The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases
Abstract
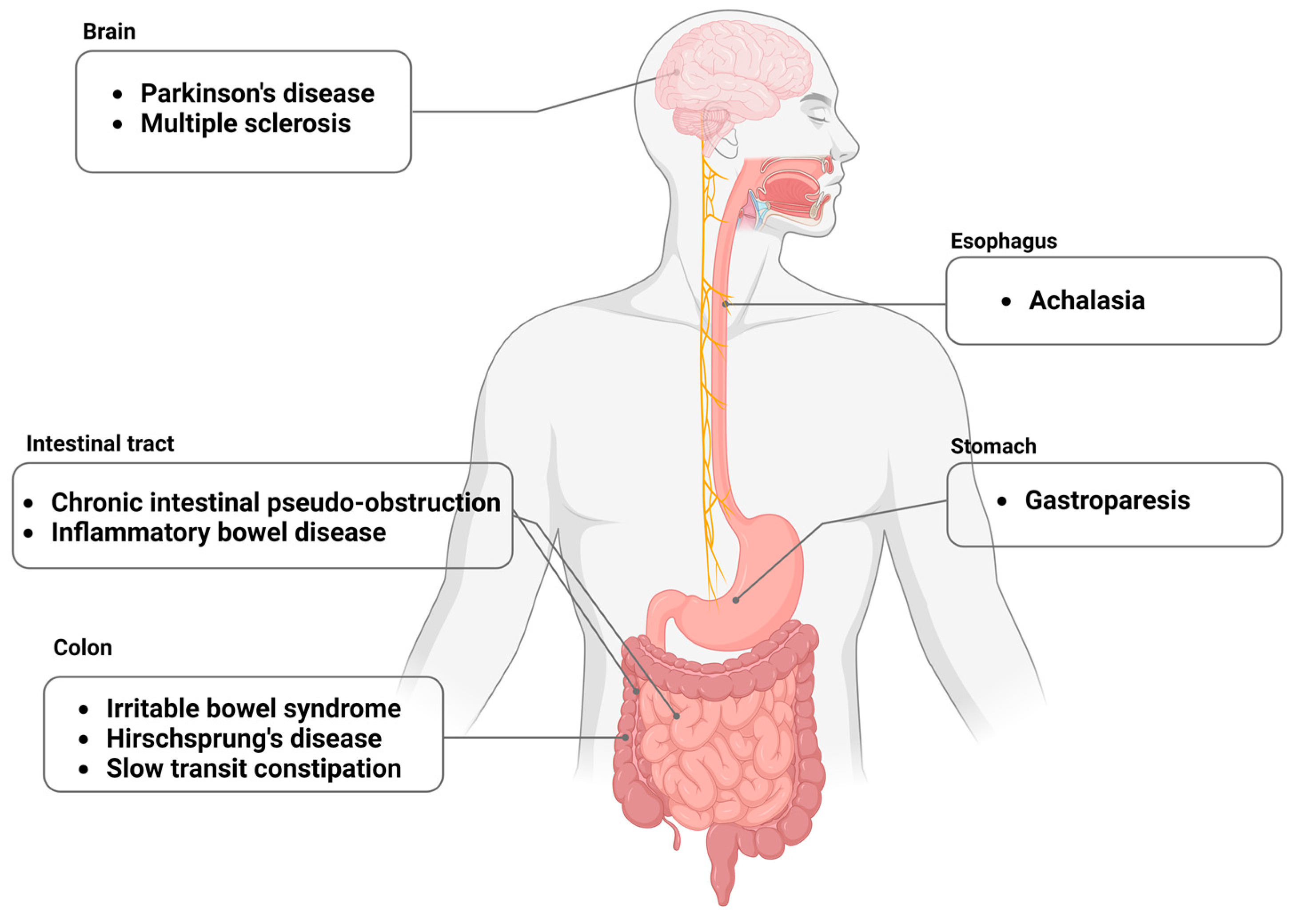
:1. Introduction
2. Aging
3. Gastroparesis
4. Irritable Bowel Syndrome
5. Esophageal Achalasia
6. Hirschsprung’s Disease
7. Parkinson’s Disease
8. Multiple Sclerosis
9. Slow-Transit Constipation
10. Chronic Intestinal Pseudo-Obstruction
11. Inflammatory Bowel Disease
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Goldstein, A.M.; Thapar, N.; Karunaratne, T.B.; De Giorgio, R. Clinical aspects of neurointestinal disease: Pathophysiology, diagnosis, and treatment. Dev. Biol. 2016, 417, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Di Lorenzo, C.; Farrugia, G.; Hamilton, F.A.; Mawe, G.M.; Pasricha, P.J.; Wiley, J.W. Functional Bowel Disorders: A Roadmap to Guide the Next Generation of Research. Gastroenterology 2018, 154, 723–735. [Google Scholar] [CrossRef]
- Gubatan, J.; Zikos, T.; Spear Bishop, E.; Wu, J.; Gottfried, A.; Becker, L.; Habtezion, A.; Neshatian, L. Gastrointestinal symptoms and healthcare utilization have increased among patients with functional gastrointestinal and motility disorders during the COVID-19 pandemic. Neurogastroenterol. Motil. 2022, 34, e14243. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Kito, Y.; Hwang, S.J.; Ward, S.M. Regulation of Gastrointestinal Smooth Muscle Function by Interstitial Cells. Physiology 2016, 31, 316–326. [Google Scholar] [CrossRef]
- Huizinga, J.D.; Hussain, A.; Chen, J.H. Interstitial cells of Cajal and human colon motility in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G552–G575. [Google Scholar] [CrossRef] [PubMed]
- López-Pingarrón, L.; Almeida, H.; Soria-Aznar, M.; Reyes-Gonzales, M.C.; Rodríguez-Moratinos, A.B.; Muñoz-Hoyos, A.; García, J.J. Interstitial Cells of Cajal and Enteric Nervous System in Gastrointestinal and Neurological Pathology, Relation to Oxidative Stress. Curr. Issues Mol. Biol. 2023, 45, 3552–3572. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ward, S.M. Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br. J. Pharmacol. 2019, 176, 212–227. [Google Scholar] [CrossRef]
- McCann, C.J.; Cooper, J.E.; Natarajan, D.; Jevans, B.; Burnett, L.E.; Burns, A.J.; Thapar, N. Transplantation of enteric nervous system stem cells rescues nitric oxide synthase deficient mouse colon. Nat. Commun. 2017, 8, 15937. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, P.J.; Grover, M.; Yates, K.P.; Abell, T.L.; Koch, K.L.; McCallum, R.W.; Sarosiek, I.; Bernard, C.E.; Kuo, B.; Bulat, R.; et al. Progress in Gastroparesis—A Narrative Review of the Work of the Gastroparesis Clinical Research Consortium. Clin. Gastroenterol. Hepatol. 2022, 20, 2684–2695. [Google Scholar] [CrossRef]
- Nguyen, V.T.T.; Taheri, N.; Chandra, A.; Hayashi, Y. Aging of enteric neuromuscular systems in gastrointestinal tract. Neurogastroenterol. Motil. 2022, 34, e14352. [Google Scholar] [CrossRef]
- Camilleri, M.; Cowen, T.; Koch, T.R. Enteric neurodegeneration in ageing. Neurogastroenterol. Motil. 2008, 20, 185–196. [Google Scholar] [CrossRef]
- Cox, N.J.; Ibrahim, K.; Sayer, A.A.; Robinson, S.M.; Roberts, H.C. Assessment and Treatment of the Anorexia of Aging: A Systematic Review. Nutrients 2019, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Chapman, I.; Luscombe-Marsh, N.; Feinle-Bisset, C.; Horowitz, M.; Soenen, S. Ageing Is Associated with Decreases in Appetite and Energy Intake—A Meta-Analysis in Healthy Adults. Nutrients 2016, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public. Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Gomez-Pinilla, P.J.; Gibbons, S.J.; Sarr, M.G.; Kendrick, M.L.; Shen, K.R.; Cima, R.R.; Dozois, E.J.; Larson, D.W.; Ordog, T.; Pozo, M.J.; et al. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol. Motil. 2011, 23, 36–44. [Google Scholar] [CrossRef]
- Truong Thuy Nguyen, V.; Taheri, N.; Choi, E.L.; Kellogg, T.A.; Linden, D.R.; Hayashi, Y. Insulin-Like Growth Factor1 Preserves Gastric Pacemaker Cells and Motor Function in Aging via ERK1/2 Activation. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 369–383. [Google Scholar] [CrossRef]
- Izbeki, F.; Asuzu, D.T.; Lorincz, A.; Bardsley, M.R.; Popko, L.N.; Choi, K.M.; Young, D.L.; Hayashi, Y.; Linden, D.R.; Kuro-o, M.; et al. Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice. J. Physiol. 2010, 588, 3101–3117. [Google Scholar] [CrossRef]
- Soenen, S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. The ageing gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 12–18. [Google Scholar] [CrossRef]
- Asuzu, D.T.; Hayashi, Y.; Izbeki, F.; Popko, L.N.; Young, D.L.; Bardsley, M.R.; Lorincz, A.; Kuro-O, M.; Linden, D.R.; Farrugia, G.; et al. Generalized neuromuscular hypoplasia, reduced smooth muscle myosin and altered gut motility in the klotho model of premature aging. Neurogastroenterol. Motil. 2011, 23, e309–e323. [Google Scholar] [CrossRef]
- Broad, J.; Kung, V.W.S.; Palmer, A.; Elahi, S.; Karami, A.; Darreh-Shori, T.; Ahmed, S.; Thaha, M.A.; Carroll, R.; Chin-Aleong, J.; et al. Changes in neuromuscular structure and functions of human colon during ageing are region-dependent. Gut 2019, 68, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Sanders, K.M. Gastroparesis. Gastroenterology 2022, 162, 68–87.e1. [Google Scholar] [CrossRef] [PubMed]
- Dilmaghani, S.; Zheng, T.; Camilleri, M. Epidemiology and Healthcare Utilization in Patients With Gastroparesis: A Systematic Review. Clin. Gastroenterol. Hepatol. 2023, 21, 2239–2251.e2. [Google Scholar] [CrossRef] [PubMed]
- Ordög, T.; Takayama, I.; Cheung, W.K.; Ward, S.M.; Sanders, K.M. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes 2000, 49, 1731–1739. [Google Scholar] [CrossRef]
- Grover, M.; Farrugia, G.; Lurken, M.S.; Bernard, C.E.; Faussone-Pellegrini, M.S.; Smyrk, T.C.; Parkman, H.P.; Abell, T.L.; Snape, W.J.; Hasler, W.L.; et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 2011, 140, 1575–1585.e8. [Google Scholar] [CrossRef]
- Horváth, V.J.; Vittal, H.; Ordög, T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes 2005, 54, 1528–1533. [Google Scholar] [CrossRef]
- Horváth, V.J.; Vittal, H.; Lörincz, A.; Chen, H.; Almeida-Porada, G.; Redelman, D.; Ordög, T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology 2006, 130, 759–770. [Google Scholar] [CrossRef]
- Lorincz, A.; Redelman, D.; Horváth, V.J.; Bardsley, M.R.; Chen, H.; Ordög, T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology 2008, 134, 1083–1093. [Google Scholar] [CrossRef]
- Hayashi, Y.; Asuzu, D.T.; Gibbons, S.J.; Aarsvold, K.H.; Bardsley, M.R.; Lomberk, G.A.; Mathison, A.J.; Kendrick, M.L.; Shen, K.R.; Taguchi, T.; et al. Membrane-to-nucleus signaling links insulin-like growth factor-1- and stem cell factor-activated pathways. PLoS ONE 2013, 8, e76822. [Google Scholar] [CrossRef]
- Hayashi, Y.; Toyomasu, Y.; Saravanaperumal, S.A.; Bardsley, M.R.; Smestad, J.A.; Lorincz, A.; Eisenman, S.T.; Cipriani, G.; Nelson Holte, M.H.; Al Khazal, F.J.; et al. Hyperglycemia Increases Interstitial Cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, Leading to Rapid Gastric Emptying. Gastroenterology 2017, 153, 521–535.e20. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Kudva, Y.; Basu, A.; Camilleri, M.; Low, P.A.; Vella, A.; Zinsmeister, A.R. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin. Gastroenterol. Hepatol. 2015, 13, 466–476.e1. [Google Scholar] [CrossRef]
- Singh, R.; Ha, S.E.; Wei, L.; Jin, B.; Zogg, H.; Poudrier, S.M.; Jorgensen, B.G.; Park, C.; Ronkon, C.F.; Bartlett, A.; et al. miR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility. Gastroenterology 2021, 160, 1662–1678.e18. [Google Scholar] [CrossRef]
- Cipriani, G.; Gibbons, S.J.; Kashyap, P.C.; Farrugia, G. Intrinsic Gastrointestinal Macrophages: Their Phenotype and Role in Gastrointestinal Motility. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 120–130.e121. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Gibbons, S.J.; Nguyen, T.V.; Stoltz, G.J.; Lurken, M.S.; Ordog, T.; Szurszewski, J.H.; Farrugia, G. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 2008, 135, 2055–2064.e2. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Kashyap, P.C.; Dutta, N.; Stoltz, G.J.; Ordog, T.; Shea Donohue, T.; Bauer, A.J.; Linden, D.R.; Szurszewski, J.H.; Gibbons, S.J.; et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology 2010, 138, 2399–2409.e1. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Irritable bowel syndrome: Treatment based on pathophysiology and biomarkers. Gut 2023, 72, 590–599. [Google Scholar] [CrossRef]
- Camilleri, M. Management Options for Irritable Bowel Syndrome. Mayo Clin. Proc. 2018, 93, 1858–1872. [Google Scholar] [CrossRef]
- Camilleri, M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA 2021, 325, 865–877. [Google Scholar] [CrossRef]
- Eijsbouts, C.; Zheng, T.; Kennedy, N.A.; Bonfiglio, F.; Anderson, C.A.; Moutsianas, L.; Holliday, J.; Shi, J.; Shringarpure, S.; Voda, A.I.; et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat. Genet. 2021, 53, 1543–1552. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Hillestad, E.M.R.; van der Meeren, A.; Nagaraja, B.H.; Bjørsvik, B.R.; Haleem, N.; Benitez-Paez, A.; Sanz, Y.; Hausken, T.; Lied, G.A.; Lundervold, A.; et al. Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J. Gastroenterol. 2022, 28, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, G.; Shi, Q.; Yang, S.; Ma, T.; Mishra, S.K.; Wen, A.; Xu, H.; Wang, Q.; Jiang, Y.; et al. Characterizing the microbiota in gastrointestinal tract segments of Rhabdophis subminiatus: Dynamic changes and functional predictions. Microbiologyopen 2019, 8, e00789. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Donato, S.; Di Pardo, A.; Maglione, V.; Filosa, S.; Crispi, S. New Therapeutic Drugs from Bioactive Natural Molecules: The Role of Gut Microbiota Metabolism in Neurodegenerative Diseases. Curr. Drug Metab. 2018, 19, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.M.; Kearney, S.M.; Smillie, C.S.; Alm, E.J. Two dynamic regimes in the human gut microbiome. PLoS Comput. Biol. 2017, 13, e1005364. [Google Scholar] [CrossRef] [PubMed]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Ther. Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Perez Ingles, D.; Myneedu, K.; Deoker, A.; Sarosiek, I.; Zuckerman, M.J.; Schmulson, M.J.; Bashashati, M. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2019, 31, e13718. [Google Scholar] [CrossRef]
- Krammer, L.; Sowa, A.S.; Lorentz, A. Mast Cells in Irritable Bowel Syndrome: A Systematic Review. J. Gastrointestin. Liver Dis. 2019, 28, 463–472. [Google Scholar] [CrossRef]
- Borriello, F.; Iannone, R.; Marone, G. Histamine Release from Mast Cells and Basophils. Handb. Exp. Pharmacol. 2017, 241, 121–139. [Google Scholar] [CrossRef]
- Shulman, R.J.; Jarrett, M.E.; Cain, K.C.; Broussard, E.K.; Heitkemper, M.M. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J. Gastroenterol. 2014, 49, 1467–1476. [Google Scholar] [CrossRef]
- Duraisamy, K.; Premkumar, K.; Selvakumar, G.P.; Thangavel, R.; Ahmed, M.E.; Zaheer, S.A.; Raikwar, S.P.; Dubova, L.; Iyer, S.S.; Zaheer, A. Mast Cells Augment Neuroinflammation and Neurodegeneration. FASEB J. 2019, 33, 791–795. [Google Scholar] [CrossRef]
- Liu, B.; Sjölander, A.; Pedersen, N.L.; Ludvigsson, J.F.; Chen, H.; Fang, F.; Wirdefeldt, K. Irritable bowel syndrome and Parkinson’s disease risk: Register-based studies. NPJ Park. Dis. 2021, 7, 5. [Google Scholar] [CrossRef]
- Eshraghian, A.; Eshraghian, H. Interstitial cells of Cajal: A novel hypothesis for the pathophysiology of irritable bowel syndrome. Can. J. Gastroenterol. 2011, 25, 277–279. [Google Scholar] [CrossRef]
- Wouters, M.M.; Gibbons, S.J.; Roeder, J.L.; Distad, M.; Ou, Y.; Strege, P.R.; Szurszewski, J.H.; Farrugia, G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology 2007, 133, 897–906. [Google Scholar] [CrossRef]
- Atkinson, W.; Lockhart, S.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 2006, 130, 34–43. [Google Scholar] [CrossRef]
- Liu, H.N.; Ohya, S.; Nishizawa, Y.; Sawamura, K.; Iino, S.; Syed, M.M.; Goto, K.; Imaizumi, Y.; Nakayama, S. Serotonin augments gut pacemaker activity via 5-HT3 receptors. PLoS ONE 2011, 6, e24928. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Sultan, S.; Chang, L.; Heidelbaugh, J.J.; Smalley, W.; Verne, G.N. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Diarrhea. Gastroenterology 2022, 163, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Sultan, S.; Lembo, A.; Verne, G.N.; Smalley, W.; Heidelbaugh, J.J. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology 2022, 163, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xiong, T.; Grabauskas, G.; Owyang, C. Mucosal Serotonin Reuptake Transporter Expression in Irritable Bowel Syndrome Is Modulated by Gut Microbiota Via Mast Cell-Prostaglandin E2. Gastroenterology 2022, 162, 1962–1974.e6. [Google Scholar] [CrossRef] [PubMed]
- Matheis, F.; Muller, P.A.; Graves, C.L.; Gabanyi, I.; Kerner, Z.J.; Costa-Borges, D.; Ahrends, T.; Rosenstiel, P.; Mucida, D. Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss. Cell 2020, 180, 64–78.e16. [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D.; Bashashati, M.; Hirota, S.A.; Gui, X.; Roberts, J.A.; MacDonald, J.A.; Muruve, D.A.; McKay, D.M.; Beck, P.L.; Mawe, G.M.; et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 2012, 18, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Der, T.; Bercik, P.; Donnelly, G.; Jackson, T.; Berezin, I.; Collins, S.M.; Huizinga, J.D. Interstitial cells of cajal and inflammation-induced motor dysfunction in the mouse small intestine. Gastroenterology 2000, 119, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J.; Boeckxstaens, G. The spectrum of achalasia: Lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology 2013, 145, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Trieu, J.A.; Dua, A.; Enofe, I.; Shastri, N.; Venu, M. Population trends in achalasia diagnosis and management: A changing paradigm. Dis. Esophagus 2021, 34, doab014. [Google Scholar] [CrossRef]
- Gregersen, H.; Pedersen, J.; Drewes, A.M. Deterioration of muscle function in the human esophagus with age. Dig. Dis. Sci. 2008, 53, 3065–3070. [Google Scholar] [CrossRef]
- Wu, M.; Van Nassauw, L.; Kroese, A.B.; Adriaensen, D.; Timmermans, J.P. Myenteric nitrergic neurons along the rat esophagus: Evidence for regional and strain differences in age-related changes. Histochem. Cell Biol. 2003, 119, 395–403. [Google Scholar] [CrossRef]
- Qualman, S.J.; Haupt, H.M.; Yang, P.; Hamilton, S.R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology 1984, 87, 848–856. [Google Scholar] [CrossRef]
- Zizer, E.; Beilke, S.; Bäuerle, T.; Schilling, K.; Möhnle, U.; Adler, G.; Fischer, K.D.; Wagner, M. Loss of Lsc/p115 protein leads to neuronal hypoplasia in the esophagus and an achalasia-like phenotype in mice. Gastroenterology 2010, 139, 1344–1354. [Google Scholar] [CrossRef]
- Savarino, E.; Gemignani, L.; Zentilin, P.; De Bortoli, N.; Malesci, A.; Mastracci, L.; Fiocca, R.; Savarino, V. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin. Gastroenterol. Hepatol. 2011, 9, 1104–1106. [Google Scholar] [CrossRef]
- Boeckxstaens, G.E. Achalasia: Virus-induced euthanasia of neurons? Am. J. Gastroenterol. 2008, 103, 1610–1612. [Google Scholar] [CrossRef] [PubMed]
- Gockel, I.; Becker, J.; Wouters, M.M.; Niebisch, S.; Gockel, H.R.; Hess, T.; Ramonet, D.; Zimmermann, J.; Vigo, A.G.; Trynka, G.; et al. Common variants in the HLA-DQ region confer susceptibility to idiopathic achalasia. Nat. Genet. 2014, 46, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Salter, A.K.; Hennig, G.W.; Koh, S.D.; Perrino, B.A.; Ward, S.M.; Baker, S.A. Responses to enteric motor neurons in the gastric fundus of mice with reduced intramuscular interstitial cells of cajal. J. Neurogastroenterol. Motil. 2014, 20, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Drumm, B.T.; Kim, M.K.; Lyu, J.H.; Baker, S.; Sanders, K.M.; Ward, S.M. Calcium transients in intramuscular interstitial cells of Cajal of the murine gastric fundus and their regulation by neuroeffector transmission. J. Physiol. 2022, 600, 4439–4463. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sanders, K.M.; Ward, S.M. Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res. 2000, 302, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.J.; Bayguinov, Y.; Sanders, K.M.; Ward, S.M. Relationship between enteric neurons and interstitial cells in the primate gastrointestinal tract. Neurogastroenterol. Motil. 2012, 24, e437–e449. [Google Scholar] [CrossRef]
- Horiguchi, K.; Sanders, K.M.; Ward, S.M. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tissue Res. 2003, 311, 299–313. [Google Scholar] [CrossRef]
- Iino, S.; Ward, S.M.; Sanders, K.M. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J. Physiol. 2004, 556, 521–530. [Google Scholar] [CrossRef]
- Sivarao, D.V.; Mashimo, H.L.; Thatte, H.S.; Goyal, R.K. Lower esophageal sphincter is achalasic in nNOS(-/-) and hypotensive in W/W(v) mutant mice. Gastroenterology 2001, 121, 34–42. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Chen, W.F.; Wang, Y.; Xu, X.Y.; Zeng, Y.G.; Lee Dillon, D.; Cheng, J.; Xu, M.D.; Zhong, Y.S.; Zhang, Y.Q.; et al. Mast cell infiltration associated with loss of interstitial cells of Cajal and neuronal degeneration in achalasia. Neurogastroenterol. Motil. 2019, 31, e13565. [Google Scholar] [CrossRef]
- Qian, H.; Wang, Y.; Chen, X.; Lin, L.; Zhang, W.; Tang, N.; Si, X.; Jiao, C.; Zhang, G.; Ye, B. “M1/M2” Muscularis Macrophages Are Associated with Reduction of Interstitial Cells of Cajal and Glial Cells in Achalasia. Dig. Dis. Sci. 2023, 68, 1260–1268. [Google Scholar] [CrossRef]
- Ambartsumyan, L.; Smith, C.; Kapur, R.P. Diagnosis of Hirschsprung Disease. Pediatr. Dev. Pathol. 2020, 23, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.C. Hirschsprung disease. Curr. Opin. Pediatr. 2013, 25, 368–374. [Google Scholar] [CrossRef]
- Ahmad, H.; Levitt, M.A.; Yacob, D.; Halleran, D.R.; Gasior, A.C.; Di Lorenzo, C.; Wood, R.J.; Langer, J.C. Evaluation and Management of Persistent Problems After Surgery for Hirschsprung Disease in a Child. Curr. Gastroenterol. Rep. 2021, 23, 18. [Google Scholar] [CrossRef]
- Tilghman, J.M.; Ling, A.Y.; Turner, T.N.; Sosa, M.X.; Krumm, N.; Chatterjee, S.; Kapoor, A.; Coe, B.P.; Nguyen, K.H.; Gupta, N.; et al. Molecular Genetic Anatomy and Risk Profile of Hirschsprung’s Disease. N. Engl. J. Med. 2019, 380, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; de Graaff, E.; Pachnis, V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron 2003, 40, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Chen, F.; Milewski, R.; Li, J.; Lu, M.M.; Epstein, J.A. Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret. J. Clin. Investig. 2000, 106, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.; Anderson, D.J. Postmigratory neural crest cells expressing c-RET display restricted developmental and proliferative capacities. Neuron 1995, 15, 527–539. [Google Scholar] [CrossRef]
- Gosain, A.; Brinkman, A.S. Hirschsprung’s associated enterocolitis. Curr. Opin. Pediatr. 2015, 27, 364–369. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Coles, M.C.; Foster, K.E.; Patel, A.; Williams, A.; Natarajan, D.; Barlow, A.; Pachnis, V.; Kioussis, D. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature 2007, 446, 547–551. [Google Scholar] [CrossRef]
- Banks, T.A.; Rouse, B.T.; Kerley, M.K.; Blair, P.J.; Godfrey, V.L.; Kuklin, N.A.; Bouley, D.M.; Thomas, J.; Kanangat, S.; Mucenski, M.L. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 1995, 155, 1685–1693. [Google Scholar] [CrossRef]
- Davis, I.A.; Rouse, B.T. Immune responsiveness of lymphotoxin-alpha-deficient mice: Two reconstitution models. Cell Immunol. 1998, 189, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kumaraguru, U.; Davis, I.A.; Deshpande, S.; Tevethia, S.S.; Rouse, B.T. Lymphotoxin alpha-/- mice develop functionally impaired CD8+ T cell responses and fail to contain virus infection of the central nervous system. J. Immunol. 2001, 166, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Druckenbrod, N.R.; Epstein, M.L. Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development 2009, 136, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; Barlow-Anacker, A.J.; Erickson, C.S.; Pierre, J.F.; Heneghan, A.F.; Epstein, M.L.; Kudsk, K.A. Impaired Cellular Immunity in the Murine Neural Crest Conditional Deletion of Endothelin Receptor-B Model of Hirschsprung’s Disease. PLoS ONE 2015, 10, e0128822. [Google Scholar] [CrossRef]
- Chen, X.; Meng, X.; Zhang, H.; Feng, C.; Wang, B.; Li, N.; Abdullahi, K.M.; Wu, X.; Yang, J.; Li, Z.; et al. Intestinal proinflammatory macrophages induce a phenotypic switch in interstitial cells of Cajal. J. Clin. Investig. 2020, 130, 6443–6456. [Google Scholar] [CrossRef]
- Coyle, D.; Kelly, D.A.; O’Donnell, A.M.; Gillick, J.; Puri, P. Use of anoctamin 1 (ANO1) to evaluate interstitial cells of Cajal in Hirschsprung’s disease. Pediatr. Surg. Int. 2016, 32, 125–133. [Google Scholar] [CrossRef]
- Gu, A.; Wu, Z.; Wang, P.; Liu, J.; Wang, J.; Wang, Q.; Chen, J. Downregulation of ICCs and PDGFRα+ cells on colonic dysmotility in hirschsprung disease. Front. Pediatr. 2022, 10, 975799. [Google Scholar] [CrossRef]
- Newman, C.J.; Laurini, R.N.; Lesbros, Y.; Reinberg, O.; Meyrat, B.J. Interstitial cells of Cajal are normally distributed in both ganglionated and aganglionic bowel in Hirschsprung’s disease. Pediatr. Surg. Int. 2003, 19, 662–668. [Google Scholar] [CrossRef]
- Bettolli, M.; De Carli, C.; Jolin-Dahel, K.; Bailey, K.; Khan, H.F.; Sweeney, B.; Krantis, A.; Staines, W.A.; Rubin, S. Colonic dysmotility in postsurgical patients with Hirschsprung’s disease. Potential significance of abnormalities in the interstitial cells of Cajal and the enteric nervous system. J. Pediatr. Surg. 2008, 43, 1433–1438. [Google Scholar] [CrossRef]
- Daniel, E.E.; Posey-Daniel, V. Neuromuscular structures in opossum esophagus: Role of interstitial cells of Cajal. Am. J. Physiol. 1984, 246, G305–G315. [Google Scholar] [CrossRef]
- Nemeth, L.; Maddur, S.; Puri, P. Immunolocalization of the gap junction protein Connexin43 in the interstitial cells of Cajal in the normal and Hirschsprung’s disease bowel. J. Pediatr. Surg. 2000, 35, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Coyle, D.; Doyle, B.; Murphy, J.M.; O’Donnell, A.M.; Gillick, J.; Puri, P. Expression of connexin 26 and connexin 43 is reduced in Hirschsprung’s disease. J. Surg. Res. 2016, 206, 242–251. [Google Scholar] [CrossRef]
- Totland, M.Z.; Rasmussen, N.L.; Knudsen, L.M.; Leithe, E. Regulation of gap junction intercellular communication by connexin ubiquitination: Physiological and pathophysiological implications. Cell. Mol. Life Sci. 2020, 77, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.M.; Yu, S.Y.; Guo, P.; Du, Y.; Hu, Y.; Piao, Y.S.; Zuo, L.J.; Lian, T.H.; Wang, R.D.; Yu, Q.J.; et al. Nonmotor symptoms in patients with Parkinson disease: A cross-sectional observational study. Medicine 2016, 95, e5400. [Google Scholar] [CrossRef]
- Breckenridge, C.B.; Berry, C.; Chang, E.T.; Sielken, R.L.; Mandel, J.S. Association between Parkinson’s Disease and Cigarette Smoking, Rural Living, Well-Water Consumption, Farming and Pesticide Use: Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151841. [Google Scholar] [CrossRef]
- Lee, A.; Gilbert, R.M. Epidemiology of Parkinson Disease. Neurol. Clin. 2016, 34, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Hilton, D.; Stephens, M.; Kirk, L.; Edwards, P.; Potter, R.; Zajicek, J.; Broughton, E.; Hagan, H.; Carroll, C. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014, 127, 235–241. [Google Scholar] [CrossRef]
- Araki, N.; Yamanaka, Y.; Poudel, A.; Fujinuma, Y.; Katagiri, A.; Kuwabara, S.; Asahina, M. Electrogastrography for diagnosis of early-stage Parkinson’s disease. Park. Relat. Disord. 2021, 86, 61–66. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Banach, T.; Zurowski, D.; Gil, K.; Krygowska-Wajs, A.; Marszałek, A.; Thor, P.J. Peripheral mechanisms of intestinal dysmotility in rats with salsolinol induced experimental Parkinson’s disease. J. Physiol. Pharmacol. 2006, 57, 291–300. [Google Scholar] [PubMed]
- Hou, M.; Mao, X.; Liu, Y.; Hou, X.; Bi, X. Could Abnormal Distribution of Interstitial Cells of Cajal be Involved in Gastrointestinal Disorders in Patients with Parkinson’s Disease? Arch. Neurol. Neurosci. 2018, 1. [Google Scholar] [CrossRef]
- Adler, C.H.; Beach, T.G. Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov. Disord. 2016, 31, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Heimrich, K.G.; Jacob, V.Y.P.; Schaller, D.; Stallmach, A.; Witte, O.W.; Prell, T. Gastric dysmotility in Parkinson’s disease is not caused by alterations of the gastric pacemaker cells. NPJ Park. Dis. 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Kenna, J.E.; Bakeberg, M.C.; Gorecki, A.M.; Chin Yen Tay, A.; Winter, S.; Mastaglia, F.L.; Anderton, R.S. Characterization of Gastrointestinal Symptom Type and Severity in Parkinson’s Disease: A Case-Control Study in an Australian Cohort. Mov. Disord. Clin. Pract. 2021, 8, 245–253. [Google Scholar] [CrossRef]
- Padhi, P.; Worth, C.; Zenitsky, G.; Jin, H.; Sambamurti, K.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Mechanistic Insights Into Gut Microbiome Dysbiosis-Mediated Neuroimmune Dysregulation and Protein Misfolding and Clearance in the Pathogenesis of Chronic Neurodegenerative Disorders. Front. Neurosci. 2022, 16, 836605. [Google Scholar] [CrossRef]
- The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019, 93, 688. [CrossRef]
- Miller, D.H.; Weinshenker, B.G.; Filippi, M.; Banwell, B.L.; Cohen, J.A.; Freedman, M.S.; Galetta, S.L.; Hutchinson, M.; Johnson, R.T.; Kappos, L.; et al. Differential diagnosis of suspected multiple sclerosis: A consensus approach. Mult. Scler. 2008, 14, 1157–1174. [Google Scholar] [CrossRef]
- Levinthal, D.J.; Rahman, A.; Nusrat, S.; O’Leary, M.; Heyman, R.; Bielefeldt, K. Adding to the burden: Gastrointestinal symptoms and syndromes in multiple sclerosis. Mult. Scler. Int. 2013, 2013, 319201. [Google Scholar] [CrossRef]
- Wunsch, M.; Jabari, S.; Voussen, B.; Enders, M.; Srinivasan, S.; Cossais, F.; Wedel, T.; Boettner, M.; Schwarz, A.; Weyer, L.; et al. The enteric nervous system is a potential autoimmune target in multiple sclerosis. Acta Neuropathol. 2017, 134, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Ding, Y.; Xue, R.; Jia, Z.; Huang, Z.; Ding, Y.; Gu, C.; Yang, J. Involvement of interstitial cells of Cajal in bladder dysfunction in mice with experimental autoimmune encephalomyelitis. Int. Urol. Nephrol. 2017, 49, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- den Braber-Ymker, M.; Heijker, S.; Lammens, M.; Croockewit, S.; Nagtegaal, I.D. Intestinal involvement in amyloidosis is a sequential process. Neurogastroenterol. Motil. 2018, 30, e13469. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Lacy, B.E. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020, 158, 1232–1249.e1233. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, K.; Busciglio, I.; Burton, D.; Breen-Lyles, M.K.; Camilleri, M. Expanding criteria for slow colonic transit in patients being evaluated for chronic constipation by scintigraphy. Neurogastroenterol. Motil. 2020, 32, e13878. [Google Scholar] [CrossRef]
- He, C.L.; Burgart, L.; Wang, L.; Pemberton, J.; Young-Fadok, T.; Szurszewski, J.; Farrugia, G. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology 2000, 118, 14–21. [Google Scholar] [CrossRef]
- Bassotti, G.; Villanacci, V.; Maurer, C.A.; Fisogni, S.; Di Fabio, F.; Cadei, M.; Morelli, A.; Panagiotis, T.; Cathomas, G.; Salerni, B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut 2006, 55, 41–46. [Google Scholar] [CrossRef]
- Xu, S.U.; Zhai, J.; Xu, K.E.; Zuo, X.; Wu, C.; Lin, T.; Zeng, L.I. M1 macrophages-derived exosomes miR-34c-5p regulates interstitial cells of Cajal through targeting SCF. J. Biosci. 2021, 46, 90. [Google Scholar] [CrossRef]
- Hayashi, Y.; Asuzu, D.T.; Bardsley, M.R.; Gajdos, G.B.; Kvasha, S.M.; Linden, D.R.; Nagy, R.A.; Saravanaperumal, S.A.; Syed, S.A.; Toyomasu, Y.; et al. Wnt-induced, TRP53-mediated Cell Cycle Arrest of Precursors Underlies Interstitial Cell of Cajal Depletion During Aging. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 117–145. [Google Scholar] [CrossRef]
- Di Nardo, G.; Di Lorenzo, C.; Lauro, A.; Stanghellini, V.; Thapar, N.; Karunaratne, T.B.; Volta, U.; De Giorgio, R. Chronic intestinal pseudo-obstruction in children and adults: Diagnosis and therapeutic options. Neurogastroenterol. Motil. 2017, 29, e12945. [Google Scholar] [CrossRef]
- Di Nardo, G.; Karunaratne, T.B.; Frediani, S.; De Giorgio, R. Chronic intestinal pseudo-obstruction: Progress in management? Neurogastroenterol. Motil. 2017, 29, e13231. [Google Scholar] [CrossRef]
- Zenzeri, L.; Tambucci, R.; Quitadamo, P.; Giorgio, V.; De Giorgio, R.; Di Nardo, G. Update on chronic intestinal pseudo-obstruction. Curr. Opin. Gastroenterol. 2020, 36, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Tchikviladzé, M.; Joly, F.; Slama, A.; Hatem, D.C.; Jardel, C.; Messing, B.; Lombès, A. Frequency of mitochondrial defects in patients with chronic intestinal pseudo-obstruction. Gastroenterology 2009, 137, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Moussa, K.; Tandon, M.; Culpepper-Morgan, J.; Proctor, D.D. Role of interstitial cells of Cajal in motility disorders of the bowel. Am. J. Gastroenterol. 2003, 98, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Porcher, C.; Baldo, M.; Henry, M.; Orsoni, P.; Julé, Y.; Ward, S.M. Deficiency of interstitial cells of Cajal in the small intestine of patients with Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 118–125. [Google Scholar] [CrossRef] [PubMed]
- He, C.L.; Soffer, E.E.; Ferris, C.D.; Walsh, R.M.; Szurszewski, J.H.; Farrugia, G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology 2001, 121, 427–434. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Miller, S.M.; El-Youssef, M.; Rodeberg, D.; Lindor, N.M.; Burgart, L.J.; Szurszewski, J.H.; Farrugia, G. Chronic intestinal pseudoobstruction associated with altered interstitial cells of cajal networks. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 492–497. [Google Scholar] [CrossRef]
- Baker, S.A.; Hwang, S.J.; Blair, P.J.; Sireika, C.; Wei, L.; Ro, S.; Ward, S.M.; Sanders, K.M. Ca2+ transients in ICC-MY define the basis for the dominance of the corpus in gastric pacemaking. Cell Calcium 2021, 99, 102472. [Google Scholar] [CrossRef] [PubMed]
- Hennig, G.W.; Spencer, N.J.; Jokela-Willis, S.; Bayguinov, P.O.; Lee, H.T.; Ritchie, L.A.; Ward, S.M.; Smith, T.K.; Sanders, K.M. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol. Motil. 2010, 22, e138–e151. [Google Scholar] [CrossRef] [PubMed]
- Iino, S.; Horiguchi, K.; Nojyo, Y. W(sh)/W(sh) c-Kit mutant mice possess interstitial cells of Cajal in the deep muscular plexus layer of the small intestine. Neurosci. Lett. 2009, 459, 123–126. [Google Scholar] [CrossRef]
- Ward, S.M.; McLaren, G.J.; Sanders, K.M. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J. Physiol. 2006, 573, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Kenny, S.E.; Vanderwinden, J.M.; Rintala, R.J.; Connell, M.G.; Lloyd, D.A.; Vanderhaegen, J.J.; De Laet, M.H. Delayed maturation of the interstitial cells of Cajal: A new diagnosis for transient neonatal pseudoobstruction. Report of two cases. J. Pediatr. Surg. 1998, 33, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007, 117, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, N.; Segnani, C.; Ippolito, C.; De Giorgio, R.; Colucci, R.; Faussone-Pellegrini, M.S.; Chiarugi, M.; Campani, D.; Castagna, M.; Mattii, L.; et al. Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in ulcerative colitis. J. Cell. Mol. Med. 2012, 16, 318–327. [Google Scholar] [CrossRef]
- Rumessen, J.J.; Vanderwinden, J.M.; Horn, T. Ulcerative colitis: Ultrastructure of interstitial cells in myenteric plexus. Ultrastruct. Pathol. 2010, 34, 279–287. [Google Scholar] [CrossRef]
- Dave, M.; Hayashi, Y.; Gajdos, G.B.; Smyrk, T.C.; Svingen, P.A.; Kvasha, S.M.; Lorincz, A.; Dong, H.; Faubion, W.A., Jr.; Ordog, T. Stem cells for murine interstitial cells of cajal suppress cellular immunity and colitis via prostaglandin E2 secretion. Gastroenterology 2015, 148, 978–990. [Google Scholar] [CrossRef]
- Sreeramkumar, V.; Fresno, M.; Cuesta, N. Prostaglandin E2 and T cells: Friends or foes? Immunol. Cell Biol. 2012, 90, 579–586. [Google Scholar] [CrossRef]
- Ferreira-Duarte, M.; Rodrigues-Pinto, T.; Sousa, T.; Faria, M.A.; Rocha, M.S.; Menezes-Pinto, D.; Esteves-Monteiro, M.; Magro, F.; Dias-Pereira, P.; Duarte-Araújo, M.; et al. Interaction between the Renin-Angiotensin System and Enteric Neurotransmission Contributes to Colonic Dysmotility in the TNBS-Induced Model of Colitis. Int. J. Mol. Sci. 2021, 22, 4836. [Google Scholar] [CrossRef]
- Altdorfer, K.; Bagaméri, G.; Donáth, T.; Fehér, E. Nitric oxide synthase immunoreactivity of interstitial cells of Cajal in experimental colitis. Inflamm. Res. 2002, 51, 569–571. [Google Scholar] [CrossRef]
- Schneider, K.M.; Blank, N.; Alvarez, Y.; Thum, K.; Lundgren, P.; Litichevskiy, L.; Sleeman, M.; Bahnsen, K.; Kim, J.; Kardo, S.; et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell 2023, 186, 2823–2838. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.L.; Taheri, N.; Tan, E.; Matsumoto, K.; Hayashi, Y. The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases. Biomolecules 2023, 13, 1358. https://doi.org/10.3390/biom13091358
Choi EL, Taheri N, Tan E, Matsumoto K, Hayashi Y. The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases. Biomolecules. 2023; 13(9):1358. https://doi.org/10.3390/biom13091358
Chicago/Turabian StyleChoi, Egan L., Negar Taheri, Elijah Tan, Kenjiro Matsumoto, and Yujiro Hayashi. 2023. "The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases" Biomolecules 13, no. 9: 1358. https://doi.org/10.3390/biom13091358
APA StyleChoi, E. L., Taheri, N., Tan, E., Matsumoto, K., & Hayashi, Y. (2023). The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases. Biomolecules, 13(9), 1358. https://doi.org/10.3390/biom13091358




