The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases
Abstract
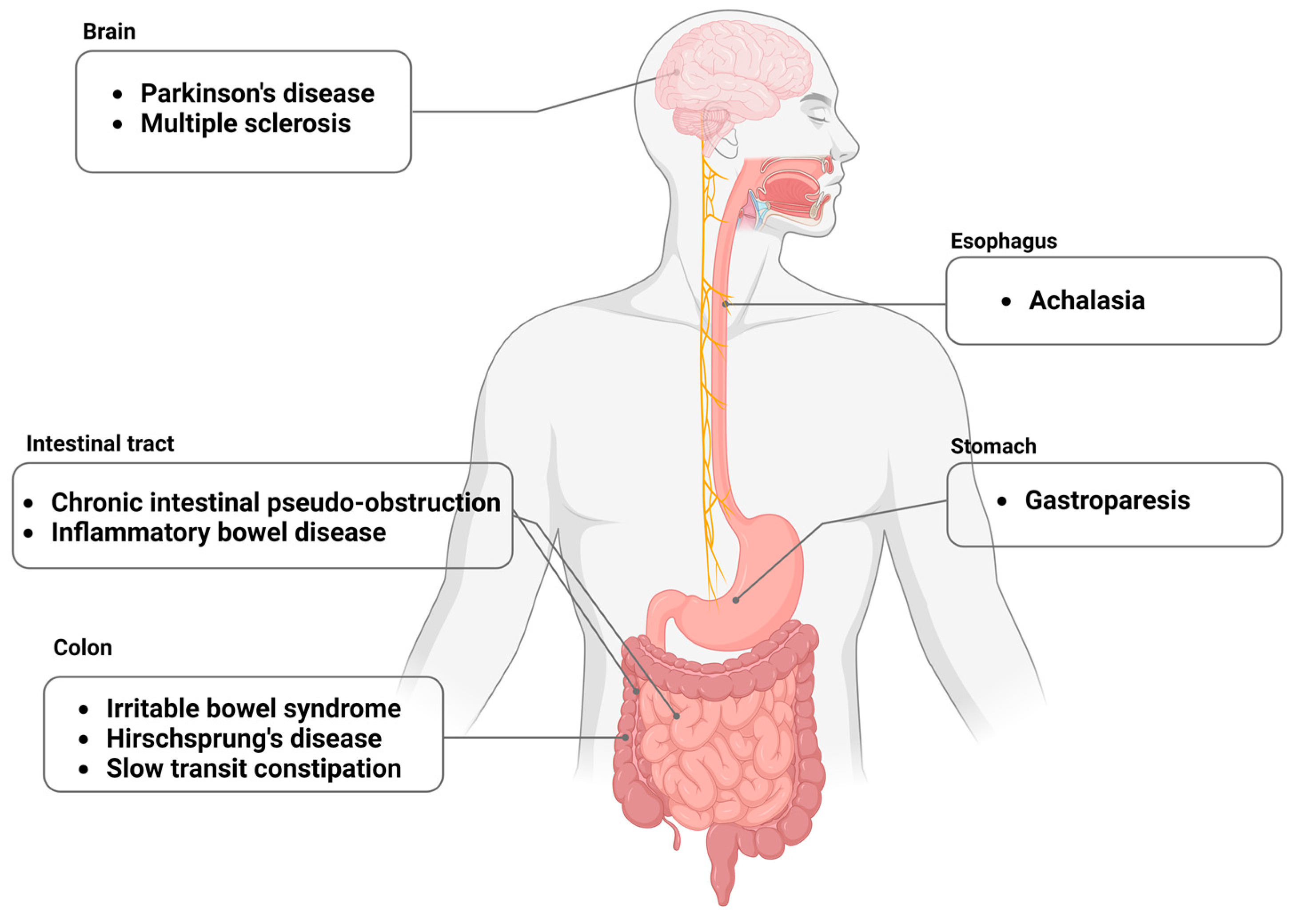
1. Introduction
2. Aging
3. Gastroparesis
4. Irritable Bowel Syndrome
5. Esophageal Achalasia
6. Hirschsprung’s Disease
7. Parkinson’s Disease
8. Multiple Sclerosis
9. Slow-Transit Constipation
10. Chronic Intestinal Pseudo-Obstruction
11. Inflammatory Bowel Disease
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Goldstein, A.M.; Thapar, N.; Karunaratne, T.B.; De Giorgio, R. Clinical aspects of neurointestinal disease: Pathophysiology, diagnosis, and treatment. Dev. Biol. 2016, 417, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Di Lorenzo, C.; Farrugia, G.; Hamilton, F.A.; Mawe, G.M.; Pasricha, P.J.; Wiley, J.W. Functional Bowel Disorders: A Roadmap to Guide the Next Generation of Research. Gastroenterology 2018, 154, 723–735. [Google Scholar] [CrossRef]
- Gubatan, J.; Zikos, T.; Spear Bishop, E.; Wu, J.; Gottfried, A.; Becker, L.; Habtezion, A.; Neshatian, L. Gastrointestinal symptoms and healthcare utilization have increased among patients with functional gastrointestinal and motility disorders during the COVID-19 pandemic. Neurogastroenterol. Motil. 2022, 34, e14243. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Kito, Y.; Hwang, S.J.; Ward, S.M. Regulation of Gastrointestinal Smooth Muscle Function by Interstitial Cells. Physiology 2016, 31, 316–326. [Google Scholar] [CrossRef]
- Huizinga, J.D.; Hussain, A.; Chen, J.H. Interstitial cells of Cajal and human colon motility in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G552–G575. [Google Scholar] [CrossRef] [PubMed]
- López-Pingarrón, L.; Almeida, H.; Soria-Aznar, M.; Reyes-Gonzales, M.C.; Rodríguez-Moratinos, A.B.; Muñoz-Hoyos, A.; García, J.J. Interstitial Cells of Cajal and Enteric Nervous System in Gastrointestinal and Neurological Pathology, Relation to Oxidative Stress. Curr. Issues Mol. Biol. 2023, 45, 3552–3572. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ward, S.M. Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br. J. Pharmacol. 2019, 176, 212–227. [Google Scholar] [CrossRef]
- McCann, C.J.; Cooper, J.E.; Natarajan, D.; Jevans, B.; Burnett, L.E.; Burns, A.J.; Thapar, N. Transplantation of enteric nervous system stem cells rescues nitric oxide synthase deficient mouse colon. Nat. Commun. 2017, 8, 15937. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, P.J.; Grover, M.; Yates, K.P.; Abell, T.L.; Koch, K.L.; McCallum, R.W.; Sarosiek, I.; Bernard, C.E.; Kuo, B.; Bulat, R.; et al. Progress in Gastroparesis—A Narrative Review of the Work of the Gastroparesis Clinical Research Consortium. Clin. Gastroenterol. Hepatol. 2022, 20, 2684–2695. [Google Scholar] [CrossRef]
- Nguyen, V.T.T.; Taheri, N.; Chandra, A.; Hayashi, Y. Aging of enteric neuromuscular systems in gastrointestinal tract. Neurogastroenterol. Motil. 2022, 34, e14352. [Google Scholar] [CrossRef]
- Camilleri, M.; Cowen, T.; Koch, T.R. Enteric neurodegeneration in ageing. Neurogastroenterol. Motil. 2008, 20, 185–196. [Google Scholar] [CrossRef]
- Cox, N.J.; Ibrahim, K.; Sayer, A.A.; Robinson, S.M.; Roberts, H.C. Assessment and Treatment of the Anorexia of Aging: A Systematic Review. Nutrients 2019, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Chapman, I.; Luscombe-Marsh, N.; Feinle-Bisset, C.; Horowitz, M.; Soenen, S. Ageing Is Associated with Decreases in Appetite and Energy Intake—A Meta-Analysis in Healthy Adults. Nutrients 2016, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public. Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Gomez-Pinilla, P.J.; Gibbons, S.J.; Sarr, M.G.; Kendrick, M.L.; Shen, K.R.; Cima, R.R.; Dozois, E.J.; Larson, D.W.; Ordog, T.; Pozo, M.J.; et al. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol. Motil. 2011, 23, 36–44. [Google Scholar] [CrossRef]
- Truong Thuy Nguyen, V.; Taheri, N.; Choi, E.L.; Kellogg, T.A.; Linden, D.R.; Hayashi, Y. Insulin-Like Growth Factor1 Preserves Gastric Pacemaker Cells and Motor Function in Aging via ERK1/2 Activation. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 369–383. [Google Scholar] [CrossRef]
- Izbeki, F.; Asuzu, D.T.; Lorincz, A.; Bardsley, M.R.; Popko, L.N.; Choi, K.M.; Young, D.L.; Hayashi, Y.; Linden, D.R.; Kuro-o, M.; et al. Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice. J. Physiol. 2010, 588, 3101–3117. [Google Scholar] [CrossRef]
- Soenen, S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. The ageing gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 12–18. [Google Scholar] [CrossRef]
- Asuzu, D.T.; Hayashi, Y.; Izbeki, F.; Popko, L.N.; Young, D.L.; Bardsley, M.R.; Lorincz, A.; Kuro-O, M.; Linden, D.R.; Farrugia, G.; et al. Generalized neuromuscular hypoplasia, reduced smooth muscle myosin and altered gut motility in the klotho model of premature aging. Neurogastroenterol. Motil. 2011, 23, e309–e323. [Google Scholar] [CrossRef][Green Version]
- Broad, J.; Kung, V.W.S.; Palmer, A.; Elahi, S.; Karami, A.; Darreh-Shori, T.; Ahmed, S.; Thaha, M.A.; Carroll, R.; Chin-Aleong, J.; et al. Changes in neuromuscular structure and functions of human colon during ageing are region-dependent. Gut 2019, 68, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Sanders, K.M. Gastroparesis. Gastroenterology 2022, 162, 68–87.e1. [Google Scholar] [CrossRef] [PubMed]
- Dilmaghani, S.; Zheng, T.; Camilleri, M. Epidemiology and Healthcare Utilization in Patients With Gastroparesis: A Systematic Review. Clin. Gastroenterol. Hepatol. 2023, 21, 2239–2251.e2. [Google Scholar] [CrossRef] [PubMed]
- Ordög, T.; Takayama, I.; Cheung, W.K.; Ward, S.M.; Sanders, K.M. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes 2000, 49, 1731–1739. [Google Scholar] [CrossRef]
- Grover, M.; Farrugia, G.; Lurken, M.S.; Bernard, C.E.; Faussone-Pellegrini, M.S.; Smyrk, T.C.; Parkman, H.P.; Abell, T.L.; Snape, W.J.; Hasler, W.L.; et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 2011, 140, 1575–1585.e8. [Google Scholar] [CrossRef]
- Horváth, V.J.; Vittal, H.; Ordög, T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes 2005, 54, 1528–1533. [Google Scholar] [CrossRef]
- Horváth, V.J.; Vittal, H.; Lörincz, A.; Chen, H.; Almeida-Porada, G.; Redelman, D.; Ordög, T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology 2006, 130, 759–770. [Google Scholar] [CrossRef]
- Lorincz, A.; Redelman, D.; Horváth, V.J.; Bardsley, M.R.; Chen, H.; Ordög, T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology 2008, 134, 1083–1093. [Google Scholar] [CrossRef]
- Hayashi, Y.; Asuzu, D.T.; Gibbons, S.J.; Aarsvold, K.H.; Bardsley, M.R.; Lomberk, G.A.; Mathison, A.J.; Kendrick, M.L.; Shen, K.R.; Taguchi, T.; et al. Membrane-to-nucleus signaling links insulin-like growth factor-1- and stem cell factor-activated pathways. PLoS ONE 2013, 8, e76822. [Google Scholar] [CrossRef]
- Hayashi, Y.; Toyomasu, Y.; Saravanaperumal, S.A.; Bardsley, M.R.; Smestad, J.A.; Lorincz, A.; Eisenman, S.T.; Cipriani, G.; Nelson Holte, M.H.; Al Khazal, F.J.; et al. Hyperglycemia Increases Interstitial Cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, Leading to Rapid Gastric Emptying. Gastroenterology 2017, 153, 521–535.e20. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Kudva, Y.; Basu, A.; Camilleri, M.; Low, P.A.; Vella, A.; Zinsmeister, A.R. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin. Gastroenterol. Hepatol. 2015, 13, 466–476.e1. [Google Scholar] [CrossRef]
- Singh, R.; Ha, S.E.; Wei, L.; Jin, B.; Zogg, H.; Poudrier, S.M.; Jorgensen, B.G.; Park, C.; Ronkon, C.F.; Bartlett, A.; et al. miR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility. Gastroenterology 2021, 160, 1662–1678.e18. [Google Scholar] [CrossRef]
- Cipriani, G.; Gibbons, S.J.; Kashyap, P.C.; Farrugia, G. Intrinsic Gastrointestinal Macrophages: Their Phenotype and Role in Gastrointestinal Motility. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 120–130.e121. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Gibbons, S.J.; Nguyen, T.V.; Stoltz, G.J.; Lurken, M.S.; Ordog, T.; Szurszewski, J.H.; Farrugia, G. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 2008, 135, 2055–2064.e2. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Kashyap, P.C.; Dutta, N.; Stoltz, G.J.; Ordog, T.; Shea Donohue, T.; Bauer, A.J.; Linden, D.R.; Szurszewski, J.H.; Gibbons, S.J.; et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology 2010, 138, 2399–2409.e1. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Irritable bowel syndrome: Treatment based on pathophysiology and biomarkers. Gut 2023, 72, 590–599. [Google Scholar] [CrossRef]
- Camilleri, M. Management Options for Irritable Bowel Syndrome. Mayo Clin. Proc. 2018, 93, 1858–1872. [Google Scholar] [CrossRef]
- Camilleri, M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA 2021, 325, 865–877. [Google Scholar] [CrossRef]
- Eijsbouts, C.; Zheng, T.; Kennedy, N.A.; Bonfiglio, F.; Anderson, C.A.; Moutsianas, L.; Holliday, J.; Shi, J.; Shringarpure, S.; Voda, A.I.; et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat. Genet. 2021, 53, 1543–1552. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Hillestad, E.M.R.; van der Meeren, A.; Nagaraja, B.H.; Bjørsvik, B.R.; Haleem, N.; Benitez-Paez, A.; Sanz, Y.; Hausken, T.; Lied, G.A.; Lundervold, A.; et al. Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J. Gastroenterol. 2022, 28, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, G.; Shi, Q.; Yang, S.; Ma, T.; Mishra, S.K.; Wen, A.; Xu, H.; Wang, Q.; Jiang, Y.; et al. Characterizing the microbiota in gastrointestinal tract segments of Rhabdophis subminiatus: Dynamic changes and functional predictions. Microbiologyopen 2019, 8, e00789. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Donato, S.; Di Pardo, A.; Maglione, V.; Filosa, S.; Crispi, S. New Therapeutic Drugs from Bioactive Natural Molecules: The Role of Gut Microbiota Metabolism in Neurodegenerative Diseases. Curr. Drug Metab. 2018, 19, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.M.; Kearney, S.M.; Smillie, C.S.; Alm, E.J. Two dynamic regimes in the human gut microbiome. PLoS Comput. Biol. 2017, 13, e1005364. [Google Scholar] [CrossRef] [PubMed]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Ther. Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Perez Ingles, D.; Myneedu, K.; Deoker, A.; Sarosiek, I.; Zuckerman, M.J.; Schmulson, M.J.; Bashashati, M. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2019, 31, e13718. [Google Scholar] [CrossRef]
- Krammer, L.; Sowa, A.S.; Lorentz, A. Mast Cells in Irritable Bowel Syndrome: A Systematic Review. J. Gastrointestin. Liver Dis. 2019, 28, 463–472. [Google Scholar] [CrossRef]
- Borriello, F.; Iannone, R.; Marone, G. Histamine Release from Mast Cells and Basophils. Handb. Exp. Pharmacol. 2017, 241, 121–139. [Google Scholar] [CrossRef]
- Shulman, R.J.; Jarrett, M.E.; Cain, K.C.; Broussard, E.K.; Heitkemper, M.M. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J. Gastroenterol. 2014, 49, 1467–1476. [Google Scholar] [CrossRef]
- Duraisamy, K.; Premkumar, K.; Selvakumar, G.P.; Thangavel, R.; Ahmed, M.E.; Zaheer, S.A.; Raikwar, S.P.; Dubova, L.; Iyer, S.S.; Zaheer, A. Mast Cells Augment Neuroinflammation and Neurodegeneration. FASEB J. 2019, 33, 791–795. [Google Scholar] [CrossRef]
- Liu, B.; Sjölander, A.; Pedersen, N.L.; Ludvigsson, J.F.; Chen, H.; Fang, F.; Wirdefeldt, K. Irritable bowel syndrome and Parkinson’s disease risk: Register-based studies. NPJ Park. Dis. 2021, 7, 5. [Google Scholar] [CrossRef]
- Eshraghian, A.; Eshraghian, H. Interstitial cells of Cajal: A novel hypothesis for the pathophysiology of irritable bowel syndrome. Can. J. Gastroenterol. 2011, 25, 277–279. [Google Scholar] [CrossRef][Green Version]
- Wouters, M.M.; Gibbons, S.J.; Roeder, J.L.; Distad, M.; Ou, Y.; Strege, P.R.; Szurszewski, J.H.; Farrugia, G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology 2007, 133, 897–906. [Google Scholar] [CrossRef]
- Atkinson, W.; Lockhart, S.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 2006, 130, 34–43. [Google Scholar] [CrossRef]
- Liu, H.N.; Ohya, S.; Nishizawa, Y.; Sawamura, K.; Iino, S.; Syed, M.M.; Goto, K.; Imaizumi, Y.; Nakayama, S. Serotonin augments gut pacemaker activity via 5-HT3 receptors. PLoS ONE 2011, 6, e24928. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Sultan, S.; Chang, L.; Heidelbaugh, J.J.; Smalley, W.; Verne, G.N. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Diarrhea. Gastroenterology 2022, 163, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Sultan, S.; Lembo, A.; Verne, G.N.; Smalley, W.; Heidelbaugh, J.J. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology 2022, 163, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xiong, T.; Grabauskas, G.; Owyang, C. Mucosal Serotonin Reuptake Transporter Expression in Irritable Bowel Syndrome Is Modulated by Gut Microbiota Via Mast Cell-Prostaglandin E2. Gastroenterology 2022, 162, 1962–1974.e6. [Google Scholar] [CrossRef] [PubMed]
- Matheis, F.; Muller, P.A.; Graves, C.L.; Gabanyi, I.; Kerner, Z.J.; Costa-Borges, D.; Ahrends, T.; Rosenstiel, P.; Mucida, D. Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss. Cell 2020, 180, 64–78.e16. [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D.; Bashashati, M.; Hirota, S.A.; Gui, X.; Roberts, J.A.; MacDonald, J.A.; Muruve, D.A.; McKay, D.M.; Beck, P.L.; Mawe, G.M.; et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 2012, 18, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Der, T.; Bercik, P.; Donnelly, G.; Jackson, T.; Berezin, I.; Collins, S.M.; Huizinga, J.D. Interstitial cells of cajal and inflammation-induced motor dysfunction in the mouse small intestine. Gastroenterology 2000, 119, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J.; Boeckxstaens, G. The spectrum of achalasia: Lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology 2013, 145, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Trieu, J.A.; Dua, A.; Enofe, I.; Shastri, N.; Venu, M. Population trends in achalasia diagnosis and management: A changing paradigm. Dis. Esophagus 2021, 34, doab014. [Google Scholar] [CrossRef]
- Gregersen, H.; Pedersen, J.; Drewes, A.M. Deterioration of muscle function in the human esophagus with age. Dig. Dis. Sci. 2008, 53, 3065–3070. [Google Scholar] [CrossRef]
- Wu, M.; Van Nassauw, L.; Kroese, A.B.; Adriaensen, D.; Timmermans, J.P. Myenteric nitrergic neurons along the rat esophagus: Evidence for regional and strain differences in age-related changes. Histochem. Cell Biol. 2003, 119, 395–403. [Google Scholar] [CrossRef]
- Qualman, S.J.; Haupt, H.M.; Yang, P.; Hamilton, S.R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology 1984, 87, 848–856. [Google Scholar] [CrossRef]
- Zizer, E.; Beilke, S.; Bäuerle, T.; Schilling, K.; Möhnle, U.; Adler, G.; Fischer, K.D.; Wagner, M. Loss of Lsc/p115 protein leads to neuronal hypoplasia in the esophagus and an achalasia-like phenotype in mice. Gastroenterology 2010, 139, 1344–1354. [Google Scholar] [CrossRef]
- Savarino, E.; Gemignani, L.; Zentilin, P.; De Bortoli, N.; Malesci, A.; Mastracci, L.; Fiocca, R.; Savarino, V. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin. Gastroenterol. Hepatol. 2011, 9, 1104–1106. [Google Scholar] [CrossRef]
- Boeckxstaens, G.E. Achalasia: Virus-induced euthanasia of neurons? Am. J. Gastroenterol. 2008, 103, 1610–1612. [Google Scholar] [CrossRef] [PubMed]
- Gockel, I.; Becker, J.; Wouters, M.M.; Niebisch, S.; Gockel, H.R.; Hess, T.; Ramonet, D.; Zimmermann, J.; Vigo, A.G.; Trynka, G.; et al. Common variants in the HLA-DQ region confer susceptibility to idiopathic achalasia. Nat. Genet. 2014, 46, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Salter, A.K.; Hennig, G.W.; Koh, S.D.; Perrino, B.A.; Ward, S.M.; Baker, S.A. Responses to enteric motor neurons in the gastric fundus of mice with reduced intramuscular interstitial cells of cajal. J. Neurogastroenterol. Motil. 2014, 20, 171–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hwang, S.J.; Drumm, B.T.; Kim, M.K.; Lyu, J.H.; Baker, S.; Sanders, K.M.; Ward, S.M. Calcium transients in intramuscular interstitial cells of Cajal of the murine gastric fundus and their regulation by neuroeffector transmission. J. Physiol. 2022, 600, 4439–4463. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sanders, K.M.; Ward, S.M. Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res. 2000, 302, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.J.; Bayguinov, Y.; Sanders, K.M.; Ward, S.M. Relationship between enteric neurons and interstitial cells in the primate gastrointestinal tract. Neurogastroenterol. Motil. 2012, 24, e437–e449. [Google Scholar] [CrossRef]
- Horiguchi, K.; Sanders, K.M.; Ward, S.M. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tissue Res. 2003, 311, 299–313. [Google Scholar] [CrossRef]
- Iino, S.; Ward, S.M.; Sanders, K.M. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J. Physiol. 2004, 556, 521–530. [Google Scholar] [CrossRef]
- Sivarao, D.V.; Mashimo, H.L.; Thatte, H.S.; Goyal, R.K. Lower esophageal sphincter is achalasic in nNOS(-/-) and hypotensive in W/W(v) mutant mice. Gastroenterology 2001, 121, 34–42. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Chen, W.F.; Wang, Y.; Xu, X.Y.; Zeng, Y.G.; Lee Dillon, D.; Cheng, J.; Xu, M.D.; Zhong, Y.S.; Zhang, Y.Q.; et al. Mast cell infiltration associated with loss of interstitial cells of Cajal and neuronal degeneration in achalasia. Neurogastroenterol. Motil. 2019, 31, e13565. [Google Scholar] [CrossRef]
- Qian, H.; Wang, Y.; Chen, X.; Lin, L.; Zhang, W.; Tang, N.; Si, X.; Jiao, C.; Zhang, G.; Ye, B. “M1/M2” Muscularis Macrophages Are Associated with Reduction of Interstitial Cells of Cajal and Glial Cells in Achalasia. Dig. Dis. Sci. 2023, 68, 1260–1268. [Google Scholar] [CrossRef]
- Ambartsumyan, L.; Smith, C.; Kapur, R.P. Diagnosis of Hirschsprung Disease. Pediatr. Dev. Pathol. 2020, 23, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.C. Hirschsprung disease. Curr. Opin. Pediatr. 2013, 25, 368–374. [Google Scholar] [CrossRef]
- Ahmad, H.; Levitt, M.A.; Yacob, D.; Halleran, D.R.; Gasior, A.C.; Di Lorenzo, C.; Wood, R.J.; Langer, J.C. Evaluation and Management of Persistent Problems After Surgery for Hirschsprung Disease in a Child. Curr. Gastroenterol. Rep. 2021, 23, 18. [Google Scholar] [CrossRef]
- Tilghman, J.M.; Ling, A.Y.; Turner, T.N.; Sosa, M.X.; Krumm, N.; Chatterjee, S.; Kapoor, A.; Coe, B.P.; Nguyen, K.H.; Gupta, N.; et al. Molecular Genetic Anatomy and Risk Profile of Hirschsprung’s Disease. N. Engl. J. Med. 2019, 380, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; de Graaff, E.; Pachnis, V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron 2003, 40, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Chen, F.; Milewski, R.; Li, J.; Lu, M.M.; Epstein, J.A. Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret. J. Clin. Investig. 2000, 106, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.; Anderson, D.J. Postmigratory neural crest cells expressing c-RET display restricted developmental and proliferative capacities. Neuron 1995, 15, 527–539. [Google Scholar] [CrossRef]
- Gosain, A.; Brinkman, A.S. Hirschsprung’s associated enterocolitis. Curr. Opin. Pediatr. 2015, 27, 364–369. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Coles, M.C.; Foster, K.E.; Patel, A.; Williams, A.; Natarajan, D.; Barlow, A.; Pachnis, V.; Kioussis, D. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature 2007, 446, 547–551. [Google Scholar] [CrossRef]
- Banks, T.A.; Rouse, B.T.; Kerley, M.K.; Blair, P.J.; Godfrey, V.L.; Kuklin, N.A.; Bouley, D.M.; Thomas, J.; Kanangat, S.; Mucenski, M.L. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 1995, 155, 1685–1693. [Google Scholar] [CrossRef]
- Davis, I.A.; Rouse, B.T. Immune responsiveness of lymphotoxin-alpha-deficient mice: Two reconstitution models. Cell Immunol. 1998, 189, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kumaraguru, U.; Davis, I.A.; Deshpande, S.; Tevethia, S.S.; Rouse, B.T. Lymphotoxin alpha-/- mice develop functionally impaired CD8+ T cell responses and fail to contain virus infection of the central nervous system. J. Immunol. 2001, 166, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Druckenbrod, N.R.; Epstein, M.L. Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development 2009, 136, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; Barlow-Anacker, A.J.; Erickson, C.S.; Pierre, J.F.; Heneghan, A.F.; Epstein, M.L.; Kudsk, K.A. Impaired Cellular Immunity in the Murine Neural Crest Conditional Deletion of Endothelin Receptor-B Model of Hirschsprung’s Disease. PLoS ONE 2015, 10, e0128822. [Google Scholar] [CrossRef]
- Chen, X.; Meng, X.; Zhang, H.; Feng, C.; Wang, B.; Li, N.; Abdullahi, K.M.; Wu, X.; Yang, J.; Li, Z.; et al. Intestinal proinflammatory macrophages induce a phenotypic switch in interstitial cells of Cajal. J. Clin. Investig. 2020, 130, 6443–6456. [Google Scholar] [CrossRef]
- Coyle, D.; Kelly, D.A.; O’Donnell, A.M.; Gillick, J.; Puri, P. Use of anoctamin 1 (ANO1) to evaluate interstitial cells of Cajal in Hirschsprung’s disease. Pediatr. Surg. Int. 2016, 32, 125–133. [Google Scholar] [CrossRef]
- Gu, A.; Wu, Z.; Wang, P.; Liu, J.; Wang, J.; Wang, Q.; Chen, J. Downregulation of ICCs and PDGFRα+ cells on colonic dysmotility in hirschsprung disease. Front. Pediatr. 2022, 10, 975799. [Google Scholar] [CrossRef]
- Newman, C.J.; Laurini, R.N.; Lesbros, Y.; Reinberg, O.; Meyrat, B.J. Interstitial cells of Cajal are normally distributed in both ganglionated and aganglionic bowel in Hirschsprung’s disease. Pediatr. Surg. Int. 2003, 19, 662–668. [Google Scholar] [CrossRef]
- Bettolli, M.; De Carli, C.; Jolin-Dahel, K.; Bailey, K.; Khan, H.F.; Sweeney, B.; Krantis, A.; Staines, W.A.; Rubin, S. Colonic dysmotility in postsurgical patients with Hirschsprung’s disease. Potential significance of abnormalities in the interstitial cells of Cajal and the enteric nervous system. J. Pediatr. Surg. 2008, 43, 1433–1438. [Google Scholar] [CrossRef]
- Daniel, E.E.; Posey-Daniel, V. Neuromuscular structures in opossum esophagus: Role of interstitial cells of Cajal. Am. J. Physiol. 1984, 246, G305–G315. [Google Scholar] [CrossRef]
- Nemeth, L.; Maddur, S.; Puri, P. Immunolocalization of the gap junction protein Connexin43 in the interstitial cells of Cajal in the normal and Hirschsprung’s disease bowel. J. Pediatr. Surg. 2000, 35, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Coyle, D.; Doyle, B.; Murphy, J.M.; O’Donnell, A.M.; Gillick, J.; Puri, P. Expression of connexin 26 and connexin 43 is reduced in Hirschsprung’s disease. J. Surg. Res. 2016, 206, 242–251. [Google Scholar] [CrossRef]
- Totland, M.Z.; Rasmussen, N.L.; Knudsen, L.M.; Leithe, E. Regulation of gap junction intercellular communication by connexin ubiquitination: Physiological and pathophysiological implications. Cell. Mol. Life Sci. 2020, 77, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.M.; Yu, S.Y.; Guo, P.; Du, Y.; Hu, Y.; Piao, Y.S.; Zuo, L.J.; Lian, T.H.; Wang, R.D.; Yu, Q.J.; et al. Nonmotor symptoms in patients with Parkinson disease: A cross-sectional observational study. Medicine 2016, 95, e5400. [Google Scholar] [CrossRef]
- Breckenridge, C.B.; Berry, C.; Chang, E.T.; Sielken, R.L.; Mandel, J.S. Association between Parkinson’s Disease and Cigarette Smoking, Rural Living, Well-Water Consumption, Farming and Pesticide Use: Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151841. [Google Scholar] [CrossRef]
- Lee, A.; Gilbert, R.M. Epidemiology of Parkinson Disease. Neurol. Clin. 2016, 34, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Hilton, D.; Stephens, M.; Kirk, L.; Edwards, P.; Potter, R.; Zajicek, J.; Broughton, E.; Hagan, H.; Carroll, C. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014, 127, 235–241. [Google Scholar] [CrossRef]
- Araki, N.; Yamanaka, Y.; Poudel, A.; Fujinuma, Y.; Katagiri, A.; Kuwabara, S.; Asahina, M. Electrogastrography for diagnosis of early-stage Parkinson’s disease. Park. Relat. Disord. 2021, 86, 61–66. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Banach, T.; Zurowski, D.; Gil, K.; Krygowska-Wajs, A.; Marszałek, A.; Thor, P.J. Peripheral mechanisms of intestinal dysmotility in rats with salsolinol induced experimental Parkinson’s disease. J. Physiol. Pharmacol. 2006, 57, 291–300. [Google Scholar] [PubMed]
- Hou, M.; Mao, X.; Liu, Y.; Hou, X.; Bi, X. Could Abnormal Distribution of Interstitial Cells of Cajal be Involved in Gastrointestinal Disorders in Patients with Parkinson’s Disease? Arch. Neurol. Neurosci. 2018, 1. [Google Scholar] [CrossRef]
- Adler, C.H.; Beach, T.G. Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov. Disord. 2016, 31, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Heimrich, K.G.; Jacob, V.Y.P.; Schaller, D.; Stallmach, A.; Witte, O.W.; Prell, T. Gastric dysmotility in Parkinson’s disease is not caused by alterations of the gastric pacemaker cells. NPJ Park. Dis. 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Kenna, J.E.; Bakeberg, M.C.; Gorecki, A.M.; Chin Yen Tay, A.; Winter, S.; Mastaglia, F.L.; Anderton, R.S. Characterization of Gastrointestinal Symptom Type and Severity in Parkinson’s Disease: A Case-Control Study in an Australian Cohort. Mov. Disord. Clin. Pract. 2021, 8, 245–253. [Google Scholar] [CrossRef]
- Padhi, P.; Worth, C.; Zenitsky, G.; Jin, H.; Sambamurti, K.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Mechanistic Insights Into Gut Microbiome Dysbiosis-Mediated Neuroimmune Dysregulation and Protein Misfolding and Clearance in the Pathogenesis of Chronic Neurodegenerative Disorders. Front. Neurosci. 2022, 16, 836605. [Google Scholar] [CrossRef]
- The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019, 93, 688. [CrossRef]
- Miller, D.H.; Weinshenker, B.G.; Filippi, M.; Banwell, B.L.; Cohen, J.A.; Freedman, M.S.; Galetta, S.L.; Hutchinson, M.; Johnson, R.T.; Kappos, L.; et al. Differential diagnosis of suspected multiple sclerosis: A consensus approach. Mult. Scler. 2008, 14, 1157–1174. [Google Scholar] [CrossRef]
- Levinthal, D.J.; Rahman, A.; Nusrat, S.; O’Leary, M.; Heyman, R.; Bielefeldt, K. Adding to the burden: Gastrointestinal symptoms and syndromes in multiple sclerosis. Mult. Scler. Int. 2013, 2013, 319201. [Google Scholar] [CrossRef]
- Wunsch, M.; Jabari, S.; Voussen, B.; Enders, M.; Srinivasan, S.; Cossais, F.; Wedel, T.; Boettner, M.; Schwarz, A.; Weyer, L.; et al. The enteric nervous system is a potential autoimmune target in multiple sclerosis. Acta Neuropathol. 2017, 134, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Ding, Y.; Xue, R.; Jia, Z.; Huang, Z.; Ding, Y.; Gu, C.; Yang, J. Involvement of interstitial cells of Cajal in bladder dysfunction in mice with experimental autoimmune encephalomyelitis. Int. Urol. Nephrol. 2017, 49, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- den Braber-Ymker, M.; Heijker, S.; Lammens, M.; Croockewit, S.; Nagtegaal, I.D. Intestinal involvement in amyloidosis is a sequential process. Neurogastroenterol. Motil. 2018, 30, e13469. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Lacy, B.E. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020, 158, 1232–1249.e1233. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, K.; Busciglio, I.; Burton, D.; Breen-Lyles, M.K.; Camilleri, M. Expanding criteria for slow colonic transit in patients being evaluated for chronic constipation by scintigraphy. Neurogastroenterol. Motil. 2020, 32, e13878. [Google Scholar] [CrossRef]
- He, C.L.; Burgart, L.; Wang, L.; Pemberton, J.; Young-Fadok, T.; Szurszewski, J.; Farrugia, G. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology 2000, 118, 14–21. [Google Scholar] [CrossRef]
- Bassotti, G.; Villanacci, V.; Maurer, C.A.; Fisogni, S.; Di Fabio, F.; Cadei, M.; Morelli, A.; Panagiotis, T.; Cathomas, G.; Salerni, B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut 2006, 55, 41–46. [Google Scholar] [CrossRef]
- Xu, S.U.; Zhai, J.; Xu, K.E.; Zuo, X.; Wu, C.; Lin, T.; Zeng, L.I. M1 macrophages-derived exosomes miR-34c-5p regulates interstitial cells of Cajal through targeting SCF. J. Biosci. 2021, 46, 90. [Google Scholar] [CrossRef]
- Hayashi, Y.; Asuzu, D.T.; Bardsley, M.R.; Gajdos, G.B.; Kvasha, S.M.; Linden, D.R.; Nagy, R.A.; Saravanaperumal, S.A.; Syed, S.A.; Toyomasu, Y.; et al. Wnt-induced, TRP53-mediated Cell Cycle Arrest of Precursors Underlies Interstitial Cell of Cajal Depletion During Aging. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 117–145. [Google Scholar] [CrossRef]
- Di Nardo, G.; Di Lorenzo, C.; Lauro, A.; Stanghellini, V.; Thapar, N.; Karunaratne, T.B.; Volta, U.; De Giorgio, R. Chronic intestinal pseudo-obstruction in children and adults: Diagnosis and therapeutic options. Neurogastroenterol. Motil. 2017, 29, e12945. [Google Scholar] [CrossRef]
- Di Nardo, G.; Karunaratne, T.B.; Frediani, S.; De Giorgio, R. Chronic intestinal pseudo-obstruction: Progress in management? Neurogastroenterol. Motil. 2017, 29, e13231. [Google Scholar] [CrossRef]
- Zenzeri, L.; Tambucci, R.; Quitadamo, P.; Giorgio, V.; De Giorgio, R.; Di Nardo, G. Update on chronic intestinal pseudo-obstruction. Curr. Opin. Gastroenterol. 2020, 36, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Tchikviladzé, M.; Joly, F.; Slama, A.; Hatem, D.C.; Jardel, C.; Messing, B.; Lombès, A. Frequency of mitochondrial defects in patients with chronic intestinal pseudo-obstruction. Gastroenterology 2009, 137, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Moussa, K.; Tandon, M.; Culpepper-Morgan, J.; Proctor, D.D. Role of interstitial cells of Cajal in motility disorders of the bowel. Am. J. Gastroenterol. 2003, 98, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Porcher, C.; Baldo, M.; Henry, M.; Orsoni, P.; Julé, Y.; Ward, S.M. Deficiency of interstitial cells of Cajal in the small intestine of patients with Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 118–125. [Google Scholar] [CrossRef] [PubMed]
- He, C.L.; Soffer, E.E.; Ferris, C.D.; Walsh, R.M.; Szurszewski, J.H.; Farrugia, G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology 2001, 121, 427–434. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Miller, S.M.; El-Youssef, M.; Rodeberg, D.; Lindor, N.M.; Burgart, L.J.; Szurszewski, J.H.; Farrugia, G. Chronic intestinal pseudoobstruction associated with altered interstitial cells of cajal networks. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 492–497. [Google Scholar] [CrossRef]
- Baker, S.A.; Hwang, S.J.; Blair, P.J.; Sireika, C.; Wei, L.; Ro, S.; Ward, S.M.; Sanders, K.M. Ca2+ transients in ICC-MY define the basis for the dominance of the corpus in gastric pacemaking. Cell Calcium 2021, 99, 102472. [Google Scholar] [CrossRef] [PubMed]
- Hennig, G.W.; Spencer, N.J.; Jokela-Willis, S.; Bayguinov, P.O.; Lee, H.T.; Ritchie, L.A.; Ward, S.M.; Smith, T.K.; Sanders, K.M. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol. Motil. 2010, 22, e138–e151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iino, S.; Horiguchi, K.; Nojyo, Y. W(sh)/W(sh) c-Kit mutant mice possess interstitial cells of Cajal in the deep muscular plexus layer of the small intestine. Neurosci. Lett. 2009, 459, 123–126. [Google Scholar] [CrossRef]
- Ward, S.M.; McLaren, G.J.; Sanders, K.M. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J. Physiol. 2006, 573, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Kenny, S.E.; Vanderwinden, J.M.; Rintala, R.J.; Connell, M.G.; Lloyd, D.A.; Vanderhaegen, J.J.; De Laet, M.H. Delayed maturation of the interstitial cells of Cajal: A new diagnosis for transient neonatal pseudoobstruction. Report of two cases. J. Pediatr. Surg. 1998, 33, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007, 117, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, N.; Segnani, C.; Ippolito, C.; De Giorgio, R.; Colucci, R.; Faussone-Pellegrini, M.S.; Chiarugi, M.; Campani, D.; Castagna, M.; Mattii, L.; et al. Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in ulcerative colitis. J. Cell. Mol. Med. 2012, 16, 318–327. [Google Scholar] [CrossRef]
- Rumessen, J.J.; Vanderwinden, J.M.; Horn, T. Ulcerative colitis: Ultrastructure of interstitial cells in myenteric plexus. Ultrastruct. Pathol. 2010, 34, 279–287. [Google Scholar] [CrossRef]
- Dave, M.; Hayashi, Y.; Gajdos, G.B.; Smyrk, T.C.; Svingen, P.A.; Kvasha, S.M.; Lorincz, A.; Dong, H.; Faubion, W.A., Jr.; Ordog, T. Stem cells for murine interstitial cells of cajal suppress cellular immunity and colitis via prostaglandin E2 secretion. Gastroenterology 2015, 148, 978–990. [Google Scholar] [CrossRef]
- Sreeramkumar, V.; Fresno, M.; Cuesta, N. Prostaglandin E2 and T cells: Friends or foes? Immunol. Cell Biol. 2012, 90, 579–586. [Google Scholar] [CrossRef]
- Ferreira-Duarte, M.; Rodrigues-Pinto, T.; Sousa, T.; Faria, M.A.; Rocha, M.S.; Menezes-Pinto, D.; Esteves-Monteiro, M.; Magro, F.; Dias-Pereira, P.; Duarte-Araújo, M.; et al. Interaction between the Renin-Angiotensin System and Enteric Neurotransmission Contributes to Colonic Dysmotility in the TNBS-Induced Model of Colitis. Int. J. Mol. Sci. 2021, 22, 4836. [Google Scholar] [CrossRef]
- Altdorfer, K.; Bagaméri, G.; Donáth, T.; Fehér, E. Nitric oxide synthase immunoreactivity of interstitial cells of Cajal in experimental colitis. Inflamm. Res. 2002, 51, 569–571. [Google Scholar] [CrossRef]
- Schneider, K.M.; Blank, N.; Alvarez, Y.; Thum, K.; Lundgren, P.; Litichevskiy, L.; Sleeman, M.; Bahnsen, K.; Kim, J.; Kardo, S.; et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell 2023, 186, 2823–2838. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.L.; Taheri, N.; Tan, E.; Matsumoto, K.; Hayashi, Y. The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases. Biomolecules 2023, 13, 1358. https://doi.org/10.3390/biom13091358
Choi EL, Taheri N, Tan E, Matsumoto K, Hayashi Y. The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases. Biomolecules. 2023; 13(9):1358. https://doi.org/10.3390/biom13091358
Chicago/Turabian StyleChoi, Egan L., Negar Taheri, Elijah Tan, Kenjiro Matsumoto, and Yujiro Hayashi. 2023. "The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases" Biomolecules 13, no. 9: 1358. https://doi.org/10.3390/biom13091358
APA StyleChoi, E. L., Taheri, N., Tan, E., Matsumoto, K., & Hayashi, Y. (2023). The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases. Biomolecules, 13(9), 1358. https://doi.org/10.3390/biom13091358




