Prebiotics Progress Shifts in the Intestinal Microbiome That Benefits Patients with Type 2 Diabetes Mellitus
Abstract
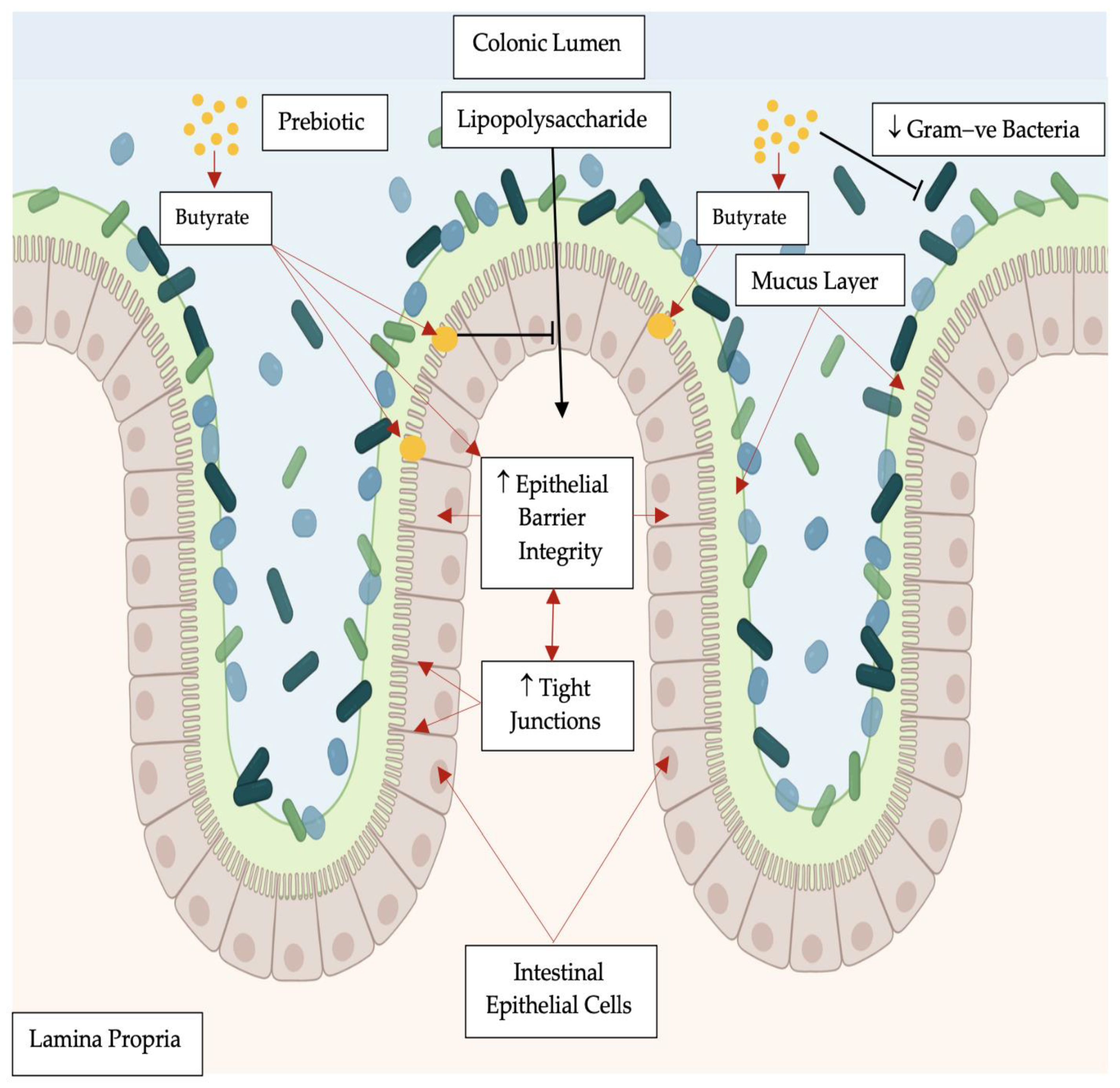
:1. Introduction
2. Pathogenesis of Diabetes Mellitus: Link between the Intestinal Microbiome Dysbiosis and Insulin Resistance
Fiber, Prebiotics, and Intestinal Bacteria
| PREBIOTICS (n) REFERENCE | TREATMENT TIME | DOSE ADMINISTERED | FLUID | FAECAL INTESTINAL MICROBIAL SHIFTS BY PREBIOTICS VERSUS CONTROLS |
|---|---|---|---|---|
| STUDIES WITH CHILDREN | ||||
| PDX/FOS * [n = 77] [67] | 2-weeks | 4.17 g PDX| 0.45 g FOS O.D. | in water | ↑ Bifidobacterium and Lactobacillus |
| AX [n = 10] [68] | 48 h | 10 g/L fluid | in water | ↑ Lactobacillus |
| STUDIES WITH ADULTS | ||||
| Inulin [n = 17 W] [69] | 8 days | 20 g/day to 40 g/day | in water | ↑ Bifidobacterium ↓ Enterococci|Enterobacteriaceae |
| Inulin [n = 10] [70] | 2-weeks | 8 g/day B.I.D. | in water | ↑ Bifidobacterium|↑ Clostridia |
| GOS [n = 18] [71] | 3-weeks | 2.5 g/day 5 g/day 10 g/day | edible chews | ↑ abundance Bifidobacterium ↑ Faecalibacterium prausnitzii ↓ Bacteroides |
| PDX [n = 20 M] [72] | 3-weeks | 21 g/day O.D. | mixed food | ↑ Faecalibacterium | Phascolarctobacterium| ↑ Dialister |
| PDX [n = 15] [73] | 3-weeks | 8 g/day O.D. | powder | ↑ Ruminococcus intestinalis| Clostridium clusters I, II and IV, ↓ abundance Lactobacillus| ↓ Enterococcus group |
| Lactulose [n = 12] [74] | 4-weeks | 20 g/day B.I.D. | mixed food | ↑ Bifidobacterium|Lactobacillus |
| Synthetic 2′-O-fucosyllactose and/or lacto-N-neotetraose [n = 44] [75] | 2-weeks | 5 10 20 g/day | Dietary bars | ↑ Actinobacteria | Bifidobacterium ↓ Firmicutes|Proteobacteria |
| Wheat Bran or Maltodextrin [n = 20] [76] | 3-weeks Cross-over study | 10 g/day | Dietary bars | ↑ Bifidobacterium adolescentis |
| PDX + SCF or placebo [n = 21] [77] | 3-weeks Cross-over study | 21 g/day PDX 21 g/day SCF | Dietary bars | With increased total corn fiber consumed from 10 g to 50 g/day Optimum mean daily consumption of 30–35 g/day Shift of the Bacteroidetes:Firmicutes ratio positively correlated to total dietary fiber intake. |
| Soluble Corn Fiber [n = 28 F] [78] | 4-weeks/dose [12-weeks] | 0 10 20 g/day | in water | ↑ Clostridium ↑ unclassified Clostridiaceae |
| XOS [n = 12 W [n = 11 M]] [79] | 8-weeks | 1.4 g/day or 2.8 g/day O.D. | capsule | ↑ Bacteroides fragilis|Bifidobacterium |
| XOS [n = 13] [80] | 3-weeks | 4 g/day | mixed food | ↑ Bifidobacterium species |
| Inulin or Maltodextrin [n = 44] [81] | 8-weeks Cross-over study | 12 g/day | in water | ↑ relative abundances Anaerostipes|Bilophila|Bifidobacterium |
| Crystalline Maize [Treat Arm 1} [n = 5 W 5 M] | 4-weeks dose escalation | 0 10 20 35 50 g/day 10 g/d to 50 g/d | powder sachets in water | Individualized effects with ↑ B. adolescentis|Parabacteroides spp.| ↑ Eisenbergiella spp. (Maize) |
| Tapioca RS4 [n = 5 W 5M] [Treat Arm 2] Digestible Corn Starch [n = 5 W 5 M] [Placebo Arm] Potato RS4 [n = 5 W 5 M] [82] [Treat Arm 3] | 4-weeks dose escalation 4-weeks dose escalation | 0 10 20 35 50 g/day 10 g/d to 50 g/d 0 10 20 35 50 g/day 10 g/d to 50 g/d | powder sachets in water powder sachets in water | ↑ E. rectale (Maize)↑ P. distasonis|Clostridiales (Tapioca extracted from cassava root) — → E. rectale → P. distasonis |
3. Functional Foods for T2DM
| PREBIOTICS (n) REFERENCE | TREATMENT TIME | DOSE ADMINISTERED | OUTCOMES |
|---|---|---|---|
| STUDIES with Prebiotics and T2DM | |||
| FOS * [n = 27 W] [47] | 8-weeks | 10 g/day O.D. | ↓ Fasting plasma glucose ↓ Glycosylated hemoglobin ↓ Interleukin-6 ↓ Tumor Necrosis Factor α ↓ Plasma Lipopolysaccharide |
| RS [n = 17] [104] | 12-weeks | 40 g/day O.D. | ↓ Postprandial Glucose (meal tolerance test) ↑ Glucagon-Like Peptide-1 ↓ Tumor Necrosis Factor |
| AX [n = 15] [105] | 5-weeks | 49.2 g/day | ↓ Fasting Serum Glucose Level. ↓ Serum Glucose ↓ Insulin Level (2 h after oral glucose intake) |
| STUDIES with Prebiotics + Pharmacotherapy and T2DM | |||
| Prebiotic Fiber + Sulfonylurea [n= 30] [28] | 24-weeks | Sulfonylurea + OZ101 an OLF 13.5 or 27 g/d T.I.D. | Low dose of 13.5 g/T.I.D. associated…
|
| Prebiotic Fiber + Metformin [n = 9 with 6 eligible] [106] | 1-week cross-over 4-week follow-up | Metformin (850 mg) O.D. increased B.I.D. + Prebiotic Fiber (BiomeBliss) O.D. | Modest shifts in microbial composition
|
4. Modulation of the Intestinal Microbiome in T2DM
| Patients with T2DM * (n) [Reference] | Faecal Microbiome Analysis | Gut Microbiome Characterizations with T2DM |
|---|---|---|
| (n = 18/36 M|T2DM) [110] | real-time quantitative PCR (qPCR) | Plasma glucose association with ↓ abundance Firmicutes phylum ↓ abundance Clostridia class ↑ abundance Betaproteobacteria class |
| subgroup (n = 20) tag-encoded amplicon pyrosequencing of the V4 region of the 16S rRNA gene | +ve association with ratios of Bacteroidetes > Firmicutes Bacteroides-Prevotella > C. coccoides-E. rectale | |
| (n = 345 and 23 T2DM) [111] | MGWAS | ↓ abundance of butyrate-producing bacteria Clostridiales sp. SS3/4|F. prausnitzii| Roseburia intestinalis|Eubacteriumrectale| Roseburia inulinivorans T2DM characterized by a moderate degree of intestinal microbial dysbiosis |
| ↑ abundance opportunistic pathogen bacteria Bacteroides caccae|Clostridium hathewayi| Clostridium symbiosum|Eggerthella lenta| Clostridium ramosum|Escherichia coli ↑ mucin-degrading bacteria Akkermansia muciniphila ↑ sulfate-reducing bacteria Desulfovibrio sp. 3_1_syn3 | ||
| (n = 2595 W| 9.5% T2DM 14.4% IGT [112] | SgSeq faecal meta-genome sample n = 145 W normal|impaired| diabetic glucose control | Further confirmation
Patients with T2DM ↑ abundance 4 Lactobacillus spp. L. gasseri highest ↓ abundance 5 Clostridium spp. Roseburia| Faecalibacterium prausnitzii|
|
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senchukova, M.A. Microbiota of the gastrointestinal tract: Friend or foe? World J. Gastroenterol. 2023, 29, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lemme-Dumit, J.M.; Polti, M.A.; Perdigón, G.; Galdeano, C.M. Probiotic bacteria cell walls stimulate the activity of the intestinal epithelial cells and macrophage functionality. Benef. Microbes 2018, 9, 153–164. [Google Scholar] [CrossRef]
- Wan, L.Y.; Chen, Z.J.; Shah, N.P.; El-Nezami, H. Modulation of Intestinal Epithelial Defense Responses by Probiotic Bacteria. Crit. Rev. Food Sci. Nutr. 2016, 56, 2628–2641. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Kim, D.; Zeng, M.Y.; Núñez, G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp. Mol. Med. 2017, 49, e339. [Google Scholar] [CrossRef] [PubMed]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Gosiewski, T.; Bulanda, M.; Kuśnierz-Cabala, B.; Gałązka, K.; Konturek, P.C. Synergic Interaction of Rifaximin and Mutaflor (Escherichia coli Nissle 1917) in the Treatment of Acetic Acid-Induced Colitis in Rats. Gastroenterol. Res. Pract. 2016, 2016, 3126280. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Ahmed, A.; Wu, D.; Liu, L.; Qiu, J.; Yan, Y.; Jin, M.; Xin, Y. Gut microbe analysis between hyperthyroid and healthy individuals. Curr. Microbiol. 2014, 69, 675–680. [Google Scholar] [CrossRef]
- Zhao, F.; Feng, J.; Li, J.; Zhao, L.; Liu, Y.; Chen, H.; Jin, Y.; Zhu, B.; Wei, Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid 2018, 28, 175–186. [Google Scholar] [CrossRef]
- Chakera, A.J.; Pearce, S.H.; Vaidya, B. Treatment for primary hypothyroidism: Current approaches and future possibilities. Drug Des. Dev. Ther. 2012, 6, 1–11. [Google Scholar]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Moreira, C.G.; Russell, R.; Mishra, A.A.; Narayanan, S.; Ritchie, J.M.; Waldor, M.K.; Curtis, M.M.; Winter, S.E.; Weinshenker, D.; Sperandio, V. Bacterial Adrenergic Sensors Regulate Virulence of Enteric Pathogens in the Gut. mBio 2016, 7, djw029. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef]
- Jaworek, J.; Tudek, B.; Kowalczyk, P.; Kot, M.; Szklarczyk, J.; Leja-Szpak, A.; Pierzchalski, P.; Bonior, J.; Dembiński, A.; Ceranowicz, P.; et al. Effect of Endotoxemia in Suckling Rats on Pancreatic Integrity and Exocrine Function in Adults: A Review Report. Gastroenterol. Res. Pract. 2018, 2018, 6915059. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Ojo, O.; Wang, X.; Ojo, O.O.; Brooke, J.; Jiang, Y.; Dong, Q.; Thompson, T. The Effect of Prebiotics and Oral Anti-Diabetic Agents on Gut Microbiome in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Nutrients 2022, 14, 5139. [Google Scholar] [CrossRef]
- Palacios, T.; Vitetta, L.; Coulson, S.; Madigan, C.D.; Lam, Y.Y.; Manuel, R.; Briskey, D.; Hendy, C.; Kim, J.N.; Ishoey, T.; et al. Targeting the Intestinal Microbiota to Prevent Type 2 Diabetes and Enhance the Effect of Metformin on Glycaemia: A Randomised Controlled Pilot Study. Nutrients 2020, 12, 2041. [Google Scholar] [CrossRef]
- Gorgani, N.N.; Kim, K.H.S.; Free, W.L.; Afkham, M.; Henson, J.D.; Shamanna, P.; Ajdari, S.; Kahn, C.R.; Hirst, T.R.; Barnett, A.H.; et al. OZ101, an oligofructose prebiotic, may prolong sulphonylurea efficacy in patients with type 2 diabetes: A pilot study. J. Curr. Med. Res. Opin. 2022, 5, 1235–1251. [Google Scholar]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar]
- Walker, A.W.; Lawley, T.D. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 2013, 69, 75–86. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Cersosimo, E.; DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., Eds.; Copyright © 2000–2023; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ağagündüz, D.; Icer, M.A.; Yesildemir, O.; Koçak, T.; Kocyigit, E.; Capasso, R. The roles of dietary lipids and lipidomics in gut-brain axis in type 2 diabetes mellitus. J. Transl. Med. 2023, 21, 240. [Google Scholar] [CrossRef]
- McClain, D.A. Glucose Toxicity. In Encyclopedia of Endocrine Diseases; Martini, L., Ed.; Elsevier Inc.: London, UK, 2004. [Google Scholar]
- Filippello, A.; Di Mauro, S.; Scamporrino, A.; Malaguarnera, R.; Torrisi, S.A.; Leggio, G.M.; Di Pino, A.; Scicali, R.; Purrello, F.; Piro, S. High Glucose Exposure Impairs L-Cell Differentiation in Intestinal Organoids: Molecular Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2021, 22, 6660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ha, S.; Lau, H.C.; Yu, J. Excess body weight: Novel insights into its roles in obesity comorbidities. Semin. Cancer Biol. 2023, 92, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Yang, S.; Lu, J.; Jin, X.; Wu, M. Diet-gut microbiota-epigenetics in metabolic diseases: From mechanisms to therapeutics. Biomed. Pharmacother. 2022, 153, 113290. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediat. Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Gordon, S. Elie Metchnikoff, the Man and the Myth. J. Innate Immun. 2016, 8, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Cai, M.; Shen, B.; Wang, Q.; Zhang, T.; Zhou, X. Synbiotics and Gut Microbiota: New Perspectives in the Treatment of Type 2 Diabetes Mellitus. Foods 2022, 11, 2438. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, N. Effects of Metformin on the Gut Microbiota in Obesity and Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 5003–5014. [Google Scholar] [CrossRef]
- Belorkar, S.A.; Gupta, A.K. Oligosaccharides: A boon from nature’s desk. AMB Express 2016, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-abadi, M. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized controlled clinical trial. Nutrition 2014, 30, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Kaul, R.; Abdellatif, B.; Arabi, M.; Upadhyay, R.; Saliba, R.; Sebah, M.; Chaari, A. The Promising Role of Microbiome Therapy on Biomarkers of Inflammation and Oxidative Stress in Type 2 Diabetes: A Systematic and Narrative Review. Front. Nutr. 2022, 9, 906243. [Google Scholar] [CrossRef]
- An, R.; Zong, A.; Chen, S.; Xu, R.; Zhang, R.; Jiang, W.; Liu, L.; Du, F.; Zhang, H.; Xu, T. Effects of oligosaccharides on the markers of glycemic control: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2022, 13, 8766–8782. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Chavan, A.R.; Singh, A.K.; Gupta, R.K.; Nakhate, S.P.; Poddar, B.J.; Gujar, V.V.; Purohit, H.J.; Khardenavis, A.A. Recent trends in the biotechnology of functional non-digestible oligosaccharides with prebiotic potential. Biotechnol. Genet. Eng. Rev. 2023, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; de Pasquale, C.; Ekinci, E.I.; Coughlan, M.T. Gut microbiome, prebiotics, intestinal permeability and diabetes complications. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101507. [Google Scholar] [CrossRef] [PubMed]
- Enam, F.; Mansell, T.J. Prebiotics: Tools to manipulate the gut microbiome and metabolome. J. Ind. Microbiol. Biotechnol. 2019, 46, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Faas, M.M.; de Vos, P.; Akkerman, R. Impact of dietary fibers in infant formulas on gut microbiota and the intestinal immune barrier. Food Funct. 2020, 11, 9445–9467. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, R.; Faas, M.M.; de Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497. [Google Scholar] [CrossRef]
- Paone, P.; Suriano, F.; Jian, C.; Korpela, K.; Delzenne, N.M.; Van Hul, M.; Salonen, A.; Cani, P.D. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes 2022, 14, 2152307. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Z.; Yang, Z.; Qiao, X. Microbiota-derived short-chain fatty acids and modulation of host-derived peptides formation: Focused on host defense peptides. Biomed. Pharmacother. 2023, 162, 114586. [Google Scholar] [CrossRef]
- Golpour, F.; Abbasi-Alaei, M.; Babaei, F.; Mirzababaei, M.; Parvardeh, S.; Mohammadi, G.; Nassiri-Asl, M. Short chain fatty acids, a possible treatment option for autoimmune diseases. Biomed. Pharmacother. 2023, 163, 114763. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; Van de Wiele, T.; Verstraete, W.; Delcour, J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Perez Santiago, J.; Iablokov, S.N.; Chopra, D.; Rodionov, D.A.; Peterson, S.N. Short-Chain Fatty Acids Modulate Healthy Gut Microbiota Composition and Functional Potential. Curr. Microbiol. 2022, 79, 128. [Google Scholar] [CrossRef] [PubMed]
- Toporovski, M.S.; de Morais, M.B.; Abuhab, A.; Crippa Júnior, M.A. Effect of Polydextrose/Fructooligosaccharide Mixture on Constipation Symptoms in Children Aged 4 to 8 Years. Nutrients 2021, 13, 1634. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Englyst, H.N.; Macfarlane, S.; Furrie, E.; Macfarlane, G.T.; McBain, A.J. Degradation of cross-linked and non-cross-linked arabinoxylans by the intestinal microbiota in children. Appl Environ. Microbiol. 2003, 69, 6354–6360. [Google Scholar] [CrossRef]
- Kleessen, B.; Sykura, B.; Zunft, H.J.; Blaut, M. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am. J. Clin. Nutr. 1997, 65, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Finlay, R.K.; Wynne, A.G.; Gibson, G.R. Human Volunteer Study on the Prebiotic Effects of HP-Inulin—Faecal Bacteria Enumerated Using Fluorescent In Situ Hybridisation (FISH). Anaerobe 2001, 7, 113–118. [Google Scholar] [CrossRef]
- Davis, L.M.; Martínez, I.; Walter, J.; Goin, C.; Hutkins, R.W. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS ONE 2011, 6, e25200. [Google Scholar] [CrossRef]
- Hooda, S.; Boler, B.M.; Serao, M.C.; Brulc, J.M.; Staeger, M.A.; Boileau, T.W.; Dowd, S.E.; Fahey, G.C., Jr.; Swanson, K.S. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J. Nutr. 2012, 142, 1259–1265. [Google Scholar] [CrossRef]
- Costabile, A.; Fava, F.; Röytiö, H.; Forssten, S.D.; Olli, K.; Klievink, J.; Rowland, I.R.; Ouwehand, A.C.; Rastall, R.A.; Gibson, G.R.; et al. Impact of polydextrose on the faecal microbiota: A double-blind, crossover, placebo-controlled feeding study in healthy human subjects. Br. J. Nutr. 2012, 108, 471–481. [Google Scholar] [CrossRef]
- Ballongue, J.; Schumann, C.; Quignon, P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand. J. Gastroenterol. Suppl. 1997, 222, 41–44. [Google Scholar] [CrossRef]
- Elison, E.; Vigsnaes, L.K.; Rindom Krogsgaard, L.; Rasmussen, J.; Sørensen, N.; McConnell, B.; Hennet, T.; Sommer, M.O.; Bytzer, P. Oral supplementation of healthy adults with 2′-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br. J. Nutr. 2016, 116, 1356–1368. [Google Scholar] [CrossRef]
- Windey, K.; De Preter, V.; Huys, G.; Broekaert, W.F.; Delcour, J.A.; Louat, T.; Herman, J.; Verbeke, K. Wheat bran extract alters colonic fermentation and microbial composition, but does not affect faecal water toxicity: A randomised controlled trial in healthy subjects. Br. J. Nutr. 2015, 113, 225–238. [Google Scholar] [CrossRef]
- Holscher, H.D.; Caporaso, J.G.; Hooda, S.; Brulc, J.M.; Fahey, G.C., Jr.; Swanson, K.S. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: Follow-up of a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 55–64. [Google Scholar] [CrossRef]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; Story, J.A.; MacDonald-Clarke, C.J.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J. Nutr. 2016, 146, 1298–1306. [Google Scholar] [CrossRef]
- Finegold, S.M.; Li, Z.; Summanen, P.H.; Downes, J.; Thames, G.; Corbett, K.; Dowd, S.; Krak, M.; Heber, D. Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota. Food Funct. 2014, 5, 436–445. [Google Scholar] [CrossRef]
- Chung, Y.C.; Hsu, C.K.; Ko, C.Y.; Chan, Y.C. Dietary intake of xylooligosaccharides improves the intestinal microbiota, fecal moisture, and pH value in the elderly. Nutr Res. 2007, 27, 756–761. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e386. [Google Scholar] [CrossRef]
- Havenaar, R. Intestinal health functions of colonic microbial metabolites: A review. Benef. Microbes 2011, 2, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Liu, J.L.; Segovia, I.; Yuan, X.L.; Gao, Z.H. Controversial Roles of Gut Microbiota-Derived Short-Chain Fatty Acids (SCFAs) on Pancreatic β-Cell Growth and Insulin Secretion. Int. J. Mol. Sci. 2020, 21, 910. [Google Scholar] [CrossRef]
- Sunkara, L.T.; Jiang, W.; Zhang, G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS ONE 2012, 7, e49558. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kasuno, K.; Takahashi, N.; Taniguchi, T.; Iwano, M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 499–505. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. T(H)2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Yan, J.; Takakura, A.; Zandi-Nejad, K.; Charles, J.F. Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes 2018, 9, 84–92. [Google Scholar] [CrossRef]
- Garde, S.; Chodisetti, P.K.; Reddy, M. Peptidoglycan: Structure, Synthesis, and Regulation. EcoSal Plus 2021, 9, 2. [Google Scholar] [CrossRef]
- Wolf, A.J.; Underhill, D.M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 2018, 18, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Boix-Amorós, A.; Selma-Royo, M.; Mira, A.; Collado, M.C. Gut Microbiota and Mucosal Immunity in the Neonate. Med. Sci. 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Rawi, M.H.; Zaman, S.A.; Pa’ee, K.F.; Leong, S.S.; Sarbini, S.R. Prebiotics metabolism by gut-isolated probiotics. J. Food Sci. Technol. 2020, 57, 2786–2799. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Zhang, X.F.; Guan, X.X.; Tang, Y.J.; Sun, J.F.; Wang, X.K.; Wang, W.D.; Fan, J.M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef]
- Liu, N.; Wang, H.; Yang, Z.; Zhao, K.; Li, S.; He, N. The role of functional oligosaccharides as prebiotics in ulcerative colitis. Food Funct. 2022, 13, 6875–6893. [Google Scholar] [CrossRef]
- Hartemink, R.; Quataert, M.C.; van Laere, K.M.; Nout, M.J.; Rombouts, F.M. Degradation and fermentation of fructo-oligosaccharides by oral streptococci. J. Appl. Bacteriol. 1995, 79, 551–557. [Google Scholar] [CrossRef]
- Hartemink, R.; Van Laere, K.M.; Rombouts, F.M. Growth of enterobacteria on fructo-oligosaccharides. J. Appl. Microbiol. 1997, 83, 367–374. [Google Scholar] [CrossRef]
- Van Laere, K.M.; Hartemink, R.; Bosveld, M.; Schols, H.A.; Voragen, A.G. Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria. J. Agric. Food Chem. 2000, 48, 1644–1652. [Google Scholar] [CrossRef]
- Li, K.-K.; Tian, P.-J.; Wang, S.-D.; Lei, P.; Qu, L.; Huang, J.-P.; Shan, Y.-J.; Li, B.-L. Targeting gut microbiota: Lactobacillus alleviated type 2 diabetes via inhibiting LPS secretion and activating GPR43 pathway. J. Funct. Foods 2017, 561–570. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Bodinham, C.L.; Smith, L.; Thomas, E.L.; Bell, J.D.; Swann, J.R.; Costabile, A.; Russell-Jones, D.; Umpleby, A.M.; Robertson, M.D. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr. Connect. 2014, 3, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; O’Dea, K. Arabinoxylan fibre improves metabolic control in people with Type II diabetes. Eur. J. Clin. Nutr. 2004, 58, 621–628. [Google Scholar] [CrossRef]
- Dixon, S.A.; Mishra, S.; Dietsche, K.B.; Jain, S.; Mabundo, L.; Stagliano, M.; Krenek, A.; Courville, A.; Yang, S.; Turner, S.A.; et al. The effects of prebiotics on gastrointestinal side effects of metformin in youth: A pilot randomized control trial in youth-onset type 2 diabetes. Front. Endocrinol. 2023, 14, 1125187. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Kosinsky, R.L. Epigenetics of Aging and Aging-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 401. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef]
- Ling, C. Epigenetic regulation of insulin action and secretion–role in the pathogenesis of type 2 diabetes. J. Intern. Med. 2020, 288, 158–167. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Artasensi, A.; Mazzolari, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Therapeutic Options. Molecules 2023, 28, 3094. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, K.; Fan, H.; Wei, M.; Xiong, Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1114424. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.G.; Costa, J.A.; Alfenas, R.C.G. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism 2017, 68, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Boroni Moreira, A.P.; de Cássia Gonçalves Alfenas, R. The influence of endotoxemia on the molecular mechanisms of insulin resistance. Nutr. Hosp. 2012, 27, 382–390. [Google Scholar]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef]
- Cox, A.J.; Zhang, P.; Bowden, D.W.; Devereaux, B.; Davoren, P.M.; Cripps, A.W.; West, N.P. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017, 43, 163–166. [Google Scholar] [CrossRef]
- Demir, E.; Ozkan, H.; Seckin, K.D.; Sahtiyancı, B.; Demir, B.; Tabak, O.; Kumbasar, A.; Uzun, H. Plasma Zonulin Levels as a Non-Invasive Biomarker of Intestinal Permeability in Women with Gestational Diabetes Mellitus. Biomolecules 2019, 9, 24. [Google Scholar] [CrossRef]
- Vitetta, L.; Bambling, M.; Strodl, E. Probiotics and Commensal Bacteria Metabolites Trigger Epigenetic Changes in the Gut and Influence Beneficial Mood Dispositions. Microorganisms 2023, 11, 1334. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef]
- Gueddouri, D.; Caüzac, M.; Fauveau, V.; Benhamed, F.; Charifi, W.; Beaudoin, L.; Rouland, M.; Sicherre, F.; Lehuen, A.; Postic, C.; et al. Insulin resistance per se drives early and reversible dysbiosis-mediated gut barrier impairment and bactericidal dysfunction. Mol. Metab. 2022, 57, 101438. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Stirling, K.; Yang, J.J.; Zhang, L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J. Diabetes. 2020, 11, 293–308. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenet. 2012, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2022, 13, 1103836. [Google Scholar] [CrossRef]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Piquer-Esteban, S.; Ruiz-Ruiz, S.; Arnau, V.; Diaz, W.; Moya, A. Exploring the universal healthy human gut microbiota around the World. Comput. Struct. Biotechnol. J. 2022, 20, 421–433. [Google Scholar] [CrossRef]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Sai Prashanthi, G.; Ali, M.H.; Sharma, S.; Tyagi, M.; Shivaji, S. Dysbiosis in the Gut Bacterial Microbiome of Patients with Uveitis, an Inflammatory Disease of the Eye. Indian J. Microbiol. 2018, 58, 457–469. [Google Scholar] [CrossRef]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef]
- Azpiroz, F.; Molne, L.; Mendez, S.; Nieto, A.; Manichanh, C.; Mego, M.; Accarino, A.; Santos, J.; Sailer, M.; Theis, S.; et al. Effect of Chicory-derived Inulin on Abdominal Sensations and Bowel Motor Function. J. Clin. Gastroenterol. 2017, 51, 619–625. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proc. Nutr. Soc. 2016, 75, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Seki, N.; Hamano, K.; Ochi, H.; Abe, F.; Masuda, K.; Iino, H. Prebiotic effect of two grams of lactulose in healthy Japanese women: A randomised, double-blind, placebo-controlled crossover trial. Benef. Microbes 2019, 10, 629–639. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int. J. Food Microbiol. 2011, 149, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Kamikado, K.; Aoki, R.; Suganuma, N.; Nishijima, T.; Nakatani, A.; Kimura, I. Bifidobacterium animalis subsp. lactis GCL2505 modulates host energy metabolism via the short-chain fatty acid receptor GPR43. Sci. Rep. 2020, 10, 4158. [Google Scholar] [CrossRef]
- Boyanova, L.; Markovska, R.; Yordanov, D.; Gergova, R.; Hadzhiyski, P. Anaerobes in specific infectious and noninfectious diseases: New developments. Anaerobe 2023, 81, 102714. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Cui, J.; Ramesh, G.; Wu, M.; Jensen, E.T.; Crago, O.; Bertoni, A.G.; Gao, C.; Hoffman, K.L.; Sheridan, P.A.; Wong, K.E.; et al. Butyrate-Producing Bacteria and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES). Diabetes 2022, 71, 2438–2446. [Google Scholar] [CrossRef]
- Cheng, H.L.; Yen, G.C.; Huang, S.C.; Chen, S.C.; Hsu, C.L. The next generation beneficial actions of novel probiotics as potential therapeutic targets and prediction tool for metabolic diseases. J. Food Drug Anal. 2022, 30, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitetta, L.; Gorgani, N.N.; Vitetta, G.; Henson, J.D. Prebiotics Progress Shifts in the Intestinal Microbiome That Benefits Patients with Type 2 Diabetes Mellitus. Biomolecules 2023, 13, 1307. https://doi.org/10.3390/biom13091307
Vitetta L, Gorgani NN, Vitetta G, Henson JD. Prebiotics Progress Shifts in the Intestinal Microbiome That Benefits Patients with Type 2 Diabetes Mellitus. Biomolecules. 2023; 13(9):1307. https://doi.org/10.3390/biom13091307
Chicago/Turabian StyleVitetta, Luis, Nick N. Gorgani, Gemma Vitetta, and Jeremy D. Henson. 2023. "Prebiotics Progress Shifts in the Intestinal Microbiome That Benefits Patients with Type 2 Diabetes Mellitus" Biomolecules 13, no. 9: 1307. https://doi.org/10.3390/biom13091307
APA StyleVitetta, L., Gorgani, N. N., Vitetta, G., & Henson, J. D. (2023). Prebiotics Progress Shifts in the Intestinal Microbiome That Benefits Patients with Type 2 Diabetes Mellitus. Biomolecules, 13(9), 1307. https://doi.org/10.3390/biom13091307







