). Biomolecular Minerals and Volcanic Glass Bio-Mimics to Control Adult Sand Flies, the Vector of Human Leishmania Protozoan Parasites. Biomolecules, 13(8), 1235. https://doi.org/10.3390/biom13081235







